Abstract
Objectives
To determine if combining behavioral urinary incontinence (UI) treatments with physical activity improves UI in frail older women.
Design
Single blinded, two-arm pilot randomized controlled trial.
Setting
Senior apartments.
Participants
42 frail women (mean age 84.9±6.4) without dementia.
Intervention
12-week program of tailored behavioral UI treatments, 150 minutes of weekly walking and twice weekly strength training classes.
Measurements
UI was measured with 3-day bladder diaries, the International Consultation on Incontinence Questionnaire (ICIQ), and UI global improvement questions. Toileting skills were measured with the Performance Oriented Timed Toileting Instrument (POTTI) and the Minnesota Toileting Skills Questionnaire (MTSQ). Physical function was measured with the Short Physical Performance Battery. UI-related quality of life was measured with the Incontinence Impact Questionnaire and Urogenital Distress Inventory.
Results
The treatment group reported a 50% reduction in daily leaks using bladder diaries, while the control group reported no change (p=.036). Although there were no group differences in total ICIQ scores (p=.664), the treatment group reported significantly greater improvement on the ICIQ item for urine leakage (p=.014). Over 81% of the treatment group vs. 36% of the control group reported improvement in UI (X2=4.84, p=.011) with a mean estimated percent improvement of 65.3±32.0 vs. 34.1±41.3, p=.027, respectively. While not statistically significant, the treatment group improved their toileting skills, while the control group declined (p=.423 POTTI, p=.115 MTSQ). Balance (p=.328) and gait (p=.241) improved more in the treatment group, while chair stands improved more in the control group (p=.144).
Conclusion
UI may be improved in frail older women by combining behavioral strategies for UI with physical activity. However, larger trails are needed to determine if these findings can be replicated and sustained.
Keywords: urinary incontinence, randomized controlled trial, frail, intervention, older women
Urinary incontinence (UI) is a prevalent, poorly treated, and costly chronic condition adversely affecting older women, who incur over half of its $16 billion in annual expenses.(1) Among community-dwelling older adults, frail women have the highest prevalence of UI (over 45%) and a disparate risk for devastating consequences including: poor physical and mental health, reduced quality of life, disabilities with activities of daily living, nursing home placement, and even death.(2, 3) Despite the high burden of UI, fewer than 30% of frail older adults seek treatment (3) and less than 10% of UI expenses target treatment.(1) Even when frail older adults seek treatment for UI, clinicians lack good evidence to guide treatment.(4)
Current treatment guidelines indicate UI should be approached as a syndrome in older adults and address genitourinary and non-genitourinary contributors.(4) For frail older adults, a significant non-genitourinary factor is functional impairment that hinders toileting. Thus treatment should include physical activity to improve toileting skills along with lifestyle and behavioral therapies for urogenital contributors.(4)
A few small studies indicate frail older women improved UI using lifestyle and behavioral therapies, such as, pelvic floor muscle exercises, bladder training, urgency suppression, modification of caffeine and fluid consumption.(4-6) UI has been improved in nursing home residents with physical activity alone (7), by combining behavioral UI treatments with physical activity (8), and by combining physical activity with prompted voiding.(9-11) Physical and occupational therapy strategies for improving toileting skills have also helped frail older women reduce UI.(12) However, no prior studies have investigated whether combining lifestyle and behavioral therapies for UI with exercise to improve toileting skills are efficacious in frail older women able to actively participate in self-care. Developing efficacious programs tailored to the abilities of this high risk group may prevent deleterious consequences of untreated UI.
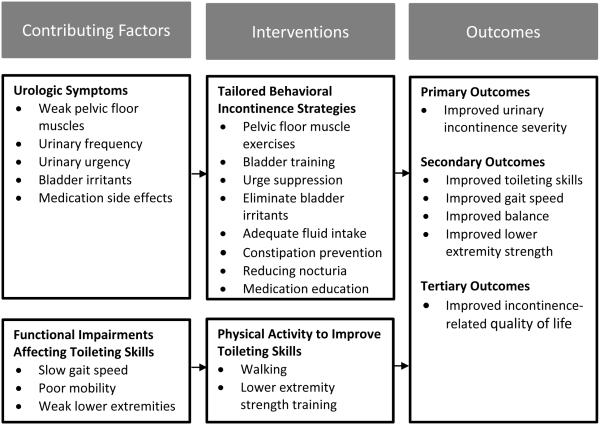
The purpose of this pilot study was to determine the preliminary efficacy of a 12-week multifactorial UI treatment program for frail older women without dementia called the Defeating Urinary Incontinence with Exercise Training (DUET) program. DUET aimed to improve UI by using lifestyle and behavioral therapies to reduce the frequency and severity of UI and by improving walking and transferring skills needed for toileting. The conceptual model for the study is provided in Figure 1. The primary hypothesis was that a multifactorial UI treatment program would result in greater improvements in UI severity. Secondary hypotheses were that the multifactorial UI treatment program would result in greater improvements in toileting skills, gait speed, balance, lower extremity strength, and UI-related quality of life (UI-QOL).
Figure 1.
The Defeating Urinary Incontinence with Exercise Training (DUET) Intervention Framework for Improving Urinary Incontinence in Frail Older Women
METHODS
Design
This pilot randomized controlled trial (RCT) enrolled 42 women living at six senior housing facilities. The study occurred from September 2012-September 2015. Participants were randomly assigned after baseline data collection using a computer generated random number list after baseline data collection to a 12-week treatment (N=23) or no-treatment control group (N=19). Outcome data was collected by trained research assistants who were registered nurses blinded to group assignment. The study protocol was approved by a University Institutional Review Board (approval number 1112S07944) and registered with ClinicalTrials.gov (Identifier: NCT02206958).
Participants
Women were recruited from independent and assisted living apartments located in an upper Midwestern metropolitan area. They were screened for eligibility via in person interviews after recruitment events. Their primary care providers were contacted to identify contra-indications to study participation. Participant inclusion criteria included:
Having UI, indicated by scoring at least one point on the International Consultation on Incontinence Questionnaire (ICIQ) (13)
Being frail, defined as being at risk for functional decline, by scoring three or more points on the Vulnerable Elders Survey (14), having a gait speed less than 0.8 meters per second (15), or using a walking assistive device
Being able to safely participate in low intensity physical activity using the Exercise Assessment and Screening for You (16)
Being cognitively intact by passing the Mini-Cog (17)
Participants were excluded if they had UI associated with a central nervous system disorder, bladder cancer, recent bladder or incontinence surgery, terminal illness, if they had an ostomy, used a pessary or urinary catheter, started or changed the dose of an anti-incontinence medication within three months or had orthopedic surgery on the lower extremities or spine in the past year.
Intervention
The 12-week intervention included bladder and physical activity components. Figure 1 describes the intervention components. Bladder interventions followed international guidelines for lifestyle and behavioral therapies for UI.(4) They were administered by a gerontological nurse practitioner (author: KMT) during four home visits lasting 20-60 minutes. The nurse practitioner did an incontinence focused history and physical exam to assess UI contributors, determine the ability to do PFMEs correctly, diagnose the type of UI, and recommend lifestyle and behavioral therapies. Participants were instructed to do pelvic floor muscle exercises (PFME) five days a week while listening to a 13 minute instructional audio CD. The PFME prescription included doing five fast strong contractions, followed by 20 slow sustained contractions held for 10 seconds. Participants selected additional strategies after the nurse practitioner discussed their UI contributors and made tailored recommendations. These additional strategies are listed in Figure 1.(18) Control group participants received one home visit from the same nurse practitioner to complete the same health history and physical exam received by the treatment group. They received the treatment group’s printed material on lifestyle and behavioral therapies after completing 12-week outcome assessments.
The physical activity program met national guidelines for older adults.(19) It included 150 minutes of moderate intensity walking (30 minutes, 5 days weekly) and twice weekly 1-hour group exercise sessions led by an exercise instructor. The group exercise sessions included 10 strength building exercises (1 set of 12-15 repetitions at moderate intensity) using TheraBand™ resistance bands targeting muscles needed for walking and transferring. Participants wore an electronic pedometer (Fitbit®) to motivate walking.
Measurements
Data collection occurred at baseline before group assignment and at the end of the 12-week intervention. Data was collected using interviewer-administered questionnaires and performance based tests in a private community room at each senior housing facility.
Demographic information was collected at baseline. UI was measured with three instruments including: three day bladder diaries (20), the ICIQ (13), and the patient global ratings of satisfaction and perceptions of improvement with UI treatment that ask about satisfaction with progress in the program (completely, somewhat, not at all), global perception of improvement (much better, better, about the same, worse, much worse), and estimated percent improvement rated from 0% (no better) to 100% (completely better).(21)
Toileting skills were measured with a modification of the Performance Oriented Timed Toileting Instrument (POTTI) (22) and the Minnesota Toileting Skills Questionnaire (MTSQ).(23) During the POTTI, participants are timed as they complete tasks that simulate toileting with higher times indicating poorer performance.(23) The MTSQ asks participants to rate the degree of difficulty (0=none, 1=a little, 2=some, 3=quite a lot, 4=cannot do) they have completing five tasks involved in toileting. Scores range from 0-20 with higher scores indicating more difficulty.(23)
Physical function was measured with the Short Physical Performance Battery (SPPB) which measures balance, time to walk eight feet, and time to rise from a chair and return to a seated position five times. Each activity is scored 0-4. Total scores range from 0-12. Lower scores represent poorer performance.(24, 25)
UI-QOL was measured with the Incontinence Impact Questionnaire (IIQ) and Urogenital Distress Inventory (UDI).(26) IIQ scores range from 0-400 and UDI scores range from 0-300 with higher scores indicated greater impact or distressing symptoms.
Daily exercise logs were completed during the intervention by treatment group participants to record the number of minutes walked daily and the number of times they did all, some, or none of the PFME. The exercise instructor recorded attendance for the group exercise classes.
Data Analysis
Descriptive statistics summarized demographic and outcome data. Differences in outcomes between groups were measured with Chi-square, t-tests, or ANCOVA models that controlled for group assignment and the baseline value of the dependent variable. Differences were considered statistically significant if p<.05. Analyses were performed using SAS, version 6.0 (SAS Institute, Inc., Cary, NC).
RESULTS
Participants were mostly white (98%), widowed or divorced (57%) women, who lived alone (83%) in independent living apartments (90.5%). Their mean age was 84.9±6.4 years; 43% lived in low-income buildings and 57% lived in regular income buildings. The most common type of UI was mixed stress and urgency UI (62%), followed by urgency UI (22%), stress UI (14%), and functional UI (2%). There were no statistically significant differences between the treatment and control groups in any baseline characteristic.
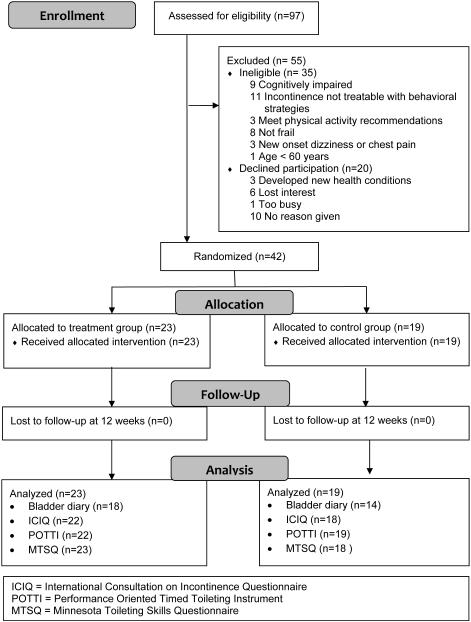
Figure 2 reports the study flow. Of the 97 women screened for study participation, 36% were ineligible, 20% refused participation, and 44% were enrolled. The most common reasons for ineligibility were having UI not amenable to lifestyle and behavioral therapies, having cognitive impairment, and not being frail. All participants completed the 12-week assessments. However, 24% had missing bladder diary data.
Figure 2.
Flow of Participants through Study
Adherence to the nurse practitioner visits was high. All but one treatment group participant completed all four of the home visits and that lone participant completed three visits. Treatment group participants did 3.0±2.3 PFME sessions weekly (5 weekly session prescribed), attended 1.2±0.6 weekly exercise classes (2 weekly classes prescribed) and walked 84.3±173.1 minutes per week (150 minutes per week prescribed). Several participants were unable to log PFME (17%) and minutes walked (22%).
Treatment group participants reported greater improvement in UI severity than control group participants on several measures. The mean number of daily leaks reported in bladder diaries decreased about 50% for the treatment group, while it remained constant for the control group (p=.036) (see Table 1). The treatment group did not report greater improvement in UI severity than the control group in ICIQ total scores (see Table 1). However, 55% of treatment group participants and 17% of control group participants reported improvement on the ICIQ item that asks “how often do you leak urine?” (X2=6.06, p=.014). Finally, the treatment group reported significant improvement on two items in the patient global ratings of satisfaction and perceptions of improvement. Over 81% of the treatment group vs. 36% of the control group reported getting better when asked “overall, do you feel that you are better?” (X2=4.84, p=.011). Using a scale of 0% (no better) to 100% (completely better), the treatment group reported a mean estimated percent improvement of 65.3±32.0 vs. 34.1±41.3 for the control group (t=2.33, p=.027).
Table 1.
Outcomes from the Defeating Urinary Incontinence with Exercise Training Intervention (N=42)
| Treatment Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|
| Baseline N=23 |
12 week N=23 |
Baseline N=19 |
12 week N=19 |
||||
| Characteristics | Mean±SD | Mean±SD | Mean±SD | Mean±SD | F(df) | d | p |
| Incontinence Variables | |||||||
| Mean daily urinary leaks via 3-day bladder diary |
1.5±2.0 N= 21 |
0.8±1.1 N= 18 |
1.7±1.8 N= 17 |
1.7±1.1 N= 14 |
6.6 (2, 20) |
−0.9a | .036 |
| ICIQ Urinary incontinence severity (normal range: 0- 21) |
7.7±2.9 | 7.2±3.8 | 9.5±3.4 | 7.7±3.7 | 1.9 (2, 38) |
−0.5a | .664 |
| Toileting Skills Variables | |||||||
| Performance Oriented Timed Toileting Instrument (in seconds) |
33.1±18.5 | 32.3±7.1 | 33.8±13.9 | 34.0±6.9 | 107.1 (2, 40) |
−1.8a | .423 |
| Minnesota Toileting Skills Questionnaire (normal range: 0-20) |
3.0±2.9 | 2.6±2.2 | 2.9±2.2 | 3.7±2.2 | 24.8 (2, 39) |
−1.1a | .115 |
| Physical Function Variables | |||||||
| SPPB total score (normal range: 0-12) |
7.0±2.4 | 7.3±1.6 | 6.9±2.6 | 7.4±1.6 | 42.0 (2, 40) |
−0.1b | .802 |
| SPPB Balance Score (normal range: 0-4) |
2.5±1.2 | 2.7±0.8 | 2.8±1.2 | 2.5±0.8 | 32.7 (2, 40) |
0.3a | .328 |
| SPPB Gait Score (normal range: 0-4) |
2.7±0.9 | 3.3±0.7 | 2.8±1.0 | 3.1±0.7 | 12.3 (2, 40) |
0.3a | .241 |
| SPPB Chair Stand Score (normal range: 0-4) |
1.8±1.4 | 1.3±1.1 | 1.2±1.1 | 1.9±1.1 | 12.2 (2, 40) |
−0.5 b | .144 |
|
Incontinence-Quality of
Life Variables |
|||||||
| Incontinence Impact Questionnaire (normal range: 0-400) |
45.8±48.8 | 39.5±31.6 | 58.8±58.8 | 40.8±31.6 | 45.7 (2, 39) |
−1.3b | .898 |
| Urinary Distress Inventory (normal range: 0-300) |
64.8±46.7 | 44.0±3 5.2 | 73.7±44.5 | 52.2±35.3 | 5.1 (2, 40) |
3.3a | .897 |
Note: Each row represents an ANCOVA model controlling for the baseline value of the outcome and group assignment. Adjusted means and standard deviations are reported for the 12-week assessments. The reference group is the control group.
The treatment group improved more at 12 weeks than the control group.
The control group improved more at 12 weeks than the treatment group.
ICIQ=International Consultation on Incontinence Questionnaire
SPPB=Short Physical Performance Battery
Table 1 displays the difference in outcomes between the treatment and control groups for the other study variables. While not statistically significant, participants in the treatment group improved their POTTI and self-reported toileting skills, while the control group deteriorated slightly. The treatment group had greater improvement in balance and gait. However, the control group had greater improvements in lower extremity strength measured with the timed chair stands. Both groups had similar improvements in UI-QOL.
DISCUSSION
This is the first study known to the authors to attempt to improve UI in frail older women without dementia by combining lifestyle and behavioral incontinence therapies with physical activity to improve toileting skills. While the small non-representative sample limits generalizability, these pilot study results provide proof of concept and suggestions for improving a larger efficacy trial. Treatment group participants reported a 50% reduction in UI episodes on bladder diaries while the control group stayed the same. This improvement meets the threshold for a clinically meaningful change of a 50-70% reduction in daily UI episodes.(27) The treatment group also reported improvements in UI severity using questions on perceived improvement to UI treatment. However, the ICIQ, a commonly used questionnaire about UI severity did not capture change in the overall score. The lack of responsiveness with the ICIQ could be related to the relatively low severity of UI reported in this sample. The mean ICIQ score was 7.7 whereas the maximum score is 20. The ICIQ item asking about frequency of UI did show a statistically significant improvement in the treatment group.
There were other challenges with measuring UI in this frail sample. During baseline assessments 9% of participants could not complete the bladder diary and this number increased to 24% at the 12-week assessment. The main reasons for having incomplete bladder diaries were visual impairment and refusing to complete the diary. This has occurred in other studies as well. McDowell et al. found that 30% of cognitively intact homebound older adults were unable to complete a bladder diary.(28) In future trials researchers may want to consider excluding participants who cannot complete bladder diaries or develop different ways of administering the diaries, such as, by telephone interviewers. Despite the volume of missing diary data, the improvement reported on the diaries corroborate with improvement reported on the other UI measures, and future studies should include more than bladder diaries to measure this outcome.
While not statistically significant the treatment group improved their self-reported and objectively measured toileting skills, while the control group declined slightly. Most likely, the small sample size explains the lack of statistical significance. Another possibility is that more time is needed to detect differences in this outcome. The treatment group also improved their gait speed and balance, which are essential skills for toileting. The lack of change in the chair stand test may be related to floor effects with the measure.
UI-QOL improved similarly in the treatment and control groups. The level of bother and distress UI caused these frail older women was low at baseline and the improvement could be attributed to regression to the mean. It may also be that UI has less impact on QOL in older adults, because they have other conditions impairing QOL more. Additionally, they may have adapted behaviorally and psychologically to UI.(29) Another possibility is these measures do not capture UI’s impact on QOL in frail populations.
Several study limitations are noteworthy. Differential attention to the treatment and control groups may have influenced treatment group participants to report greater improvements in UI. The improvements reported on bladder diaries are only valid for women who completed them. Participant logging difficulties hindered monitoring treatment fidelity of the walking and PFME activities. Non-study related exercise of control participants may explain the limited improvements in function. The sample was mostly White, but it is the typical demographic composition in the urban Midwest. Diversity was enhanced by having similar enrollment from low and regular income buildings. Reducing the burden of logging may improve future treatment fidelity monitoring. The PFME instructions could be provided on a computer tablet that tracks access. Participants’ walking goals could be based on steps rather than minutes, so pedometers can track walking.
CONCLUSION
In this pilot RCT, frail older women reported statistically and clinically significant improvements in UI severity after completing a 12-week program that combined lifestyle and behavioral UI therapies with physical activity. While not statistically significant, they also improved their toileting skills, gait, and balance. The program did not improve UI-QOL. More definitive clinical trials are needed to determine if these effects occur in larger diverse samples and if they can be sustained past 12 weeks. Future research is also needed to create UI and UI-QOL measures that are responsive to frail older women.
Acknowledgements
The authors would like to thank Patricia L. Schaber PhD, OTR/L, Associate Professor in the University of Minnesota Program for Occupational Therapy, for her consultation on developing the intervention and Michelle Mathiason Moore, MS for statistical support.
Funding: This work was supported by award number UL1TR000114 from The National Center for Advancing Translational Sciences of the National Institutes of Health, by grant number K12HD055887 from the Building Interdisciplinary Research Careers in Women’s Health Program of the National Institutes of Child Health and Human Development, by the University of Minnesota Academic Health Center Seed grant program, and by the Hartford Center for Geriatric Nursing Excellence at Iowa.
Footnotes
| Elements of Financial/Personal Conflicts |
KMT | JFW | UB | BJOK | TCM | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
|
Employment or
Affiliation |
X | X | X | X | X | |||||
| Grants/Funds | X | X | X | X | X | |||||
| Honoraria | X | X | X | X | X | |||||
| Speaker Forum | X | X | X | X | X | |||||
| Consultant | X | X | X | X | X | |||||
| Stocks | X | X | X | X | X | |||||
| Royalties | X | X | X | X | X | |||||
| Expert Testimony | X | X | X | X | X | |||||
| Board Member | X | X | X | X | X | |||||
| Patents | X | X | X | X | X | |||||
| Personal Relationship | X | X | X | X | X | |||||
Author Contributions: KMT: study concept and design, acquisition of subjects and data, data analysis and interpretation, drafting the manuscript. JFW: study concept and design, data interpretation, critical review of the manuscript. UB: study concept and design, data interpretation, critical review of the manuscript. BJOK: study concept and design, data interpretation, critical review of the manuscript. TCM: study concept and design, data interpretation, critical review of the manuscript.
Sponsor’s Role: The funders had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
References
- 1.Wilson L, Brown JS, Shin GP, et al. Annual direct cost of urinary incontinence. Obstet Gynecol. 2001;98(3):398–406. doi: 10.1016/s0029-7844(01)01464-8. [DOI] [PubMed] [Google Scholar]
- 2.Holroyd-Leduc JM, Mehta KM, Covinsky KE. Urinary incontinence and its association with death, nursing home admission, and functional decline. J Am Geriatr Soc. 2004;52(5):712–8. doi: 10.1111/j.1532-5415.2004.52207.x. [DOI] [PubMed] [Google Scholar]
- 3.Luo X, Chuang CC, Yang E, et al. Prevalence, management and outcomes of medically complex vulnerable elderly patients with urinary incontinence in the United States. Int J Clin Pract. 2015;69(12):1517–1524. doi: 10.1111/ijcp.12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagg A, Gibson W, Ostaszkiewicz J, et al. Urinary incontinence in frail elderly persons: Report from the 5th International Consultation on Incontinence. Neurourol Urodyn. 2015;34(5):398–406. doi: 10.1002/nau.22602. [DOI] [PubMed] [Google Scholar]
- 5.Stenzelius K, Molander U, Odeberg J, et al. The effect of conservative treatment of urinary incontinence among older and frail older people: A systematic review. Age Ageing. 2015;44(5):736–744. doi: 10.1093/ageing/afv070. [DOI] [PubMed] [Google Scholar]
- 6.Talley KM, Wyman JF, Shamliyan TA. State of the science: Conservative interventions for urinary incontinence in frail community-dwelling older adults. Nurs Outlook. 2011;59(4):215–20. doi: 10.1016/j.outlook.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinsnes AG, Helbostad JL, Nyronning S, et al. Effect of physical training on urinary incontinence: A randomized parallel group trial in nursing homes. Clin Interv Aging. 2012;7:45–50. doi: 10.2147/CIA.S25326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tak EC, van Hespen A, van Dommelen P, et al. Does improved functional performance help to reduce urinary incontinence in institutionalized older women? A multicenter randomized clinical trial. BMC Geriatr. 2012;12:51. doi: 10.1186/1471-2318-12-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouslander JG, Griffiths PC, McConnell E, et al. Functional incidental training: A randomized, controlled, crossover trial in Veterans Affairs nursing homes. J Am Geriatr Soc. 2005;53(7):1091–100. doi: 10.1111/j.1532-5415.2005.53359.x. [DOI] [PubMed] [Google Scholar]
- 10.Schnelle JF, MacRae PG, Ouslander JG, et al. Functional Incidental Training, mobility performance, and incontinence care with nursing home residents. J Am Geriatr Soc. 1995;43(12):1356–62. doi: 10.1111/j.1532-5415.1995.tb06614.x. [DOI] [PubMed] [Google Scholar]
- 11.Schnelle JF, Alessi CA, Simmons SF, et al. Translating clinical research into practice: A randomized controlled trial of exercise and incontinence care with nursing home residents. J Am Geriatr Soc. 2002;50(9):1476–83. doi: 10.1046/j.1532-5415.2002.50401.x. [DOI] [PubMed] [Google Scholar]
- 12.van Houten P, van Houten W, Achterberg M, et al. Urinary incontinence in disabled elderly women: A randomized clinical trial on the effect of training mobility and toileting skills to achieve independent toileting. Gerontology. 2007;53(4):205–10. doi: 10.1159/000100544. [DOI] [PubMed] [Google Scholar]
- 13.Avery K, Avery J, Donovan T, et al. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn. 2004;23(4):322–30. doi: 10.1002/nau.20041. [DOI] [PubMed] [Google Scholar]
- 14.Min L, Min W, Yoon J, et al. The Vulnerable Elders-13 Survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070–6. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Middleton A, Fritz SL, Lusardi M. Walking speed: The functional vital sign. J Aging Phys Act. 2015;23(2):314–22. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Resnick B, Ory M, Hora K, et al. A proposal for a new screening paradigm and tool called Exercise Assessment and Screening for You (EASY) J Aging Phys Act. 2008;16(2):215–33. doi: 10.1123/japa.16.2.215. [DOI] [PubMed] [Google Scholar]
- 17.Borson S, Borson J, Scanlan M, et al. The Mini-Cog: A cognitive 'vital signs' measure for dementia screening in multi-lingual elderly. Int J Geriatr Psychiatry. 2000;15(11):1021–7. doi: 10.1002/1099-1166(200011)15:11<1021::aid-gps234>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Engberg S, Kincade J, Thompson D. Future directions for incontinence research with frail elders. Nurs Res. 2004;53(6 Suppl):S22–9. doi: 10.1097/00006199-200411006-00004. [DOI] [PubMed] [Google Scholar]
- 19.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc. 2007;39(8):1435–45. doi: 10.1249/mss.0b013e3180616aa2. [DOI] [PubMed] [Google Scholar]
- 20.Jimenez-Cidre MA, Lopez-Fando L, Esteban-Fuertes M, et al. The 3-day bladder diary is a feasible, reliable and valid tool to evaluate the lower urinary tract symptoms in women. Neurourol Urodyn. 2015;34(2):128–32. doi: 10.1002/nau.22530. [DOI] [PubMed] [Google Scholar]
- 21.Burgio KL, Goode PS, Richter HE, et al. Global ratings of patient satisfaction and perceptions of improvement with treatment for urinary incontinence: Validation of three global patient ratings. Neurourol Urodyn. 2006;25(5):411–7. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 22.Ouslander JG, Morishita L, Blaustein J, et al. Clinical, functional, and psychosocial characteristics of an incontinent nursing home population. J Gerontol. 1987;42(6):631–7. doi: 10.1093/geronj/42.6.631. [DOI] [PubMed] [Google Scholar]
- 23.Talley KM, Wyman JF, Olson-Kellogg BG, et al. Reliability and validity of two measures of toileting skills in frail older women without dementia. J Gerontol Nurs. 2016;3:1–5. doi: 10.3928/00989134-20160531-02. [DOI] [PubMed] [Google Scholar]
- 24.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 25.Puthoff ML. Outcome measures in cardiopulmonary physical therapy: Short physical performance battery. Cardiopulm Phys Ther J. 2008;19(1):17. [PMC free article] [PubMed] [Google Scholar]
- 26.Shumaker SA, Wyman JF, Uebersax JS, et al. Health-related quality of life measures for women with urinary incontinence: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Qual Life Res. 1994;3(5):291–306. doi: 10.1007/BF00451721. [DOI] [PubMed] [Google Scholar]
- 27.Shamliyan T, Wyman J, Kane RL. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Agency for Healthcare Research and Quality (US); Rockville (MD): 2012. Report No:11(12)-EHC074-EF. [PubMed] [Google Scholar]
- 28.McDowell BJ, Engberg S, Sereika S, et al. Effectiveness of behavioral therapy to treat incontinence in homebound older adults. J Am Geriatr Soc. 1999;47(3):309–18. doi: 10.1111/j.1532-5415.1999.tb02994.x. [DOI] [PubMed] [Google Scholar]
- 29.Tannenbaum C, Corcos J. Outcomes in urinary incontinence: Reconciling clinical relevance with scientific rigour. Eur Urol. 2008;53(6):1151–61. doi: 10.1016/j.eururo.2008.02.013. [DOI] [PubMed] [Google Scholar]