Abstract
OBJECTIVE
To test whether structured physical activity is associated with a greater reduction in major mobility disability (MMD) among older persons with versus without metabolic syndrome (MetS).
DESIGN
Data from the Lifestyle Interventions and Independence for Elders (LIFE) study, a multi-center randomized trial of 1,635 persons, with assessments every 6 months (average 2.7 years).
SETTING
8 U.S. centers.
PARTICIPANTS
1,535 sedentary men and women, aged 70 to 89 years, with functional limitations. 100 participants were excluded because of missing MetS data.
INTERVENTION
Participants were randomized to moderate-intensity physical activity program (n = 766) or health education program (n = 769).
MEASUREMENTS
MetS was defined according to the 2009 multi-agency harmonized criteria. Outcomes included incident MMD (loss of ability to walk 400 m) and persistent MMD (two consecutive MMD diagnoses or one MMD diagnosis followed by death).
RESULTS
763 (49.7%) participants met criteria for MetS. Relative to health education, physical activity reduced incident MMD among participants with MetS (hazard ratio [HR], 0.72 [95%CI, 0.57–0.91]; P=0.007), but not among those without MetS (HR, 0.96 [95%CI, 0.73–1.25]; P=0.75); the test for statistical interaction was not significant (P=0.13). Physical activity reduced the risk of persistent MMD among participants with MetS (HR, 0.57 [95% CI, 0.41–0.79]; P< 0.001), but not among those without MetS (HR, 0.97 [95% CI, 0.67–1.41]; P= 0.87). The test for statistical interaction was significant (P=0.037).
CONCLUSIONS
Among older persons with functional limitations, moderate-intensity physical activity substantially reduces the risk of persistent MMD in those with, but not without, MetS. Comparable results were observed for incident MMD. The LIFE physical activity program may be an effective strategy for reducing mobility disability among vulnerable older persons with MetS.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01072500
INTRODUCTION
Metabolic syndrome (MetS)1 has been identified in approximately 50% of older adults2 in the US. Sedentary behavior is a risk factor for MetS,3 while increasing levels of physical activity may slow the progression towards MetS, independent of multiple confounding factors.4 Previous studies have documented an association between MetS and subsequent decline in multiple measures of functional status, including mobility.5,6 These adverse effects are highly predictive of disability, hospitalization, nursing home admission, and mortality.7 Thus, the relationships of MetS and physical activity, individually and together, with subsequent mobility disability are of upmost clinical and public health importance.
Structured physical activity is effective in preventing mobility disability among older adults with functional limitations.8 Prior studies indicate that the mobility-preserving effect of physical activity may be modified in the presence of obesity, a well-documented precursor of MetS. However, the direction of the association is still unclear: while some studies found no mobility benefit9 or an attenuated benefit10 of physical activity in the presence of obesity, others found a greater benefit,11,12 suggesting a potential differential effect of physical activity on mobility disability according to metabolic status. The objective of the current study was to determine whether baseline MetS modifies the beneficial effect of a structured physical activity intervention on mobility disability in a sample of vulnerable older adults with functional limitations.
METHODS
Study Design and Participants
The Lifestyle Interventions and Independence for Elders (LIFE) study was a Phase 3, single-blind, multi-center, parallel randomized-controlled trial, which compared the effects of a moderate-intensity physical activity (PA) program with a health education (HE) program on the incidence of major mobility disability (MMD; defined as inability to walk 400 meters in less than 15 minutes) in 1,635 adults, 70 to 89 years old, who had functional limitations. The study design, inclusion/exclusion criteria, randomization procedure (see Supplementary Appendix S1 for study flow), and intervention protocol have been described in detail elsewhere.8,13
Briefly, potential participants were considered eligible if they were sedentary (i.e., <20 minutes/week of structured physical activity in the past month and reported ≤125 minutes/week of moderate physical activity),14 had functional limitations (i.e., Short Physical Performance Battery (SPPB) score ≤9),7 and were able to walk 400m in <15 min, unassisted, without sitting or leaning. The exclusion criteria were designed to identify persons who were likely incapable of fully-participating in the interventions because of comorbid conditions or cognitive impairment and those for whom PA would be unsafe.8
This article presents results for a secondary post-hoc analysis that was not pre-specified in the study protocol but was pre-specified in a proposal that was approved by the LIFE Publications and Presentations Committee prior to initiation.
Analytic Sample
Of the 1,635 LIFE participants, 818 were randomized to the PA group and 817 to the HE group. The present study excluded 100 participants (52 from PA and 48 from HE) for whom baseline MetS status could not be determined because of missing data, resulting in an analytic sample of 1,535 participants.
Intervention and Follow-up Schedule
The two interventions, including adherence, have been described in detail elsewhere.8,13 Briefly, the PA intervention required attendance at 2 center-based visits per week and home-based activities 3–4 times per week. The program included walking, with a goal of 30 minutes/session of moderate intensity walking or 150 minutes/week, as well as flexibility, strength, and balance training. The physical activity group attended an average of 63% of the scheduled sessions (median [IQR], 71% [50%–83%]).
The HE program involved attending weekly workshops focused on successful aging topics for the first 26 weeks and monthly workshops thereafter. The HE group also participated in a 5- to 10-minute instructor-led program of stretching exercises. HE participants attended an average of 73% of the scheduled sessions (median [IQR], 82% [63%–90%]).
Measures
Major Mobility Disability (MMD)
The two outcomes included incident MMD and persistent MMD. MMD was defined as the inability to complete a usual-pace 400-meter walk without sitting or the help of another person or walker (use of a straight cane was permitted). Participants were assessed every 6 months for up to 42 months. As previously described,8 for participants who were unable to come to the clinic, MMD definition was based on objective inability to walk 4 meters in < 10 seconds, or documented inability to walk across a room. Deaths were ascertained through regular surveillance. Incident MMD was defined as newly-developed MMD. Because newly-disabled individuals may recover,15 we also evaluated persistent MMD, denoting more severe and/or non-reversible mobility disability. Persistent MMD was defined as two consecutive MMD diagnoses or one MMD diagnosis followed by death. The number needed to treat for the main outcome (MMD) in the LIFE Study was 51, 26, and 17 for intervention durations of 1, 2, and 3 years.
Metabolic Syndrome (MetS)
MetS was measured at baseline using the harmonized criteria from the 2009 Joint Interim Statement,1 as the presence of ≥ 3 components from the following: abdominal obesity (waist circumference ≥102 cm in men and ≥88 cm in women); low HDL-cholesterol (<40 mg/dl for men and <50 mg/dl for women or drug treatment for low HDL); elevated triglycerides (TG) (≥150 mg/dl or drug treatment for elevated TG); hypertension (systolic pressure ≥130 and/or diastolic pressure ≥85 mm Hg or antihypertensive drug treatment with a history of physician-diagnosed hypertension); and elevated fasting plasma glucose (≥100 mg/dl or drug treatment for diabetes). MetS was coded as absent/present.
Other Measures
Details about the main baseline assessments (summarized in Table 1), including sociodemographic and health status information, have been previously described.8,16
Table 1.
Descriptive Characteristics of Study Participants, According to Metabolic Syndrome (MetS) Status and Intervention Group
| Characteristics | MetS (n=763) | Non-MetS (n=772) | ||
|---|---|---|---|---|
| PA (n=386) | HE (n=377) | PA (n=380) | HE (n=392) | |
| Baseline | ||||
| Demographics | ||||
| Age, mean(SD), years | 77.9 (5.0) | 78.0 (4.9) | 79.5 (5.4) | 80.1 (5.3) |
| Female, n(%) | 257 (66.6) | 248 (65.8) | 250 (65.8) | 267 (68.1) |
| Non-White, n(%) | 86 (22.3) | 74 (19.7) | 81 (21.4) | 70 (17.9) |
| Education, high-school or less, n(%) | 147 (38.1) | 139 (36.9) | 106 (28.0) | 109 (28.0) |
| MetS criteria,a n (%) | ||||
| Abdominal obesity | 359 (93.2) | 348 (92.3) | 188 (49.9) | 214 (55.4) |
| Low HDL-C | 160 (41.8) | 161 (44.0) | 10 (2.6) | 7 (1.8) |
| High triglycerides | 205 (53.4) | 195 (53.7) | 25 (6.6) | 23 (5.9) |
| Hypertension | 359 (93.0) | 352 (93.4) | 245 (64.5) | 248 (63.3) |
| High fasting glucose | 321 (83.6) | 312 (82.8) | 73 (19.2) | 69 (17.6) |
| MetS criteria count, n (%) | ||||
| 0 | 47 (12.4) | 41 (10.5) | ||
| 1 | 125 (32.9) | 141 (36.0) | ||
| 2 | 208 (54.7) | 210 (53.6) | ||
| 3 | 208 (53. 9) | 205 (54.4) | ||
| 4 | 110 (28.5) | 107 (28.4) | ||
| 5 | 68 (17.6) | 65 (17.2) | ||
| Health Status | ||||
| Diabetes diagnosis, n(%) | 176 (45.6) | 192 (50.9) | 34 (8.9) | 41 (10.5) |
| BMI, mean(SD) | 32.2 (5.5) | 32.6 (6.0) | 28.0 (5.3) | 28.1 (5.7) |
| BMI category, n(%) | ||||
| Underweight (< 18.5) | 0 (0.0) | 1 (0.3) | 3 (0.8) | 3 (0.8) |
| Normal (18.5 to <25.0) | 23 (6.0) | 22 (5.8) | 107 (28.2) | 126 (32.1) |
| Overweight (25.0 to <30.0) | 124 (32.1) | 115 (30.5) | 158 (41.6) | 145 (37.0) |
| Obese (≥ 30.0) | 239 (61.9) | 239 (63.4) | 112 (29.5) | 118 (30.1) |
| Comorbidities,b mean(SD) | 0.7 (0.9) | 0.7 (0.9) | 0.6 (0.8) | 0.7 (0.8) |
| 3MSE score, mean(SD) | 91.7 (5.4) | 91.6 (5.5) | 91.6 (5.4) | 91.7 (5.2) |
| Physical Performance | ||||
| SPPB score | 7.4 (1.6) | 7.2 (1.6) | 7.4 (1.6) | 7.4 (1.5) |
| 400m walking speed, mean (SD), m/s | 0.81 (0.2) | 0.80 (0.2) | 0.84 (0.2) | 0.84 (0.2) |
| Physical Activity (baseline) | ||||
| CHAMPS score, mean(SD) | 16.9 (33.5) | 16.7 (32.8) | 15.7 (31.1) | 20.1 (35.5) |
| Accelerometry,c mean(SD), min/wk | 182.1 (151.0) | 180.7 (181.1) | 197.4 (166.2) | 208.2 (180.1) |
| Follow-up | ||||
| Intervention adherence | ||||
| % of session attended | 63.8 (26.6) | 74.0 (23.7) | 63.3 (26.5) | 73.0 (25.2) |
| Number MMD assessments, mean(SD) | 6.0 (1.7) | 6.1 (1.7) | 5.9 (1.8) | 6.0 (1.6) |
| Attrition and Follow-up | ||||
| Follow-up time, mean(SD), years | 2.7 (0.6) | 2.7 (0.6) | 2.7 (0.6) | 2.7 (0.5) |
| Died, n(%) | 24 (6.2) | 17 (4.5) | 23 (6.1) | 22 (5.6) |
| Dropped-out/lost to follow-up, n(%) | 15 (3.9) | 8 (2.1) | 15 (3.9) | 16 (4.1) |
Abbreviations: MetS – metabolic syndrome; PA – physical activity group; HE – health education group; MMD – major mobility disability; BMI - Body-Mass Index; 3MSE - Modified Mini-Mental Examination score (range 0–100; higher score indicates better cognitive function); SPPB – Short Physical Performance Battery (range: 0–9 per inclusion criteria; higher score indicates better performance); CHAMPS - Community Healthy Activities Model Program for Seniors physical activity score (range 0–120; higher score indicates higher levels of physical activity); HDL-C - High-Density Lipoprotein Cholesterol.
Missing values account for the small discrepancies between total n (in each column) and the values listed under each descriptive characteristic.
Abdominal obesity: waist circumference ≥ 102 cm in men and ≥ 88 cm in women; low HDL-C: < 40 mg/dl for men and < 50 mg/dl for women or drug treatment for low HDL; elevated triglycerides: ≥ 150 mg/dl or drug treatment for elevated triglycerides; hypertension: systolic pressure ≥ 130 and/or diastolic pressure ≥ 85 mm Hg or antihypertensive drug treatment with a history of physician-diagnosed hypertension; and elevated fasting plasma glucose: ≥ 100 mg/dl or drug treatment for diabetes. MetS defined as the presence of 3 or more criteria.
Comorbidities (range 0–6) include participants’ reports of physician-diagnosed angina/myocardial infarction, congestive heart failure, stroke, cancer, and lung disease (absent/present for all 5 conditions), and peripheral arterial disease (absent if ankle-brachial index ≥ 0.9 and present if < 0.9).
Accelerometry based on the 760 counts/minute cut-point.17
Statistical Analysis
Baseline characteristics were summarized by MetS status and intervention group using means/standard deviations for continuous variables and counts/percentages for categorical variables. Intervention adherence was calculated as percentage of scheduled intervention sessions attended. In addition, minutes spent in activity associated with more than 760 counts/min (by accelerometry)17 were analyzed using mixed-effects analysis of covariance models for repeatedly measured outcomes with an unstructured parameterization for longitudinal covariance.
For primary analyses, incident MMD and persistent MMD were summarized according to intervention group and MetS status. Cox regression models were used to assess the potential modifying effect of MetS status on the effectiveness of the PA intervention in preventing incident and persistent MMD, respectively. Discrete time-to-failure was measured from the date of randomization. The proportion of event-free participants over time was described using Kaplan-Meier estimates. Participants who remained event-free until death or the last follow-up visit were censored at their last available assessment. All models included the main effects of MetS status and intervention group and their interaction. Study design variables (gender and clinical site) were used as stratification factors in all models. Finally, because of earlier reports showing associations between the severity of MetS and mobility disability,6 we performed supplementary analyses with MetS as an ordinal variable (range 0–5), denoting the number of MetS components identified in each participant.
All analyses were performed using SAS 9.4 (Cary, NC) and p-values less than 0.05 were considered statistically significant.
RESULTS
Participants’ demographic and health characteristics are summarized in Table 1. As shown, 763 (49.7%) participants had 3 or more MetS criteria, thus fulfilling the requirements for MetS (i.e., MetS subgroup), while 772 (50.3%) had 2 or fewer criteria (i.e., non-MetS subgroup). Baseline characteristics were similar between PA and HE participants within each MetS subgroup.
Intervention Adherence and Attrition
PA participants in both the MetS and non-MetS subgroups attended an average of about 63% of the scheduled sessions; HE participants attended an average of 73% (MetS subgroup) and 74% (non-MetS subgroup) of the sessions. Follow-up and attrition indicators were similar between the MetS/intervention subgroups. Differences in accelerometer-based minutes of physical activity between the PA and HE groups within each MetS group were maintained over time (Supplementary Appendix S2).
Outcomes
Incident MMD was experienced by 129 participants in the MetS/PA subgroup (33.4%) vs. 158 (41.9%) in the MetS/HE subgroup; in the non-MetS group, 103 PA (27.1%) and 112 HE participants (28.6%) experienced new-onset MMD. Persistent MMD was observed in 59 participants in the MetS/PA subgroup (15.3%) vs. 95 in the MetS/HE subgroup (25.2%); in the non-MetS group, persistent MMD was observed in 54 PA (14.2%) and 57 HE participants (14.4%).
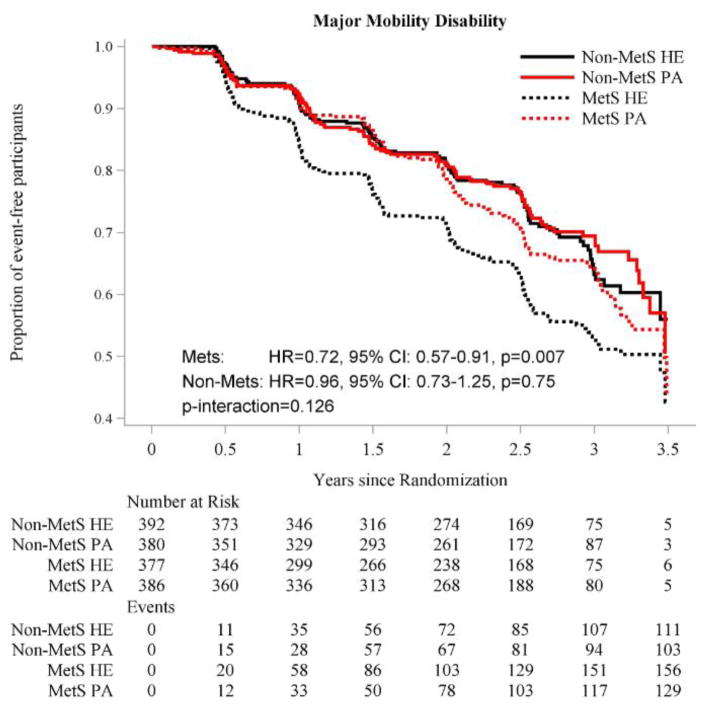
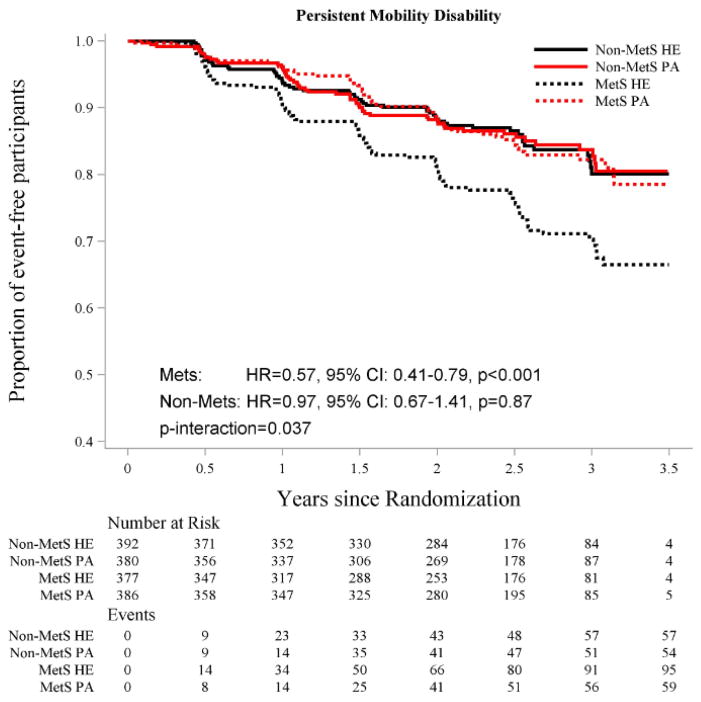
The Kaplan-Meier curves for incident and persistent MMD are provided in Figures 1 and 2, respectively. The PA intervention was beneficial in reducing incident MMD among participants with MetS (HR=0.72, 95%CI: [0.57, 0.91]), but not among those without MetS (HR=0.96, 95%CI: [0.73, 1.25]), although the test for statistical interaction was not significant (P=0.13) (Figure 1). In contrast, the test for statistical interaction was significant for persistent MMD (P=0.037) (Figure 2). Relative to HE, PA reduced the risk of persistent MMD among participants with MetS (HR=0.57, 95% CI: [0.41, 0.79]), but not among those without MetS (HR=0.97, 95% CI: [0.67, 1.41]).
Figure 1.
Effect of Physical Activity Intervention vs. Health Education on Incident Major Mobility Disability, According to Metabolic Syndrome Status.
Abbreviations: HR-hazard ratio; MetS – metabolic syndrome; PA – physical activity; HE – health education
The graph for incident MMD was truncated at 3.5 years; the non-MetS/HE subgroup had one additional MMD event and the MetS/HE subgroup had 2 additional MMD events between 3.5 and 3.6 years of follow-up.
Number of events represents cumulative events and adjusted HRs and P values are from proportional hazards regression models adjusted for gender and clinical site, as described in the Statistical Analysis section.
Figure 2.
Effect of Physical Activity Intervention vs. Health Education on Persistent Major Mobility Disability, According to Metabolic Syndrome Status.
Abbreviations: HR-hazard ratio; MetS – metabolic syndrome; PA – physical activity; HE – health education
The graph for persistent MMD was truncated at 3.5 years. No additional events were recorded between 3.5 and 3.6 years of follow-up.
Number of events represents cumulative events and adjusted HRs and P values are from proportional hazards regression models adjusted for gender and clinical site, as defined in the Statistical Analysis section.
Supplementary analyses with MetS as an ordinal variable showed a differential treatment effect of the PA intervention (vs. HE) according to MetS severity (i.e., increasing number of MetS criteria) for persistent MMD (P-interaction=0.027), but not for incident MMD (P-interaction=0.23). For both outcomes, the effect of the PA intervention (relative to HE) was stronger at higher MetS counts compared with lower MetS counts, with each one-unit increase in MetS criteria count associated with a 19% and 8% corresponding decrease in HR for persistent and incident MMD, respectively.
DISCUSSION
In this analysis of data from a large clinical trial, structured physical activity compared with health education substantially reduced the risk of persistent MMD among older persons with MetS, but not among those without MetS. The results were similar for incident MMD, although the difference in risk reduction between the MetS subgroups did not achieve statistical significance. The large reduction in major mobility disability in the MetS group is of particular clinical and public health importance given the high prevalence of MetS, the health and economic consequences of losing independent mobility, and the scarcity of proven interventions to avert mobility disability in this vulnerable older population.
Consistent with prior estimates,2 MetS was highly prevalent (~ 50%) in our sample of functionally-impaired older persons. The supplementary results, with metabolic dysfunction ranging from 0 (none of criteria present) to 5 (all criteria present), were consistent with those from the primary analyses; a stronger treatment effect was observed at higher number of MetS components for both persistent and incident MMD, although the formal test for statistical interaction was not significant for the latter.
Although the number (in absolute terms) of persistent and incident MMD cases was smaller in the non-MetS vs. MetS groups, the intervention showed a larger benefit in the MetS group for both mobility outcomes. Our finding of a greater benefit in reducing persistent MMD (43% reduction in risk) than incident MMD (28% reduction) suggests that physical activity facilitates recovery among older persons with MetS who have lost independent mobility. The difference between the number of incident and persistent events among MetS participants (Figures 1 and 2) indicates that 70 of 129 (54.3%) MetS/PA participants recovered their mobility after a first MMD event, compared with 95 of 158 (39.8%) in the MetS/HE subgroup. In contrast, among non-MetS participants, although a relatively large number of incident MMD cases did not become persistent cases, the likelihood of recovery from newly-developed mobility disability appears to be similar among PA and HE participants. These findings call for further investigation to identify factors that promote recovery from newly-developed mobility disability among older persons without MetS.
Despite the substantial benefit of physical activity in preventing a first episode of MMD in the MetS subgroup, the difference in risk reduction between the MetS and non-MetS subgroups did not reach statistical significance in either the primary or supplementary analyses. The LIFE study was powered to detect clinically meaningful reductions in MMD according to intervention group for all participants,8 but not within specific subgroups. Because our analyses were not pre-specified in the original study protocol and 100 participants were omitted because of missing baseline MetS data, we cannot exclude the possibility that a larger sample or longer follow-up would have allowed us to identify statistically significant subgroup differences.
Several possible explanations for the observed benefit of physical activity in reducing mobility disability among participants with but not without MetS merit comment. First, the results may reflect differences in the biological processes contributing to mobility disability in the two metabolic groups. The mechanisms responsible for mobility impairments among individuals with MetS have been extensively investigated. MetS and some of its components (in particular the combination of abdominal obesity and hyperglycemia)6 have been linked to muscle deficiency,18,19 chronic inflammatory and oxidative stress processes,20,21 low cardiorespiratory fitness,22 and biomechanical leg stress.19 In turn, prior observational and intervention studies have demonstrated robust beneficial effects of physical activity on these processes.23–25
In contrast, our findings suggest that mobility disability in persons without MetS may result from processes that are not responsive to moderate physical activity, such as central and peripheral neuroaging,26,27 age-related sensory impairments,28 and loss of proprioception.29 It is unclear whether the contribution of these factors to mobility disability differs between persons with and without MetS. Because physical activity may provide other health benefits to persons without MetS, our findings should not be interpreted to mean that persons without MetS should not engage in physical activity. Additional work is needed to elucidate the mechanisms underlying mobility disability in individuals without MetS, as a first step towards identifying mobility-preserving interventions for this group.
It is unlikely that the differential benefit of physical activity between the MetS and non-MetS subgroups can be explained by differences in adherence to the PA intervention since the percentage of sessions attended within each intervention arm were similar between the two subgroups (Table 1) and the difference in objectively-assessed minutes of physical activity between PA and HE participants in the MetS and non-MetS groups was maintained over time (Supplementary Appendix S2).
To our knowledge, this is the first study assessing the effectiveness of a structured physical activity intervention in individuals with and without MetS at high-risk for mobility disability. Our study has important strengths, including an objectively-measured outcome of high clinical relevance in older adults;30 assessment of MetS based on updated harmonized criteria,1 allowing for comparison across studies; high retention and intervention adherence rates; and a larger sample and longer follow-up relative to other randomized trials of physical activity in older populations.8 In addition to being a post-hoc analysis and not being powered to detect subgroup differences, our study had at least three other limitations. First, information on the duration of MetS, which may be associated with the risk of incident and persistent mobility disability and with the likelihood of benefit from the physical activity intervention, was not available. Second, the 2.7 years of average follow-up is short relative to the 9-year life expectancy of the LIFE cohort.8 Third, because our target population included sedentary older persons with functional limitations, the results may not be generalizable to younger and/or more functionally intact persons.
In conclusion, structured moderate-intensity physical activity substantially reduces the risk for persistent major mobility disability among sedentary, functionally-impaired older adults with MetS, but not among those without MetS. Although not statistically significant, comparable results were observed for incident major mobility disability. These findings, coupled with the demonstrated safety of the physical activity program, support further evaluation of this program as a cost-effective strategy for reducing mobility disability among vulnerable older persons with MetS. Additional research is needed to clarify the mechanisms underlying mobility disability among metabolically-healthy older persons and to identify feasible and effective interventions aimed at reducing the risk for disability in this group.
Supplementary Material
CONSORT Study Flow Diagram (online material)
Accelerometry-assessed adherence (online material)
LIFE Study Acknowledgements (online material)
Conflict of Interest Disclosure Form.
Acknowledgments
Funding:
The LIFE Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement # U01AG22376 and a supplement from the National Heart, Lung and Blood Institute (#U01AG022376). It is sponsored in part by the Intramural Research Program, National Institute on Aging, National Institutes of Health. Complete acknowledgments and funding information are shown in Supplementary Appendix S3 and are available at: https://www.thelifestudy.org/secure/documents
Dr. Botoseneanu was funded by grants AG024824 from the University of Michigan - Claude D. Pepper Older Americans Independence Center; UL1TR000433 from the Michigan Institute for Clinical and Health Research; and grant # T32AG019134 from the National Institute on Aging.
Dr. Gill is the recipient of an Academic Leadership Award (K07AG043587) from the National Institute on Aging and is supported by the Yale Claude D. Pepper Older Americans Independence Center (P30AG21342).
Footnotes
All authors meet the criteria for authorship stated in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals.
Conflict of Interest Disclosure: The authors are not aware of any potential conflict of interest. Please see COI form attached as Supplementary Appendix S4.
Authors’ Contributions:
Botoseneanu, Gill: concept and design. Chen, Ambrosius: statistical analyses. Botoseneanu, Gill, Chen, Ambrosius, Allore: interpretation of results. Botoseneanu, Chen, Gill: drafting the manuscript. Gill, King: acquisition of data. Botoseneanu, Gill, Chen, Ambrosius, Allore, Anton, Folta, King, Nicklas, Spring, Strotmeyer: critical review and revision of manuscript. All authors have approved the submitted version for publication.
Data Integrity Statement: Haiying Chen and Walter A. Ambrosius had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Sponsor’s Role: None
References
- 1.Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Ford ES, Li C, Zhao G. Prevalence and correlates of metabolic syndrome based on a harmonious definition among adults in the US. J Diab. 2010;2(3):180–193. doi: 10.1111/j.1753-0407.2010.00078.x. [DOI] [PubMed] [Google Scholar]
- 3.Healy GN, Wijndaele K, Dunstan DW, et al. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab) Diabetes Care. 2008;31(2):369–371. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 4.Ekelund U, Brage S, Franks PW, Hennings S, Emms S, Wareham NJ. Physical activity energy expenditure predicts progression toward the metabolic syndrome independently of aerobic fitness in middle-aged healthy Caucasians: The Medical Research Council Ely Study. Diabetes Care. 2005;28(5):1195–1200. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 5.Blazer DG, Hybels CF, Fillenbaum GG. Metabolic syndrome predicts mobility decline in a community-based sample of older adults. J Am Geriatr Soc. 2006;54(3):502–506. doi: 10.1111/j.1532-5415.2005.00607.x. [DOI] [PubMed] [Google Scholar]
- 6.Carriere I, Peres K, Ancelin ML, et al. Metabolic syndrome and disability: Findings from the Prospective Three-City Study. J Gerontol A Biol Sci Med Sci. 2014;69(1):79–86. doi: 10.1093/gerona/glt101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol A Biol Sci Med Sci. 1994;49(2):85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 8.Pahor M, Guralnik JM, Ambrosius WT, et al. Effect of structured physical activity on prevention of major mobility disability in older adults: The LIFE study randomized clinical trial. JAMA. 2014;311(23):2387–2396. doi: 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rejeski WJ, Brubaker PH, Goff DC, et al. Translating weight loss and physical activity programs into the community to preserve mobility in older, obese adults in poor cardiovascular health. Arch Intern Med. 2011;171(10):880–6. doi: 10.1001/archinternmed.2010.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manini TM, Newman AB, Fielding R, et al. Effects of exercise on mobility in obese and nonobese older adults. Obesity. 2010;18(6):1168–1175. doi: 10.1038/oby.2009.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brach JS, VanSwearingen JM, FitzGerald SJ, Storti KL, Kriska AM. The relationship among physical activity, obesity, and physical function in community-dwelling older women. Prev Med. 2004;39(1):74–80. doi: 10.1016/j.ypmed.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 12.Di Francesco V, Zamboni M, Zoico E, et al. Relationships between leisure-time physical activity, obesity and disability in elderly men. Aging Clin Exp Res. 2005;17(3):201–206. doi: 10.1007/BF03324597. [DOI] [PubMed] [Google Scholar]
- 13.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: Design and methods. J Gerontol A Biol Sci Med Sci. 2011;66(11):1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Med Sci Sports Exerc. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. Am J Epidemiol. 2005;161(6):575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- 16.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle Interventions and Independence for Elders Study: Recruitment and baseline characteristics. J Gerontol A Biol Sci Med Sci. 2013;68(12):1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37(11):512–522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 18.Cawthon PM, Fox KM, Gandra SR, et al. Clustering of strength, physical function, muscle, and adiposity characteristics and risk of disability in older adults. J Am Geriatr Soc. 2011;59(5):781–787. doi: 10.1111/j.1532-5415.2011.03389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: Results from the InCHIANTI study. Int J Obes. 2009;33(6):635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beavers KM, Hsu F, Houston DK, et al. The role of metabolic syndrome, adiposity, and inflammation in physical performance in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2013;68(5):617–623. doi: 10.1093/gerona/gls213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stenholm S, Koster A, Alley DE, et al. Joint association of obesity and metabolic syndrome with incident mobility limitation in older men and women--results from the Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci. 2010;65(1):84–92. doi: 10.1093/gerona/glp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brien SE, Janssen I, Katzmarzyk PT. Cardiorespiratory fitness and metabolic syndrome: US National Health and Nutrition Examination Survey 1999–2002. Appl Physiol Nutr Metab. 2007;32(1):143–147. doi: 10.1139/h06-090. [DOI] [PubMed] [Google Scholar]
- 23.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Starr KNP, McDonald SR, Bales CW. Obesity and physical frailty in older adults: A scoping review of lifestyle intervention trials. J Am Med Dir Assoc. 2014;15(4):240–250. doi: 10.1016/j.jamda.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beavers KM, Ambrosius WT, Nicklas BJ, Rejeski WJ. Independent and combined effects of physical activity and weight loss on inflammatory biomarkers in overweight and obese older adults. J Am Geriatr Soc. 2013;61(7):1089–1094. doi: 10.1111/jgs.12321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guttmann CR, Benson R, Warfield SK, et al. White matter abnormalities in mobility-impaired older persons. Neurology. 2000;54(6):1277–1283. doi: 10.1212/wnl.54.6.1277. [DOI] [PubMed] [Google Scholar]
- 27.Ward RE, Boudreau RM, Caserotti P, et al. Sensory and motor peripheral nerve function and incident mobility disability. J Am Geriatr Soc. 2014;62(12):2273–2279. doi: 10.1111/jgs.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: Implications for health and functioning. Am J Public Health. 2004;94(5):823–829. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang JT, Ganz DA. Quality indicators for falls and mobility problems in vulnerable elders. J Am Geriatr Soc. 2007;55(s2):327–334. doi: 10.1111/j.1532-5415.2007.01339.x. [DOI] [PubMed] [Google Scholar]
- 30.Newman AB, Simonsick EM, Naydeck BL, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295(17):2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Study Flow Diagram (online material)
Accelerometry-assessed adherence (online material)
LIFE Study Acknowledgements (online material)
Conflict of Interest Disclosure Form.