Abstract
Objective
To determine the feasibility of delivering inhaled treprostinil during mechanical ventilation and spontaneous unassisted ventilation using the Tyvaso Inhalation System (TIS), and t Vibrating Mesh Nebulizer (VMN). We sought to compare differences in fine particle fraction (FPF), and absolute inhaled treprostinil mass delivered to neonatal, pediatric, and adult models affixed with a facemask, conventional, and high frequency ventilation between TIS and with different nebulizer locations between TIS and VMN.
Design
FPF was first determined via cascade impaction with both the TIS and VMN. Next, a test lung, configured with neonatal, pediatric, and adult mechanics and a filter to capture medication was attached to a realistic face model during spontaneous breathing or an ETT during conventional ventilation and HFOV. Inhaled treprostinil was then nebulized with both the TIS, and VMN, and the filter was analyzed via HPLC. Testing was done in triplicate. Independent two-sample t-tests were used to compare mean FPF, and inhaled mass between devices. ANOVA with Tukey post-hoc tests were used to compare within device differences.
Setting
Academic children’s hospital aerosol research laboratory.
Measurements and Main Results
FPF was not different between the TIS and VMN (0.78 ± 0.04 vs 0.77 ± 0.08, respectively, P = 0.79). The VMN delivered the same or greater inhaled treprostinil than the TIS in every simulated model and condition. When using the VMN, delivery was highest when using HFOV in the neonatal and pediatric models, and with the nebulizer in the distal position in the adult model.
Conclusion
The VMN is a suitable alternative to the TIS for inhaled treprostinil delivery. FPF is similar between devices, and VMN delivery meets or exceeds delivery of the TIS. Delivery for infants and children during HFOV with the VMN may result in higher than expected dosages.
Keywords: treprostinil, aerosol drug therapy, nebulizers, mechanical ventilation, high-frequency ventilation, pulmonary hypertension
Introduction
Pulmonary Arterial Hypertension (PAH) is a rare condition that affects infants, children, and adults. This disease is characterized by pulmonary arteriolar constriction, pulmonary vasculature remodeling and consequent elevation in pulmonary vascular resistance leading to increased right heart strain and ultimately failure (1). In patients affected with PAH in the United States, initial inpatient costs are substantial with average costs reported over $80,000 per patient in the first year of treatment (2). Selective inhaled pulmonary vasodilators may help to reverse some of the effects of PAH and promote improved ventilation and perfusion matching (3).
Inhaled nitric oxide has been shown to improve oxygenation, and decrease the need for ECMO in infants with hypoxic respiratory failure (4) but it is costly and requires specialized equipment. Inhaled epoprostenol is a less costly alternative and has been shown to Improve the oxygenation index while maintaining systemic arterial pressure in neonates with persistent pulmonary hypertension of the newborn that are refractory to inhaled nitric oxide therapy (5). However, epoprostenol is not approved for inhaled use, is an alkaline sticky solution which can clog nebulizers (6) and even a brief discontinuation in nebulization could result in rebound pulmonary hypertension. This drug is also unstable at room temperature and has a short serum half-life (<6 minutes) (7). Inhaled iloprost has a longer serum half- life (20–30 minutes) than epoprostenol (< 6 min) which allows it to be delivered with intermittent nebulization (8), and has been shown to be as effective as inhaled nitric oxide at improving ventilation and perfusion matching (9). Inhaled treprostinil has been shown to be effective and safe in both adults (10–11) and children (12) and has reportedly fewer systemic side effects than inhaled iloprost. Treprostinil has a half-life up to four hours (8), which allows therapy to be given less frequently than iloprost. Currently, the only FDA approved nebulizer for delivery is via the Tyvaso (treprostinil) Inhalation System (TIS) (United Therapeutics Corp, Silver Springs, MD) which incorporates an ultrasonic nebulizer (OPTINEB®-ir, NEBU-TEC, Elsenfeld, Germany) as the aerosol generator. This system provides automatic timed actuations of the nebulized medication and is meant for awake and cooperative patients as the patient must inhale when alerted to do so by the device. As such, the TIS is not intended to be used during mechanical ventilation and there are currently no recommendations for selecting aerosol delivery devices or how those devices should be configured within the patient circuit to efficiently deliver inhaled treprostinil during mechanical ventilation. Delivery with a jet nebulizer has been reported (13), but this adds gas flow into the system which may affect ventilator parameters as well as the patient’s ability to trigger the ventilator (14). Many institutions are using Vibrating Mesh Nebulizers (VMN) to deliver inhaled medications during mechanical ventilation because it does not affect ventilator parameters or function (14); however, there are no published in-vitro or in-vivo studies describing the efficacy of inhaled treprostinil delivery with a VMN in mechanically ventilated or spontaneous breathing patients.
We sought to explore the possibility of using a VMN (Aeroneb®Solo, Aerogen, Galway, Ireland) to deliver inhaled treprostinil in a variety of simulated conditions and in-vitro patient models including an infant, pediatric, and adult model. The objectives of this study were to determine whether there were differences in treprostinil drug delivery between VMN and TIS with respect to: 1) respirable particle size or fine particle fraction (FPF); 2) lung model filter mass during spontaneous breathing and two different forms of invasive mechanical ventilation; and 3) lung model filter mass at different nebulizer circuit positions/locations during mechanical ventilation.
Materials and Methods
Fine particle fraction (FPF)
The total mass of inhaled treprostinil delivered within the respirable particle range of the human respiratory tract was quantified with the VMN and TIS using a multi-staged Next Generation Impactor (NGI, Copley Scientific, Nottingham, UK) (Supplemental Digital Content 1 describes technical details). Each type of nebulizer was tested in triplicate with a new nebulizer used each time. To ensure detectable amounts of drug at the various stages of the NGI, larger than clinically recommended doses were used. Inhaled treprostinil was placed in the VMN (0.84 ml, 504μg) and it was nebulized through the NGI throat with an occlusive rubber fitting adapter until dry; no residual medication was noted. Inhaled treprostinil was then placed in the TIS and 84 inhalations (6 μg per inhalation, 504μg total) were actuated. Any residual medication left in the cup of the TIS was collected and measured to determine total dose delivered. Drug was eluted from the NGI throat and collection cups using Ethanol (20 ml for the throat and 10 ml for the trays) and placed into labeled collection cups. The collection cups were immediately washed and dried following sample recovery.
Lung Model
A test lung (ASL 5000, Ingmar Medical with software package (version 3.2) was configured for term neonate, small child, and adult models used with spontaneously breathing and ventilator assisted breathing, as shown in Table 1.
Table 1. Testing Parameters.
Table 1 shows the testing parameters and settings for the test lung (ASL 5000), conventional ventilator, and high-frequency oscillator ventilator for each model type (neonatal, pediatric, adult).
| Testing Parameters for Each Lung Model | ||||
|---|---|---|---|---|
| Testing Parameter | Neonatal (4kg) | Pediatric (20 kg) | Adult (70 kg) | |
| Test Lung Settings | Resistance (cmH2O/L/s) | 50 | 25 | 5 |
| Compliance (ml/cmH2O) | 4 | 20 | 70 | |
| Pleural Pressure (spontaneous only) (cmH2O) | 8 | 10 | 11 | |
| Rate (bpm) | 30 | 20 | 15 | |
| Upper airway (spontaneous only) | One month old infant | 5 year old child | Adult | |
| Lower Airway | 3.5 ET tube | 4.5 ET tube | 7.5 ET tube | |
| Conventional Ventilator Settings | Ventilator | SERVO-i | SERVO-i | SERVO-i |
| Circuit Size | Infant | Ped/Adult | Ped/Adult | |
| Mode | PRVC | PRVC | PRVC | |
| Tidal Volume (ml) | 24 | 140 | 560 | |
| RR (bpm) | 30 | 20 | 15 | |
| Ti (s) | 0.40 | 0.75 | 1.0 | |
| FiO2 | 1.00 | 1.00 | 1.00 | |
| PEEP (cmH2O) | 5 | 5 | 5 | |
| Bias Flow (LPM) | 0.5 | 2 | 2 | |
| High-Frequency Oscillator Ventilation Settings | Ventilator | 3100 A | 3100 A | 3100 B |
| Amplitude (ΔP) | 36 | 46 | 66 | |
| MAP (cmH2O) | 14 | 20 | 30 | |
| HZ | 10 | 8 | 6 | |
| Bias Flow (LPM) | 15 | 20 | 30 | |
| IT (%) | 33 | 33 | 33 | |
| FiO2 | 1.00 | 1.00 | 1.00 | |
Two bacterial/viral electret filters (Respirgard-II, Vital Signs; Englewood, Colorado) were connected in series between the distal tip of the endotracheal tube (or at the end of the realistic upper airway model during mask delivery) and the lung model. One filter was used to collect the drug delivered while the second filter was used to protect the internal components of the lung model.
Ventilator and Humidification
Two ventilators, a SERVO-i (Marquet, Solna, Sweden) conventional critical care ventilator and a Sensor Medics 3100A or 3100 B high frequency oscillator ventilator (HFOV; Carefusion, Yorba Linda, CA), equipped with respective heated wire circuits were used for this study. A Fisher and Paykel MR730 heater (Auckland, NZ) was used for humidification with all ventilators and the temperature was allowed to stabilize for 30 minutes prior to testing. The choice of setup and settings were based on what is commonly used in the clinical setting at our institutions. A ventilator pre-use check was performed per manufacturer recommendations prior to testing. Table 1 shows the ventilator settings used during conventional and HFOV ventilation.
Nebulizers
An Aeroneb®Solo vibrating mesh nebulizer (VMN) was used for testing. The VMNs were placed in-line using the specialized Aerogen adaptors provided for use with mechanical ventilation. A Pro-X controller unit (Aerogen, Galway, Ireland) in continuous mode was used to power the nebulizers (Supplemental Digital Content 2 provides further device description). A Tyvaso® Inhalation System (TIS) was also used for testing. The TIS is a OPTINEB®-ir (NEBUTEC med; Elsenfeld, Germany) Microprocessor controlled ultrasonic nebulizer configured for delivery of inhaled treprostinil during timed inhalation periods. Since the TIS is not designed for use with mechanical ventilation it required use of several adapters to be placed in the circuit.
Nebulizer Position during Mechanical Ventilation
Two nebulizer conditions (Figure 1A) were tested during conventional ventilation, Proximal (nebulizer placed between the patient wye connecter and the inspiratory limb), and Distal, (nebulizer placed on the inlet of the humidifier). During HFOV, nebulizer placement in the Distal Position has been shown to deliver negligible medication (15) thus only delivery in the Proximal Position was tested (nebulizer placed between patient wye connector and ETT) (Figure 1B).
Figure 1. Experimental Set-up for Conventional and High Frequency Ventilation.
Figure 1A shows the different nebulizer positions used within the heated circuit when testing drug delivery between the two devices during conventional ventilation with the Servo-I ventilator. Figure 1B shows the nebulizer position used within the heated circuit when testing drug delivery between the two devices during high frequency oscillatory ventilation. For simplicity, this figure shows only the VMN nebulizer but the TIS was also used in testing (Figure modified from reference 27).
Medication Delivery during Mechanical Ventilation
The nebulizers were placed in their respective positions for the condition being tested and the system heat and humidification was allowed to stabilize 20 minutes prior to testing at each condition. When testing was performed with the TIS, one ampule of inhaled treprostinil (2.9 ml, 1.74mg) was placed in the medication cup and 9 inhalations at 6μg per inhalation (54μg total) were delivered in each condition. In CMV conditions, the manual breath key on the SERVO-i was used to coordinate ventilator breaths with TIS inhalations. During HFOV there was no attempted coordination of nebulizer actualization with inhalations due to the rapid respiratory rate. The medication cup was discarded, nebulizer components were replaced, and adapters were rinsed with sterile water and allowed to dry completely between each testing condition.
The TIS nebulizes only periodically in time intervals that may, with difficulty be synchronized with a ventilator; whereas, the VMN will nebulize continuously, thus drug delivery during exhalation is considered to be negligible.(14) To accommodate for this increase in waste with the VMN, the 54μg dosage was adjusted to account for total cycle time spent in inspiration vs expiration using the equation 54μg x (I + E) / 600mcg/mL, assuming an I:E ratio of 1:3, then 0.36 mL (216 μg) was used with the VMN.
The adjusted inhaled treprostinil dose was drawn up into a 1 ml filter needle syringe, placed into the VMN and nebulized until dry. Special care was taken to position the filter superior to the endotracheal tube to avoid fluid condensate from accumulating in the tube and falling into the filter when a heated humidifier was used (mechanical ventilation). Testing was completed when all of the solution was nebulized. The bacterial/viral electret filter at the end of the endotracheal tube was removed, labeled and recorded in a laboratory notebook and placed into a refrigerator. Between each testing condition the VMN and ET tubes were rinsed with sterile water and allowed to dry completely to reduce the delivery of residual medication delivery with subsequent testing or the likelihood of large drug molecules reaching the filter.
Medication Delivery via Facemask
The VMN and TIS were attached to a realistic anatomic face/upper airway and spontaneously breathing lung model configured for a newborn, small child, and adult using a tight fitting mask (Figure 2). The realistic airway models used in the current study have been described elsewhere (16). A Vital Signs™ Adult size 5 mask (BD, Franklin Lakes, NJ) was placed on an adult airway model; Vital Signs™ Toddler size 3 mask was placed on a Child (5 yr) model and Vital Signs™ neo size 1 mask was placed on an infant nasal model. We selected non-vented masks because it is suggested that eye and skin contact of iTre should be avoided in patients. The same dosing and drug delivery strategy for the VMN and TIS nebulizer delivery was used during the mask spontaneous breathing condition as those used in the ventilation conditions.
Figure 2. Experimental Set-up for Spontaneous breathing with Face Mask.
Figure 2 shows one example of treprostinil face mask delivery in the pediatric small child model. For simplicity, this figure shows only the VMN nebulizer but the TIS was also used in testing.
Inhaled Treprostinil Measurement
Inhaled treprostinil was recovered from the bacterial/viral electret filter following nebulization and quantified using high-performance liquid chromatography. (Supplemental Digital Content 3 provides technical details). Testing was done in triplicate for each nebulizer type (using a different nebulizer each time), patient model, and nebulizer condition for a total of 72 measurements.
Data Analysis
Inhaled treprostinil concentrations were recorded in an Excel (Microsoft, Redmond, WA) spread sheet and expressed as the absolute mass (μg) that adhered to and was recovered from the respective filters or collection cups (NGI), and was recovered from the filter or collection cup of the impactor. FPF was calculated as the ratio of inhaled fine particle mass to the total mass of drug inhaled as aerosol (17). In this experiment, particles were considered fine if they adhered to collection cups on stages 3–7 of a NGI, corresponding with a respirable particle size range of 0.98–5.39 μg median diameter (D50).
All data were expressed as mean ± SD (n = 3 tests for each model, condition, and device). Statistical analyses were performed using R Statistical Software (version 3.1.2; R Foundation for Statistical Computing, Vienna, Austria). An independent T-test was done to compare the mean fine particle fraction of inhaled treprostinil between the TIS and VMN. Independent T tests were also performed to compare differences between mean inhaled treprostinil mass (μg) from the two nebulizers, stratified by condition and lung model. Within nebulizer types, the differences in inhaled treprostinil mass (μg) in-between nebulizer conditions were explored via one-way ANOVA with post-hoc Tukey tests. Statistical significance was set a priori at P < 0.05.
Results
Fine particle fraction
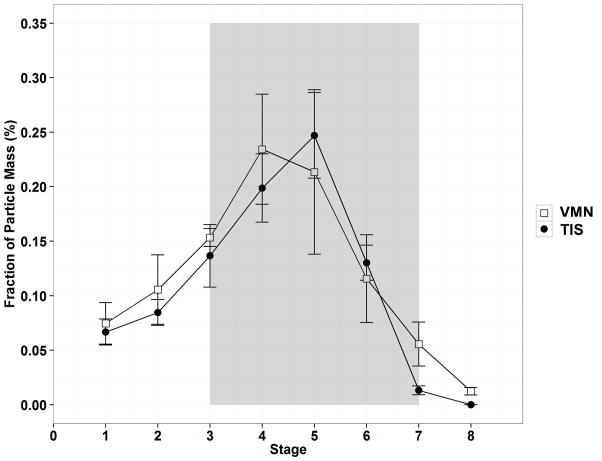
The fraction of aerosol particles mass that are considered fine (deposited in stage 3–7 of the NGI) between the two nebulizers is shown in Figure 3. The total fine particle fraction of inhaled treprostinil was not different between the VMN and TIS (0.78 ± 0.04 vs 0.77 ± 0.08, respectively, P = 0.79).
Figure 3. Fraction of Respirable Fine Particle Mass in Lung Impactor.
Figure 3 shows the Fraction of Particle Mass (%) between nebulizer types across the different stages of the next generation aerosol impactor. The shaded grey area (stages 3–7; MMAD) represents the fraction of fine particle mass delivered to the collection cups of the impactor.
Delivery between devices
The inhaled treprostinil mass (μg) for each condition is shown in Figure 4. The VMN delivered greater inhaled treprostinil than the TIS in every simulated model and nebulizer condition (P < 0.05), except when no statically significant differences occurred between the TIS and VMN when using the pediatric model with the nebulizers in the distal position (34.6 ± 0.6 and 25.5 ± 4.4 μg, respectively, P = 0.07), face mask condition (16.1 ± 2.5 and 14.2 ± 0.4 μg, respectively, P = 0.33), and when using the adult model with the nebulizers in the proximal position (30.1 ± 3.2 and 33.3 ± 4.6, respectively, P = 0.39).
Figure 4. Lung Model Deposition from Nebulizers used in Different Facemask and Ventilator Configurations.
Figure 4 shows Inhaled treprostinil mass (μg) deposited to the filter between nebulizer types across the different lung model types and nebulizer conditions. For comparison between nebulizers significant symbols are as follows:* P < 0.05, ** P < 0.01. For comparisons within nebulizers across different location conditions, significant values are as follows: § dose was higher at this position than all other positions P < 0.05, || dose was higher at this position that the distal and mask positions P < 0.05, † dose was higher at this position than the mask position P < 0.05 ‡ dose was higher at this position than the proximal and mask positions P < 0.05.
Delivery between nebulizer conditions (TIS)
In the neonatal model, delivery was highest during HFOV (11.6 ± 0.8 μg, P < 0.01), whereas in the pediatric and adult model delivery was highest with the nebulizer in the distal positions (36.6 ± 0.6 and 50.1 ± 4.5 μg, respectively, P < 0.01). The TIS in the proximal position delivered more drug than the distal and mask position only in the neonatal model (7.1 ± 1.8 μg, versus 3.1 ± 0.6 and 1.2 ± 0.1 μg, respectively, P < 0.01). In the adult model, placing the nebulizer in the proximal or HFOV position delivered more drug than in the mask position (30.1 ± 3.2 and 22.7 ± 4.1 μg, respectively, versus 6.7 ± 0.8 μg, P < 0.01).
Delivery between nebulizer conditions (VMN)
In the neonatal and pediatric model, delivery was greatest during HFOV (68.2 ± 6.3 and 63.1 ± 2.3 μg, respectively, P < 0.01). In the adult model, delivery was greatest with either HFOV or the distal position (47.5 ± 5.5 and 61.5 ± 2.3 μg, respectively, P < 0.01). Delivery was higher in the distal position than the mask position in the pediatric model (25.3 ± 4.4 μg versus 14.2 ± 0.4 μg, P < 0.01). In the adult model delivery was higher with the nebulizer in the proximal position than the mask position (33.3 ± 4.6 μg versus 19.5 ± 0.6 μg, P < 0.01).
Discussion
The efficacy of inhaled drugs is highly dependent on the deposition of medication throughout the lungs (18). Our data suggests that when inhaled treprostinil is nebulized with either the VMN or the TIS, the fine particle fraction is similar between devices and that the majority of the particles are sufficiently small to be adequately deposited in the lung. Our reported fine particle fraction of 0.78 with the TIS, and 0.77 with the VMN should be sufficiently high enough to provide adequate lung deposition as previous work has been done to demonstrate a reasonable correlation between in-vitro fine particle fraction (< 5.8 μg) and in-vivo whole lung deposition (19). Other investigators have reported inhaled treprostinil fine particle fraction with some differing results. Patel et al. (13) reported a higher inhaled treprostinil fine particle fraction of 0.98 when using the Aerotech II small volume nebulizer placed inline with a ventilator, while Sidler-Moix et al. (20). reported a lower fine particle fraction of 0.47–0.54 when nebulizing albuterol with a VMN. Differences in reported fine particle fractions may be expected however as it has been shown that device differences or the physiochemical properties of a substance may affect fine particle fraction (21) We are the first to report to report fine particle fraction of inhaled treprostinil when using a VMN and these results should be confirmed, and ideally with in-vivo/in-vitro correlations using the VMN with the target adult maintenance dosage of 54μg.
We quantified the amount of inhaled treprostinil delivered when using the VMN versus the TIS in a variety of conditions and models. Overall the VMN delivered as much if not more inhaled treprostinil mass as the TIS in every condition and patient model. We used an adjusted dose based on Total Cycle Time (54μg x (I + E) / 600mcg/mL) for the VMN to account for drug loss when nebulizing continuously during the expiratory phase to achieve a target filter dose of 54μg which is the target maintenance dose per the label claim. Patel et al. (13) used a similar approach when nebulizing inhaled treprostinil, although the algorithm differed slightly and was calculated as (number of breaths needed = 29 seconds/Inspiratory Time), so for inspiratory times shorter than 1 second, the number of breaths needed would be higher than 29. However, the lowest inspiratory time they reported was 0.7 seconds, whereas our lowest inspiratory time was 0.4 seconds during conventional ventilation. In general their adjusted dosing and our adjusted dosing were intentionally higher to offset losses that occurred during exhalation. When using their dosing algorithm Patel also delivered higher than target dosages in some of their ventilator conditions which were similar to our findings. There are some concerns in regards to the potential for overdosing with inhaled treprostinil with an adjusted dose with a VMN. The label claims a target single treatment maintenance dose of inhaled treprostinil is 54μg 4 times a day in adults; however, the recommendation is to start with a lower dose of 18μg and titrating up slowly as tolerated over several weeks to the maintenance dose to avoid systemic adverse effects. Nelsen and Zaccardelli (22) reported in healthy adults that when delivering a 90μg as a single dose to healthy adults there were adverse events of chest pain, chest discomfort, nausea, and vomiting. It is for this reason that the maximum tolerable dose was determined to be 84μg. The highest dosages we achieved were during HFOV and they were all below the maximal tolerable dose found by Nelsen et al. (22) however the maximal tolerable dose of 84μg for adults may be lower in children, and our reported doses of 74μg and 65μg that occurred during HFOV for our neonatal and pediatric models, respectively may exceed or approach the limit for a tolerable dose, thus we recommend to approach dosing cautiously when using HFOV for neonates and pediatrics. Takatsuki et al. (12) are the only investigators to report a few adverse events of mild or moderate hypotension in children with inhaled treprostinil use and these cases resolved without any interventions. However, further work should be done to describe these adverse events in infants and children particularly when given during high-frequency ventilation when there is a greater chance for overdosing.
We characterize differences in inhaled treprostinil delivery with both the VMN and TIS when placing the nebulizer in different conditions (face mask, proximal and distal positions during mechanical ventilations, and HFOV). Our in-vitro results demonstrate that nebulizer condition plays a key role in inhaled treprostinil delivery across different patient models for the VMN and TIS. Nebulizer position during conventional ventilation impacts aerosol delivery greatly, and appears to be related to circuit size, bias flow, and tidal volume, where the optimal nebulizer position may vary across different patient and model sizes and conditions (23–27). Our results are similar to Ari et al. (23) in that we did not find a significant difference between the proximal and distal positions of the VMN with the pediatric model, but we did find larger delivery with the nebulizer in the distal position with the adult model. Our data also confirms similar findings with DiBlasi et al. (27) who observed greater nebulized iloprost delivery in the proximal than distal position for their neonatal model. Generally as model size increases drug delivery tends to increase as well in in-vitro studies due to the advantages of increased tidal volumes, and larger bore circuitry (15, 23). We speculate that the larger observed differences in drug delivery in the distal position with adult testing may be best explained by the reservoir-like effect of the aerosol gathering in the inspiratory limb during exhalation which may result in a larger bolus of drug delivered to the lungs than when the nebulizer is placed proximally. This relationship is different with infant conventional ventilator testing due to high respiratory rates, short inspiratory times and small tidal volumes. These effects are described in greater detail elsewhere (23–28). Our results also demonstrate superior aerosol delivery when using a VMN during HFOV in our infant and pediatric models compared with conventional ventilation which confirms reports by DiBlasi (27) and Alzahrani (28).
Limitations
As with other in-vitro studies the results of this study must be approached cautiously. The amount of drug delivered to the filter represents the total mass of available drug delivered distal to the artificial airway, but it does not take into account the amount of aerosol that would normally be exhaled. The total cycle time adjusted dose for the VMN is under the assumption that the TIS actuations would all be synchronized during inspiration, but the TIS inspiration time is static at 2.5 seconds so any inspiratory time shorter than 2.5 seconds will result in some waste where the TIS is nebulizing during the expiratory phase of a ventilator breath. In these instances where inspiratory time is shorter, the VMN would be given an advantage over the TIS when increasing the dose of the VMN alone as we did in this study. Additional limitations include using only one particular brand of critical care conventional and high-frequency ventilator, humidifier, circuit, lung model setting, and ventilator settings.
Conclusion
Given these findings, it would seem that the VMN is a suitable alternative to the TIS for inhaled treprostinil delivery with both mechanical ventilation and spontaneous breathing. The VMN provides a sufficiently high fine particle fraction suitable for lung deposition, and when using an adjusted inhaled treprostinil dose calculated based upon the total cycle time using the equation: Dose x (I + E) / 600μg/mL. VMN delivery meets or exceeds delivery of the TIS. For optimal inhaled treprostinil delivery during conventional ventilation we recommend placing the VMN proximal to the patient wye in an infant circuit and distal to the humidifier in a pediatric circuit. Placing the VMN distal to the humidifier during conventional ventilation or proximal to the endotracheal tube during HFOV may result in higher than expected doses. In these cases, patients should be monitored closely for adverse effects. We used the target maintenance dose of 54ug per treatment session in this in-vitro study; however; for clinical use, the health care professionals should refer to the Full Prescribing Information.
Supplementary Material
Acknowledgments
United Therapeutics provided inhaled treprostinil medication and TIS supplies for this research. Funding for writing support, travel, and research time was provided through an investigator research grant from United Therapeutics, but no contribution was made in terms of study design, analysis of the data, or the writing of this paper. Robert DiBlasi has received research funding from Draeger Medical, Mallinckrodt Medical, and Aerogen Pharma. The University of Colorado contracts with United Therapeutics for Dr. Ivy to be a consultant. Donna Parker has received consulting fees and has served on advisory panels for Mallinckrodt Medical and is a KOL for Aerogen. None of the other authors have any conflicts of interest to disclose.
Footnotes
Work was performed in the department of respiratory care at Seattle Children’s Hospital, Seattle, WA under the supervision of the last author (RMD).
No reprints will be ordered.
Copyright form disclosure:
Ms. Parker’s institution received funding from United Therapeutics Corporation; she disclosed other funding support for salary support, travel, for obtaining measurements, statistical analysis, and for writing assistance; she has received consulting fees, has served on advisory panels for Mallinckrodt Medical, and is a KOL for Aerogen; she disclosed off-label product use an Aeroneb nebulizer (designed for use with mechanical ventilation) being used to deliver Treprostinil (currently, aerosolized inhaled treprostinil is only FDA approved for delivery via the Tyvaso [treprostinil] Inhalation System [TIS] [United Therapeutics Corp, Silver Springs, MD]). Dr. Shen disclosed work for hire. Dr. Ivy received support for article research from the National Institutes of Health, and from the Jayden de Luca Foundation and the Frederick and Margaret Weyerhaeuser Foundation, who support pulmonary hypertension research at the University of Colorado; his institution received funding from United Therapeutics (consultant), Actelion, Gilead, and Eli Lilly; and he disclosed off-label product use of Treprostinil, which is only indicated for adult pulmonary hypertension treatment. Mr. Hotz disclosed off-label product use of inhaled treprostinil, which is only intended for use in the outpatient setting (this study describes its use during mechanical ventilation). Mr. DiBlasi’s institution received funding from United Therapeutics Corp; he received funding from Mallinckrodt Medical (paid for research and speaker honoraria), Draeger Medical (provided research funds and speaker honararia), and Aerogen Pharma; he disclosed funding in the form of a grant from Aerogen Pharma to study aerosolized surfactant in his lab; and he disclosed off-label product use regarding a device that is approved for aerosolized drugs during medication delivery, but not necessarily for medication delivery with the drug described in this study. The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Barst RJ, Ertel SI, Beghetti M, et al. Pulmonary arterial hypertension: a comparison between children and adults. Eur Respir J. 2011;37:665–677. doi: 10.1183/09031936.00056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sikirica M, Iorga SR, Bancroft T, et al. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Health Serv Res. 2014;676:1–11. doi: 10.1186/s12913-014-0676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ivy DD. Prostacyclin in the intensive care setting. Pediatr Crit Care Med. 2011;11:S41–S45. doi: 10.1097/PCC.0b013e3181d10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 5.Kelly LK, Porta NFM, Goodman DM, et al. Inhaled prostacyclin for term infants with persistent pulmonary hypertension refractory to inhaled nitric oxide. J Pediatr. 2002;141:830–832. doi: 10.1067/mpd.2002.129849. [DOI] [PubMed] [Google Scholar]
- 6.MAUDE Adverse Event Report: Aerogen LTD Aeroneb professional nebulizer system Aeroneb Solo Convenience 10/PK. Available at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/detail.cfm?mdrfoi__id=2577645.
- 7.Voswinckel R, Rubin LJ. Inhaled treprostinil: a therapeutic review. Drug Des Devel Ther. 2012;6:19–28. doi: 10.2147/DDDT.S19281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stream AR, Bull TM. Experiences with treprostinil in the treatment of pulmonary arterial hypertension. Ther Adv Respir Dis. 2014;6:269–276. doi: 10.1177/1753465812455368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winterhalter M, Simon A, Fischer S, et al. Comparison of Inhaled Iloprost and Nitric Oxide in Patients With Pulmonary Hypertension During Weaning From Cardiopulmonary Bypass in Cardiac Surgery: A Prospective Randomized Trial. J Cardiothorac Vasc Anesth. 2008;22:406–413. doi: 10.1053/j.jvca.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Channick RN, Olschewski H, Seeger W, et al. Safety and Efficacy of Inhaled Treprostinil as Add-On Therapy to Bosentan in Pulmonary Arterial Hypertension. J Am Coll Cardiol. 2006;48:1433–1437. doi: 10.1016/j.jacc.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 11.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J Am Coll Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 12.Takatsuki S, Parker DK, Doran AK, et al. Acute pulmonary vasodilator testing with inhaled treprostinil in children with pulmonary arterial hypertension. Pediatr Cardiol. 2014;34:1006–1012. doi: 10.1007/s00246-012-0597-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patel RB, Smaldone GC, Cuccia AD, et al. In vitro delivery of aerosolized treprostinil via modern mechanical ventilation. J Aerosol Med Pulm Drug Deliv. 2013;26:200–207. doi: 10.1089/jamp.2012.1013. [DOI] [PubMed] [Google Scholar]
- 14.DiBlasi RM. Clinical Controversies in Aerosol Therapy for Infants and Children. Respir Care. 2015;60:894–914. doi: 10.4187/respcare.04137. [DOI] [PubMed] [Google Scholar]
- 15.Fang T, Lin H, Chiu S, et al. Aerosol Delivery Using Jet Nebulizer and Vibrating Mesh Nebulizer during High Frequency Oscillatory Ventilation: An In Vitro Comparison. J Aerosol Med Pulm Drug Deliv. 2016;29:1–7. doi: 10.1089/jamp.2015.1265. [DOI] [PubMed] [Google Scholar]
- 16.White CC, Crotwell DN, Shen S, et al. Bronchodilator Delivery during Simulated Pediatric Noninvasive Ventilation. Respir Care. 2013;58:1459–1466. doi: 10.4187/respcare.02171. [DOI] [PubMed] [Google Scholar]
- 17.Finlay WH, Stapleton KW, Zuberbuhler P. Fine particle fraction as a measure of mass depositing in the lung during inhalation of nearly isotonic nebulized aerosols. J Aerosol Sci. 1997;28:1301–1309. [Google Scholar]
- 18.Heyder J. Deposition of inhaled particles in the human respiratory tract and consequences for regional targeting in respiratory drug delivery. Proc Am Thorac Soc. 2004;1:315–320. doi: 10.1513/pats.200409-046TA. [DOI] [PubMed] [Google Scholar]
- 19.Newman SP, Chan H. In Vitro / In Vivo Comparisons in Pulmonary Drug Delivery. J Aerosol Med Pulm Drug Deliv. 2008;21(1):77–84. doi: 10.1089/jamp.2007.0643. [DOI] [PubMed] [Google Scholar]
- 20.Sidler-Moix A, Di Paolo ER, Dolci U, et al. Physicochemical aspects and efficiency of albuterol nebulization: comparison of three aerosol types in an in vitro pediatric model. Respir Care. 2015;60:38–46. doi: 10.4187/respcare.02490. [DOI] [PubMed] [Google Scholar]
- 21.Ghazanfari T, Elhissi MA, Ding Z, et al. The influence of fluid physicochemical properties on vibrating-mesh nebulization. Int J Pharm. 2007;339:103–111. doi: 10.1016/j.ijpharm.2007.02.035. [DOI] [PubMed] [Google Scholar]
- 22.Nelsen AC, Laliberte KJ, Zaccardelli DSGS. Pharmacokinetics of Inhaled Treprostinil Sodium in Healthy Volunteers. Am J Respir Crit Care Med. 2010;181:A338. [Google Scholar]
- 23.Ari A, Telli O, Harwood R, et al. Influence of Nebulizer Type, Position, and Bias Flow on Aerosol Drug Delivery in Simulated Pediatric and Adult Lung Models during Mechanical Ventilation. Respir Care. 2010;55:845–851. [PubMed] [Google Scholar]
- 24.Ari A, Areabi H, Fink JB. Evaluation of Aerosol Generator Devices at 3 Locations in Humidified and Non-humidified Circuits during Adult Mechanical Ventilation. Respir Care. 2010;55:837–844. [PubMed] [Google Scholar]
- 25.Berlinski A, Willis JR. Albuterol Delivery by 4 Different Nebulizers Placed in 4 Different Positions in a Pediatric Ventilator In-Vitro Model. Respir Care. 2013;58:1124–1133. doi: 10.4187/respcare.02074. [DOI] [PubMed] [Google Scholar]
- 26.Berlinski A, Willis JR. Effect of Tidal Volume and Nebulizer Type and Position on Albuterol Delivery in a Pediatric Model of Mechanical Ventilation. Respir Care. 2015;60:1424–1430. doi: 10.4187/respcare.04013. [DOI] [PubMed] [Google Scholar]
- 27.Diblasi RM, Crotwell DN, Shen S, et al. Iloprost drug delivery during infant conventional and high-frequency oscillatory ventilation. Pulm Circ. 2016;6:63–69. doi: 10.1086/685080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alzahrani WA. Comparison of Albuterol Delivery between High Frequency Oscillatory Ventilation and Conventional Mechanical Ventilation in a Simulated Adult Lung Model using Different Compliance Levels (abstract) Respir Care. 2010;55:1576. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.