Introduction
Chronic Pancreatitis (CP) is a debilitating disease that classically presents with recurrent bouts of acute pancreatitis, chronic abdominal pain as well as evidence of end organ damage. This is a result of extensive fibrosis and inflammation within the gland that eventually leads to both exocrine and endocrine insufficiency. The pathogenesis of disease remains controversial and several theories have been proposed to explain its pathophysiology1. The Necrosis-Fibrosis theory is the most widely accepted which essentially states that chronic fibrotic changes ensue after a series of recurrent acute insults to the periductal areas1. While no theory has been conclusively proven, it is likely that the pathogenesis of disease is a complex interworking of multiple etiologies and cofactors.
CP poses many challenges to clinicians. One of the biggest dilemmas is establishing a clear diagnosis. The arsenal of tests and imaging modalities available to providers is vast and this speaks to the often cumbersome task of making the diagnosis as there is usually not one image or test that reliably establishes CP. Diagnosis can be particularly elusive in patients with early chronic pancreatitis (also known as minimal change chronic pancreatitis3) given that these patients classically present with clinical symptoms suggestive of chronic pancreatitis but lack definitive radiographic abnormalities. Other objective parameters that assist in diagnosis, such as indirect pancreatic function tests (PFTs), can often be normal for years after the onset of symptoms. Conversely, there have been subsets of patients described that have evidence of pancreatic fibrosis with no clinical symptomatology indicative of CP. This suggests that the degree of fibrosis is not directly correlated with the degree of exocrine & endocrine dysfunction8. Many studies have been undertaken to improve diagnostics in chronic pancreatitis, but this has been significantly limited by the lack of a gold standard. ERCP had once been touted as a potential gold standard for chronic pancreatitis but it only evaluates ductal anatomy and studies have shown it can have a propensity of attributing ductal changes to chronic pancreatitis when in-fact they are secondary to environmental, obesity, or age-related changes20, 21. Currently, histology is the gold standard but obtaining it safely and routinely is not currently available. However, even when autopsy tissue is reviewed for CP diagnosis there are profound age-related findings that may be confused for changes related to chronic pancreatitis22. A true diagnosis of chronic pancreatitis may not be made simply by clinical history, imaging or function testing alone, but rather by evidence gathered by a combination of these diagnostic tools. Since management largely focuses on both delaying progression and treating symptoms of the disease, rather than cure, providers need to be certain prior to rendering this diagnosis.
Disease Presentation and Risk Factors
Common presentations of disease include chronic abdominal pain, steatorrhea, diabetes, and weight loss of unexplained etiology. Initial evaluation in patients presenting with signs and symptoms concerning for CP should include a thorough history and screening for key risk factors for CP, especially alcohol and tobacco use given these risk factors will increase the pre-test probability of disease. In the United States the most common etiology of chronic pancreatitis remains alcohol6. Yadav et al found that the prevalence of heavy drinking for men (defined as 2–5 drinks/day) and women (defined as 1–5 drinks a day) was 38.4% and 11.0% for CP, respectively. This was compared to 10.0% and 3.6% for controls. This led to an odds ratio of 3.10 for heavy drinking in CP. Importantly this study also concluded that cigarette smoking was an independent, dose-dependent risk factor for CP as well as recurrent acute pancreatitis7. Smoking ≥ 1 pack per day equated to 3.3 times greater odds to develop chronic pancreatitis7.
Two major classification systems have been established to help assess risk factors in the development of CP: TIGAR-O and MANNHEIM (table 1) and are helpful in guiding providers as to when to initiate testing for CP. Current testing modalities are generally in two categories: Imaging (CT, MRI, EUS, ERCP) and pancreatic function tests (further divided into direct and indirect tests). Each of these tests have unique roles in establishing the diagnosis and it is important that clinicians follow an outlined approach in the workup of CP in order to avoid unnecessary testing and misdiagnosis. This section aims at going over the initial evaluation as well as describing how imaging and PFTs can be used in the process of evaluating a patient with suspected CP.
Table 1.
Major Classification Systems of Etiologies for Chronic Pancreatitis
| Classification for CP Etiology |
|---|
| Traditional |
|
|
| Alcohol, idiopathic, hereditary, obstructive, hyperlipidemia |
|
|
| TIGAR-O |
|
|
| Toxic-metabolic: alcohol, tobacco smoking, hypercalcemia, hyperlipidemia, chronic renal failure, medications, toxins |
| Idiopathic: early onset, late onset, tropical |
| Genetic mutations: PRSS1, CFTR, SPINK1, others |
| Autoimmune: isolated, syndromic |
| Recurrent and severe AP-associated CP: postnecrotic (severe AP), vascular disease/ischemic, postirradiation |
| Obstructive: pancreas divisum, sphincter of Oddi disorders, duct obstruction (eg, tumor), posttraumatic pancreatic duct scars |
|
|
| MANNHEIM |
|
|
| M indicates multiple risk factors including: |
| Alcohol consumption: excessive (>80 g/d), increased (20–80 g/d), moderate (<20 g/d) |
| Nicotine consumption |
| Nutritional factors: high caloric proportion of fat and protein, hyperlipidemia |
| Hereditary factors: hereditary, familial, idiopathic (early onset, late onset), tropical |
| Efferent duct factors: pancreas divisum, annular pancreas and other congenital abnormalities of the pancreas, pancreatic duct obstruction (eg, tumors), posttraumatic pancreatic duct scars, sphincter of Oddi dysfunction |
| Immunological factors: autoimmune pancreatitis |
| Miscellaneous and rare metabolic disorders: hypercalcemia, hyperparathyroidism, chronic renal failure, drugs, toxins |
Imaging for Chronic Pancreatitis
Computed Tomography
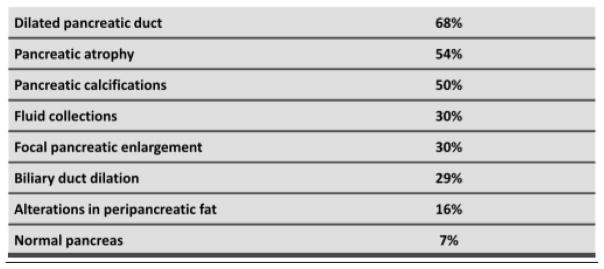
Computed tomography (CT) is considered the best initial imaging test in the workup for CP. Advantages of CT scans are that they are widely accessible, show a detailed view of pancreatic morphology changes seen in CP, and are especially useful in detecting changes seen in advanced disease. It can also quickly assess extra-pancreatic pathology that may explain various presentations mimicking chronic pancreatitis. Another advantage of CT is its ability to detect various complications of acute and chronic pancreatitis such as pseudo cysts, biliary or duodenal obstruction, venous thrombosis, pseudoaneursyms and pancreatico-pleural fistulas4. There are three findings classically seen on CT in CP, including, a dilated pancreatic duct (68%), pancreatic atrophy (54%), and pancreatic classifications (50%)9 (see figure 1). Note that normal pancreatic morphology may also be observed on CT imaging in CP, making the diagnosis particularly difficult in certain population of patients. Additionally, while pancreatic atrophy is visualized in a large proportion of patients with CP, this is not a specific finding and can also be seen with normal aging. Furthermore, as shown in Figure 1, pancreatic enlargement may also be visualized in chronic pancreatitis. While changes of pancreatic parenchyma in late CP are visualized, CT fails to visualize classic changes seen within the pancreatic ducts, thus making it unreliable to diagnose early CP.
Figure 1.
Computed Tomography findings in CP [4]
MRCP
While CT has seen profound improvements over the last 3 decades (such as multidector and multi-phase imaging with contrast), there are significant limitations in diagnosing CP, particularly in evaluation of pancreatic ductal anatomy as well as assisting in diagnosis of early CP. MRCP & secretin enhanced MRCP (sMRCP) are both sensitive and specific for characteristic changes seen in CP. Specific advantages of MRCP evaluation in CP are better visualization of both the pancreatic parenchyma and ducts. Parenchymal changes that are visualized via MRI include pancreatic atrophy, depressed T1 signal, irregular contour of head or body, heterogeneous parenchyma, and delayed gadolinium enhancement of the pancreas after administration6. Ductal changes include intraductal filling defects often indicative of calculi, main pancreatic duct dilation, side branch dilation, irregular duct contour, and decreased compliance after administration of secretin6. While there are no standardized criteria for diagnosing chronic pancreatitis with the use of MRCP, pancreatic image grading systems have been proposed that describe changes seen in normal to severe CP (see table 2). In general, the Cambridge Classification can be modified to classify MRCP findings. With the increasing use of sMRCP, a formal grading system is needed that evaluates both parenchymal and ductal changes as this may better guide clinicians in early diagnosis of CP.
Table 2.
M-ANNHEIM pancreatic imaging Criteria for Chronic Pancreatitis
| Cambridge Grading | CT, US, MRI/MRCP | EUS |
|---|---|---|
| Normal | Quality Study depicting whole gland without abnormal features (0 points) | |
| Equivocal | One abnormal feature (1 point) | Four or fewer abnormal Features (no differentiation between equivocal and mild) (1 point) Five or more abnormal features (no differentiation between moderate and marked) (3 points) |
| Mild changes | Two or more abnormal features, but normal main pancreatic duct | |
| Moderate changes | Two or more abnormal features, including minor main pancreatic duct abnormalities (either enlargement between 2 and 4 mm or increased echogenicity of the duct wall) (3 points) | |
| Marked changes | As above with one ore more of the required features of marked changes (4 points) |
• Abnormal Features: Main pancreatic duct enlargement (2–4 mm), slight gland enlargement (up to 2× normal), heterogeneous parenchyma, small cavities (10 mm), irregular ducts, focal acute pancreatitis, increased echogenicity of the main pancreatic duct wall, irregular head/body contour
• Marked Changes: Large cavities (>10 mm), gross gland enlargement (>2× normal), intraductal filling defects or calculi, duct obstruction, structure or gross irregularity, contiguous organ invasion
Secretin enhanced MRCP allows for a non-invasive approach to evaluate for pancreatic exocrine function. Secretin is a peptide that prompts cells within the pancreatic duct to release bicarbonate rich fluid into the small bowel. Similar to direct pancreatic function tests (discussed later), this method uses IV secretin and subsequently observes the T2 intensity changes seen within the pancreatic duct. This is used as a surrogate marker for pancreatic exocrine function [11]. sMRCP allows for better visualization of pancreatic ducts as well as side branches compared to conventional MRCP. Prior to sMRCP, ERCP had been considered the gold standard for diagnosis because of its ability to detect subtle changes within the pancreatic ducts and the side branches. This is due to the retrograde administration of contrast that leads to over-distension of the pancreatic ducts11. While this over-distension does not occur with the physiologic filling of the pancreatic ducts, adequate visualization of the main pancreatic duct, side branches and accessory pancreatic ducts (if present) does occur with sMRCP11.
On average, healthy individuals will distend the pancreatic duct to approximately 2/3 of its diameter in response to secretin administration11. This decreases as severity of disease worsens, which is likely due to the amount of fibrosis within the gland11. Another measure of pancreatic function that can be elicited from sMRCP are pancreatic duct flow rates. One study evaluated 76 patients with CP and measured changes in small bowel water volume. This was quantified, plotted against time and flow rate was subsequently derived. Flow rates were compared from patients with pancreatic disease of varying degrees to people with normal pancreas tissue. Normal patients were found to have flow rates of 7.4 ± 2.9 ml/min, compared to 5.3 ± 2.4 ml/min in severe CP, 3.8 ± 3.1 ml/min in pancreatic atrophy, and 5.3 ± 2.4 ml/min in subjects with stone obstruction (all statistically significant)13. These results suggest that flow rates through the pancreatic duct diminish with increasing pancreatic fibrosis and atrophy. In addition, this study showed that sMRCP could potentially be used to evaluate a spectrum of pancreatic pathology, not just CP.
Endoscopic Ultrasound
Endoscopic Ultrasound (EUS) is another tool that has increasingly been used in the workup of chronic pancreatitis. As discussed earlier, the diagnosis of chronic pancreatitis is typically not in doubt in those individuals with severe disease, as they will have classic symptoms, risk factors and usually pathologic imaging features on CT or MRCP. In early CP, the diagnosis is rarely straightforward and may be confused with other disease entities. It is in this subset of patients where EUS has the most potential to assist in diagnosis, as it is rarely needed for diagnostic purposes in advanced CP.
Like MRCP, EUS evaluates for both parenchymal and ductal changes in the pancreas 6,14 for diagnoses of CP. A total of nine EUS criteria (4 parenchymal and 5 ductal) have been proposed by the International Working Group in the diagnosis of CP16 (see table 3). The presence of greater than five findings provides a definitive diagnosis of CP where as two or less effectively rules out this disease. Patients with 2–5 criteria have an indeterminate diagnosis and should be thoroughly worked up further with pancreatic function testing. The nine criteria have been linked to distinct histological changes noted from specimens collected after EUS evaluation17. Changes seen with EUS can be nonspecific and seen in healthy patients, as shown by Rajan et al 15. In this study, EUS findings were reviewed in 120 patients with no known pancreatic pathology and it was shown that as regular aging ensues, people are more likely to develop at least 1 parenchymal and 1 ductal EUS abnormality. This was observed 23% of the time in patients <40, 25% in ages 40–60 and 39% in patients >60 years old.
Table 3.
| EUS Criteria | Histologic Correlate |
|---|---|
|
| |
| Parenchymal freatures | |
|
|
|
| Hyperechoic foci | Focal Fibrosis |
|
|
|
| Hyperechoic strands | Bridging Fibrosis |
|
|
|
| Lobular contour | Interlobular Fibrosis |
|
|
|
| Cysts | Cyst/pseudocyst |
|
|
|
| Ductal Features | |
|
|
|
| Main duct dilation (mm) | >3 head, > 2 body, >1 tail |
|
|
|
| Duct irregularity | Focal dilation/narrowing |
|
|
|
| Hyperechoic margins | Periductal Fibrosis |
|
|
|
| Visible side branches | Side branch dilation |
|
|
|
| Stones | Calcified stones |
|
|
|
While these criteria are helpful in the workup of CP, there has been much debate as to the interpretation of findings, as again, these may be the result of normal aging, smoking, or obesity and unrelated to CP. Furthermore, EUS is operator dependent, making it potentially vulnerable to inter-endosonographer variability. This is a major disadvantage of EUS and remains as one of the biggest hurdles for its use in the diagnosis of CP. One study compared EUS interpretations across 11 experienced endosonographers. There findings showed moderately good agreement for a final diagnosis of CP as well as for two of the features (duct dilation and lobularity), however there was poor agreement for the other seven features of EUS18. Given the lack of standardization across EUS interpretation in the context of CP, the Rosemont criteria were developed. These criteria represented the consensus opinion of 32 endosongraphers with the goal to create a more standard approach in the interpretation of EUS findings in CP14. At the conclusion of the meeting, major and minor criteria were developed. Major criteria were divided in A and B. Major A criteria included hyperechoic foci with shadowing and main pancreatic duct calculi. Major B criteria included lobularity with honeycombing. Minor criteria included: dilated ducts (greater than 3.5 mm), presence of pancreatic cysts, irregular pancreatic duct, dilated side branches (greater than 1 mm), hyperechoic duct wall, strands, non-shadowing hyperechoic foci and lobularity with noncontiguous lobules14. Direct comparison of Rosemont to standard criteria shows little benefit at diagnosing CP when compared to pancreas function testing.
In summary, EUS has potential to be useful for diagnosis of early CP since it is highly sensitive for detecting pancreatic abnormalities; however, there are important limitations including only fair interobserver agreement and low specificity of some findings (2–5). The Rosemont criteria are the most widely used diagnostic criteria for CP, but have suboptimal accuracy, particularly for early CP. Considering the large number of potential explanations for EUS abnormalities of the pancreas this test should not be used in isolation to establish a clinical diagnosis of CP. Additional research is needed to optimize this imaging modality, including assessment of novel imaging techniques such as EUS elastography and assessment of pancreatic duct compliance following secretin stimulation.
Endoscopic Retrograde Cholangiopancreatography
Endoscopic retrograde cholangiopancreatography (ERCP) is a modality seldom used for diagnosis of CP at the present time. It allows for a detailed pancreatogram, which can show some changes related to chronic fibrosis and atrophy. However following the advent of CT, MRCP, and EUS, its use is typically limited to therapeutic interventions rather than purely diagnostic.
Historically, specific findings described in a retrograde pancreatogram include the main pancreatic duct caliber & contour, clear definitions of its side branches, intraductal filling defects, strictures and cavity formation. Normal MPD caliber & contour is typically described as a smooth, consistent tapering from head to tail23. Normal MPD size is more difficult to definitively determine as it varies with age, race and gender. In a series done in 1982, average sizes of the main duct were 3.6 (head), 2.7 (body) and 1.6 mm (tail)24 with the upper limit of normal up to 5–6 mm. Historically, significant disagreements occurred over interpretations of pancreatogram findings. The Cambridge criteria were an attempt to standardize interpretations of various pancreatogram findings. During this International workshop, pancreatic ductal changes were classified as equivocal, mild, moderate, or severe (see table 5)23. A normal pancreatogram was one without any abnormalities in the MPD or its side branches, an equivocal one would be with <3 side branch abnormalities, where as mild would be 3 or more side branch abnormalities. Moderate and severe would describe findings involving both the main pancreatic duct and side branches. Additionally, pancreatograms with large cavities, filling defects or strictures, irregularity in ductal contour, calcification, and/or organ invasion were classified as severe. However, it is important to note that the Cambridge classification system soley refers to the pancreatic ductal anatomy and does not describe the clinical stage of CP23.
Table 5.
Cambridge Criteria
| Grade | Main Pancreatic Duct | Branch Ducts |
|---|---|---|
| Normal | Normal | Normal |
| Cambridge 1 (equivocal) | Normal | <3 abnormal |
| Cambridge 2 (mild) | Normal | ≥3 abnormal |
| Cambridge 3 (moderate) | Abnormal | >3 abnormal |
| Cambridge 4 (severe) | Abnormal * | >3 abnormal |
Including large cavity >10 mm, Intraductal filling defects, duct obstruction (stricture), duct dilation or irregularity, calculi/pancreatic calcification, or contiguous organ invasion
Although ERCP is sensitive for detection of changes in the pancreatic duct, there are several drawbacks when used for the diagnosis of CP. First, like EUS, it is operator dependent and prone to interobserver variability. Differences in ERCP lie not just in quality of the pancreatogram but also in the interpretation of the images. Next, pancreatograms do not provide assessment of the classic CP changes within the pancreatic parenchyma. Finally, ERCP is the most invasive diagnostic modality and carries post-procedural risks, most notably, post-ERCP pancreatitis. For these reasons, the American Society for Gastrointestinal Endoscopy (ASGE) recommended that ERCP only be used for diagnosing CP, once other imaging methods have been exhausted19.
Pancreatic Function Tests
Pancreatic Function Tests (PFTs) are typically classified as indirect (noninvasive) or direct (invasive)32. Indirect PFTs refer to the evaluation of pancreatic exocrine function without direct hormonal stimulation of the gland. Examples of indirect PFTs include serum trypsinogen, fecal elastase, and fecal fat measurements. Direct PFTs involve hormonal stimulation of the pancreas by either secretin or cholcystekinin (CCK).
Advantages of indirect PFTs are that they are inexpensive, non-invasive, and easy to perform tests that can be performed on an outpatient basis. One such test is the 72-hour fecal fat collection. Fecal fat collection is not typically used in the diagnostic algorithm for chronic pancreatitis, rather it’s utility lies in grading the degree of exocrine dysfunction in patients with established CP and response to pancreatic enzyme supplementation25. When performed correctly, this is an excellent test at quantifying steatorrhea. However, due to large need for patient cooperation for stool collection and coordination with the laboratory many clinicians avoid this test, particularly in the diagnostic evaluation of CP. A more commonly utilized indirect PFT is the fecal pancreatic elastase-1. In 1999, Gullo et al sought to measure fecal elastase-1 levels in 53 healthy individuals, 44 patient with confirmed CP (further stratified as severe, moderate or mild) and 43 patients with non-pancreatic GI disorders. All healthy subjects and all but 3 subjects with non-pancreatic GI disorders had elastase levels >190 as compared to only 10 of the 44 in the CP group. Of note, 22/22 of the severe chronic pancreatitis had levels <190 mcg/g as compared to only 2/9 classified as mild CP, showing that sensitivity precipitously declines in early CP as compared to severe CP27. It is important to know that other conditions such as small bacterial overgrowth and submission of a non-formed stool specimen can cause false positive test results28. Finally, trypsin is a serologic test that can help detect the underlying etiology of a patient’s steatorrhea. Jacobson et al found that at levels <20 ng/ml, serum trypsinogen was a specific marker of steatorrhea caused by a pancreatic etiology29 and at very high levels (>150 ng/ml) indicate active pancreatic inflammation25. In short, indirect PFTs are helpful as adjunct tests along with imaging suggestive of disease. Alone, intermediate results should not be interpreted as diagnostic for CP6.
Direct PFTs allow for evaluation of both acinar and ductal pancreatic cells by aspirating duodenal contents after stimulation by either CCK or secretin, respectively30. When stimulated by CCK, pancreatic enzymes are measured in the duodenal contents, and bicarbonate concentration is measured from the fluid following secretin stimulation. In one large retrospective study, patients who had symptoms concerning for CP but no radiographic evidence of disease, had sPFTs (secretin PFTs) completed and subsequently were followed over a number of years to see if they would develop CP. A positive sPFT was defined as <75 mEq/l in duodenal fluid collections (collected at 15, 30, 45 and 60 minutes after stimulation). Seventy patients that were studied had negative sPFT and 20 patients had positive sPFTs30. Of those with positive sPFTs, 9 later developed CP where as of the 70 with negative sPFTs, only 2 patients later developed chronic pancreatitis30. Sensitivity & specificity of diagnosing CP with sPFTs were 82% and 86%, respectively whereas the PPV and NPV were 45% and 97%, respectively30. This suggests primary utility of secretin-stiumlated PFT lies in ruling out CP in patients who present with signs & symptoms concerning for CP, such as chronic abdominal pain.
Direct PFTs and EUS may have the greatest benefit in helping to diagnose early CP. PFTs may be able to detect exocrine dysfunction suspicious for CP prior to classic imaging changes and EUS, as discussed, may detect early ductal and parenchymal changes suggestive of CP. A study done by Stevens et al in 2009 sought to compare EUS with direct PFTs in the diagnosis of minimal change CP. They showed a 72% concordance between EUS and direct PFT testing in early CP. They also concluded that direct PFTs (specifically sPFT) were more likely to be abnormal earlier on in disease as compared to EUS (using a cut off of >3 criteria)31. Finally, it has been suggested that using both EUS and direct PFTs, in combination, may add more specificity in diagnosis and PFTs may add clarity to those patients with indeterminate EUS findings (2–4 EUS features).
Differential Diagnosis of Chronic Pancreatitis
A critical clinical dilemma occurs in the presence of focal abnormalities in the pancreas, with the primary considerations being pancreatic adenocarcinoma (PDAC), focal CP, and autoimmune pancreatitis. Although CP may potentially develop in any location, this commonly occurs in the pancreatic head. The term groove pancreatitis describes the anatomic variant of CP that characteristically involves the pancreatic head, duodenum, and pancreatioduodenal groove32. Focal CP allows for parenchymal sparing which can also be confused with PDAC or other causes of a pancreatic mass33. Many patients with groove pancreatitis develop elevated serum CA19-9 levels due to biliary obstruction or acute inflammation, which further confuses the clinical scenario. Particularly in these scenarios a high index of clinical suspicion for PDAC must be maintained, and patients often require surgical resection due to the inability to exclude malignancy. However, the duct-penetrating sign may provide a means of discriminating between focal CP and PDAC. This sign refers to a non-constricted, patent main pancreatic duct that courses into the area of focal pancreatic enlargement; the presence of a duct-penetrating sign suggests CP rather than PDAC33. Also, pancreatic parenchymal calcifications and an irregular MPD favor CP rather than PDAC6. Patients with focal AIP may have a dramatically elevated serum IgG4 level and/or the presence of characteristic extrapancreatic disease, but undoubtedly have negative fine needle aspiration of the mass and dramatic response to steroid therapy.
Summary
In summary, diagnosing CP can range from routine in those with severe disease and obvious calcifications on CT imaging to elusive in those patients with early changes of CP. The workup of suspected CP should follow a progressively non-invasive to more invasive STEP-wise approach in a patient with a suspicious clinical presentation and risk factors that raise their pre-test probability of disease. After a thorough history and physical exam, basic labs should be obtained such as lipase, amylase, metabolic panel and indirect PFTs (fecal elastase-1, serum trypsin). Computed tomography remains the best initial imaging modality to obtain as it has good sensitivity for severe CP and may obviate the need for other diagnostic tests. When equivocal, an MRCP should be obtained for a more detailed evaluation of the both the pancreatic parenchyma and ducts. If the diagnosis remains in doubt, EUS should be performed with or without pancreas function testing. ERCP remains a last line diagnostic test and seldom should be used outside of therapeutic purposes. Future advances should target optimizing current diagnostic tools to more accurately diagnose early CP, as it is in this population where the benefits of delaying progression of CP may have the most profound effect. Likely the best way at establishing a diagnosis in these patients are via pancreatic function testing in the setting of indeterminate EUS results. Biomarker studies of pancreas fluid may supplement diagnosis.
Table 4.
Rosemont Criteria14
| Consistent with Chronic Pancreatitis |
|---|
| 1 Major A feature and 3 or more minor features |
| 1 Major A feature and 1 major B feature |
| 2 Major A features |
| Probable Chronic Pancreatitis |
| 1 Major A feature and less than 3 minor features |
| 1 Major B feature and 3 or more minor features |
| 5 or more minor features |
| Indeterminate for Chronic Pancreatitis |
| 3–4 minor features, no major features |
| Major B feature alone or with less than 3 minor features |
| Normal Pancreas |
| Less than 2 minor features, no major features |
Acknowledgments
Grant Support: Research reported in this publication was supported by the National Cancer Institute and National Institute of Diabetes And Digestive and Kidney Diseases (NIDDK) under award number U01DK108327 (PH, DC). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- CP
chronic pancreatitis
- CCK
cholecystokinin
- PDAC
pancreatic ductal adenocarcinoma
- PFT
pancreatic function testing
- MRCP
magnetic resonance cholangiopancreatography
- ERCP
Endoscopic Retrograde Cholangiopancreatogram
- CT
Computed Tomography
Footnotes
Potential conflicts of interest/disclosures: Hart (Abbvie, Inc., honorarium for speaking and KC Specialty Therapeutics, LLC, consulting fees)
Resources
- 1.Stevens T, Conwell DL, Zuccaro G. Pathogenesis of chronic pancreatitis: an evidence-based review of past theories and recent developments. The American journal of gastroenterology. 2004;99:2256–2270. doi: 10.1111/j.1572-0241.2004.40694.x. [DOI] [PubMed] [Google Scholar]
- 2.Forsmark CE. The early diagnosis of chronic pancreatitis. Clin Gastroenterol Hepatol. 2008;6:1291–1293. doi: 10.1016/j.cgh.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Forsmark CE. The diagnosis of chronic pancreatitis. Gastrointestinal endoscopy. 2000;52:293–298. doi: 10.1067/mge.2000.106889. [DOI] [PubMed] [Google Scholar]
- 4.Conwell DL, Wu BU. Chronic pancreatitis: making the diagnosis. Clin Gastroenterol Hepatol. 2012;10:1088–1095. doi: 10.1016/j.cgh.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi AJ, Miller F. Chronic pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Seminars in ultrasound, CT, and MR. 2007;28:384–394. doi: 10.1053/j.sult.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Conwell DL, Lee LS, Yadav D, Longnecker DS, Miller FH, Mortele KJ, Levy MJ, Kwon R, Lieb JG, Stevens T, Toskes PP, Gardner TB, Gelrud A, Wu BU, Forsmark CE, Vege SS. American Pancreatic Association Practice Guidelines in Chronic Pancreatitis: evidence-based report on diagnostic guidelines. Pancreas. 2014;43:1143–1162. doi: 10.1097/MPA.0000000000000237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav D, Hawes RH, Brand RE, Anderson MA, Money ME, Banks PA, Bishop MD, Baillie J, Sherman S, DiSario J, Burton FR, Gardner TB, Amann ST, Gelrud A, Lawrence C, Elinoff B, Greer JB, O’Connell M, Barmada MM, Slivka A, Whitcomb DC. Alcohol consumption, cigarette smoking, and the risk of recurrent acute and chronic pancreatitis. Archives of internal medicine. 2009;169:1035–1045. doi: 10.1001/archinternmed.2009.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bozkurt T, Braun U, Leferink S, Gilly G, Lux G. Comparison of pancreatic morphology and exocrine functional impairment in patients with chronic pancreatitis. Gut. 1994;35:1132–1136. doi: 10.1136/gut.35.8.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luetmer PH, Stephens DH, Ward EM. Chronic pancreatitis: reassessment with current CT. Radiology. 1989;171:353–357. doi: 10.1148/radiology.171.2.2704799. [DOI] [PubMed] [Google Scholar]
- 10.Karasawa E, Goldberg HI, Moss AA, Federle MP, London SS. CT pancreatogram in carcinoma of the pancreas and chronic pancreatitis. Radiology. 1983;148:489–493. doi: 10.1148/radiology.148.2.6867347. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal R, Stevens T, Novak E, Veniero JC. Secretin-enhanced MRCP: review of technique and application with proposal for quantification of exocrine function. AJR American journal of roentgenology. 2012;198:124–132. doi: 10.2214/AJR.10.5713. [DOI] [PubMed] [Google Scholar]
- 12.Cappeliez O, Delhaye M, Deviere J, Le Moine O, Metens T, Nicaise N, Cremer M, Stryuven J, Matos C. Chronic pancreatitis: evaluation of pancreatic exocrine function with MR pancreatography after secretin stimulation. Radiology. 2000;215:358–364. doi: 10.1148/radiology.215.2.r00ma10358. [DOI] [PubMed] [Google Scholar]
- 13.Gillams AR, Lees WR. Quantitative secretin MRCP (MRCPQ): results in 215 patients with known or suspected pancreatic pathology. European radiology. 2007;17:2984–2990. doi: 10.1007/s00330-007-0708-9. [DOI] [PubMed] [Google Scholar]
- 14.Catalano MF, Sahai A, Levy M, Romagnuolo J, Wiersema M, Brugge W, Freeman M, Yamao K, Canto M, Hernandez LV. EUS-based criteria for the diagnosis of chronic pancreatitis: the Rosemont classification. Gastrointestinal endoscopy. 2009;69:1251–1261. doi: 10.1016/j.gie.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 15.Rajan E, Clain JE, Levy MJ, Norton ID, Wang KK, Wiersema MJ, Vazquez-Sequeiros E, Nelson BJ, Jondal ML, Kendall RK, Harmsen WS, Zinsmeister AR. Age-related changes in the pancreas identified by EUS: a prospective evaluation. Gastrointestinal endoscopy. 2005;61:401–406. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 16.Wallace MB, Hawes RH, Durkalski V, Chak A, Mallery S, Catalano MF, Wiersema MJ, Bhutani MS, Ciaccia D, Kochman ML, Gress FG, Van Velse A, Hoffman BJ. The reliability of EUS for the diagnosis of chronic pancreatitis: interobserver agreement among experienced endosonographers. Gastrointestinal endoscopy. 2001;53:294–299. doi: 10.1016/s0016-5107(01)70401-4. [DOI] [PubMed] [Google Scholar]
- 17.Varadarajulu S, Eltoum I, Tamhane A, Eloubeidi MA. Histopathologic correlates of noncalcific chronic pancreatitis by EUS: a prospective tissue characterization study. Gastrointestinal endoscopy. 2007;66:501–509. doi: 10.1016/j.gie.2006.12.043. [DOI] [PubMed] [Google Scholar]
- 18.Wallace MB, Hawes RH. Endoscopic ultrasound in the evaluation and treatment of chronic pancreatitis. Pancreas. 2001;23:26–35. doi: 10.1097/00006676-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Adler DG, Lichtenstein D, Baron TH, Davila R, Egan JV, Gan SL, Qureshi WA, Rajan E, Shen B, Zuckerman MJ, Lee KK, VanGuilder T, Fanelli RD. The role of endoscopy in patients with chronic pancreatitis. Gastrointestinal endoscopy. 2006;63:933–937. doi: 10.1016/j.gie.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Heij HA, Obertop H, van Blankenstein M, Nix GA, Westbroek DL. Comparison of endoscopic retrograde pancreatography with functional and histologic changes in chronic pancreatitis. Acta radiologica (Stockholm, Sweden : 1987) 1987;28:289–293. [PubMed] [Google Scholar]
- 21.Schmitz-Moormann P, Himmelmann GW, Brandes JW, Folsch UR, Lorenz-Meyer H, Malchow H, Soehendra LN, Wienbeck M. Comparative radiological and morphological study of human pancreas. Pancreatitis like changes in postmortem ductograms and their morphological pattern. Possible implication for ERCP. Gut. 1985;26:406–414. doi: 10.1136/gut.26.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy: a systematic study of 112 autopsies in patients without known pancreatic disease. Human pathology. 1984;15:677–683. doi: 10.1016/s0046-8177(84)80294-4. [DOI] [PubMed] [Google Scholar]
- 23.Axon AT, Classen M, Cotton PB, Cremer M, Freeny PC, Lees WR. Pancreatography in chronic pancreatitis: international definitions. Gut. 1984;25:1107–1112. doi: 10.1136/gut.25.10.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeny PC, Lawson TL. Radiology of the Pancreas. New York, NY: Springer New York; 1982. [Google Scholar]
- 25.Lieb JG, 2nd, Draganov PV. Pancreatic function testing: here to stay for the 21st century. World journal of gastroenterology. 2008;14:3149–3158. doi: 10.3748/wjg.14.3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez J, Laveda R, Trigo C, Frasquet J, Palazon JM, Perez-Mateo M. Fecal elastase-1 determination in the diagnosis of chronic pancreatitis. Gastroenterologia y hepatologia. 2002;25:377–382. doi: 10.1016/s0210-5705(02)70269-0. [DOI] [PubMed] [Google Scholar]
- 27.Gullo L, Ventrucci M, Tomassetti P, Migliori M, Pezzilli R. Fecal elastase 1 determination in chronic pancreatitis. Dig Dis Sci. 1999;44:210–213. doi: 10.1023/a:1026691209094. [DOI] [PubMed] [Google Scholar]
- 28.Nousia-Arvanitakis S. Fecal elastase-1 concentration: an indirect test of exocrine pancreatic function and a marker of an enteropathy regardless of cause. Journal of pediatric gastroenterology and nutrition. 2003;36:314–315. doi: 10.1097/00005176-200303000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Jacobson DG, Curington C, Connery K, Toskes PP. Trypsin-like immunoreactivity as a test for pancreatic insufficiency. The New England journal of medicine. 1984;310:1307–1309. doi: 10.1056/NEJM198405173102007. [DOI] [PubMed] [Google Scholar]
- 30.Hart PA, Topazian M, Raimondo M, Cruz-Monserrate Z, Fisher WE, Lesinski GB, Steen H, Conwell DL. Endoscopic Pancreas Fluid Collection: Methods and Relevance for Clinical Care and Translational Science. The American journal of gastroenterology. 2016;111:1258–1266. doi: 10.1038/ajg.2016.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens T, Dumot JA, Parsi MA, Zuccaro G, Vargo JJ. Combined endoscopic ultrasound and secretin endoscopic pancreatic function test in patients evaluated for chronic pancreatitis. Dig Dis Sci. 2010;55:2681–2687. doi: 10.1007/s10620-009-1084-x. [DOI] [PubMed] [Google Scholar]
- 32.Hart PA, Conwell DL. Diagnosis of Exocrine Pancreatic Insufficiency. Current treatment options in gastroenterology. 2015;13:347–353. doi: 10.1007/s11938-015-0057-8. [DOI] [PubMed] [Google Scholar]
- 33.Coakley FV, Hanley-Knutson K, Mongan J, Barajas R, Bucknor M, Qayyum A. Pancreatic imaging mimics: part 1, imaging mimics of pancreatic adenocarcinoma. AJR American journal of roentgenology. 2012;199:301–308. doi: 10.2214/AJR.11.7907. [DOI] [PubMed] [Google Scholar]
- 34.Ichikawa T, Sou H, Araki T, Arbab AS, Yoshikawa T, Ishigame K, Haradome H, Hachiya J. Duct-penetrating sign at MRCP: usefulness for differentiating inflammatory pancreatic mass from pancreatic carcinomas. Radiology. 2001;221:107–116. doi: 10.1148/radiol.2211001157. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich CF, Ignee A, Braden B, Barreiros AP, Ott M, Hocke M. Improved differentiation of pancreatic tumors using contrast-enhanced endoscopic ultrasound. Clin Gastroenterol Hepatol. 2008;6:590–597. e591. doi: 10.1016/j.cgh.2008.02.030. [DOI] [PubMed] [Google Scholar]