Abstract
Objectives
To evaluate in-hospital adverse events associated with typical and atypical antipsychotic drugs after cardiac surgery.
Design
Retrospective cohort study
Setting
United States nationwide inpatient database, 2003–2014
Participants
3,706 patients (mean age, 70 years) newly treated with oral atypical antipsychotic drugs (N=2,580) vs. oral typical antipsychotic drugs (N=1,126) after coronary artery bypass grafting or valve surgery.
Measurements
In-hospital mortality, arrhythmia, pneumonia, use of brain imaging (surrogate for over-sedation and neurologic events), and length of stay after drug initiation
Results
In the propensity-score matched cohort, the median treatment duration was 3 days (interquartile range [IQR], 1 to 6 days) for atypical drugs and 2 days (IQR, 1 to 3 days) for typical drugs. There were no large differences in in-hospital mortality (atypical vs. typical drugs: 5.4% vs. 5.3%; risk difference [RD], 0.1%; 95% confidence interval [CI], −2.1 to 2.3), arrhythmia (2.0% vs. 2.2%; RD, 0.0%; 95% CI, −1.4 to 1.4), pneumonia (16.1% vs. 14.5%; RD, 1.6%; 95% CI, −1.9 to 5.0), and length of stay (9.9 days vs. 9.3 days; mean difference, 0.5 days; 95% CI, −1.2 to 2.2). Use of brain imaging, however, was more common after initiating atypical drugs relative to typical drugs (17.3% vs. 12.4%; RD, 4.9%; 95% CI, 1.4 to 8.4).
Conclusion
In hospitalized patients who underwent cardiac surgery, short-term use of typical antipsychotic drugs was associated with similar risks of adverse events compared with atypical drugs. Moreover, increased use of brain imaging associated with atypical drugs suggests that these drugs may cause over-sedation or adverse neurologic events. Due to the relatively low event rates, however, our analysis could not exclude modest differences in adverse events between atypical and typical drugs.
Keywords: pharmacoepidemiology, antipsychotics, delirium, cardiac surgery
INTRODUCTION
Delirium affects 35–60% of patients after cardiac surgery. It is associated with greater mortality, postoperative outcomes, and health care costs.1–4 Approximately 10–50% of patients with delirium develop agitation4–6 that often requires antipsychotic medication (APM) treatment. The American Geriatrics Society Clinical Practice Guideline recommends use of the lowest effective dose of an APM for the shortest duration, acknowledging its unclear effectiveness and numerous harms, including the effects on central nervous system (e.g., over-sedation, extrapyramidal symptoms), cardiovascular system (e.g., QTc prolongation, arrhythmia), and pneumonia.7
Current evidence on the safety of APMs mainly comes from studies of long-term use in community-dwelling older adults and nursing home residents with dementia. These studies have consistently shown that typical APMs confer higher risk of mortality than atypical APMs when used to treat behavioral symptoms of dementia.8–14 However, little is known about the safety of APMs when they are used for a short duration to treat delirium-related agitation in hospitalized patients. Several randomized controlled trials of APMs to treat delirium were underpowered to examine adverse clinical events (i.e., sample size <100).7 Although a few small observational studies in the medical inpatient setting found no increase in adverse events associated with APMs vs. no use,15–17 there is a paucity of safety data in cardiac surgery patients who may be susceptible to cardiac and other postoperative adverse events that have been linked to APMs. In addition, most untreated patients have hypoactive delirium in which APMs are not indicated, and they are already at increased risk of adverse events. Thus, comparing treated vs. untreated patients with delirium may be less useful to clinicians who are considering an APM to treat delirium-related agitation.
The objective of our study was to investigate in-hospital adverse events associated with a short-term use of oral atypical vs. typical APMs in a national inpatient database of patients who underwent cardiac surgery. We hypothesized that patients treated with oral atypical APMs have a lower risk of in-hospital mortality, arrhythmia, pneumonia, neurologic events, and prolonged hospitalization than patients treated with oral typical APMs after cardiac surgery.
METHODS
Data Source and Study Population
We conducted a retrospective cohort study using the Premier database that contains complete billing and coding information on approximately 20% of hospital discharges in the United States. The database includes all inpatients treated at the participating hospitals and contains information on demographic characteristics, admission characteristics, discharge diagnoses and status, date-stamped log of all billed items (e.g., medications, procedures, and diagnostic tests), and hospital-level characteristics. Data are routinely audited, reconciled, and validated to ensure that the use of supplies and hospital resources is within an acceptable range. The database has been used to study effectiveness and safety of medications in the perioperative setting.18–21 The Partners Institutional Review Board approved this research.
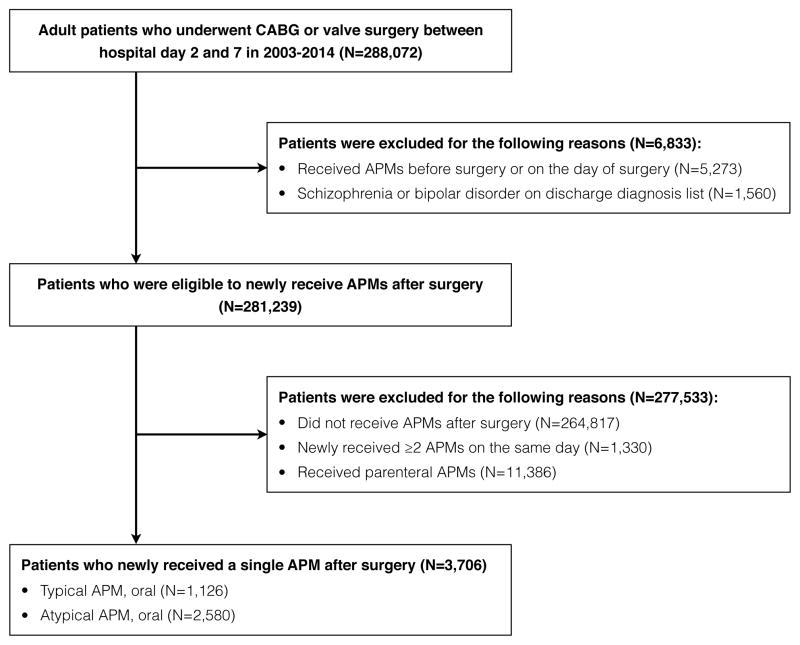
Although delirium is under-coded in an administrative database, delirium constitutes a majority of indications for APM initiation in the hospital.22,23 Thus, we assume that patients who did not receive APMs on admission and were newly treated with the drugs after cardiac surgery had postoperative delirium with agitation requiring pharmacologic intervention. The eligible cohort consisted of 205,274 adult patients who underwent coronary artery bypass grafting (International Classification of Diseases 9th revision [ICD-9] diagnosis codes 36.1x) or valve replacement (ICD-9 diagnosis codes 35.2x) between hospital day 2 and day 7 in the period from January 1, 2003 to December 31, 2014. Since outpatient medications are not recorded in the database, we required at least 1 hospital day before surgery to obtain preoperative drug exposure. We restricted to patients who underwent surgery within 7 hospital days, because those with prolonged hospitalization before surgery (i.e., operated after 7 hospital days) might have a different risk of adverse events. To exclude chronic APM users, patients who received APMs before surgery (n=5,273) or had a diagnosis of schizophrenia or bipolar disorder (n=1,560) were excluded. We also excluded those who never received APMs (n=264,817); started ≥2 different drugs on the same day (n=1,330); or received parenteral drugs (n=11,386). The latter 2 excluded groups generally represent those with greater severity of illness. Our study cohort, therefore, included 3,706 patients who initiated a single oral APM after cardiac surgery (Figure).
Figure. Creation of Study Population in the Premier Database, 2003–2014.
Abbreviations: APM, antipsychotic medication; CABG, coronary artery bypass grafting.
Exposure and Study Outcomes
We defined daily exposure to atypical vs. typical APMs using charge codes (see the generic names in Supplemental Table S1). We converted the total APM dose administered on the day of initiation to chlorpromazine-equivalent dose24; low dose was defined as chlorpromazine-equivalent dose ≤100 mg/day (median dose of typical APM group), which corresponds to haloperidol ≤2 mg/day, olanzapine ≤5 mg/day, quetiapine ≤75 mg/day, or risperidone ≤2 mg/day.
The outcomes were in-hospital mortality and clinical adverse events related to APMs, defined using a combination of discharge diagnoses and date-stamped charge codes for medications, procedures, and diagnostic tests that appeared after initiation of APMs: 1) arrhythmia (cardiopulmonary resuscitation or cardioversion); 2) pneumonia (discharge diagnosis of pneumonia, identified by ICD-9 diagnosis codes 481–486 and 507, plus initiation of ≥2 antibiotics); 3) use of brain imaging (computed tomography or magnetic resonance imaging of the brain) as a surrogate marker of over-sedation, altered mental status, and other neurologic events; 4) length of stay after initiation of APMs. In our validation study based on medical record review (Supplemental Method S1), we found that 81% of patients who underwent brain imaging after cardiac surgery had indications of altered mental status or neurological abnormalities (Supplemental Table S2). Follow-up began on the day following APM initiation and ended on the discharge day, regardless of treatment duration (analogous to the “intention-to-treat” analysis).
Measurement of Patient-Level and Hospital-Level Covariates
We considered patient-level, procedure-related, and hospital-level characteristics as listed in Supplemental Table S3. The Romano modification of the Charlson Comorbidity Index was created using the discharge diagnosis information.25 All procedures and medications were measured from admission date until the day before APM initiation.
Statistical Analysis
To adjust for the differences in patient-level and hospital-level characteristics between the treatment groups, we performed propensity score matching. We estimated the propensity score of receiving atypical APMs vs. typical APMs using logistic regression. The logistic models included all patient-level and hospital-level variables listed above, as well as the number of days between surgery and drug initiation to make the onset of delirium-related agitation comparable between the treatment groups. We performed 1:1 nearest neighbor matching with a caliper that corresponded to 0.2 of the standard deviation of the logit propensity score.26 The covariate balance between the treatment groups was evaluated using standardized mean differences before and after matching. An absolute standardized mean difference <0.1 was considered as appropriate level of balance.
The main analysis was to estimate the risk difference (RD) and risk ratio (RR) with their 95% confidence interval (CI) for in-hospital mortality, arrhythmia, pneumonia, and use of brain imaging associated with initiation of atypical vs. typical APMs in the matched cohort. We used binomial regression for RD, modified Poisson regression for RR (which avoids convergence problems of log binomial regression),27 and linear regression for the mean difference in length of stay after drug initiation. Generalized estimating equations with robust standard errors were used to account for clustering patients within hospitals.28
As secondary analyses, we examined whether the comparative safety profile differs by age (<65, 65–74, or ≥75 years), comorbidity burden (Charlson Comorbidity Index ≤4 or >4), and level of care (ward or intensive care) at the time of APM initiation. Because adverse events that occurred several days after treatment initiation may not be attributable to the drug itself, we examined the events that occurred within 3 days, 5 days, and 7 days of initiation. We performed comparisons by dose categories (i.e., low-dose atypical APMs vs. low-dose typical APMs and high-dose atypical APMs vs. high-dose typical APMs) and pair-wise comparisons for olanzapine, quetiapine, and risperidone vs. haloperidol (reference group). Propensity scores were estimated for each pairwise comparison using haloperidol as reference and 1:1 nearest neighbor matching was performed as in the main analysis. In addition, we carried out several sensitivity analyses to test robustness of our results to several assumptions that we made in outcome definition, analytical approaches, and unmeasured confounding (see Supplemental Method S2). All analyses were performed in SAS version 9.3 and R version 3.2.3. A 2-sided p-value <0.05 was considered statistically significant.
RESULTS
Use of APMs After Cardiac Surgery
The proportion of patients who were newly treated with any APMs after cardiac surgery was 5.8%. Treated patients had higher in-hospital mortality than untreated patients (6.3% vs. 2.7%). Among 3,706 patients treated with an oral APM, 2,580 (69.6%) received atypical drugs (3 most common exposures were quetiapine 50.2%, risperidone 22.5%, and olanzapine 21.0%) and 1,126 (30.4%) received typical drugs (haloperidol accounted for 84.5%, followed by mesoridazine 9.7%). Treatment was initiated within a median of 4 days after surgery (interquartile range: 2 to 8 days).
Compared with 1,126 patients treated with typical APMs, 2,580 patients treated with atypical APMs were younger and more likely to be black and undergo valve surgery (Table 1). The severity of illness and comorbidity burden were higher in those receiving atypical APMs, as evidenced by greater Charlson Comorbidity Index, longer duration of critical care and ventilation support, and higher use of blood transfusion and dialysis. They were more likely to be treated at larger, non-teaching hospitals with higher procedure volume and in the South.
Table 1.
Characteristics of Patients Treated with Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery in the Premier Database, 2003–2014a
| Characteristics | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| Atypical APM(n=2,580) | Typical APM(n=1,126) | SMD(atypical – typical) | Atypical APM(n=832) | Typical APM(n=832) | SMD(atypical – typical) | |
| Patient-level characteristics | ||||||
| Age, years | 68.9 (11.4) | 70.9 (10.4) | −0.18 | 70.8 (10.6) | 69.9 (11.0) | 0.09 |
| Male | 67.2 | 69.7 | −0.05 | 69.4 | 69.4 | 0.00 |
| Race | ||||||
| White | 73.4 | 74.5 | −0.03 | 72.2 | 74.9 | 0.06 |
| Black | 6.8 | 4.3 | 0.11 | 5.0 | 4.7 | 0.02 |
| Other | 19.8 | 21.2 | −0.03 | 22.7 | 20.4 | 0.06 |
| Payor | ||||||
| Medicare | 68.8 | 71.5 | −0.06 | 71.9 | 73.3 | −0.03 |
| Medicaid | 6.9 | 6.4 | 0.02 | 7.7 | 5.6 | 0.08 |
| Commercial | 18.8 | 17.8 | 0.03 | 16.1 | 16.3 | −0.01 |
| Uninsured | 4.3 | 3.7 | 0.03 | 3.7 | 3.8 | −0.01 |
| Others | 1.2 | 0.6 | 0.06 | 0.6 | 0.8 | −0.03 |
| Admission status | ||||||
| Elective | 28.9 | 31.8 | −0.06 | 30.8 | 31.6 | −0.02 |
| Urgent | 31.7 | 30.6 | 0.02 | 30.5 | 31.5 | −0.02 |
| Emergent | 38.7 | 33.1 | 0.12 | 37.1 | 35.7 | 0.03 |
| Others | 0.7 | 4.4 | −0.24 | 1.6 | 1.2 | 0.03 |
| Hospital days before surgery | 3.7 (1.7) | 3.6 (1.6) | 0.03 | 3.7 (1.6) | 3.7 (1.6) | −0.02 |
| Surgery type | ||||||
| CABG alone | 66.2 | 72.3 | −0.13 | 70.8 | 70.8 | 0.00 |
| CABG and valve surgery | 18.9 | 17.5 | 0.04 | 17.2 | 18.1 | −0.03 |
| Valve surgery alone | 14.9 | 10.1 | 0.15 | 12.0 | 11.1 | 0.03 |
| Intensive care unit, days | 7.1 (8.6) | 4.9 (6.3) | 0.29 | 6.0 (8.3) | 5.7 (6.9) | 0.03 |
| Mechanical ventilation, days | 5.1 (8.3) | 3.4 (5.9) | 0.22 | 3.9 (7.1) | 4.1 (6.5) | −0.02 |
| Blood transfusion | 26.9 | 19.4 | 0.18 | 21.9 | 23.0 | −0.03 |
| Dialysis | 11.1 | 5.8 | 0.19 | 7.8 | 7.2 | 0.02 |
| Charlson Comorbidity Index | 3.9 (1.9) | 2.6 (1.7) | 0.19 | 2.7 (1.8) | 2.7 (1.9) | −0.02 |
| Hospital characteristics | ||||||
| Number of beds | 577 (271) | 511 (200) | 0.28 | 512 (223) | 503 (215) | 0.04 |
| Annual procedure volume | ||||||
| CABG | 317 (214) | 288 (186) | 0.14 | 284 (202) | 285 (205) | −0.00 |
| Valve surgery | 117 (103) | 91 (76) | 0.29 | 92 (86) | 92 (82) | −0.01 |
| Teaching hospital | 58.2 | 65.0 | −0.14 | 59.7 | 59.3 | 0.01 |
| Urban hospital | 95.2 | 94.3 | 0.04 | 94.0 | 93.4 | 0.03 |
| Geographic location | ||||||
| South | 51.2 | 36.5 | 0.30 | 45.0 | 44.0 | 0.02 |
| West | 15.0 | 18.1 | −0.08 | 17.9 | 19.8 | −0.05 |
| Midwest | 12.7 | 19.0 | −0.17 | 14.7 | 13.6 | 0.03 |
| Northeast | 21.0 | 26.4 | −0.13 | 22.5 | 22.6 | −0.00 |
Abbreviations: APM, antipsychotic medications; CABG, coronary artery bypass grafting; SNRI, serotonin-norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor
Data were presented in mean (standard deviation) or proportions.
Adverse Events Associated with Atypical vs. Typical APMs
In the 832 propensity score-matched pairs, the differences in patient-level and hospital-level characteristics were minimal (Table 1). The median duration of treatment was 3 days (interquartile range, 1 to 6 days) for atypical drugs and 2 days (1 to 3 days) for typical drugs; switching to a different class or parenteral drugs was observed in 5.0% and 10.6%, respectively.
Before propensity score matching, adverse events were more common in patients treated with atypical APMs than those treated with typical APMs (Table 2). After matching, there was no significant difference between the treatment groups in terms of in-hospital mortality (5.4% vs. 5.3%; RD, 0.1%, 95% CI, −2.1 to 2.3; RR, 1.02, 95% CI, 0.68 to 1.54), arrhythmia (2.0% vs. 2.2%; RD, 0.0%, 95% CI, −1.4 to 1.4; RR, 1.00, 95% CI, 0.51 to 1.98), pneumonia (16.1% vs. 14.5%; RD, 1.6%, 95% CI, −1.9 to 5.0; RR: 1.11, 95% CI, 0.89 to 1.38), and length of stay (9.9 days vs. 9.3 days; mean difference, 0.5 days). However, these differences are imprecisely estimated due to the relatively small sample size and low event rates, and the possibility of modest differences in these events cannot be excluded. We found increased use of brain imaging after initiating atypical drugs relative to typical drugs (17.3% vs. 12.4%; RD, 4.9%, 95% CI, 1.4 to 8.4; RR, 1.40, 95% CI, 1.10 to 1.78): this suggests that one in 20 patients treated with atypical drugs vs. typical drugs may suffer from over-sedation or adverse neurologic events requiring brain imaging.
Table 2.
Adverse Events Associated with Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery in the Premier Database, 2003–2014
| Outcomes | Before Propensity Score Matching | After Propensity Score Matching | ||||||
|---|---|---|---|---|---|---|---|---|
| Atypical APM (n=2,580) | Typical APM (n=1,126) | Risk or Mean Difference % or d (95% CI) | Risk Ratio (95% CI) | Atypical APM (n=832) | Typical APM (n=832) | Risk or Mean Difference % or d (95% CI) | Risk Ratio (95% CI) | |
| Primary outcome | ||||||||
| In-hospital mortality, n (%) | 183 (7.1) | 49 (4.4) | 2.8 (1.3, 4.4) | 1.67 (1.22, 2.26) | 45 (5.4) | 44 (5.3) | 0.1 (−2.1, 2.3) | 1.02 (0.68, 1.54) |
| Secondary outcomes | ||||||||
| Arrhythmia, n (%) | 77 (3.0) | 21 (1.9) | 1.1 (0.1, 2.1) | 1.59 (0.99, 2.57) | 17 (2.0) | 18 (2.2) | 0.0 (−1.4, 1.4) | 1.00 (0.51, 1.98) |
| Pneumonia, n (%) | 547 (21.2) | 143 (12.7) | 8.1 (5.5, 10.7) | 1.62 (1.36, 1.93) | 134 (16.1) | 121 (14.5) | 1.6 (−1.9, 5.0) | 1.11 (0.89, 1.38) |
| Use of brain imaging, n (%) | 445 (17.2) | 132 (11.7) | 5.3 (2.8, 7.8) | 1.44 (1.20, 1.74) | 144 (17.3) | 103 (12.4) | 4.9 (1.4, 8.4) | 1.40 (1.10, 1.78) |
| LOS, d, mean (sd) | 11.5 (14.9) | 8.7 (17.9) | 2.7 (1.5, 3.9) | NA | 9.9 (14.3) | 9.3 (19.8) | 0.5 (−1.2, 2.2) | NA |
Abbreviations: APM, antipsychotic medications; CI, confidence interval; d, days; LOS, length of stay; SD, standard deviation.
Secondary Analyses
We did not find a meaningful variation by age, comorbidity burden, level of care, or follow-up time (Table 3 and Supplemental Table S4), with a few exceptions: the association of atypical APMs with use of brain imaging seemed somewhat stronger in patients 75 years or older (RD, 8.5%, 95% CI, 3.0 to 13.9; RR, 1.84, 95% CI, 1.23 to 2.76) and in those who were in the intensive care unit (RD, 8.6%, 95% CI, 3.0 to 14.2; RR, 1.56, 95% CI, 1.16 to 2.09). Increased use of brain imaging was more remarkable among those treated with low-dose APMs (RD, 4.7%, 95% CI, 0.1 to 9.3; RR, 1.41, 95% CI, 1.01 to 1.98) and with quetiapine vs. haloperidol (RD, 5.0%, 95% CI, 1.0 to 8.9; RR, 1.43, 95% CI, 1.07 to 1.92) (Supplemental Table S4). Due to limited sample size, we were unable to determine whether this association differed from that for olanzapine or risperidone.
Table 3.
Adverse Events Associated with Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery by Subgroups and Follow-Up Time in the Premier Database, 2003–2014
| Analyses | Exposure | N | In-HospitalMortalityn (%) | Arrhythmian (%) | Pneumonian (%) | Use of Brain Imagingn (%) | Length of Stayd, mean ± sd |
|---|---|---|---|---|---|---|---|
| 1. Age | |||||||
| < 65 years | Atypical APM | 212 | 11 (5.2) | 4 (1.9) | 39 (18.4) | 28 (13.2) | 9.7 ± 12.6 |
| Typical APM | 212 | 7 (3.3) | 3 (1.4) | 39 (18.4) | 23 (10.9) | 7.9 ± 9.7 | |
| RD or MD, % or d (95% CI) | 1.9 (−1.9, 5.7) | 0.5 (−2.0, 2.9) | 0.8 (−6.3, 7.8) | 2.1 (−4.3, 8.5) | 1.8 (−0.4, 3.9) | ||
| RR (95% CI) | 1.57 (0.62, 3.98) | 1.32 (0.30, 5.83) | 1.04 (0.71, 1.54) | 1.19 (0.70, 2.04) | NA | ||
| 65–74 years | Atypical APM | 282 | 23 (8.2) | 10 (3.5) | 52 (18.4) | 43 (15.2) | 9.3 ± 13.9 |
| Typical APM | 282 | 16 (5.7) | 8 (2.8) | 41 (14.5) | 39 (13.8) | 10.9 ± 32.1 | |
| RD or MD, % or d (95% CI) | 2.2 (−2.0, 6.5) | 0.6 (−2.4, 3.5) | 3.9 (−2.0, 9.9) | 1.6 (−4.2, 7.4) | −1.6 (−5.7, 2.4) | ||
| RR (95% CI) | 1.37 (0.75, 2.50) | 1.18 (0.48, 2.91) | 1.27 (0.88, 1.84) | 1.12 (0.75, 1.66) | NA | ||
| ≥ 75 years | Atypical APM | 325 | 15 (4.6) | 6 (1.8) | 51 (15.7) | 60 (18.5) | 9.3 ± 10.5 |
| Typical APM | 325 | 20 (6.2) | 7 (2.2) | 44 (13.5) | 33 (10.2) | 9.1 ± 10.4 | |
| RD or MD, % or d (95% CI) | −1.3 (−4.8, 2.2) | −0.5 (−3.0, 1.9) | 2.1 (−3.2, 7.5) | 8.5 (3.0, 13.9) | 0.2 (−1.4, 1.8) | ||
| RR (95% CI) | 0.78 (0.40, 1.52) | 0.75 (0.20, 2.84) | 1.16 (0.80, 1.67) | 1.84 (1.23, 2.76) | NA | ||
| 2. Comorbidity burden | |||||||
| CCI ≤4 | Atypical APM | 738 | 39 (5.3) | 24 (3.3) | 104 (14.1) | 109 (14.8) | 9.1 ± 13.3 |
| Typical APM | 738 | 31 (4.2) | 17 (2.3) | 97 (13.1) | 95 (12.9) | 9.1 ± 20.6 | |
| RD or MD, % or d (95% CI) | 1.1 (−1.1, 3.3) | 0.9 (−1.0, 2.8) | 1.0 (−2.5, 4.5) | 1.7 (−1.9, 5.4) | 0.0 (−1.8, 1.8) | ||
| RR (95% CI) | 1.26 (0.78, 2.02) | 1.43 (0.68, 3.03) | 1.08 (0.83, 1.39) | 1.14 (0.87, 1.49) | NA | ||
| CCI >4 | Atypical APM | 100 | 5 (5.0) | 3 (3.0) | 23 (23.0) | 19 (19.0) | 10.2 ± 9.8 |
| Typical APM | 100 | 13 (13.0) | 3 (3.0) | 22 (22.0) | 17 (17.0) | 12.0 ± 18.4 | |
| RD or MD, % or d (95% CI) | −7.9 (−15.8, 0.0) | −0.1 (−4.9, 4.6) | 1.2 (−10.3, 12.7) | 0.8 (−9.7, 11.3) | −3.5 (−11.0, 4.0) | ||
| RR (95% CI) | 0.40 (0.15, 1.07) | 0.96 (0.20, 4.62) | 1.05 (0.63, 1.76) | 1.05 (0.58, 1.89) | NA | ||
| 3. Level of care | |||||||
| Ward | Atypical APM | 430 | 13 (3.0) | 7 (1.6) | 39 (9.1) | 43 (10.0) | 6.8 ± 12.2 |
| Typical APM | 430 | 11 (2.6) | 6 (1.4) | 24 (5.6) | 39 (9.1) | 5.9 ± 8.2 | |
| RD or MD, % or d (95% CI) | 0.5 (−1.7, 2.7) | 0.2 (−1.4, 1.9) | 3.5 (0.0, 6.9) | 0.9 (−3.0, 4.8) | 0.9 (−0.4, 2.3) | ||
| RR (95% CI) | 1.19 (0.54, 2.60) | 1.17 (0.40, 3.43) | 1.62 (0.99, 2.65) | 1.10 (0.73, 1.65) | NA | ||
| Intensive care | Atypical APM | 389 | 39 (10.0) | 16 (4.1) | 90 (23.1) | 93 (23.9) | 12.9 ± 12.5 |
| Typical APM | 389 | 25 (6.4) | 10 (2.6) | 90 (23.1) | 60 (15.4) | 13.0 ± 27.9 | |
| RD or MD, % or d (95% CI) | 3.5 (−0.3, 7.4) | 1.7 (−0.8, 4.2) | −0.1 (−6.1, 5.9) | 8.6 (3.0, 14.2) | 0.0 (−3.0, 2.9) | ||
| RR (95% CI) | 1.55 (0.95, 2.52) | 1.66 (0.78, 3.54) | 0.99 (0.77, 1.29) | 1.56 (1.16, 2.09) | NA | ||
Abbreviations: APM, antipsychotic medications; CCI, Charlson Comorbidity Index; CI, confidence interval; d, days; MD, mean difference; NA, not applicable; RD, risk difference; RR, risk ratio; sd, standard deviation.
Sensitivity Analysis
When we redefined the neurologic event as use of brain imaging without a discharge diagnosis of stroke or transient ischemic attack, the association was slightly stronger (RD, 4.2%; 95% CI, 1.5 to 6.9; RR, 1.62; 95% CI, 1.18 to 2.21), which supports possible adverse effects of atypical APMs on neurological events other than stroke. Alternative analytical approaches, such as conditional logistic regression, restriction to patients who received ≥2 consecutive days of initial APM class, and Cox regression, showed similar estimates to those from the main analysis (Supplemental Table S5, analysis 2 through 4). The as-treated analysis produced inconclusive estimates due to the small number of events (analysis 5). Excluding outcomes that occurred within 1–2 days did not meaningfully change the association (analysis 6 and 7). Our sensitivity analysis for unmeasured confounder suggests that a prevalence difference >30% or an association between the confounder and outcome >3.0 would be needed to change the RRs by more than 10% (Supplemental Figure S1).
DISCUSSION
In a national sample of older patients who newly received an APM after cardiac surgery, we found no large difference in in-hospital mortality, arrhythmia, pneumonia, and length of stay between patients treated with atypical vs. typical drugs. However, the relatively low event rates did not preclude modest differences in the risk of adverse events. Nonetheless, the possible adverse effect of atypical drugs on the central nervous system (e.g., over-sedation), as reflected by increased use of brain imaging, is noteworthy.
Currently, there is abundant evidence on the harms of long-term APM treatment. Several observational studies8–14 and meta-analyses of randomized controlled trials29,30 have shown that long-term treatment is associated with increased mortality in older adults with dementia. In these studies, the mortality risk was higher immediately after drug initiation and attenuated over time.10,11 Typical APMs conferred higher risk of mortality than atypical APMs8–14: quetiapine seemed to have the lowest risk.11,12 The higher risk was partly explained by an excess mortality due to cardiovascular, respiratory, and infectious causes associated with typical drugs.14
It is important to recognize that hospitalized older adults undergoing cardiac surgery are less frail than community-dwelling or institutionalized older adults who need APMs for dementia-related behavioral problems. The treatment is usually short-term (i.e., several days) when APMs are used to treat delirium-related agitation. Therefore, the frequency and impact of adverse events may differ in cardiac surgery patients. In our study, atypical APMs were more frequently used than typical drugs (69.6% vs. 30.4%) and more likely to be used in sicker patients, as reflected by higher disease burden (Table 1) and risks of adverse events before propensity score matching (Table 2). These observations suggest that physicians presume atypical APMs are safer than typical APMs.
In fact, there are little data on the safety of short-term APM treatment. A few observational studies conducted in hospitalized patients had a sample that was too small to allow comparison among different APMs.15–17 In a single-center study of non-surgical patients with delirium in cardiac care unit, treatment with haloperidol or quetiapine was not associated with mortality and QTc prolongation compared with no treatment.17 Considering that APMs are not indicated for hypoactive delirium, such analysis may represent the comparison of hyperactive vs. hypoactive delirium. Although our analysis did not include untreated patients, the risk of adverse events in our study population treated with oral APMs was higher than the previously reported risk of mortality (3.1%),31 pneumonia (3.1–4.5%),32,33 and arrhythmia (1.7%)34 in cardiac surgery patients. We found that short-term use of typical APMs does not appear to increase the risk of in-hospital mortality and arrhythmia compared with atypical drugs in these patients. Although the indications for brain imaging were not available in the Premier database, our validation study suggests that it is a reliable surrogate for altered mental state and adverse neurologic events. Unless physicians who had preference for atypical APMs were more likely to obtain a brain imaging, increased use of brain imaging after initiating atypical APMs relative to typical drugs is likely due to the immediate negative effect of atypical drugs on the central nervous system (e.g., over-sedation) through their anti-histamine properties. The differential associations for individual atypical drugs vs. haloperidol (Supplemental Table S4) make it less likely that general prescribing preference for atypical APMs was associated with lower threshold to order a brain imaging. Rather, quetiapine has a more sedating profile than other drugs, which might explain this association. The possibility of administering an APM to sedate the patient to obtain a brain imaging is also unlikely, because our sensitivity analyses of restricting to those who received APMs for ≥2 consecutive days and excluding the events occurring within 1–2 days of initiation showed persistent associations. We found that the positive association of atypical APMs with use of brain imaging seemed slightly stronger in the low-dose comparison (RR: 1.41; 95% CI: 1.01 to 1.98) than in the high-dose comparison (RR: 1.28; 95% CI: 0.85 to 1.93). Although the smaller sample size receiving high-dose APMs was, in part, responsible for lack of statistically significant difference, we speculate that the risk of sedation may become less pronounced between atypical and typical APMs used in high doses. In addition, APMs may cause aspiration pneumonia by impairing swallowing function.35 A meta-analysis on the pneumonia risk showed the pooled odds ratio of 1.68 for typical APMs and 1.98 for atypical APMs.36 However, our study was underpowered to detect a small risk difference in pneumonia by APM class.
Our results are generally consistent with the risks of adverse events reported in randomized controlled trials of APMs (Supplemental Table S6). Although the risk varied widely according to the study population (e.g., critical care, inpatient, or postoperative settings), sedation was a common adverse event that seemed to occur more often with atypical APMs (quetiapine: 5–42%; olanzapine: 0–33%; risperidone: 15–29%) than typical APMs (haloperidol: 0–29%). The sedating effect is an important consideration in treatment of delirium-related agitation because APMs may simply convert hyperactive delirium to hypoactive delirium without improving clinical outcomes. A meta-analysis of randomized controlled trials showed no difference in in-hospital mortality and length of stay between APM classes.37 Trial evidence on arrhythmia and extrapyramidal symptoms was heterogeneous; pneumonia was rarely reported.
Strengths and Limitations
We analyzed a national inpatient database, which enhances generalizability. To minimize confounding, we applied restriction in the form of active comparator and new-user design38 and propensity score matching. Restriction is superior to statistical adjustment in reducing confounding by health status and frailty.39
Our study also has important limitations to consider. Despite use of a national database, our analyses did not have sufficient power to detect modest associations and the 95% CI of our estimate for in-hospital mortality contained the estimates from studies of long-term treatment in older adults with dementia. Thus, we are unable to conclude that safety profiles of these drugs in hospitalized patients are different from those in older adults with dementia. As most patients were treated for a median of 2–3 days, we were unable to evaluate the risk associated with a longer treatment. In the Premier database, the onset of non-fatal adverse events was determined based on date-stamped charges for services (e.g., antibiotics or brain imaging). If there was a “lag” time between the actual onset of adverse events and use of these services, the adverse events might have preceded APM use. Our sensitivity analysis showed that the potential lag time of 1–2 days was unlikely to change our main results.
We assumed that APMs were used for delirium-related agitation. In hospitalized older adults, the most common indication of APM initiation was delirium (65–83%).22,23 Considering that delirium is poorly documented in clinical notes (32–56% against a gold-standard assessment),40 we think that use of APMs for indications unrelated to delirium is probably uncommon. Although the use of brain imaging had a positive predictive value of 0.81 for altered mental status and neurologic events in our medical record review, this estimate was based on the physician practice in a large academic health system and might not be generalizable to other hospitals. Outcome definitions based on discharge diagnosis and charge codes have high specificity and moderate sensitivity (e.g., sensitivity 55–70% and specificity 99% for pneumonia).41 Accordingly, RR estimates should be unbiased, but RD estimates might have been underestimated. As in any observational study, a possibility of unmeasured confounding exists. Although the severity of delirium and illness were unavailable in the Premier database, large imbalance (i.e., prevalence difference >30%) between the treatment groups or a strong independent effect (i.e., RR >3.0) on outcomes is unlikely in an active-comparator study that adjusted for an extensive list of covariates. Finally, in the Premier database that only contains inpatient information, we were unable to examine persistent APM use before admission or adverse events after discharge.
Conclusions
Our analysis of a national sample of cardiac surgery patients suggests that short-term use of oral typical APM may cause less sedation and adverse neurologic events, without large increase in in-hospital mortality, arrhythmia, pneumonia, or length of stay, compared with oral atypical APMs. However, due to the low event rates, we cannot exclude modest, but potentially clinically important differences in adverse events, including mortality. Our findings, therefore, need to be interpreted with caution, particularly given the existing literature associating typical APMs with an increased risk of death in other settings. More research in a larger sample of patients undergoing different surgeries is needed to confirm our findings.
Supplementary Material
Supplemental Method S1. Medical Record Review to Evaluate the Indications for Brain Imaging After Cardiac Surgery
Supplemental Method S2. Statistical Analysis for Sensitivity Analyses
Supplemental Table S1. List of Antipsychotic Medications
Supplemental Table S2. Indications for Brain Imaging After Cardiac Surgery Based on Medical Record Review
Supplemental Table S3. List of Potential Confounders
Supplemental Table S4. Adverse Events Associated with Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery According to Follow-Up Time, Dose, and Individual Antipsychotic Drugs in the Premier Database, 2003–2014
Supplemental Table S5. Alternative Analytical Approaches to Compare Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery
Supplemental Table S6. Adverse Events of Antipsychotic Drugs in Randomized Controlled Trials for Delirium Prevention and Treatment
Supplemental Figure S1. Influence of Unmeasured Confounder in Comparing Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery
Acknowledgments
Funding Sources:
DHK: Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies
KFH: grant K01MH099141 from the National Institute of Mental Health
YP: Pharmacoepidemiology Program at Harvard T. H. Chan School of Public Health which is supported by PhRMA Foundation
BTB: grant K08HD075831 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development
ERM: grants R01AG030618, P01AG031720, and a Mid-Career Investigator Award (K24AG035075) from the National Institute on Aging
DHK is supported by the Paul B. Beeson Clinical Scientist Development Award in Aging (K08AG051187) from the National Institute on Aging, American Federation for Aging Research, The John A. Hartford Foundation, and The Atlantic Philanthropies. KFH is supported by grant K01MH099141 from the National Institute of Mental Health. YP is supported by the Pharmacoepidemiology Program at Harvard T. H. Chan School of Public Health which is supported by PhRMA Foundation. BTB is supported by grant K08HD075831 from the Eunice Kennedy Shriver National Institute of Child Health & Human Development. ERM was funded by grants R01AG030618, P01AG031720, and a Mid-Career Investigator Award (K24AG035075) from the National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Meeting Presentations:
The Gerontological Society of America Annual Meeting, Orlando, FL, in November 2015
The International Conference on Pharmacoepidemiology and Therapeutic Risk Management, Boston, MA, in August 2015
Conflict of Interest: DHK and BTB are consultants to the Alosa Foundation, a nonprofit educational organization with no relationship to any drug or device manufacturers; EP is consultant to Aetion, Inc.; BTB and KFH are investigators on grants to Brigham and Women’s Hospital from Pfizer and Lilly and BTB on a grant from Baxalta, for projects unrelated to the present study. BTB is a consultant to Optum, Inc. Other authors declare no conflict of interest.
Author Contributions: DHK designed the study, analyzed and interpreted data, and prepared and revised the manuscript. KH, EP, YP, AA, and BTB designed the study, interpreted data, and revised the manuscript for intellectual content. ERM interpreted data and revised the manuscript for intellectual content. RL performed the statistical analysis and revised the manuscript for intellectual content.
Sponsor’s Role: The funding sources did not have any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
References
- 1.Rudolph JL, Marcantonio ER. Review articles: postoperative delirium: acute change with long-term implications. Anesth Analg. 2011;112(5):1202–1211. doi: 10.1213/ANE.0b013e3182147f6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saczynski JS, Marcantonio ER, Quach L, et al. Cognitive trajectories after postoperative delirium. N Engl J Med. 2012;367(1):30–39. doi: 10.1056/NEJMoa1112923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie DL, Marcantonio ER, Zhang Y, Leo-Summers L, Inouye SK. One-year health care costs associated with delirium in the elderly population. Arch Intern Med. 2008;168(1):27–32. doi: 10.1001/archinternmed.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPherson JA, Wagner CE, Boehm LM, et al. Delirium in the cardiovascular ICU: exploring modifiable risk factors. Crit Care Med. 2013;41(2):405–413. doi: 10.1097/CCM.0b013e31826ab49b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liptzin B, Levkoff SE. An empirical study of delirium subtypes. Br J Psychiatry. 1992;161:843–845. doi: 10.1192/bjp.161.6.843. [DOI] [PubMed] [Google Scholar]
- 6.van den Boogaard M, Schoonhoven L, van der Hoeven JG, van Achterberg T, Pickkers P. Incidence and short-term consequences of delirium in critically ill patients: A prospective observational cohort study. Int J Nurs Stud. 2012;49(7):775–783. doi: 10.1016/j.ijnurstu.2011.11.016. [DOI] [PubMed] [Google Scholar]
- 7.American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142–150. doi: 10.1111/jgs.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 9.Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438–445. doi: 10.1001/jamapsychiatry.2014.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 11.Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71–79. doi: 10.1176/appi.ajp.2011.11030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huybrechts KF, Gerhard T, Crystal S, et al. Differential risk of death in older residents in nursing homes prescribed specific antipsychotic drugs: population based cohort study. Bmj. 2012;344:e977. doi: 10.1136/bmj.e977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rochon PA, Normand SL, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168(10):1090–1096. doi: 10.1001/archinte.168.10.1090. [DOI] [PubMed] [Google Scholar]
- 14.Setoguchi S, Wang PS, Alan Brookhart M, Canning CF, Kaci L, Schneeweiss S. Potential causes of higher mortality in elderly users of conventional and atypical antipsychotic medications. J Am Geriatr Soc. 2008;56(9):1644–1650. doi: 10.1111/j.1532-5415.2008.01839.x. [DOI] [PubMed] [Google Scholar]
- 15.Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253–262. doi: 10.1002/gps.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elie M, Boss K, Cole MG, McCusker J, Belzile E, Ciampi A. A retrospective, exploratory, secondary analysis of the association between antipsychotic use and mortality in elderly patients with delirium. Int Psychogeriatr. 2009;21(3):588–592. doi: 10.1017/S1041610209008977. [DOI] [PubMed] [Google Scholar]
- 17.Naksuk N, Thongprayoon C, Park JY, et al. Clinical impact of delirium and antipsychotic therapy: 10-Year experience from a referral coronary care unit. Eur Heart J Acute Cardiovasc Care. 2015 doi: 10.1177/2048872615592232. [DOI] [PubMed] [Google Scholar]
- 18.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 19.Schneeweiss S, Seeger JD, Landon J, Walker AM. Aprotinin during coronary-artery bypass grafting and risk of death. N Engl J Med. 2008;358(8):771–783. doi: 10.1056/NEJMoa0707571. [DOI] [PubMed] [Google Scholar]
- 20.Bateman BT, Bykov K, Choudhry NK, et al. Type of stress ulcer prophylaxis and risk of nosocomial pneumonia in cardiac surgical patients: cohort study. Bmj. 2013;347:f5416. doi: 10.1136/bmj.f5416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patorno E, Neuman MD, Schneeweiss S, Mogun H, Bateman BT. Comparative safety of anesthetic type for hip fracture surgery in adults: retrospective cohort study. Bmj. 2014;348:g4022. doi: 10.1136/bmj.g4022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothberg MB, Herzig SJ, Pekow PS, Avrunin J, Lagu T, Lindenauer PK. Association between sedating medications and delirium in older inpatients. J Am Geriatr Soc. 2013;61(6):923–930. doi: 10.1111/jgs.12253. [DOI] [PubMed] [Google Scholar]
- 23.Herzig SJ, Rothberg MB, Guess JR, et al. Antipsychotic Use in Hospitalized Adults: Rates, Indications, and Predictors. J Am Geriatr Soc. 2016;64(2):299–305. doi: 10.1111/jgs.13943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry. 2003;64(6):663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]
- 25.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075–1079. doi: 10.1016/0895-4356(93)90103-8. discussion 1081–1090. [DOI] [PubMed] [Google Scholar]
- 26.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 28.Yelland LN, Salter AB, Ryan P. Performance of the modified Poisson regression approach for estimating relative risks from clustered prospective data. Am J Epidemiol. 2011;174(8):984–992. doi: 10.1093/aje/kwr183. [DOI] [PubMed] [Google Scholar]
- 29.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. Jama. 2005;294(15):1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 30.Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553–563. doi: 10.1111/j.1532-5415.1990.tb02407.x. [DOI] [PubMed] [Google Scholar]
- 31.Shroyer AL, Coombs LP, Peterson ED, et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75(6):1856–1864. doi: 10.1016/s0003-4975(03)00179-6. discussion 1864–1855. [DOI] [PubMed] [Google Scholar]
- 32.Allou N, Bronchard R, Guglielminotti J, et al. Risk factors for postoperative pneumonia after cardiac surgery and development of a preoperative risk score*. Crit Care Med. 2014;42(5):1150–1156. doi: 10.1097/CCM.0000000000000143. [DOI] [PubMed] [Google Scholar]
- 33.Kilic A, Ohkuma R, Grimm JC, et al. A novel score to estimate the risk of pneumonia after cardiac surgery. J Thorac Cardiovasc Surg. 2016;151(5):1415–1420. doi: 10.1016/j.jtcvs.2015.12.049. [DOI] [PubMed] [Google Scholar]
- 34.El-Chami MF, Sawaya FJ, Kilgo P, et al. Ventricular arrhythmia after cardiac surgery: incidence, predictors, and outcomes. J Am Coll Cardiol. 2012;60(25):2664–2671. doi: 10.1016/j.jacc.2012.08.1011. [DOI] [PubMed] [Google Scholar]
- 35.Rudolph JL, Gardner KF, Gramigna GD, McGlinchey RE. Antipsychotics and oropharyngeal dysphagia in hospitalized older patients. J Clin Psychopharmacol. 2008;28(5):532–535. doi: 10.1097/JCP.0b013e318184c905. [DOI] [PubMed] [Google Scholar]
- 36.Nose M, Recla E, Trifiro G, Barbui C. Antipsychotic drug exposure and risk of pneumonia: a systematic review and meta-analysis of observational studies. Pharmacoepidemiol Drug Saf. 2015;24(8):812–820. doi: 10.1002/pds.3804. [DOI] [PubMed] [Google Scholar]
- 37.Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic Medication for Prevention and Treatment of Delirium in Hospitalized Adults: A Systematic Review and Meta-Analysis. J Am Geriatr Soc. 2016;64(4):705–714. doi: 10.1111/jgs.14076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. doi: 10.1093/aje/kwg231. [DOI] [PubMed] [Google Scholar]
- 39.McGrath LJ, Ellis AR, Brookhart MA. Controlling Time-Dependent Confounding by Health Status and Frailty: Restriction Versus Statistical Adjustment. Am J Epidemiol. 2015;182(1):17–25. doi: 10.1093/aje/kwu485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hope C, Estrada N, Weir C, Teng CC, Damal K, Sauer BC. Documentation of delirium in the VA electronic health record. BMC Res Notes. 2014;7:208. doi: 10.1186/1756-0500-7-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aronsky D, Haug PJ, Lagor C, Dean NC. Accuracy of administrative data for identifying patients with pneumonia. Am J Med Qual. 2005;20(6):319–328. doi: 10.1177/1062860605280358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Method S1. Medical Record Review to Evaluate the Indications for Brain Imaging After Cardiac Surgery
Supplemental Method S2. Statistical Analysis for Sensitivity Analyses
Supplemental Table S1. List of Antipsychotic Medications
Supplemental Table S2. Indications for Brain Imaging After Cardiac Surgery Based on Medical Record Review
Supplemental Table S3. List of Potential Confounders
Supplemental Table S4. Adverse Events Associated with Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery According to Follow-Up Time, Dose, and Individual Antipsychotic Drugs in the Premier Database, 2003–2014
Supplemental Table S5. Alternative Analytical Approaches to Compare Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery
Supplemental Table S6. Adverse Events of Antipsychotic Drugs in Randomized Controlled Trials for Delirium Prevention and Treatment
Supplemental Figure S1. Influence of Unmeasured Confounder in Comparing Atypical vs. Typical Antipsychotic Drugs After Cardiac Surgery