Abstract
OBJECTIVES
To examine associations of adiposity and adiposity change (loss, stable, gain) with subsequent longitudinal cognitive performance among African Americans in mid- and late-life.
DESIGN
Cohort study using linear mixed models.
SETTING
Genetic Epidemiology Network of Arteriopathy
PARTICIPANTS
African American hypertensive sibships (n=1108) in Jackson, Mississippi.
MEASUREMENTS
Waist circumference and body mass index (BMI) were measured at two exams, five years apart. Stable adiposity was defined as values within ±5% of the first measure. A composite cognitive Z-score was derived from individual cognitive test Z-scores at two study visits, six years apart.
RESULTS
Higher waist circumference was associated with a greater rate of cognitive decline during follow-up (beta= −0.0009 per year, p=0.001); neither BMI, change in waist circumference, nor change in BMI was associated with rate of decline. Loss of adiposity in mid-life was associated with a higher cognitive Z-score among the middle-aged, while loss of adiposity in late-life was associated with a lower Z-score among the older adults (p=0.01 for waist circumference-x-age group interaction and p=0.04 for BMI-x-age group interaction). Simultaneous inclusion of waist circumference and BMI measures in the cross-sectional model suggests an association between higher waist circumference and poorer cognitive performance (beta= −0.009; p=0.006) and between higher BMI and better cognitive performance (beta= 0.014; p=0.06).
CONCLUSION
Results suggest a differential pattern of the adiposity-cognition relationship by age (mid- or late-life) and regional distribution of adiposity.
Keywords: body mass index, waist circumference, cognitive decline, ethnicity, longitudinal
INTRODUCTION
Obesity is an emerging but complex factor associated with cognitive decline and dementia. Accumulating evidence from epidemiological studies suggests a link between increased adiposity at mid-life and dementia (1). In contrast, elevated body mass index (BMI) measured in late-life has been associated with reduced dementia risk (2–4). A well-known prodromal weight loss phenomenon preceding a diagnosis of dementia (5) suggests that the timing of adiposity measures during the lifecourse complicates the relationship between adiposity and cognitive decline.
Another important consideration in the relationship between body adiposity and cognitive decline is the regional distribution of adiposity, such as central versus overall adiposity. Increasing evidence suggests that waist circumference, a measure of central adiposity, better estimates risk of obesity-related disease than measures of overall adiposity such as BMI (6).
African Americans are disproportionately affected by both obesity and late-onset dementia (7, 8). Despite the higher prevalence of dementia and obesity in this race/ethnic population, few studies of adiposity and dementia have included substantial numbers of African Americans.
The likely modification of obesity and dementia risk by age, the potential divergent relationships of waist circumference and BMI, and the increased risk of dementia and greater prevalence of obesity among African Americans highlight the need for longitudinal studies of adiposity and cognitive function among African Americans across the adult life span. The aim of this study was to investigate the relationship of central and overall adiposity as well as longitudinal changes in adiposity to cognitive decline in African American adults in mid- and late-life.
METHODS
Study Population
Data are from the African American cohort of the Genetic Epidemiology Network of Arteriopathy (GENOA) study, a community-based study of hypertensive sibships; although whites were recruited in GENOA, they were not offered repeat measures of cognition so cognitive decline was not available. The GENOA study enrolled hypertensive African Americans and their siblings from Jackson, Mississippi between 1997 and 1999 (Visit 1). At least two siblings were required to have essential hypertension before age 60 years; non-hypertensive siblings were also invited to participate. Detailed neurocognitive testing was conducted on the cohort concurrent with and shortly after GENOA Visit 2 (2001–2006) and repeated in 2009 to 2011 (Visit 3).
Adiposity measurements for this analysis were obtained at Visit 1 and Visit 2. Cognitive function tests were obtained at Visit 2 and Visit 3, such that the first cognitive exam coincided with the second adiposity measure. All measures used standardized protocols. Of the 1482 African American participants enrolled at Visit 2 in GENOA, 292 were missing cognitive test results at both time-points, 40 were missing adiposity measures at Visit 2, and 31 were younger than age 35. To reduce possible measurement error, we further excluded 11 outliers with waist circumference measurements < 70 or > 150 cm, resulting in 1,108 for the analytic cohort. The study protocol was approved by the human studies Institutional Review Board at the University of Mississippi Medical Center. All participants provided informed consent.
Cognitive Outcomes
Neurocognitive tests were offered to all participants and were administered in the same sequence using standardized protocols. The test battery assessed global mental status and 4 cognitive domains: memory, language, processing speed, and executive function. The Mini-Mental State Examination was used to assess global cognitive function (9). Processing speed was measured by the Wechsler Adult Intelligence Scale Revised Digit Symbol Substitution Task (10) and the Trail Making Test A (11). The Rey Auditory Verbal Learning Test assessed memory utilizing multiple learning trials of a 15-item word list followed by 30-minute delayed recall of the word list (11). Language was measured by the Word Fluency Test (letters F, A, and S) and the Animal Naming Task (11, 12). The Trail Making Test B test was administered to measure executive function using time and error counts (11). For Trails A and B, because higher (slower) times indicate poorer performance, times were multiplied by −1 for analyses so that higher numbers represented better performance in all measures.
A composite measure was created by converting each individual test within a domain into a Z-score using the mean and standard deviation from the baseline cognitive evaluation of all participants, then averaging the Z-scores as previously described (13–15). The composite global cognitive Z-score was calculated by averaging the Z-scores of each cognitive domain, and then standardizing using the global Z mean and global Z standard deviation from Visit 2. Thus, a global cognitive Z-score of −1 would describe cognitive performance that is 1 standard deviation below the global cognitive Z mean score at Visit 2.
Measures of Adiposity
Standardized protocols for adiposity measurements were followed at Visits 1 and 2. Height was measured by stadiometer and weight by electronic balance. BMI was calculated as weight (kilograms)/height2 (meters). Waist circumference was measured to the nearest 0.1 centimeter at the umbilicus on the skin or lightweight, non-constricting underwear at the end of exhalation. The tape measure was held horizontal to the floor. A full-length mirror was used to verify the participant was standing erect, tape was horizontal, and weight was equally distributed on both feet.
Waist circumference was analyzed as a continuous variable as well as a categorical variable; with tertiles categorized as 70.0–96.7 cm, 96.7–107.7 cm, and 107.7–150.0 cm.
Change in BMI and change in waist circumference were calculated as difference scores (Visit 2 measure minus Visit 1 measure) and then categorized into three adiposity change groups: lost ≥5% of their initial adiposity measure, gained ≥5% of their initial measurement, or remained stable (lost or gained less than 5% of their initial measurement).
Covariates
All covariates were assessed at Visit 2. Age was categorized into two groups to distinguish between mid-life (ages 35–60 years) and late-life (ages 60+ years). Education was categorized as less than high school, high school, some college, or at least a college degree. Blood pressure, measured three times in a seated, rested state with appropriately sized cuffs, was defined as the average of the 2nd and 3rd measurements. A diagnosis of diabetes was self-reported. History of stroke was self-reported. ‘Ever’ smoked was defined as having smoked more than 100 cigarettes during lifetime.
Statistical Analysis
Linear mixed models with random intercepts were used to examine the association of Visit 2 adiposity or adiposity change with longitudinal cognitive performance. The linear mixed model accounts for correlation between multiple visits and between sibships. Separate models were fit for waist circumference, BMI, change in waist circumference, and change in BMI. We included a two-way interaction term (adiposity-by-age group) in each model to examine differences in cognitive performance associated with adiposity in mid-life compared to late-life. After stratifying the models by age group, we also fit each model with a two-way interaction term (adiposity-by-time) to determine if the rate of cognitive decline differed among the adiposity groups. Models were adjusted for baseline age (continuous), education, sex, duration of time between the measures of adiposity, and the duration of time between cognitive testing. In a second model, we further adjusted for baseline systolic blood pressure, diabetes status, history of stroke, and smoking status which may be mediators between adiposity and cognitive performance.
RESULTS
Participant characteristics for the analytic sample at Visit 2 (the first cognitive exam) and by age group (mid- or late-life) are shown in Table 1. The age range was 35–86 years (mean=58 years). Participants were predominantly women (71%), had limited to intermediate education (68% had a high school degree or less), were obese (mean BMI = 31.3), and, by study design, predominantly hypertensive (73%). There was an average of 5.1 years between adiposity measures and an average of 6.2 years between cognitive measures. Compared to mid-life participants, late-life participants had less education, smaller BMI, larger waist circumference, higher systolic blood pressure, greater prevalence of hypertension, lower baseline Mini-Mental State Examination scores, and a shorter duration between the study visits. Late-life individuals were also less likely to have gained >5% of their initial waist circumference or BMI. There were no significant differences between the mid- and late-life participants in sex distribution, smoking status, diabetes or stroke prevalence. The rate of cognitive decline in late-life was significantly greater than the rate in mid-life (0.08 SD vs 0.06 SD per year, p=0.002).
Table 1.
Characteristics of study participants at the baseline cognitive exam (Visit 2) by age group (mid-life = 35–60 years and late-life = 60+ years).
| Characteristic | Total (N=1108) |
Mid-life (N=653) |
Late-life (N=455) |
P |
|---|---|---|---|---|
| Age, years | 57.9 (9.0) | 52.1 (6.1) | 66.3 (4.7) | <0.001 |
| Female, n (%) | 888 (71%) | 470 (72%) | 318 (70%) | 0.45 |
| Education level, n (%) | <0.001 | |||
| No high school | 358 (37%) | 137 (25%) | 221 (53%) | |
| High school | 305 (31%) | 213 (38%) | 92 (22%) | |
| Some college | 28 (3%) | 17 (3%) | 11 (3%) | |
| College degree + | 280 (29%) | 187 (34%) | 93 (22%) | |
| Waist circumference, cm, median (IQR) | 101.9 (17.8) | 101.2 (18.2) | 103.0 (16.9) | 0.05 |
| Change in waist circumference | 0.02 | |||
| Lost ≥5% | 213 (19%) | 114 (17%) | 99 (22%) | |
| Stable Gained ≥5% | 546 (49%) | 319 (49%) | 227 (50%) | |
| 349 (32%) | 220 (34%) | 129 (28%) | ||
| Body mass index, kg/m2, median (IQR) | 30.6 (7.7) | 30.8 (8.0) | 30.0 (7.5) | 0.04 |
| Change in BMI | <0.001 | |||
| Lost ≥5% | 127 (11%) | 65 (10%) | 62 (14%) | |
| Stable | 688 (62%) | 379 (58%) | 309 (68%) | |
| Gained ≥5% | 293 (26%) | 209 (32%) | 84 (18%) | |
| Weight, kg | 88.8 (17.6) | 90.5 (18.2) | 86.5 (16.4) | <0.001 |
| Weight change/year, kg | 0.2 (1.0) | 0.3 (1.0) | 0.0 (1.1) | <0.001 |
| Ever smoked, n (%) | 436 (39.8%) | 265 (41.2%) | 171 (37.8%) | 0.27 |
| Systolic blood pressure, mmHg | 134 (22) | 131 (20) | 138 (23) | <0.001 |
| Hypertension, n (%) | 808 (73%) | 425 (68%) | 363 (80%) | <0.001 |
| Diabetes, n (%) | 297 (27%) | 176 (27%) | 121 (27%) | 0.89 |
| Stroke, n (%) | 54 (5%) | 28 (4%) | 26 (6%) | 0.28 |
| Time between adiposity measures, years | 5.1 (1.3) | 5.6 (1.1) | 4.4 (1.2) | <0.001 |
| Time between cognitive tests, years | 6.2 (1.2) | 6.4 (0.9) | 5.9 (1.5) | <0.001 |
| MMSE score | 27.0 (2.5) | 27.5 (2.1) | 26.2 (2.9) | <0.001 |
Data are means (standard deviation) unless otherwise stated.
MMSE: Mini Mental State Examination
Waist circumference
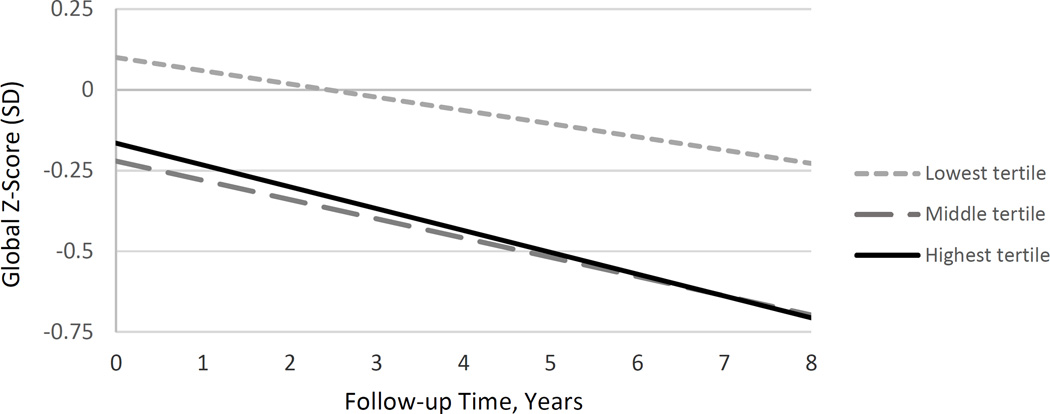
Higher waist circumference at Visit 2 was associated with a greater rate of cognitive decline during follow-up (beta= −0.0009 per year, p=0.001) for all participants (mid- and late-life combined). The test of the interaction term between age group and waist circumference yielded p=0.87, suggesting no modification of this association by age. Persons in the lowest waist circumference tertile had slower rates of decline compared to persons in the middle or highest tertiles (Figure 1, low vs high tertile: beta= 0.026, p=0.001; low vs middle tertile: beta= 0.025, p=0.002; middle vs high tertile: beta= 0.001, p=0.88). These results were essentially unchanged after further adjustment for systolic blood pressure, diabetes status, history of stroke, and smoking history (data not shown).
Figure 1.
Decline in cognitive Z-score by waist circumference tertile, adjusted for age, sex, and education.
Among the waist circumference change groups (lost, stable, or gained), the rate of cognitive decline was not significantly different in either mid- or late-life (p > 0.10 for each change group-by-time interaction term). The test of the interaction term between age group and waist circumference change group yielded beta= 0.17 and p= 0.01 suggesting that loss of waist circumference in mid-life was associated with a higher Z-score among the middle-aged, while loss of waist circumference in late-life was associated with a lower Z-score among the older adults (Table 2). During mid-life, the test for trend of decreasing cognitive Z-scores across the ordered levels of lost, stable, and gain in waist circumference yielded p=0.08. The test for trend of increasing cognitive Z-scores across the ordered change groups in late-life yielded p=0.13. Pairwise comparisons between the waist circumference change groups showed no significant differences in mid-life. In late-life, significantly lower cognitive Z-scores were observed among the loss group compared to the stable group (difference in Z-scores= −0.18, p= 0.05) and in the loss group compared to the gain group (difference in Z-scores= −0.21, p= 0.04).
Table 2.
Associations between adiposity change groups (loss, stable, or gain) and their subsequent mean cognitive Z-scores in mid- and late-life.
| Change group | Mean Z-score* (95% CI) | Pairwise comparison |
Difference in Z-scores between groups* (95% CI) |
p |
|---|---|---|---|---|
| Waist Circumference in Mid-life | ||||
| Loss (n=114) | −0.15 (−0.32, 0.02) | Loss vs stable | 0.04 (−0.14, 0.21) | 0.67 |
| Stable (n=319) | −0.19 (−0.30, −0.07) | Loss vs gain | 0.12 (−0.06, 0.31) | 0.20 |
| Gain (n=220) | −0.27 (−0.40, −0.14) | Stable vs gain | 0.08 (−0.05, 0.22) | 0.23 |
| P for trend | 0.08 | |||
| Waist Circumference in Late-life | ||||
| Loss (n=99) | −0.35 (−0.52, −0.17) | Loss vs stable | −0.18 (−0.36, 0.0009) | 0.05 |
| Stable (n=227) | −0.16 (−0.30, −0.03) | Loss vs gain | −0.21 (−0.42, −0.01) | 0.04 |
| Gain (n=129) | −0.13 (−0.29, 0.03) | Stable vs gain | −0.03 (−0.20, 0.14) | 0.71 |
| P for trend | 0.13 | |||
| Body Mass Index in Mid-life | ||||
| Loss (n=65) | −0.18 (−0.38, 0.03) | Loss vs stable | −0.01 (−0.22, 0.20) | 0.92 |
| Stable (n=379) | −0.17 (−0.27, −0.06) | Loss vs gain | 0.12 (−0.10, 0.35) | 0.27 |
| Gain (n=209) | −0.30 (−0.44, −0.16) | Stable vs gain | 0.13 (−0.00, 0.27) | 0.05 |
| P for trend | 0.11 | |||
| Body Mass Index in Late-life | ||||
| Loss (n=62) | −0.47 (−0.68, −0.27) | Loss vs stable | −0.36 (−0.57, −0.16) | <0.001 |
| Stable (n=309) | −0.11 (−0.23, 0.01) | Loss vs gain | −0.24 (−0.49, 0.01) | 0.06 |
| Gain (n=84) | −0.24 (−0.42, −0.05) | Stable vs gain | 0.13 (−0.06, 0.31) | 0.18 |
| P for trend | 0.14 | |||
Adjusted for baseline age (continuous), sex, education, time between cognitive tests, and time between adiposity measures.
Body mass index
BMI at Visit 2 was not significantly associated with rate of cognitive decline in mid- or late-life (BMI-by-time interaction: p>0.10 in both mid- and late-life). The BMI change group-by-age group interaction yielded beta=0.17 and p=0.04 suggesting that loss of BMI in mid-life was associated with a higher Z-score among the middle-aged, while loss of BMI in late-life was associated with a lower Z-score among the older adults (Table 2). During mid-life, the test for trend of decreasing cognitive Z-scores across the ordered levels of loss, stable, and gain in BMI yielded p=0.11. The test for trend of increasing cognitive Z-scores across the ordered change groups in late-life yielded p=0.14. Pairwise comparisons between the BMI change groups in mid-life showed a significantly higher mean Z-score among the stable BMI group compared to the gain group (difference in Z-scores = 0.13; p=0.05). In late-life, significantly lower cognitive Z-scores were observed among the BMI loss group compared to the stable group (difference in Z-scores= −0.36, p<0.001) and moderately lower Z-scores among the loss group compared to the gain group (difference in Z-scores= −0.24, p=0.06).
Joint associations of waist circumference and body mass index
In another set of analyses, both the waist circumference and BMI variables (obtained at Visit 2) were simultaneously included in the model to examine associations of abdominal obesity with cognitive performance while accounting for overall obesity. Table 3 shows a significant inverse relationship between waist circumference and baseline cognitive Z-score at mid-life, at late-life, and among the combined age groups after adjustment for BMI. In contrast, there was a weak positive association with BMI and baseline cognitive Z-score after adjustment for waist circumference.
Table 3.
The association of adiposity measures with baseline global cognitive Z-score in mid-life, late-life, and combined age groups.
| Model term* | Mid-life | Late-life | Combined age groups | |
|---|---|---|---|---|
| beta (SE); p | beta (SE); p | beta (SE); p | ||
| Model 1 | ||||
| Waist circumference, cm | −0.004 (0.002); 0.08 | −0.004 (0.003); 0.20 | −0.004 (0.002); 0.03 | |
| Model 2 | ||||
| Body mass index, kg/m2 | −0.004 (0.005); 0.47 | −0.002 (0.007); 0.81 | −0.004 (0.004); 0.40 | |
| Model 3 | ||||
| Waist circumference, cm | −0.010 (0.005); p=0.03 | −0.010 (0.005); p=0.06 | −0.009 (0.003); p=0.006 | |
| Body mass index, kg/m2 | 0.014 (0.010); p=0.15 | 0.017 (0.013); p=0.17 | 0.014 (0.008); p=0.06 | |
| Model 4 | ||||
| Waist circumference, cm | −0.008 (0.005); p=0.07 | −0.010 (0.005); p=0.06 | −0.009 (0.003); p=0.008 | |
| Body mass index, kg/m2 | 0.014 (0.010); p=0.17 | 0.020 (0.013); p=0.11 | 0.016 (0.008); p=0.04 | |
Model 1: waist circumference only, with adjustment for age, education, and sex
Model 2: body mass index only, with adjustment for age, education, and sex
Model 3: both waist circumference and body mass index, with adjustment for age, education, and sex
Model 4: model 3 plus systolic blood pressure, diabetes, stroke, and smoking status
DISCUSSION
Results from this population-based study of African Americans with prevalent cardiovascular risk factors suggest the following three key findings: First, higher central adiposity in mid- or late-life was associated with a significantly greater rate of cognitive decline; neither overall adiposity nor changes in central or overall adiposity were associated with the rate of decline. Second, change in adiposity (loss, stable, or gain) showed divergent relationships with cognitive performance in mid-life vs late-life such that loss (gain) of adiposity in mid-life was associated with a higher (lower) cognitive Z-score among the middle-aged, while loss (gain) of adiposity in late-life was associated with a lower (higher) Z-score among the older adults. Third, cross-sectional analysis suggested that central and overall adiposity had independent and divergent relationships with cognitive performance. Simultaneous inclusion of waist circumference and BMI measures in our model suggests an association of higher waist circumference with significantly poorer baseline cognitive performance, while those with higher BMI had better baseline cognitive performance. The combination of waist circumference and BMI might be more predictive of cognitive performance than either measure alone.
Studies of adiposity with cognitive outcomes in African Americans are limited and have primarily focused on overall adiposity in late-life, finding higher risk for dementia with losses in adiposity (16, 17). This is in agreement with our results and with the well-known prodromal weight loss phenomenon that precedes a diagnosis of dementia (5, 18). We expand upon existing studies by contrasting relationships of overall and central adiposity and investigating changes in adiposity as well as the joint associations of overall and central adiposity with cognitive performance in mid- and late-life. The importance of these results is accentuated by the relatively short follow-up period for cognitive decline, especially among our middle-aged subcohort, as longer periods of follow-up are often required during younger ages to detect relationships with cognitive decline.
Our findings that central adiposity is associated with risk of subsequent cognitive decline is in agreement with extant literature (19–21). A potential explanation for more robust associations of central compared to overall adiposity with cognitive decline include a greater relevance of central adiposity to stroke, diabetes, and cardiovascular disease (22), which may lie on the causal pathway between obesity and cognitive decline. However, our adjustment for systolic blood pressure, diabetes, and stroke did not substantially abate the association between central adiposity and cognitive decline, suggesting that something inherent to central adiposity may increase the risk of cognitive decline. Adiposity and the trajectory of adiposity over the life course have been associated with brain atrophy, white matter changes, and blood brain barrier integrity, which in turn, have been associated with increased risk of cognitive decline and late-onset dementia (23–27). Other proposed candidate mediators that link adiposity and dementia are inflammatory markers and adipokines. Inflammatory markers are closely linked with adiposity and adipokines (28, 29) and are associated with poorer cognitive function (30–35). This requires further study, particular in African Americans who are reported to have more adverse adipokineprofiles than whites despite having similar adiposity levels (36).
Our finding of no difference in cognitive performance between individuals in late-life who gained adiposity or whose adiposity remained stable is in in agreement with the Women’s Health Initiative Study of Cognitive Aging, which also reported no difference between weight and waist circumference gain and stable groups and subsequent mean levels of global or domain-specific cognitive performance during follow-up among older women (37). One explanation could be that higher central adiposity poses persisting risk for poorer cognition regardless of additional changes in central adiposity. Additionally, the follow-up period may have been too short to observe substantial cognitive change.
Fewer studies have reported on the joint effects of central and overall adiposity on cognitive function or decline. Our findings show a positive association of overall adiposity and, simultaneously, a negative association of central adiposity with baseline cognitive performance in both mid- and late-life. This result suggests that the combination of BMI and waist circumference might be more predictive of cognitive performance than either measure alone. Similar results were reported for a cohort of older Mexican-Americans in a longitudinal study of cognitive impairment (20) and in the Women’s Health Initiative Memory Study where central adiposity, defined by waist-to-hip ratio, was associated with greater risk of cognitive impairment among normal-weight or overweight but not obese postmenopausal women (38).
This study has several strengths including longitudinal assessments of cognitive performance and adiposity and the ascertainment of two clinically available measures of adiposity, waist circumference and BMI, in African Americans, a group disproportionately burdened by dementia. Many participants also had cardiovascular risk factors associated with risk of dementia and cognitive decline; this may have enabled detection of relationships even in middle-age. Cognitive performance was evaluated with a standard battery of cognitive tests across several domains. Potentially confounding and possible mediators were considered in the analyses.
A limitation of this study is that cognitive function was not an initial focus of the GENOA study and currently lacks adjudication of dementia, which restricts interpretations of these findings to cognitive function and cognitive decline as outcomes. Regardless, cognitive decline is a risk factor for incident dementia (39) and a required component of the dementia syndrome (40). We were also unable to differentiate lean and fat components of the anthropometric measurements. The relation of anthropometric measures to cognitive function in mid- and late-life may also be confounded by cancer, HIV, menopausal status (among women) and other conditions that can cause significant weight fluctuations. We had insufficient data to explore the relationships between these conditions, weight change, and cognitive decline. As such, residual confounding may have affected our study results. Additionally, we were only able to measure weight and weight change during mid- and late-life; thus, our results may not be generalizable to adiposity and adiposity change during childhood or early adulthood. Further, because the study population is African American and primarily hypertensive, these findings may not be generalizable to other populations. However, the number of African Americans in the US is projected to increase by over 40% in the next few decades with similar or larger increases in other non-white race/ethnic groups (41), many of whom are at increased risk for dementia (8) and hypertension (42). Therefore these findings could have major public health relevance at the population level. The observational design prohibits causal inferences.
In summary, higher waist circumference, but not BMI, was associated with a faster rate of cognitive decline during both mid- and late-life, even over a relatively brief follow-up period. Loss or gain in adiposity showed divergent relationships with cognitive performance in mid-life vs late-life.
Acknowledgments
Funding Sources: This work was supported by NIH grants U01-HL054463, R01-HL87660, HL-81331, M01 RR00585 and National Institute on Aging (R01AG045255).
Sponsor’s Role: The sponsors had no role in the design, methods, subject recruitment, data collection, or analysis and preparation of the paper.
Footnotes
Conflict of Interest: The authors have no conflicts of interest
Author Contributions:
Nancy West conceptualized and designed the study, carried out the analysis, made interpretation of the data, drafted the initial manuscript, and approved the final manuscript.
Seth T. Lirette made interpretation of the data, reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Victoria A. Cannon made interpretation of the data, reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Stephen T. Turner made interpretation of the data, reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Thomas H. Mosley, Jr. made interpretation of the data, reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
Gwen Windham conceptualized and designed the study, made interpretation of the data, reviewed and revised the manuscript for important intellectual content, and approved the final manuscript as submitted.
REFERENCES
- 1.Emmerzaal TL, Kiliaan AJ, Gustafson DR. 2003–2013: a decade of body mass index, Alzheimer's disease, and dementia. Journal of Alzheimer's disease : JAD. 2015;43(3):739–755. doi: 10.3233/JAD-141086. [DOI] [PubMed] [Google Scholar]
- 2.Atti AR, Palmer K, Volpato S, et al. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. Journal of the American Geriatrics Society. 2008;56(1):111–116. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]
- 3.Luchsinger JA, Patel B, Tang MX, et al. Measures of adiposity and dementia risk in elderly persons. Arch Neurol. 2007;64(3):392–398. doi: 10.1001/archneur.64.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Power BD, Alfonso H, Flicker L, et al. Body adiposity in later life and the incidence of dementia: the health in men study. PloS one. 2011;6(3):e17902. doi: 10.1371/journal.pone.0017902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart R, Masaki K, Xue Q-L, et al. A 32-year prospective study of change in body weight and incident dementia: The Honolulu-Asia Aging Study. Arch Neurol. 2005;62(1):55–60. doi: 10.1001/archneur.62.1.55. [DOI] [PubMed] [Google Scholar]
- 6.Pi-Sunyer F, Becker D, Bouchard C, et al. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. The National Institutes of Health; 1998. [PubMed] [Google Scholar]
- 7.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA : the journal of the American Medical Association. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 8.Plassman BL, Langa KM, Fisher GG, et al. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29(1–2):125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 10.Lezak MD. Orientation and attention. Neuropsychological Assessment: Third Edition. (Third) 1995 [Google Scholar]
- 11.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. 2nd. xvi. New York: Oxford University Press; 1998. p. 736. [Google Scholar]
- 12.Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 13.Chibnik LB, Shulman JM, Leurgans SE, et al. CR1 is associated with amyloid plaque burden and age-related cognitive decline. Annals of neurology. 2011;69(3):560–569. doi: 10.1002/ana.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massaro JM, D'Agostino RB, Sr, Sullivan LM, et al. Managing and analysing data from a large-scale study on Framingham Offspring relating brain structure to cognitive function. Statistics in medicine. 2004;23(2):351–367. doi: 10.1002/sim.1743. [DOI] [PubMed] [Google Scholar]
- 15.Wilson RS, Beckett LA, Barnes LL, et al. Individual differences in rates of change in cognitive abilities of older persons. Psychology and aging. 2002;17(2):179–193. [PubMed] [Google Scholar]
- 16.Gao S, Nguyen JT, Hendrie HC, et al. Accelerated weight loss and incident dementia in an elderly African-American cohort. Journal of the American Geriatrics Society. 2011;59(1):18–25. doi: 10.1111/j.1532-5415.2010.03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu H, Gao S, Hall KS, et al. Optimal blood pressure for cognitive function: findings from an elderly African-American cohort study. Journal of the American Geriatrics Society. 2013;61(6):875–881. doi: 10.1111/jgs.12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barrett-Connor E, Edelstein SL, Corey-Bloom J, Wiederholt WC. Weight loss precedes dementia in community-dwelling older adults. Journal of the American Geriatrics Society. 1996;44(10):1147–1152. doi: 10.1111/j.1532-5415.1996.tb01362.x. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson DR, Backman K, Waern M, et al. Adiposity indicators and dementia over 32 years in Sweden. Neurology. 2009;73(19):1559–1566. doi: 10.1212/WNL.0b013e3181c0d4b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.West NA, Haan MN. Body adiposity in late life and risk of dementia or cognitive impairment in a longitudinal community-based study. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A(1):103–109. doi: 10.1093/gerona/gln006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitmer RA, Gustafson DR, Barrett-Connor E, et al. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71(14):1057–1064. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]
- 22.Balkau B, Deanfield JE, Despres JP, et al. International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation. 2007;116(17):1942–1951. doi: 10.1161/CIRCULATIONAHA.106.676379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Annals of neurology. 2010;68(2):136–144. doi: 10.1002/ana.22062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho AJ, Raji CA, Becker JT, et al. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiology of Aging. 2010;31(8):1326–1339. doi: 10.1016/j.neurobiolaging.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Isaac V, Sim S, Zheng H, et al. Adverse associations between visceral adiposity, brain structure and cognitive performance in healthy elderly. Frontiers in Aging Neuroscience. 2011;3 doi: 10.3389/fnagi.2011.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jagust W, Harvey D, Mungas D, Haan M. Central obesity and the aging brain. Arch Neurol. 2005;62(10):1545–1548. doi: 10.1001/archneur.62.10.1545. [DOI] [PubMed] [Google Scholar]
- 27.Ward M, Carlsson C, Trivedi M, et al. The effect of body mass index on global brain volume in middle-aged adults: a cross sectional study. BMC Neurology. 2005;5(1):23. doi: 10.1186/1471-2377-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barinas-Mitchell E, Kuller LH, Sutton-Tyrrell K, et al. Effect of weight loss and nutritional intervention on arterial stiffness in type 2 diabetes. Diabetes Care. 2006;29(10):2218–2222. doi: 10.2337/dc06-0665. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg Y, Boaz M, Matas Z, et al. Weight loss induced by nutritional and exercise intervention decreases arterial stiffness in obese subjects. Clinical Nutrition. 2009;28(1):21–25. doi: 10.1016/j.clnu.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Engelhart MJ, Geerlings MI, Meijer J, et al. Inflammatory proteins in plasma and the risk of dementia: the Rotterdam Study. Arch Neurol. 2004;61(5):668–672. doi: 10.1001/archneur.61.5.668. [DOI] [PubMed] [Google Scholar]
- 31.Galimberti D, Fenoglio C, Lovati C, et al. Serum MCP-1 levels are increased in mild cognitive impairment and mild Alzheimer's disease. Neurobiol Aging. 2006;27(12):1763–1768. doi: 10.1016/j.neurobiolaging.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 32.Ravaglia G, Forti P, Maioli F, et al. Blood inflammatory markers and risk of dementia: The Conselice Study of Brain Aging. Neurobiol Aging. 2007;28(12):1810–1820. doi: 10.1016/j.neurobiolaging.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt R, Schmidt H, Curb JD, et al. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Annals of neurology. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 34.Trollor JN, Smith E, Baune BT, et al. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dementia and geriatric cognitive disorders. 2010;30(6):569–578. doi: 10.1159/000322092. [DOI] [PubMed] [Google Scholar]
- 35.Yaffe K, Lindquist K, Penninx BW, et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- 36.Degawa-Yamauchi M, Dilts JR, Bovenkerk JE, et al. Lower serum adiponectin levels in African-American boys. Obesity research. 2003;11(11):1384–1390. doi: 10.1038/oby.2003.187. [DOI] [PubMed] [Google Scholar]
- 37.Driscoll I, Espeland MA, Wassertheil-Smoller S, et al. Weight change and cognitive function: Findings from the Women's Health Initiative Study of Cognitive Aging. Obesity. 2011;19(8):1595–1600. doi: 10.1038/oby.2011.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerwin DR, Gaussoin SA, Chlebowski RT, et al. Interaction between body mass index and central adiposity and risk of incident cognitive impairment and dementia: Results from the Women's Health Initiative Memory Study. Journal of the American Geriatrics Society. 2011;59(1):107–112. doi: 10.1111/j.1532-5415.2010.03219.x. [DOI] [PubMed] [Google Scholar]
- 39.Tifratene K, Robert P, Metelkina A, et al. Progression of mild cognitive impairment to dementia due to AD in clinical settings. Neurology. 2015;85(4):331–338. doi: 10.1212/WNL.0000000000001788. [DOI] [PubMed] [Google Scholar]
- 40.American Psychiatric Association., American Psychiatric Association. Diagnostic and statistical manual of mental disorders : DSM-5. 5th. xliv. Washington, D.C.: American Psychiatric Association; 2013. DSM-5 Task Force; p. 947. [Google Scholar]
- 41.Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: U.S. Census Bureau; 2015. [Google Scholar]
- 42.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart Disease and Stroke Statistics—2015 Update: A Report From the American Heart Association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]