Abstract
Fifty percent of diabetics (7% of general population) suffer from peripheral arterial occlusive disease, which may lead to amputation due to critical limb ischemia (CLI). The aim of our study was to prevent major limb amputation (MLA) in this group of patients using a local application of autologous bone marrow stem cells (ABMSC) concentrate. A total of 96 patients with CLI and foot ulcer (FU) were randomized into groups I and II. Patients in group I (n = 42, 36 males, 6 females, 66.2 ± 10.6 years) underwent local treatment with ABMSC while those in group II (n = 54, control, 42 males, 12 females, 64.1 ± 8.6 years) received standard medical care. The frequency of major limb amputation in groups I and II was 21% and 44% within the 120 days of follow up, respectively (p < 0.05). Only in salvaged limbs of group I both toe pressure and toe brachial index increased (from 22.66 ± 5.32 to 25.63 ± 4.75 mmHg and from 0.14 ± 0.03 to 0.17 ± 0.03, respectively, mean ± SEM). The CD34+ cell counts in bone marrow concentrate (BMC) decreased (correlation, p = 0.024) with age, even though there was no correlation between age and healing. An unexpected finding was made of relative, bone marrow lymphopenia in the initial bone marrow concentrates in patients who failed ABMSC therapy (21% of MLA). This difference was statistically significant (p < 0.040). We conclude ABMSC therapy results in 79% limb salvage in patients suffering from CLI and FU. In the remaining 21% lymphopenia and thrombocytopenia were identified as potential causative factors, suggesting that at least a partial correction with platelet supplementation may be beneficial.
Keywords: Critical limb ischemia (CLI), Diabetic foot ulcer, Autologous bone marrow stem cells (ABMSC), Lymphopenia of bone marrow
INTRODUCTION
In diabetic patients, nonhealing cutaneous ulcers are a significant clinical, social, and healthcare problem. Based on more than 10 million diabetic patients in the US and an estimated prevalence of 15% for chronic cutaneous ulcers, there are approximately 1.5 million patients with this problem. Peripheral arterial occlusive disease (PAOD) has been recognized as a significant factor in this population. For example, in European Study Group on Diabetes and Lower Extremity (Eurodiale) 49% of patients presenting with new diabetic foot ulcer had PAOD. Critical limb ischemia (CLI) develops suddenly and causes 50–67% of all nontraumatic lower extremity amputations. Fifty-two percent of diabetics with CLI die during the 4.5 year follow up (35,36).
Standard treatment of chronic wounds, and especially those secondary to CLI, includes surgical revascularization (distal crural or pedal bypass), endovascular therapy (recanalization by percutaneous transluminal angioplasty), or maximum podiatric wound care (hyperbaric oxygen, antibiotics, vasodilators). Despite the available therapies, 25% of patients still progress to amputation. The outcomes are even worse in diabetics, with multicausal disease, where neuropathy, poor healing, and peripheral arterial occlusive disease occur simultaneously. Thirty percent of such patients require amputation, and 25% of them die (8, 15). The introduction of novel experimental therapies using recombinant growth factor therapy combined with tissue grafting has shown minimal benefit over standard treatment (23, 24).
Building on the existing experience with autologous stem cell progenitors and various platelet preparations, we present a potential explanation for the difficulties plaguing cellular therapies and introduce an improved modality for multicellular therapy (3,20,38,40). The pro cess of angiogenesis requires not only the initial induction of tissue degradation followed by vascular sprouting, but also the stabilization of early vascular sprouts by pericytes and remodeling and reconnection to the surrounding ischemic tissue to the periphery of new vessel wall. Thus, the process of vessel maturation and maintenance requires spatially and temporally controlled re lease of different pro- and antiangiogenenic growth factors in the local milieu of the wound. While the initial vascular sprout is dependent on a strong VEGF concentration gradient, as vessels mature, their dependence on VEGF decreases. The recruitment of pericytes and sub sequent stabilization of vascular wall and perivascular healing is dependent mainly on PDGF and angiopoietin 1 (5–7,10).
Wound healing and tissue remodeling may take up to 8–12 weeks depending on degree of the initial tissue ischemia and hypoxia. Here we show that this process can be accelerated by the autologous bone marrow stem cell injection, resulting in marked improvement of outcomes. The autologous nature of cell therapy described herein appears to be effective for reperfusion up to certain degree of ischemic tissues (30,44,46). Given that tissue healing and remodeling is associated with angiogenesis, and that angiogenesis requires an orchestrated interaction of different cells, we postulate that an injection of bone marrow concentrate enriched not only in progenitor cells, but also supportive cells may be required for efficacy. The concentrate supports all of the reparative processes as the multitude of supportive inflammatory cells, such as granulocytes, lymphocytes, and platelets, emulate the microenvironment of a healing wound.
MATERIALS AND METHODS
Study Design and Treatment Allocation
The study design and informed consent was approved by the Czech republic IRB-SUKL (sukls38464/2008-31.12.2008) and the University Hospital Ostrava Human Ethical Committee (1374b/2008 3.9.2008).
A total of 96 patients with CLI and foot ulcer were recruited over the period of 9 months (May 7, 2008 to February 10, 2009) and randomized into groups I or II. Patients in group I (n = 42, 36 males, 6 females, 66.2 ± 10.6 years) underwent local treatment with ABMSC while group II (n = 54, 42 males, 12 females, 64.1 ± 8.6 years) received a standard care and served as controls.
Patients and Follow-up
All patients were examined prior (at 0) and at 90 and 120 days following the therapeutic procedures (ABMSC therapy or standard treatment). The number of patients with diabetes, hyperlipidemia, and ischemic heart dis ease were significantly higher in group II than in group I (p = 0.040, p = 0.011, p = 0.044, respectively) (Table 1). Wound defects were assessed according to the University of Texas Wound Classification and equally distributed between treatment groups (Table 2).
Table 1.
Demographic Data of 96 Patients Included in the Study
| Risk Factor | Group I (ABMC) (n = 42) |
Group II (Standard Care) (n = 54) |
p-Value |
|---|---|---|---|
| Age (years) | 66.2 ± 10.6 | 64.1 ± 8.6 | 0.109 |
| Diabetes | 88.1% | 98.2% | 0.041 |
| Hypertension | 83.3% | 90.9% | 0.262 |
| Hyperlipidemia | 54.8% | 80.0% | 0.011 |
| Stroke | 21.4% | 12.7% | 0.253 |
| Ischemic heart disease | 57.1% | 76.4% | 0.044 |
| Carcinoma | 4.8% | 1.8% | 0.577 |
| Renal insufficiency | 40.5% | 40.0% | 0.962 |
| Smoking | 45.2% | 40.0% | 0.605 |
Number of patients with diabetes, hyperlipidemia, and ischemic heart disease, respectively, were significantly higher in group II than in group I (p = 0.040, p = 0.011, p = 0.044, respectively).
Table 2.
Wound Degree Classification in CLI Patients (n = 96) With Foot Ulcer by the University of Texas Wound Classification
| Stage | Grade 0 | Grade I | Grade II | Grade III |
|---|---|---|---|---|
| A | 1 | |||
| B | 1 | 7 | 5 | |
| C | 1 | 9 | 1 | 9 |
| D | 4 | 2 | 56 |
There is an important group of 64 patients (66.7%) with severe grade of foot ulcer and critical limb ischemia in grade III/C,D out of the total number of 96 patients.
Patient inclusion criteria were: (a) CLI as defined by ankle/brachial index ≤0.4, and ankle systolic pressure ≤50 mmHg or toe systolic pressure ≤30 mmHg; (b) over 18 years of age suffering from chronic and critical limb ischemia according to the TASC classification Rutherford 4–6, Fontaine IV, (c) with signed informed consent, (d) failed basic conservative and revascularization treatment (surgical or endovascular).
Patient exclusion criteria were: (a) estimated survival less than 6 months, (b) known bone marrow diseases (e.g., lymphoma, leukemia, myelodysplastic syndrome, metastasis in the bone marrow), (c) end stage renal failure and on dialysis, (d) acute stage of severe limb ischemia with severe inflammatory process affecting the patient’s life that requires limb amputation to avert grave outcome.
The primary endpoints for both treatment groups were major limb amputation (MLA) during 120 days and a degree of pain and function (assessed by clinical examination at pretreatment (day 0) and at a 90 and 120 day follow-up intervals.
The secondary endpoints were only performed in group I and included measurements of circulatory and blood parameters, such as Laser Doppler flowmetry with PeriFlux System 5000 (Perimed AB, Järfälla, Stockholm, Sweden), measurements of transcutaneous oxygen (TcpO2), CO2 (TcpCO2) levels with challenge test (O2 inhalation 5l/min/10 min), ankle/brachial index (ABI), toe pressure (TP, mmHg), toe brachial index (TBI), and skin perfusion pressure (SPP, mmHg) (39). They were measured at baseline prior (day 0) and 90 and 120 days after the ABMSC therapy. All measurements were done according to the standard procedure described by She-field et al. (39) 1 cm above the wound, avoiding veins. In addition, peripheral blood tests such as white blood cell counts, differential counts, fibrinogen, CRP, and platelets count levels were performed. In patients (group I) bone marrow aspirate (BMA) and bone marrow concentrate (BMC) were also analyzed to assess the number of CD34+ progenitor cells (×109/L), platelets, neutrophils, lymphocytes, monocytes, eosinophils, and basophils. External wound characteristics were documented by photography and clinical examination (swelling, color, diameter) and correlated with the degree of initial ischemia, hypoxia, pain level, and claudication interval using EQ-50 quality of life questionnaire. Follow-up angiography was performed at 120 postoperative days. The term full healing refers to reestablishment of skin barrier with no exposure of subepidermal tissues. Wound refers to any, even minimal exposure of subepidermis. Minor amputation refers to amputation that does not affect weight-bearing capability. Nonhealing refers to the defect remaining the same size or progressing in depth or diameter.
ABMSC Therapeutic Procedure
The ABMSC procedure included surgical preparation, harvesting of bone marrow (BM), isolation of ABMSCs from the bone marrow aspirate, and their local application. Bone marrow was collected from both iliac bone crests according to the standard Jamshidi puncture aspiration technique, with patients under analgosedation using a propophol intravenous bolus. Perioperative monitoring of vital signs, including noninvasive blood pressure, pulse rate, ECG, and pO2 saturation, were recorded and adjusted according to needs. A total of 240 ml of bone marrow was aspirated into an aspiration set pre-filed with ACD-A anticoagulant (30 ml) and processed using the cell separator (SmartPreP2). The separator uses gradient density centrifugation to separate from the bone marrow aspirate blood elements, including white cells, platelets, and red cells within 15 min. The process of bone marrow concentration also decreases the proportion of RBCs in the final concentrate (18). Thereafter, 40 ml of final bone marrow concentrate (BMC) was drawn from centrifuge containers into a syringe and immediately applied by 40 injections, each 1 ml, into the ischemic limb along the posterior and anterior tibial artery. The entire treatment procedure took less than 1 h.
Statistical Analysis
Statistical analyses of all data set were performed with SPSS software version 15.0 for Windows (SPSS Inc., CR). A two-sided value of p < 0.05 was considered statistically significant for all analyses. Continuous variables are presented as mean ± SEM or as median (inter-quartile) in box-and-whisker plots. The differences in the major endpoints between groups I and II were compared by Mann-Whitney Rank Sum Test. Categorical variables are stated with frequency and percentage. Chi-square test or Fisher’s Exact Test were used to compare categorical data. The statistical analysis included simple (Pearson’s) correlation and nonparametric correlation by means of Spearman’s rho.
RESULTS
Patients were randomized to the respective treated (group I) and control (group II) groups, as indicated and the former were subjected to the ABMSC treatment procedure. At 120 days of follow-up the frequency of major limb amputations (MLAs) was significantly (p < 0.05) lower in group I than in group II (21% vs. 44%, respectively). Interestingly MLA (21%) was necessary only in those group I patients suffering from significant lymphopenia and thrombocytopenia in BMC at the time of treatment. Forty-two patients in group I finished 90 days follow-up and 37 patients in group II finished 120 days of follow-up. Five patients in group I and eight in group II died of causes unrelated to the therapy or CLI, mostly coronary heart disease. No patient was lost or withdrawn from the study.
Characteristics of Patients in Group I
Out of 42 patients, 37 were diabetics; from those, 31 (73.8%) were on insulin, 1 (2.38%) was on oral hypoglycemic agents, and 5 (11.9%) patients were managed conservatively. Patients had history of 50 revascularization procedures such as proximal bypasses (7 aortofemoral or femoropopliteal), distal bypasses (4 crural or pedal), proximal PTA with stent procedures (9), crural or pedal PTA recanalizations (28), and lumbar sympatectomies (2) before ABMSC therapy. In addition some of these patients had history of contralateral major limb amputation (17 patients), contralateral minor amputation (1 patient), and index limb minor amputation (17 patients). In this group 17 patients were in stage DIII according to University of Texas Wound Classification.
Characteristics of Patients in Group II (Controls)
Fifty-four patients in this group suffered from CLI and diabetic foot ulcer; 39 of them were in stage DIII according to University of Texas Wound Classification. Out of 54 patients, 46 patients had a history of revascularisation procedure such as endovascular recanalization (39), aortobifemoral bypass (1), distal pedal bypass (1), femoropopliteal bypass (4), and embolectomy (1).
Group I ABMSC Patients
Based on the degree of ischemia on day 0 (assessed by Laser Doppler flow), patients in group I were divided into light (4.76%), moderate (64.29%), and severe (30.95%) ischemia group. Similarly, hypoxia measurement (by TcpO2 measurements) resulted in subdivision to normal (7.14%), light (19.05%), moderate (59.52%), and severe (14.29%) hypoxia groups. The results are presented as a comparison between healing and nonhealing patients. Patients in group I who healed showed significant improvements (p < 0.05) in SPP, LDP alone, and LDP heat values at 90 days compared to baseline measurements (Table 3). Correspondingly, toe pressure and toe brachial index increased from baseline of (22.66 ± 5.32 and 0.14 ± 0.03 mmHg, respectively, to 25.63 ± 4.75 and 0.17 ± 0.03, respectively, at 90 days. While there were no significant differences in TcpCO2 levels between healed and not healed limbs at 90 days, the nonhealing group had worse TcpCO2 following that interval, and exceeded 40 mmHg.
Table 3.
Assessment of Ischemic Limb Function Healing
| Day | LDP (pu) Baseline Healing |
LDP (pu) Heat Healing |
TcpO2 (mmHg) Baseline Healing |
TcpO2 (mmHg) O2 Challenge Healing |
TcpCO2 (mmHg) Baseline Healing |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Day 0 | 14.15 ± 1.94 | 12.07 ± 1.61 | 48.3 ± 4.5 | 35.26 ± 4.14† | 37.67 ± 4.43 | 35.83 ± 8.96 | 88.86 ± 9.06 | 77.22 ± 17.20 | 35.17 ± 2.08 | 37.97 ± 5.3 |
| Day 90 | 9.04 ± 1.06* | 9.58 ± 2.34 | 29.25 ± 2.13* | 29.33 ± 13.2 | 41.21 ± 5.06 | 24.15 ± 9.43 | 74.95 ± 6.55 | 46.43 ± 14.25 | 37.84 ± 1.11 | 41.06 ± 6.84 |
| Day | TcpCO2 (mmHg) O2 Challenge Healing |
TP (mmHg) Healing |
TBI Healing |
SPP (mmHg) Healing |
ABI Healing |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Day 0 | 35.13 ± 2.08 | 36.62 ± 4.84 | 22.66 ± 5.32 | 14.78 ± 5.3 | 0.14 ± 0.03 | 0.10 ± 0.03 | 137.86 ± 11.16 | 77.86 ± 19.25† | 0.91 ± 0.07 | 0.57 ± 0.13† |
| Day 90 | 36.76 ± 1.32 | 38.17 ± 6.26 | 25.63 ± 4.75 | 12.43 ± 6.44 | 0.17 ± 0.03 | 0.08 ± 0.04 | 111.19 ± 11.44* | 68.57 ± 20.87 | 0.78 ± 0.70 | 0.40 ± 0.13 |
Values are mean ± SEM for healing (Yes) and nonhealing (No). Patients treated with ABMSC (Group I) were evaluated using skin perfusion pressure test (SPP), laser Doppler perfusion test (LDP), transcutaneous capillary pressure of oxygen test (TcpO2), toe pressure test (TP), and transcutaneous capillary pressure of CO2 test (TcpCO2). Both SPP and ankle brachial index (ABI) were significantly (p < 0.05) higher at baseline in patients who healed versus those who did not. There was gradual improvement in SPP, LDP, and ABI that became statistically significant (p < 0.05) at 90 days following ABMSC treatment in patients who healed versus those who did not (Group I).
p < 0.05 versus baseline value.
p < 0.05 versus healed group of patients.
Neutrophil counts in peripheral blood samples in group I patients were not significantly different in patients who healed versus those who did not. In contrast, C-reactive protein was significantly elevated (p < 0.05) in patients with poorly healed limb 30 days postintervention. Lymphocyte counts dropped significantly (p < 0.05) immediately following the procedure (day 3, 7, 14) and remained low 30 days later (Table 4).
Table 4.
Peripheral Blood Analysis
| Day | Leucocyte (×109/L Healing |
Neutrophil (×109/L) Healing |
Lymphocyte (×109/L) Healing |
Platelets (×103/μl) Healing |
Fasting Blood Glucose (g/L) Healing |
C-Reactive Protein (mg/L) Healing |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| 0 | 9.15 ± 0.79 | 8.91 ± 0.61 | 5.87 ± 0.78 | 6.17 ± 0.47 | 2.24 ± 0.18 | 1.55 ± 0.17 | 269.11 ± 13.67 | 328.21 ± 39.03 | 4.07 ± 0.14 | 4.13 ± 0.26 | 29.61 ± 9.52 | 48.86 ± 11.78 |
| 3 | 8.90 ± 0.66 | 9.09 ± 0.78 | 5.89 ± 0.58 | 6.27 ± 0.59 | 2.05 ± 0.21 | 1.49 ± 0.16 | 263.25 ± 16.25 | 323.93 ± 41.51 | 4.06 ± 0.12 | 4.02 ± 0.26 | 26.92 ± 9.91 | 58.29 ± 14.10 |
| 7 | 8.70 ± 0.68 | 8.08 ± 0.77 | 5.53 ± 0.54 | 5.33 ± 0.66 | 2.10 ± 0.14 | 1.67 ± 0.17 | 290.43 ± 24.28 | 321.64 ± 39.41 | 4.05 ± 0.16 | 4.37 ± 0.27 | 17.92 ± 3.70 | 58.51 ± 16.42 |
| 14 | 9.85 ± 0.81 | 8.81 ± 0.79 | 6.49 ± 0.73 | 6.18 ± 0.64 | 2.34 ± 0.22 | 1.60 ± 0.17 | 303.15 ± 18.41 | 342.50 ± 34.62 | 4.20 ± 0.29 | 4.14 ± 0.30 | 29.45 ± 9.80 | 46.38 ± 13.50 |
| 30 | 9.41 ± 0.56 | 8.34 ± 0.74 | 6.25 ± 0.46 | 5.66 ± 0.63 | 2.27 ± 0.15 | 1.50 ± 0.14 | 312.62 ± 25.38 | 319.85 ± 42.94 | 3.95 ± 0.16 | 4.24 ± 0.24 | 10.19 ± 1.82 | 33.18 ± 9.39 |
Values are mean ± SEM for healing (Yes) and nonhealing (No). Analysis of peripheral blood was done at baseline and at 0, 3, 7, 14, and 30 days. Significant differences were found in the number of lymphocytes, and CRP between healing and nonhealing patients. Circulating C-reactive protein levels were significantly higher on day 3, 7, and 30 (p = 0.029, p = 0.009, and p = 0.010, respectively) in patients who did not heal. Lymphocyte counts were significantly lower on day 0, 3, 14, and 30 (p = 0.018, p = 0.036, p = 0.021, and p = 0.002, respectively) in the early stages of treatment, than those of patients that healed well (Group I).
There was a significant correlation between low CD34+ cell counts in the bone marrow concentrate (BMC) and increasing age (Spearman’s rho = −0.348, p = 0.024), even though there was no difference in CD34+ cell counts between patients who healed or those who did not (Fig. 1). The SmartPreP2 centrifuge gradient density centrifugation resulted in at least a fivefold concentration of CD34+ cells in the bone marrow concentrate and more than 3.8-fold concentration of platelets (compared to a sample of BMA aspirate before centrifugation). A detailed analysis of cells counts in BMC after centrifugation is shown in Table 5. Patients who healed poorly exhibited significant lymphopenia (p < 0.05) in the initial BMC (Fig. 2A), even though no correlation between lymphopenia in BMC and diabetes was appreciated (Fig. 2B).
Figure 1.
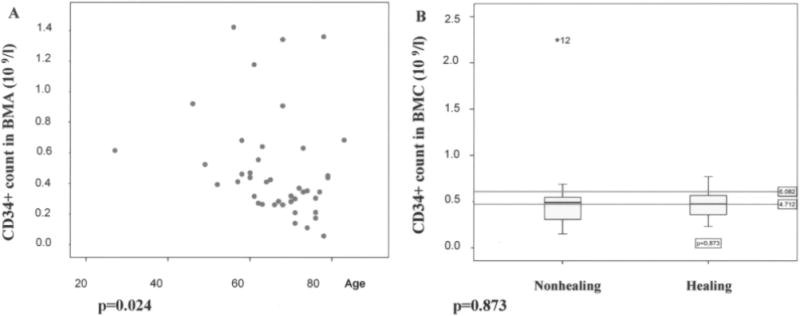
CD34+ counts decrease with age (A) but the decrease in CD34+ cell count does not correlate with wound nonhealing (B). A significant correlation between decreasing CD34+ in BMA and advancing age was found and was consistent with the previously published data on SC aging and SC count lowering with age (Spearman’s rho = −0.348, p = 0.024). Yet, we did not find the often extrapolated effect on healing. There was no statistical difference between the healing and nonhealing group and CD34+ counts (p = 0.873) in group I. We observed one extreme outlayer (marked as *12 in B).
Table 5.
Bone Marrow Cell Counts Before and After Gradient Density Centrifugation
| Cell Type | Bone Marrow Aspirate | Bone Marrow Concentrate | Fold Difference |
|---|---|---|---|
| CD34+ (×109/L) | 0.10 ± 0.01 | 0.49 ± 0.05 | 5.04 ± 0.46 |
| Platelets (×103/μl) | 337.22 ± 42.92 | 1087.14 ± 129.95 | 3.88 ± 0.30 |
| WBC (×109/L) | 17.01 ± 0.83 | 71.07 ± 0.62 | 4.20 ± 0.17 |
| Lymphocytes (×109/L) | 2.71 ± 0.16 | 11.30 ± 0.90 | 4.23 ± 0.24 |
| Monocytes (×109/L) | 1.18 ± 0.92 | 4.80 ± 0.35 | 4.27 ± 0.27 |
| Neutrophils (×109/L) | 12.85 ± 0.71 | 50.97 ± 3.61 | 3.99 ± 0.16 |
The values are mean ± SEM from 42 patients (Group I). Total cell counts were performed in bone marrow aspirates and concentrates produced with SmartPReP2 gradient density centrifugation.
Figure 2.
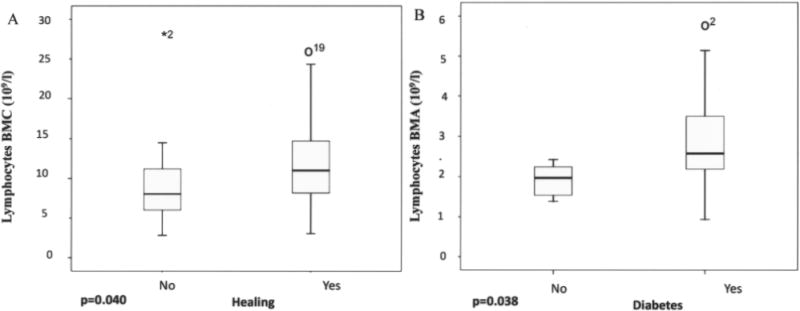
Lymphopenia in the initial BMC was evident in patients who did not heal despite ABMSC transplantation. Only patients with significant baseline lymphopenia and thrombocytopenia (p < 0.05) in the bone marrow aspirate and concentrate went on to a major limb amputation (21% of group 1), compared to healed patients (79%) (A). The low lymphocyte counts were not due to the underlying diabetes (B) and represented a distinct immune defect. We observed one extreme outlayer marked as *2 and outlayers o19 and o2 with higher lymphocytes count.
In the majority of patients with the nonhealing limb, platelet and CD34+ cells were low in the initial bone marrow aspirate, and did not show the direct monotonous (nonlinear) dependence of CD34+ cell concentration and platelets. In healing patients a higher CD34+ cell concentration corresponds to higher concentration of platelets (Spearman’s rho = 0.679, p = 0.006) (Fig. 3A, B). Patients with low platelet counts in the group of patients that healed had higher levels of VEGF than patients with low platelet counts in the nonhealing group. Thus, there was a statistically significant correlation of VEGF (pg/ml) and concentration of platelets in BMC (r = −0.549, p = 0.010) of patients with a healing limb, while it did not exist in patients with a poorly healed limb (Spearman’s rho = −0.324, p = 0.434) (Fig. 4A, B).
Figure 3.
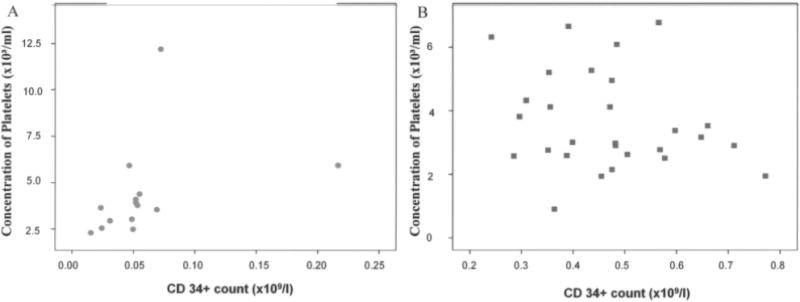
Correlation between number of CD34+ cells and number of platelets in bone marrow concentrate. A comparison of a subgroup of patients treated with ABMSCs that healed versus those that did not revealed a significant direct monotonous (nonlinear) dependence of platelets on CD34+ cell concentration; that is, a higher CD34+ cell concentrations correspond to higher concentration of platelets (Spearman’s rho = 0.679, p = 0.006) (B). In addition, the majority of patients that did not heal clustered in lower left corner, indicating low platelet and CD34+ cells. In contrast, the group of patients that healed showed a wide distribution of relative ratios of platelets and CD34+ cells (A).
Figure 4.
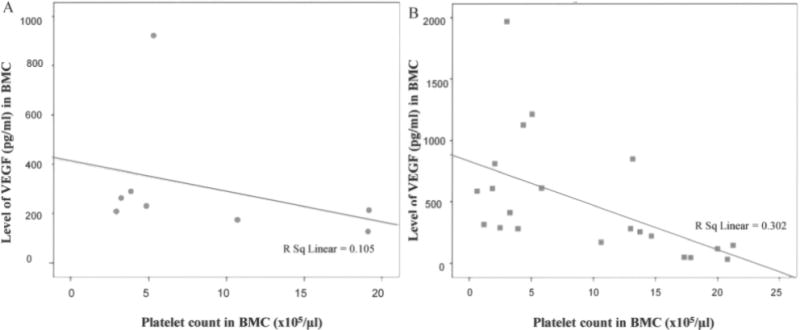
Patients with significant lymphopenia, thrombocytopenia, and low levels of VEGF in BMC did not respond well to ABMSC therapy. All limbs in this patient population were amputated (21%) within 120 days after ABMSC treatment. As evident, the combination of low platelet counts and low VEGF levels is associated with poor healing (A). Statistically significant correlation (r = −0.549, p = 0.010) only exists between the number of platelets and VEGF level in the group of patients treated with ABMSCs who had improved circulation and healed, but not in those without healing (r = −0.324, p = 0.434) (B).
As shown in representative images of the character and wound diameter of healing limbs, a complete wound closure and skin integrity was achieved by 90 days (Fig. 5). Capillary angiogenesis is demonstrated by a decrease in the degree of initial ischemia/hypoxia. Improvement in overall vascularity was further demonstrated by follow-up angiography at 120 days (Fig. 6).
Figure 5.
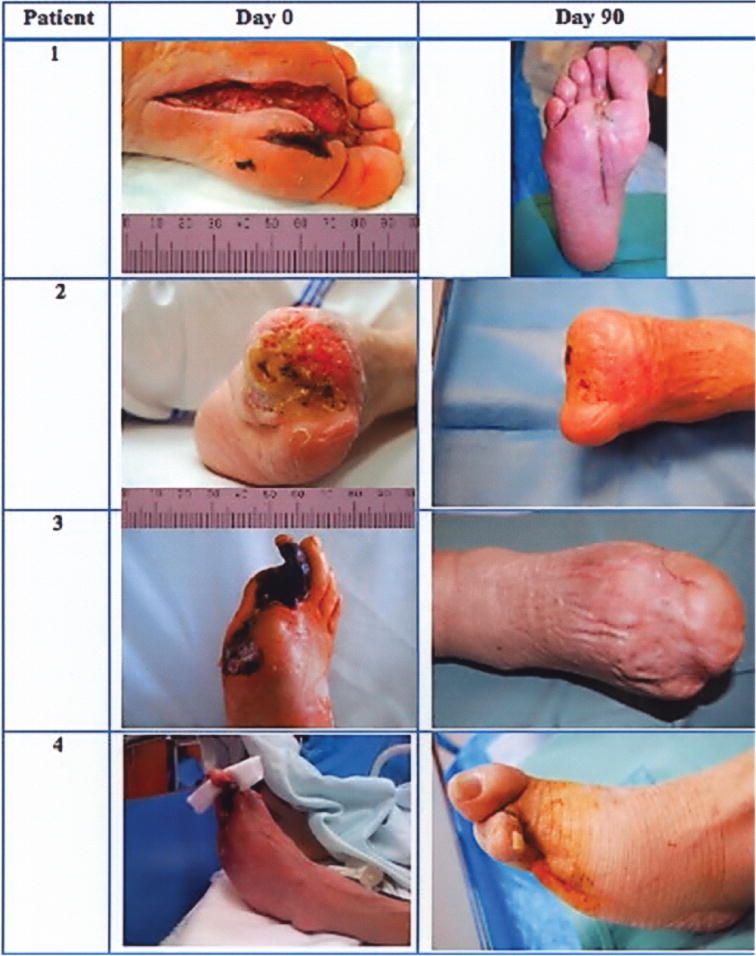
Representative photographs of limb ulcers before and 90 days after ABMSC therapy. During 90 days after ABMSC therapy all ulcers associated with CLI healed in those patients (100%) with normal lymphocyte and thrombocyte counts in BMC at the time of procedure.
Figure 6.
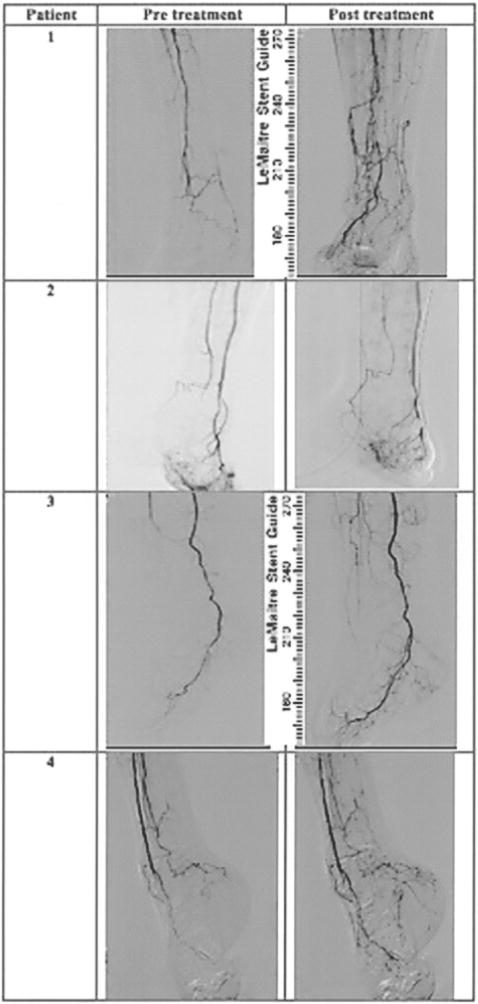
Representative angiography of limbs before and 120 days after the ABMSC therapy. Follow-up angiography reveals clear areas of neovascularization and collateral vessel formation along the calf vessels at the day 120 following administration of ABMSCs. The images correspond to the patients presented in Figure 5 with healed wounds.
Pain was evaluated using the previously validated EQ-50 quality of life questionnaire. Patients undergoing ABMSC procedure reported a subjective improvement in ischemic pain within 72 h following the cellular therapy. However, only patients with healing limbs experienced a sustained long-term (180 days) control of their pain, and this difference was statistically significant at 90 days (p < 0.05).
DISCUSSION
Cell therapies with pure progenitor cells or with mononuclear cell concentrates have shown significant improvement in the healing of chronic ulcers (1,2,22,26, 31–33,43). While the outcomes for mononuclear cell therapy were noticeably better than for therapy using only one cell type, the mechanism for this enhancement of wound healing was unclear. Our results suggest that multicellular therapy using ABMSC from bone marrow aspirate maximizes the reciprocal interactions between the various transplanted cells of the bone marrow, as well as the interaction of these cells with the extracellular matrix (ECM). The principle of “dynamic reciprocity dialogue” between the cells and their microenvironment acts as a circuitry by which tissue architecture and function become integrated (34). Thus, even a relative deficiency in one of the cellular components can result in severe defect in the wiring of this circuitry, and derail the signaling axis that transmits through, and is regulated by, the interaction of the individual cell cytoskeleton and the integrins of the underlying matrix.
The tissue microenvironment undergoes extensive remodeling during tissue regeneration and involves the deposition, degradation, and structural reorganization of ECM components. The influence of ECM on the individual cell components within the microenvironment provides morphogenic cues to control cell survival, proliferation, migration, polarization, and differentiation. Similarly, each of the cell components provides specific cues for the restructuring of the extracellular matrix. A bone marrow cell concentrate, such as the one used in our study, is enriched in platelets, which provide the vehicle for cell–cell interaction. Intact platelets provide a source of angiogenesis growth regulators, which can be released in response to tissue-specific stimuli (21,25). The sequential, spatially, and temporally controlled re lease of stimulators and inhibitors of angiogenesis from platelets may explain the improved outcomes with complete cellular therapy compared to CD34+ cell pure therapy, mononuclear cell therapy, or therapy with recombinant proteins (4,11,19,24). The majority of nonhealing patients clustered at low concentrations for both platelets and CD34+ cells (Fig. 3), suggesting that there was not an optimal concentration that would permit healing. The ratio of platelets and CD34+ cells was highly variable, but the majority of healing patients were gathered at moderate concentrations of both platelets and CD34+ cells in proportional concentrations rather than at either of the extremes. Viewed in combination with the observation that as long as patients with low platelet count also have high concentration of VEGF (Fig. 4) they can heal, a model of dynamic reciprocity compensating for individual deficiencies of growth factors emerges.
The analysis of peripheral blood and bone marrow revealed significant differences between patients with healing and nonhealing limbs. Circulating levels of C-reactive protein were significantly higher in patients who did not heal, particularly in the early postprocedural period (Table 4). This may be a reflection of an underlying chronic inflammatory state of the patient, or an excessive inflammatory response. Because the levels of C-reactive proteins were high even on day 0, an underlying chronic inflammatory state is more likely. This would be consistent with the finding of significantly lower initial lymphocytes levels in patients that did not heal well (Table 4). A corresponding statistically significant (p < 0.05) lymphopenia and thrombocytopenia was evident in the BMC of patients that healed poorly versus those that healed well (Figs. 2A and 3), yielding further support to importance of these cellular factors in wound healing and regeneration. While the role of platelets in adhesion, maturation, and differentiation of stem cell progenitors has been well documented, the role of lymphocytes in the local wound microenvironment has only been suspected (12,28,29,42). Whereas an immune defect is often correlated with an underlying chronic condition, in this study the lymphopenia in the initial BMC did not correlate with the presence of diabetes (Fig. 2A, B), and may represent a distinct immune defect. We show, possibly for the first time, that the lymphopenia is not a feature of diabetes, but rather a prognostic factor evident at baseline (27).
Our findings on the role of platelets in progenitor engraftment are supported by preclinical studies in mice with critical limb ischemia where the administration of peripheral blood mononuclear cells (PBMNC) along with platelets resulted in significantly improved perfusion than that achieved by PBMNC, platelets, or polymorphonuclear cells alone (16,17). As shown by Langer et al., endothelial cell progenitors require platelets for their homing, adhesion, and differentiation (28), strongly supporting our evidence for the dynamic interaction of endothelial cell progenitors and platelets (Fig. 3). The gradient density centrifugation increased the concentration of platelets about 3.9-fold while preserving the relative concentrations of other cells in the host BMC (Table 5), providing the perfect milieu for the local enrichment of the wound upon BMC transplantation (18).
Even though we found a statistically significant decrease of CD34+ cell counts with age, advancing age did not appear to influence wound healing as would be expected (Fig. 1). This is a frequently observed phenomenon in tissue regeneration and supports our above-stated hypothesis that it is not the absolute number of the cells present, but rather their ability to interact with other cells in the microenvironment and the availability of other collaborating cells (9,37,45).
In diabetic patients commonly used methods such as ankle/brachial index may be less informative because of significant atherosclerosis and vessel calcification rendering the vessels incompressible. For this reason we have used a number of methods to evaluate the improvements in ischemic limb perfusion and healing, such as skin perfusion pressure (SPP), laser Doppler perfusion (LDP), transcutaneous capillary pressure of oxygen (TcpO2), toe pressure (TP), and transcutaneous capillary pressure of CO2 (TcpCO2) (Table 3). Skin perfusion pressure measurement is more accurate in diabetic patients than ABI. Among all of the methods used in our study, the combination of LDP and TcpO2 provided excellent information about the local blood perfusion and oxygenation and reflected improvements in limb healing better than other methods. TcpCO2 may provide quantifiable information about metabolic status of the leg and predict a catabolic risk.
There were some differentiating characteristics between patients who healed and those that did not. Patients with high circulating neutrophil and platelet counts prior to treatment improved transiently as documented by improvements in local perfusion (laser Doppler flow and TcpO2 in Table 3) and reduction in ischemic pain, but they ultimately deteriorated, and an eventual amputations could not be prevented. The acute inflammatory response manifested by an initial elevation of neutrophils and thrombocytes in circulation did not compensate for the preexisting lymphopenia and thrombocytopenia in the BMC (Table 4). At the end, all of the amputations after AMBSC therapy in our study were secondary to infections. Based on these prognostic factors, it may be possible to clearly identify early, at the institution of ABMSC therapy, a subset of patients who are more likely to fail to cell therapy. In those patients aggressive debridement, antibiotics, grafting, and potentially pharmacological modulation of the immune system may be of more benefit than ABMSC treatment.
The analgesic effect of ABMSC application was seen within 72 h after procedure in all patients, and was most likely due to neurotransmitters (neurotrophins) release (13,14,41). Patients who had a true improvement of limb perfusion following ABMSC application, and went on to heal, had a sustained pain relief and total eradication of ischemic pain. All of the control patients and in patients where limb did not heal despite ABMSC treatment, pain was prominent feature throughout entire follow-up interval of 120 days.
The results of several clinical studies suggested that assessing degree of CLI at the baseline before ABMSC therapy would identify patients who would go on to heal well. However, group I treated patients with normal lymphocyte and platelet count in BMC prior ABMSC therapy (79%) healed well, while major limb amputation could not be avoided in those with relative lymphopenia and thrombocytopenia (21%). This relative difference in lymphocyte counts was statistically different (p = 0.04 for BMC, p = 0.038 for BMA, and p = 0.002 for peripheral lymphocyte counts on day 30) (for more details see Table 4 and Fig. 2). The outcome was independent of the severity of CLI and degree of diabetes at baseline, suggesting that intrinsic host deficiencies rather than the degree of disease progression was decisive.
In conclusion, we have identified a potential mechanism in which multicellular therapies, and bone marrow concentrates in particular, facilitate wound healing. The validation of the relative ratios and the physiological integrity of cells contributing to a wound ECM is likely to lead to further improvements in the future. We have also suggested a number of early prognosticators, identifiable at baseline, that can guide the management of patients with an underlying defect in lymphocyte numbers and/or function or inadequate platelet counts. We surmise that wound healing and tissue regeneration, an area of an as of yet unmet medical need, should benefit from effective cellular therapy and lead to a consequential medical advancement.
Acknowledgments
This clinical study was supported by the grant from the Moravian-Silesian Region Government (MSK-99/OVZ/08/006-1).
References
- 1.Amann B, Luedemann C, Ratei R, Schmidt-Lucke JA. Autologous bone marrow cell transplantation increases leg perfusion and reduces amputations in patients with advanced critical limb ischemia due to peripheral artery disease. Cell Transplant. 2009;18(3):371–380. doi: 10.3727/096368909788534942. [DOI] [PubMed] [Google Scholar]
- 2.Asahara T, Kawamoto A. Endothelial progenitor cells for postnatal vasculogenesis. Am J Physiol Cell Physiol. 2004;287(3):C572–579. doi: 10.1152/ajpcell.00330.2003. [DOI] [PubMed] [Google Scholar]
- 3.Asahara T, Masuda H, Takahashi T, Kalka C, Past-ore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85(3):221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 4.Bhattacharya V, McSweeney PA, Shi Q, Bruno B, Ishida A, Nash R, Storb RF, Sauvage LR, Hammond WP, Wu MH. Enhanced endothelialization and microvessel formation in polyester grafts seeded with CD34(+) bone marrow cells. Blood. 2000;95(2):581–585. [PubMed] [Google Scholar]
- 5.Buschmann I, Schaper W. Arteriogenesis versus angiogenesis: Two mechanisms of vessel growth. News Physiol Sci. 1999;14:121–125. doi: 10.1152/physiologyonline.1999.14.3.121. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438(7070):932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- 7.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6(4):389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 8.Cavanagh PR, Lipsky BA, Bradbury AW, Botek G. Treatment for diabetic foot ulcers. Lancet. 2005;366(9498):1725–1735. doi: 10.1016/S0140-6736(05)67699-4. [DOI] [PubMed] [Google Scholar]
- 9.Cho RH, Sieburg HB, Muller-Sieburg CE. A new mechanism for the aging of hematopoietic stem cells: Aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood. 2008;111(12):5553–5561. doi: 10.1182/blood-2007-11-123547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82(7):2031–2037. [PubMed] [Google Scholar]
- 11.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438(7070):937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 12.Daub K, Langer H, Seizer P, Stellos K, May AE, Goyal P, Bigalke B, Schonberger T, Geisler T, Siegel-Axel D, Oostendorp RA, Lindermann S, Gawaz M. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006;20(14):2559–2561. doi: 10.1096/fj.06-6265fje. [DOI] [PubMed] [Google Scholar]
- 13.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19(9):1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 14.Emanueli C, Schratzberger P, Kirchmair R, Madeddu P. Paracrine control of vascularization and neurogenesis by neurotrophins. Br J Pharmacol. 2003;140(4):614–619. doi: 10.1038/sj.bjp.0705458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438(7070):967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg DA, Jin K. From angiogenesis to neuropathology. Nature. 2005;438(7070):954–959. doi: 10.1038/nature04481. [DOI] [PubMed] [Google Scholar]
- 18.Hermann PC, Huber SL, Herrler T, von Hesler C, Andrassy J, Kevy SV, Jacobson MS, Heeschen C. Concentration of bone marrow total nucleated cells by a point-of-care device provides a high yield and preserves their functional activity. Cell Transplant. 2008;16(10):1059–1069. [PubMed] [Google Scholar]
- 19.Iba O, Matsubara H, Nozawa Y, Fujiyama S, Amano K, Mori Y, Kojima H, Iwasaka T. Angiogenesis by implantation of peripheral blood mononuclear cells and platelets into ischemic limbs. Circulation. 2002;106(15):2019–2025. doi: 10.1161/01.cir.0000031332.45480.79. [DOI] [PubMed] [Google Scholar]
- 20.Isner JM, Asahara T. Angiogenesis and vasculogenesis as therapeutic strategies for postnatal neovascularization. J Clin Invest. 1999;103(9):1231–1236. doi: 10.1172/JCI6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL. Angiogenesis is regulated by a novel mechanism: Pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajiguchi M, Kondo T, Izawa H, Kobayashi M, Yamamoto K, Shintani S, Numaguchi Y, Naoe T, Takamatsu J, Komori K, Murohara T. Safety and efficacy of autologous progenitor cell transplantation for therapeutic angiogenesis in patients with critical limb ischemia. Circ J. 2007;71(2):196–201. doi: 10.1253/circj.71.196. [DOI] [PubMed] [Google Scholar]
- 23.Kalka C, Asahara T, Krone W, Isner JM. Angiogenesis and vasculogenesis. Therapeutic strategies for stimulation of postnatal neovascularization. Herz. 2000;25(6):611–622. doi: 10.1007/pl00001974. [DOI] [PubMed] [Google Scholar]
- 24.Kalka C, Takahashi T, Masuda H, Asahara T, Isner JM. Vascular endothelial factor (VEGF): therapeutic angiogenesis and vasculogenesis in the treatment of cardio vascular disease. Med Klin (Munich) 1999;94(4):193–201. doi: 10.1007/BF03044854. [DOI] [PubMed] [Google Scholar]
- 25.Klement GL, Yip TT, Cassiola F, Kikuchi L, Cervi D, Podust V, Italiano JE, Wheatley E, Abou-Slaybi A, Bender E, Almong N, Kieran MW, Folkman J. Platelets actively sequester angiogenesis regulators. Blood. 2009;113(12):2835–2842. doi: 10.1182/blood-2008-06-159541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo FA, Nishibe T, Nishibe M, Yasuda K. Autologous transplantation of peripheral blood endothelial progenitor cells (CD34+) for therapeutic angiogenesis in patients with critical limb ischemia. Int Angiol. 2003;22(4):344–348. [PubMed] [Google Scholar]
- 27.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, Ten S, Sanz M, Exley M, Wilson B, Porcelli S, Maclaren N. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109(1):131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Langer HF, May AE, Vestweber D, De Boer HC, Hatzopoulos AK, Gawaz M. Platelet-induced differentiation of endothelial progenitor cells. Semin Thromb Hemost. 2007;33(2):136–143. doi: 10.1055/s-2007-969026. [DOI] [PubMed] [Google Scholar]
- 29.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow- derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203(5):1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matoba S, Tatsumi T, Murohara T, Imaizumi T, Katsuda Y, Ito M, Saito Y, Uemura S, Suzuki H, Fukumoto S, Yamamoto Y, Onodera R, Teramukai S, Fukushima M, Matsubara H, TACT Follow-up Study Investigators Long-term clinical outcome after intramuscular implantation of bone marrow mononuclear cells (Therapeutic Angiogenesis by Cell Transplantation [TACT] trial) in patients with chronic limb ischemia. Am Heart J. 2008;156(5):1010–1018. doi: 10.1016/j.ahj.2008.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Messner H. Stem cells: The challenge and opportunities. Bone Marrow Transplant. 2008;42(Suppl 1):S57–S59. doi: 10.1038/bmt.2008.116. [DOI] [PubMed] [Google Scholar]
- 32.Murasawa S, Asahara T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- 33.Murayama T, Tepper OM, Silver M, Ma H, Losordo DW, Isner JM, Asahara T, Kalka C. Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol. 2002;30(8):967–972. doi: 10.1016/s0301-472x(02)00867-6. [DOI] [PubMed] [Google Scholar]
- 34.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: Engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin Cancer Biol. 2005;15(5):342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Bell K, Caporusso J, Durand-Zaleski I, Komori K, Lammer J, Liapis C, Novo S, Razavi M, Robbs J, Schaper N, Shigematsu H, Sapoval M, White C, White J, Clement D, Creager M, Jaff M, Mohler E, 3rd, Rutherford RB, Sheehan P, Sillesen H, Rosenfield K. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Prompers L, Huijberts M, Schaper N, Apelqvist J, Bakker K, Edmonds M, Holstein P, Jude E, Jirkovska A, Mauricio D, Piaggesi A, Reike H, Spraul M, Van Acker K, Van Baal S, Van Merode F, Uccioli L, Urbancic V, Ragnarson TG. Resource utilisation and costs associated with the treatment of diabetic foot ulcers. Prospective data from the Eurodiale Study. Diabetologia. 2008;51(10):1826–1834. doi: 10.1007/s00125-008-1089-6. [DOI] [PubMed] [Google Scholar]
- 37.Rossi DJ, Bryder D, Weissman IL. Hematopoietic stem cell aging: Mechanism and consequence. Exp Gerontol. 2007;42(5):385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Saigawa T, Kato K, Ozawa T, Toba K, Makiyama Y, Minagawa S, Hashimoto S, Furukawa T, Nakamura Y, Hanawa H, Kodama M, Yoshimura N, Fujiwara H, Namura O, Sogawa M, Hayashi J, Aizawa Y. Clinical application of bone marrow implantation in patients with arteriosclerosis obliterans, and the association between efficacy and the number of implanted bone marrow cells. Circ J. 2004;68(12):1189–1193. doi: 10.1253/circj.68.1189. [DOI] [PubMed] [Google Scholar]
- 39.Sheffield PJ, Buckley CJ. Laser doppler flowmetry. A sophisticated tool for assessing regional arterial perfusion and potential for wound healing. In: Sheffield PJ, Smith APS, Eife CE, editors. Wound care practice. 1st. Flagstaff, AZ: Best Publishing Co; 2004. pp. 137–156. [Google Scholar]
- 40.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103(6):897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 41.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92(6):735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 42.Stellos K, Langer H, Daub K, Schoenberger T, Gauss A, Geisler T, Bigalke B, Mueller I, Schumm M, Schaefer I, Seizer P, Kraemer BF, Siegel-Axel D, May AE, Lindemann S, Gawaz M. Platelet-derived stromal cell-derived factor-1 regulates adhesion and promotes differentiation of human CD34+ cells to endothelial progenitor cells. Circulation. 2008;117(2):206–215. doi: 10.1161/CIRCULATIONAHA.107.714691. [DOI] [PubMed] [Google Scholar]
- 43.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: A pilot study and a randomised controlled trial. Lancet. 2002;360(9331):427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 44.Tepper OM, Capla JM, Galiano RD, Ceradini DJ, Callaghan MJ, Kleinman ME, Gurtner GC. Adult vasculogenesis occurs through in situ recruitment, proliferation, and tubulization of circulating bone marrow-derived cells. Blood. 2005;105(3):1068–1077. doi: 10.1182/blood-2004-03-1051. [DOI] [PubMed] [Google Scholar]
- 45.Warren LA, Rossi DJ. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2009;130(1–2):46–53. doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94(2):230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]


