Abstract
Varroa destructor, a key biotic threat to the Western honey bee, has played a major role in colony losses over the past few years worldwide. Overuse of traditional acaricides, such as tau-fluvalinate and flumethrin, on V. destructor has only increased its tolerance to them. Therefore, the application of essential oils in place of traditional pesticides is an attractive alternative, as demonstrated by its high efficiency, lack of residue and tolerance resistance. To study the acaricidal activity of essential oils, we used clove oil (Syzygium aromaticum L.), a typical essential oil with a wide range of field applications, and examined its effects on the enzyme activities of Ca2+-Mg2+-ATPase, glutathione-S-transferase (GST) and superoxide dismutase (SOD) and its effects on the water-soluble protein content of V. destructor body extracts after exposure to 0.1 μl and 1.0 μl of clove oil for 30 min. Our results showed that the water-soluble protein content significantly decreased after the treatments, indicating that the metabolism of the mites was adversely affected. The bioactivity of GSTs increased significantly after a low dosage (0.1 μl) exposure but decreased at a higher dosage (1.0 μl), while the activities of SOD and Ca2+-Mg2+-ATPase were significantly elevated after treatments. These results suggest that the protective enzyme SOD and detoxifying enzymes Ca2+-Mg2+-ATPase and GST contributed to the stress reaction of V. destructor to the essential oils and that the detoxification ability of V. destructor via GST was inhibited at higher dosages. Our findings are conducive to understanding the physiological reactions of V. destructor to treatment with essential oils and the underlying mechanisms behind the acaricidal activities of these natural products.
Keywords: Clove oil, Varroa destructor, Enzyme activity, Glutathione-S-transferase, Ca2+-Mg2+-ATPase, Superoxide dismutase
1. Introduction
The currently declining health status of the Western honey bee (Apis mellifera) has caused great concern globally over the past few years (Neumann and Carreck, 2010). Varroa destructor Anderson and Trueman (Arachnida: Acari: Varroidae), an ectoparasite mite of honey bees, is considered the most severe threat to colony health (Dietemann et al., 2012). V. destructor feeds on the haemolymph of honey bees, causing several detrimental effects to its host, including attenuated lifespan, disorientation and decreased immunity (Amdam et al., 2004, Kralj et al., 2007, Rosenkranz et al., 2010, Nazzi et al., 2012). More grave is that these adverse effects of V. destructor are compounded by bee viruses, such as DWV and IAPV, thus proving a fatal role in colony collapse (Oldroyd, 2007, Martin et al., 2012). Previous research has shown that untreated varroa-infested colonies usually die within six months to two years (Le Conte et al., 2010). To maintain control of the V. destructor population within domesticated A. mellifera colonies, various acaricides have been applied in the past: organophosphate coumaphos; pyrethroids, such as tau-fluvalinate and flumethrin; and formamidine amitraz (Milani and Barbattini, 1988, Milani and Iob, 1998). While these acaricides were effective for a time, V. destructor mites have rapidly grown resistant to these drugs due to their overuse and the single active composition of traditional miticides (Elzen et al., 1999, Thompson et al., 2002). Furthermore, wide attention has been drawn to the fact that traces of these pesticides have been found in bee products, causing additional risk to human health (Bogdanov et al., 1998, Wallner, 1999).
Essential oils are secondary metabolism products that are produced during the course of plant growth (Wallace, 2004). They facilitate the primary metabolism of plants and provide certain defences against herbivores, pests and pathogens to keep the plants healthy (Isman, 2000). Previous research has shown that over 150 of these essential oils have demonstrated varied efficacies in terms of controlling V. destructor (Lindberg et al., 2000, Gashout and Guzmán-Novoa, 2009, Su et al., 2012). As an alternative to traditional acaricides, essential oil products present certain advantages, such as high toxicity to mites, low toxicity to bees, few residues in bee products and no drug resistance (Rosenkranz et al., 2010).
The efficacy of clove Syzygium aromaticum (L.) merr. et perry (Myrtaceae) oil as a treatment for V. destructor mites has been previously demonstrated both in the laboratory and in the field. In a 20-ml glass-vial residual test, 0.75 mg of clove oil caused a 96% mortality rate in V. destructor (Gashout and Guzmán-Novoa, 2009). A 2012 study by Su et al. showed that clove oil caused a 60% mortality rate in V. destructor mites at a dosage of 1.0 μl for 48 h, a level that is considered safe for bees. Additionally, eugenol, the principal constituent of clove oil, was discovered in beeswax during a two-week period under semi-field conditions, which supports the idea that medicinal treatment with clove oil is both stable and sustainable (Girisgin et al., 2014). Mahmood et al. (2014) also demonstrated the effectiveness of clove oil and regarded it as a promising alternative to treat V. destructor in the field. Despite the promising results of prior studies, the underlying acaricidal mechanisms of treatment with essential oils have yet to be elucidated. In this study, we determined the effects of clove oil on the enzyme activities of Ca2+-Mg2+-ATPase, glutathione-S-transferase (GST) and superoxide dismutase (SOD), as well as on the total protein content in the body extracts of V. destructor mites. Our aim is to understand the physiological reactions of Varroa mites to essential oils and the associated underlying mechanisms behind its acaricidal effects.
2. Materials and methods
2.1. Extraction of clove essential oil
Clove leaves were purchased from Tongrentang Chinese Medicine-Since. Essential oil was extracted according to steam distillation methods described in Chinese Pharmacopoeia (edition 2010). Briefly, 500 g of air-dried clove leaf powder and 5 L of distilled water were combined in a round-bottom distillation flask. The flask was connected to a condenser tube, and heated to a steady boil for five hours. The extracted essential oil was collected into Eppendorf tubes (1.5 ml) and stored at 4 °C until use. According to our previous study, this essential oil consists of 62.28% eugenol, 20.79% caryophyllene, 2.48% α-caryophyllene and 6.03% phenol-2-methoxy-4-(2-propenyl)-acetate (Su et al., 2012).
2.2. Mites collection
A. mellifera colonies that were highly infested with V. destructor mites and had been untreated for approximately six months prior served as the V. destructor donor colonies. Adult female mites from capped worker or drone brood combs were collected with a small soft brush, placed on wet tissue paper and fed five fifth instar worker bee larvae. Treatments on these mites began within one hour after feeding.
2.3. Exposure to clove oil
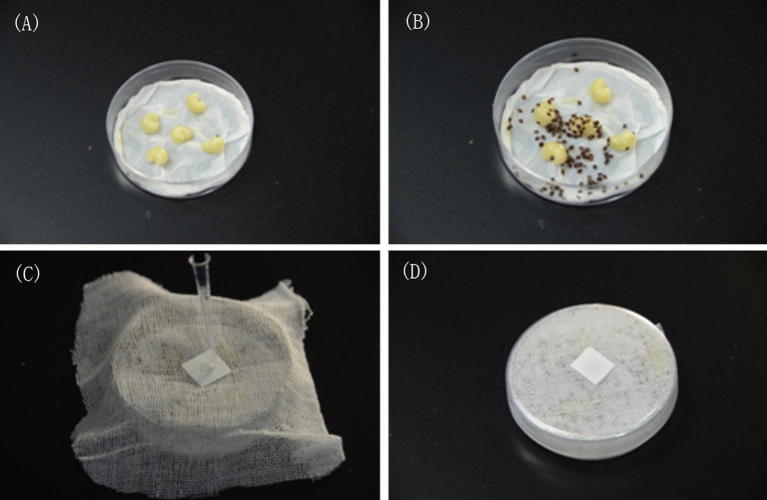
A group of V. destructor mites (100 mg, ≈350 mites) was gathered and placed into a petri dish (Ø = 60 mm) and fed five fifth instar worker bee larvae (Fig. 1A, B). Wet tissue papers were laid at the bottom of the petri dishes to maintain humidity. A piece of gauze was placed inside the cover of the petri dish. We then applied some clove oil to a small piece of filter paper and secured it between the gauze and the cover (Fig. 1C). According to Su et al. (2012), 1.0 μl clove oil is the maximum dosage that does not cause acute toxicity to the honey bee; however, 20% of V. destructor mites died when exposed to this amount for 4 h. Therefore, this dosage was selected for our study. A dosage of 0.1 μl was added to the study to investigate the effects of a lower dosage. The negative control consisted of a test group that was not exposed to the clove oil. The petri dishes were sealed with a membrane to avoid the volatilization of essential oils (Fig. 1D). Three replicates were included in each group; therefore, approximately 1050 mites were used. The petri dishes containing mites and clove oil were incubated (34.5 °C, 70%RH) for 30 minutes. The petri dishes were then frozen (−80 °C) immediately after removal from the incubator.
Fig. 1.
Experimental setup of the study. (A) The varroa mites were fed five fifth instar worker bee larvae in a petri dish. (B) A group of V. destructor mites (100 mg, ≈350 mites) were collected and placed in a petri dish. (C) Clove oil was dropped on a small piece of filter paper and secured between the gauze and the cover of the petri dish. (D) The petri dishes containing varroa mites were sealed with a membrane to prevent the volatilization of essential oils.
2.4. Determination of protein content and enzyme activities
Treated mites were washed three times with normal saline (0.9% w/v of NaCl solution). Samples were homogenized in 900 μl of normal saline and centrifuged at 2500 r/min (4 °C) for 10 min. The supernatant was used to determine the water-soluble protein concentration, using a total protein quantification assay kit (A045-3, Nanjing Jiancheng Bioengineering Institute, China). Briefly, the proteins reduced cupric ion to cuprous ion, which was further adducted with bicinchoninic acid (BCA). The product has a maximum absorbance at 562 nm. The protein content was calculated based on the absorbance of the solution.
The enzyme bioactivities of GST, SOD and Ca2+-Mg2+-ATPase were evaluated using a GST assay kit (catalog no. A004, Nanjing Jiancheng Bioengineering Institute, China), an SOD assay kit (catalog no. A001-3, Nanjing Jiancheng Bioengineering Institute, China) and a Ca2+-Mg2+-ATPase assay kit (catalog no. A070-3, Nanjing Jiancheng Bioengineering Institute, China) according to the manufacturer’s instructions.
2.5. Statistical analysis
The total protein content and the enzyme bioactivities of the different groups were analysed using a one-way ANOVA in SPSS (version 17.0).
3. Results
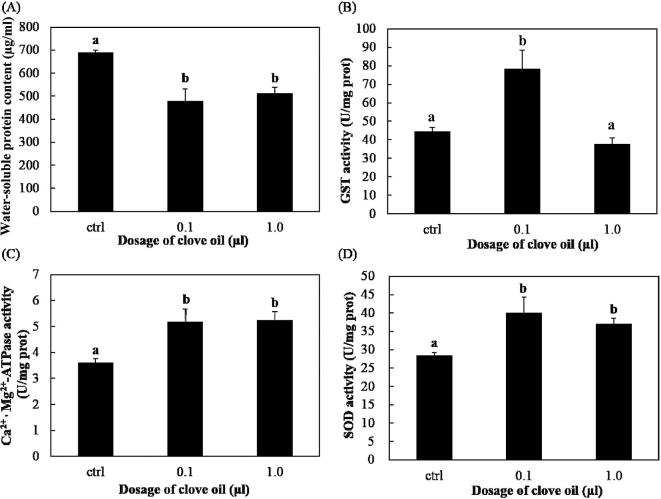
The water-soluble protein content of the V. destructor mites decreased significantly with exposure to clove oil: control group (690.84 ± 8.95 μg/ml), 0.1 μl clove oil (479.43 ± 51.35 μg/ml) and 1.0 μl clove oil (513.52 ± 25.63 μg/ml) (df = 2, F = 37.303, p0.1 vs control < 0.05, p1.0 vs control < 0.05, Fig. 2A). However, no significant difference was observed between the two treatment groups (p0.1 vs 1.0 = 0.466). As for the GST bioactivity of mites, it significantly increased at a dosage of 0.1 μl (78.42 ± 9.84 U/mg protein) compared with that of the control group (44.59 ± 2.15 U/mg protein, p0.1 vs control < 0.05); however, when treated with 1.0 μl of clove oil, the activity of this enzyme decreased (37.71 ± 3.21 U/mg protein), but the difference was not significant compared to the control group (p1.0 vs control = 0.424, Fig. 2B). The bioactivity of Ca2+-Mg2+-ATPase was significantly elevated when treated with 0.1 μl (5.19 ± 0.47 U/mg protein) and 1.0 μl (5.25 ± 0.31 U/mg protein) compared with the control group (3.62 ± 0.145 U/mg protein). There was no significant difference between the two treatment groups (df = 2, F = 22.271, p0.1 vs control < 0.05, p1.0 vs control < 0.05, p0.1 vs 1.0 = 0.981, Fig. 2C). A similar trend was observed for the enzyme activity of SOD, which was significantly elevated at 0.1 μl (40.11 ± 5.23 U/mg protein) and 1.0 μl (37.10 ± 1.46 U/mg protein) compared with that of the control group (28.52 ± 0.65 U/mg protein, df = 2, F = 15.924, p0.1 vs control < 0.05, p1.0 vs control < 0.05, p0.1 vs 1.0 = 0.393, Fig. 2D).
Fig. 2.
Water-soluble protein content and enzyme bioactivities of V. destructor with and without clove oil treatment. Mean values ± S.D. are shown. Different letters above the columns indicate significant differences among the groups (p < 0.05).
4. Discussion
In some countries, clove oil has been used to treat V. destructor in the field. However, an explanation of the biochemical mechanisms behind its acaricidal effects is lacking thus far. By determining the water-soluble protein content and enzyme activities of V. destructor when exposed to different amounts of clove oil, we explored its physiological effects on V. destructor mites.
The V. destructor mite feeds on the haemolymph of honey bees. Lacking proteinase, the mite makes use of the host’s undigested haemolymph proteins to promote oogenesis. As the major tissue type of the open circulatory system of lower arthropods (Wyatt, 1961), haemolymph is the site of metabolic processes and the exchange and storage of substances, such as proteins (Gudderra et al., 2002), and it plays a vital role in immunity and defence mechanisms (Theopold et al., 2004). Because proteins are important functional components of living organs, our study examined the water-soluble protein content of V. destructor. Our results revealed that it significantly decreased after clove oil treatment, indicating that the metabolism and the immunity system of the mites were possibly affected. This may have been caused by a decrease in feeding activity or an increase in the digestion of proteins by the mites.
In oxidative metabolism, reactive oxygen species (ROS), including superoxide anion (O2−), hydrogen peroxide (H2O2) and hydroxyl radicals (HO−) contribute to (Livingstone, 2001) oxidative stress, leading to amino acid and DNA damage (Imlay, 2003, Stadtman and Levine, 2003). Antioxidant enzymes, such as superoxide dismutase (SOD) (Dubovskiy et al., 2008), help prevent ROS-induced damage by removing oxygen free radicals; therefore, they play an important role in the antioxidant defence system (Park et al., 2009). In our study, SOD activity significantly increased after clove oil treatments, suggesting that the antioxidant reaction of V. destructor is triggered by essential oils.
Calcium-magnesium ATPase (Ca2+-Mg2+-ATPase), which is universally considered an indicator of cell health (Haya and Waiwood, 1983, Atli and Canli, 2013, Eroglu and Canli, 2013), acts as a membrane-bound enzyme and is responsible for the maintenance of calcium homoeostasis in cells (Carafoli, 1991, Nadukuru and Yallapragada, 2015). Changes in ATPase activity, caused by a disruption in transmembrane cation transport, are identified as key factors in cellular dysfunction (Stephenson, 1985). While a temporary increase in intracellular calcium ion concentrations is necessary to activate physiological functions of tissues, a sustained increase will lead to cell death (Orrenius et al., 1989). Therefore, it is critical to protect calcium homoeostasis against interference from any extraneous toxins. The bioactivities of Ca2+-Mg2+-ATPase were significantly elevated at both clove oil dosages, suggesting that clove oil may influence the cell function of V. destructor by inducing an excessive intracellular calcium ion concentration.
The glutathione-S-transferase enzymes are a major family of enzymes that detoxify cytosol (Ranson et al., 1997) by protecting against endogenous and exogenous toxic chemicals (Sheehan et al., 2001). GSTs protect other endogenous substances, such as proteins and nucleic acids, by catalysing the conjugation of activated xenobiotics and endogenous glutathione (GSH) to a water-soluble substrate. Elevated levels of GST activity are associated with insecticide resistance, such as DDT resistance in Anopheles gambiae (Ranson et al., 1997). In this study, the bioactivity of GSTs increased significantly after treatment with 0.1 μl of clove oil, indicating that the self-protection mechanism of V. destructor mites was triggered by exposure to a low dosage of essential oil. In contrast, it was interesting to see that GST bioactivity decreased significantly when the mites were treated with a relatively high dosage. This indicates that the self-protection mechanism of V. destructor mites may not work when the mites are exposed to a high dosage of clove oil. Moreover, these inverse trends of GST activity indicate that the amount of clove oil used could affect its acaricidal effects.
5. Conclusion
In this study, we determined the effects of clove oils on the water-soluble protein content and enzyme bioactivities of V. destructor mites. Our results show that water-soluble protein content and the activities of enzymes related with detoxification/protection were influenced. The significant change of these parameters after the treatment of 0.1 μl clove oil suggested that the related physiological process were affected/activated even no acute toxicity occurred to mites. The decease of GST activity after treatment with 1.0 μl clove oil indicated dysfunction of this detoxification enzyme may be related to the acute toxicity caused by clove oil.
Acknowledgements
This work was supported by the Modern Agro-industry Technology Research System (No.CARS-45), the Science and Technology Department of Zhejiang Province, China (2012C12906-19), National Natural Science Foundation of China (31372254) and the Science and Technology Innovation Plan of University Students in Zhejiang Province (No. 2015R401266).
Footnotes
Peer review under responsibility of King Saud University.
References
- Amdam G.V., Hartfelder K., Norberg K., Hagen A., Omholt S.W. Altered physiology in worker honey bees (Hymenoptera: Apidae) infested with the mite Varroa destructor (Acari: Varroidae): a factor in colony loss during overwintering? J. Econ. Entomol. 2004;97(3):741–747. doi: 10.1093/jee/97.3.741. [DOI] [PubMed] [Google Scholar]
- Atli G., Canli M. Metals (Ag+, Cd2+, Cr6+) affect ATPase activity in the gill, kidney, and muscle of freshwater fish Oreochromis niloticus following acute and chronic exposures. Environ. Toxicol. 2013;28(12):707–717. doi: 10.1002/tox.20766. [DOI] [PubMed] [Google Scholar]
- Bogdanov S., Kilchenmann V., Imdorf A. Acaricide residues in some bee products. J. Apicult. Res. 1998;37(2):57–67. [Google Scholar]
- Carafoli E. The calcium pumping ATPase of the plasma membrane. Annu. Rev. Physiol. 1991;53(1):531–547. doi: 10.1146/annurev.ph.53.030191.002531. [DOI] [PubMed] [Google Scholar]
- Committee N.P. Chinese Medicine Science and Technology Press; Beijing, China: 2010. The People’s Republic of China Pharmacopoeia. [Google Scholar]
- Dietemann V., Pflugfelder J., Anderson D., Charrière J., Chejanovsky N., Dainat B., de Miranda J., Delaplane K., Dillier F., Fuch S., Gallmann P., Gauthier L., Imdorf A., Koeniger N., Kralj J., Meikle W., Pettis J., Rosenkranz P., Sammataro D., Smith D., Yañez O., Neumann P. Varroa destructor: research avenues towards sustainable control. J. Apicult. Res. 2012;51(1):125–132. [Google Scholar]
- Dubovskiy I.M., Martemyanov V.V., Vorontsova Y.L., Rantala M.J., Gryzanova E.V., Glupov V.V. Effect of bacterial infection on antioxidant activity and lipid peroxidation in the midgut of Galleria mellonella L. larvae (Lepidoptera, Pyralidae) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2008;148(1):1–5. doi: 10.1016/j.cbpc.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Elzen P.J., Eischen F.A., Baxter J.R., Elzen G.W., Wilson W.T. Detection of resistance in US Varroa jacobsoni Oud. (Mesostigmata: Varroidae) to the acaricide fluvalinate. Apidologie. 1999;30:13–18. [Google Scholar]
- Eroglu A., Canli M. Effects of Cd, Zn and Cd+ Zn combination on ATPase activitiy in the gill and muscle of tilapia (Oreochromis niloticus) Bull. Environ. Contam. Toxicol. 2013;91(4):420–425. doi: 10.1007/s00128-013-1076-6. [DOI] [PubMed] [Google Scholar]
- Gashout H.A., Guzmán-Novoa E. Acute toxicity of essential oils and other natural compounds to the parasitic mite, Varroa destructor, and to larval and adult worker honey bees (Apis mellifera L.) J. Apicult. Res. 2009;48(4):263–269. [Google Scholar]
- Girisgin A.O., Barel S., Barzilai D.Z., Girisgin O. Determining the stability of clove oil (eugenol) for use as an acaricide in beeswax. Isr. J. Vet. Med. 2014;69:4. [Google Scholar]
- Gudderra N.P., Sonenshine D.E., Apperson C.S., Roe R.M. Hemolymph proteins in ticks. J. Insect Physiol. 2002;48(3):269–278. doi: 10.1016/s0022-1910(02)00050-1. [DOI] [PubMed] [Google Scholar]
- Haya K., Waiwood B.A. Adenylate energy charge and ATPase activity: potential biochemical indicators of sublethal effects caused by pollutants in aquatic animals. Adv. Environ. Sci. Technol. 1983;13:307–333. [Google Scholar]
- Imlay J.A. Pathways of oxidative damage. Annu. Rev. Microbiol. 2003;57(1):395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- Isman M.B. Plant essential oils for pest and disease management. Crop Prot. 2000;19(8):603–608. [Google Scholar]
- Kralj J., Brockmann A., Fuchs S., Tautz J. The parasitic mite Varroa destructor affects non-associative learning in honey bee foragers, Apis mellifera L. J. Comp. Physiol. A. 2007;193(3):363–370. doi: 10.1007/s00359-006-0192-8. [DOI] [PubMed] [Google Scholar]
- Le Conte Y., Ellis M., Ritter W. Varroa mites and honey bee health: can Varroa explain part of the colony losses? Apidologie. 2010;41(3):353–363. [Google Scholar]
- Lindberg C.M., Melathopoulos A.P., Winston M.L. Laboratory evaluation of miticides to control Varroa jacobsoni (Acari: Varroidae), a honey bee (Hymenoptera: Apidae) parasite. J. Econ. Entomol. 2000;93(2):189–198. doi: 10.1603/0022-0493-93.2.189. [DOI] [PubMed] [Google Scholar]
- Livingstone D.R. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar. Pollut. Bull. 2001;42(8):656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- Mahmood R., Asad S., Raja S., Ul Moshin A., Wagchoure E.S., Sarwar G., Islam N., Ahmad W. Control of Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) by using plant oils and extract. Pakistan J. Zool. 2014;46(3):609–615. [Google Scholar]
- Martin S.J., Highfield A.C., Brettell L., Villalobos E.M., Budge G.E., Powell M., Nikaido S., Schroeder D.C. Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336(6086):1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- Milani N., Barbattini R. Effectiveness of Apistan (fluvalinate) in the control of Varroa jacobsoni Oude-mans and its tolerance by Apis mellifera Linnaeus. Apicoltura. 1988;4:39–58. [Google Scholar]
- Milani N., Iob M. Plastic strips containing organophosphorous acaricides to control Varroa jacobsoni: a preliminary experiment. Am. Bee J. 1998;138(8):612–615. [Google Scholar]
- Nadukuru N., Yallapragada P.R. In vitro and in vivo inhibition of Ca2+-Mg2+-ATPase activity by cadmium in post larvae of Penaeus monodon. Chem. Ecol. 2015;31(5):446–454. [Google Scholar]
- Nazzi F., Brown S.P., Annoscia D., Del Piccolo F., Di Prisco G., Varricchio P., Della Vedova G., Cattonaro F., Caprio E., Pennacchio F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honey bee colonies. PLoS Pathog. 2012;8(6):e1002735. doi: 10.1371/journal.ppat.1002735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann P., Carreck N.L. Honey bee colony losses. J. Apicult. Res. 2010;49(1):1–6. [Google Scholar]
- Oldroyd B.P. What's killing American honey bees? PLoS Biol. 2007;5(6):e168. doi: 10.1371/journal.pbio.0050168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrenius S., McConkey D.J., Bellomo G., Nicotera P. Role of Ca2+ in toxic cell killing. Trends Pharmacol. Sci. 1989;10(7):281–285. doi: 10.1016/0165-6147(89)90029-1. [DOI] [PubMed] [Google Scholar]
- Park H.S., Kim S.R., Lee Y.C. Impact of oxidative stress on lung diseases. Respirology. 2009;14(1):27–38. doi: 10.1111/j.1440-1843.2008.01447.x. [DOI] [PubMed] [Google Scholar]
- Ranson H., Prapanthadara L., Hemingway J. Cloning and characterization of two glutathione S-transferases from a DDT-resistant strain of Anopheles gambiae. Biochem. J. 1997;324(1):97–102. doi: 10.1042/bj3240097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz P., Aumeier P., Ziegelmann B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Sheehan D., Meade G., Foley V.M., Dowd C.A. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001;360(1):1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E.R., Levine R.L. Free radical-mediated oxidation of free amino acids and amino acid residues in proteins. Amino Acids. 2003;25(3–4):207–218. doi: 10.1007/s00726-003-0011-2. [DOI] [PubMed] [Google Scholar]
- Stephenson E.W. Excitation of skinned muscle fibers by imposed ion gradients. II. Influence of quercetin and ATP removal on the Ca2+-insensitive component of stimulated 45Ca efflux. J. Gen. Physiol. 1985;86(6):833–852. doi: 10.1085/jgp.86.6.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X., Zheng H., Fei Z., Hu F. Effectiveness of herbal essential oils as fumigants to control Varroa destructor in laboratory assays. Chin. J. Appl. Entomol. 2012;5:014. [Google Scholar]
- Theopold U., Schmidt O., Soderhall K., Dushay M.S. Coagulation in arthropods: defence, wound closure and healing. Trends Immunol. 2004;25(6):289–294. doi: 10.1016/j.it.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Thompson H., Brown M., Ball R.F., Bew M.H. First report of Varroa destructor resistance to pyrethroids in the UK. Apidologie. 2002;33(4):357–366. [Google Scholar]
- Wallace R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004;63(04):621–629. doi: 10.1079/pns2004393. [DOI] [PubMed] [Google Scholar]
- Wallner K. Varroacides and their residues in bee products. Apidologie. 1999;30:235–248. [Google Scholar]
- Wyatt G.R. The biochemistry of insect hemolymph. Annu. Rev. Entomol. 1961;6(1):75–102. [Google Scholar]