Abstract
Animals, including humans, require a highly coordinated and flexible system of social behavior and threat evaluation. However, trauma can disrupt this system, with the amygdala implicated as a mediator of these impairments in behavior. Recent evidence has further highlighted the context of infant trauma as a critical variable in determining its immediate and enduring consequences, with trauma experienced from an attachment figure, such as occurs in cases of caregiver-child maltreatment, as particularly detrimental. This review focuses on the unique role of caregiver presence during early-life trauma in programming deficits in social behavior and threat processing. Using data primarily from rodent models, we describe the interaction between trauma and attachment during a sensitive period in early life, which highlights the role of the caregiver’s presence in engagement of attachment brain circuitry and suppressing threat processing by the amygdala. These data suggest that trauma experienced directly from an abusive caregiver and trauma experienced in the presence of caregiver cues produce similar neurobehavioral deficits, which are unique from those resulting from trauma alone. We go on to integrate this information into social experience throughout the lifespan, including consequences for complex scenarios, such as dominance hierarchy formation and maintenance.
Keywords: Development, Threat, Amygdala, Social behavior, Dominance hierarchy
1. Introduction
We have known for half a century that the brain and behavior of altricial species, including humans and rodents, continues to develop after birth, and that genetics and experience interact to guide the intricate process of constructing the brain (Andersen and Teicher, 2008, De Bellis and Thomas, 2003, Fisher, 1955, Landers and Sullivan, 2012, Levine, 1957, Levine, 2005, Mainardi et al., 1965). This open system enables early-life experiences to sculpt the brain and optimize behaviors to more closely fit diverse environments to enhance survival (Bock et al., 2014). However, this same open system can permit developmental perturbations to produce vulnerability to psychiatric disorders and maladaptive behaviors that reduce access to resources, especially during critical periods for programming complex cognition and behavior (Andersen and Teicher, 2008, Opendak and Sullivan, 2016). In particular, trauma experienced from a caregiver during a sensitive window in early life can produce life-long deficits in threat processing and social behavior across many species (Amaral, 2003, Callaghan et al., 2014, McEwen, 2003, Moriceau et al., 2006, Tang et al., 2014, Tzanoulinou and Sandi, 2017, Zeanah et al., 2003). Modeling this in rodents suggests that repeated pairing of cues associated with the caregiver and with trauma can disrupt the typical developmental trajectory of brain areas important for both forming attachments and learning about threat (Opendak and Sullivan, 2016, Raineki et al., 2012). In particular, we have observed that trauma experienced in the presence of the caregiver has similar neurobehavioral consequences as trauma experienced directly from an abusive caregiver; these socially anchored traumas produce unique and profound effects that go beyond those of trauma alone.
Understanding trauma effects and the importance of a social context on brain development has been challenging, not only because the process of building a brain is complex, but because many of the effects of early life perturbations are often not expressed until a later stage of development (Ainsworth, 1969, Gunnar et al., 2007, Landers and Sullivan, 2012, Raineki et al., 2012). Threat processing has been a major focus in studying disordered attachment because of the link between trauma and aberrant detection of danger, vigilance, and regulation of emotion in mental illness in children and adults (Bremne and Vermetten, 2001, Caron et al., 2015, Cirulli et al., 2009, Drury et al., 2012, Elzinga et al., 2003, Jedd et al., 2015, Teicher et al., 2003, Tottenham and Sheridan, 2009). We rely on animal research to highlight mechanisms, which has shown that caregiver abuse in early life produces structural and functional changes in the amygdala and a changed threat system (Bagot et al., 2009, Bock et al., 2014, Caldji et al., 1998, Ivy et al., 2008, Maestripieri et al., 1999, Raineki et al., 2012, Roth and Sullivan, 2005, Sanchez et al., 2001, Tang et al., 2014). Drury et al. (2015) have recently written a review comparing different animal models used to assess mechanisms of early life adversity (Drury et al., 2015).
Although there is a broad literature documenting multiple forms of early-life trauma across many species, the focus of this review will be species-atypical abusive maternal care using data from rodent models. While translating rodent data to humans can be challenging, the attachment system and its response to trauma have considerable convergence between species. Indeed, studies in rodents, primates, and humans have identified a sensitive period in early life during which abusive care from an attachment figure interacts with heightened neural plasticity to program long-term impairments in social behavior through effects on amygdala development (Opendak and Sullivan, 2016, Humphreys and Zeanah, 2015, Drury et al., 2015). We will begin to review attachment-trauma effects on amygdala development with an exploration of neurobiology of attachment in typical rearing conditions. We will then expand our discussion to disordered attachments that form following abusive caregiving in rodents. Finally, we will discuss the long-term consequences of these atypical attachment-trauma experiences for behavior in highly complex social scenarios in the wild.
2. Developmental trajectory of brain areas important for social behavior and threat processing
The effects of early life experience on the brain involve changes at nearly every level of analysis, from cellular signaling to behavioral expression. Indeed, through the decades, countless brain regions and nearly every neurotransmitter have been implicated in the etiology of psychopathology following early life experiences, including changes in receptors, epigenetics, brain structure, the microbiome, immune system, and homeostasis maintenance (Andersen and Teicher, 2008, Bale, 2015, Blakemore and Mills, 2014, Callaghan et al., 2016, Drury et al., 2012, Gold et al., 2016, Green et al., 2016, Halevi et al., 2016, Hartley and Lee, 2015, Heim and Binder, 2012, Humphreys et al., 2016, Kane et al., 2016, Kennedy et al., 2016, Knudsen, 2004, Lawler et al., 2016, Nelson et al., 2014, Pechtel et al., 2014, Poulos et al., 2014, Puetz et al., 2016, Reuben et al., 2016, Troller-Renfree et al., 2016, Umemori et al., 2015, Werker and Hensch, 2015, Zannas and Binder, 2014, Zeanah and Sonuga-Barke, 2016). Research on rodents and nonhuman primates promptly identified the hypothalamic-pituitary-adrenal (HPA) axis as one mediator for how experience disrupts development (Rincon-Cortes and Sullivan, 2014, Sanchez, 2006). This system is involved in the body’s response to allostatic load; chronic over-activation of the HPA axis in response to early life trauma can produce long-term adaptations in the stress response, and these changes are thought to underlie the development of disorders such as PTSD, depression, and anxiety (Graham et al., 1999). While the HPA axis has remained a focus as the mediator of early life trauma, additional mechanisms have been implicated in the complexity of early life experiences on brain programming, including a critical role for learning (Moriceau et al., 2006).
Although it is beyond the scope of this review to describe brain development in detail, a few basic concepts are helpful for the present discussion (for additional reading on brain development, see Casey et al., 2005, Houston et al., 2014). First, the brain continues to develop throughout early life, with different brain areas each having their own trajectory of development and maturation (Berdel et al., 1997, Brummelte and Teuchert-Noodt, 2006, Chareyron et al., 2012a, Chareyron et al., 2012b, Cunningham et al., 2002, Ehrlich et al., 2012, Knudsen, 2004, Van Eden and Uylings, 2004, Wakefield and Levine, 1985). Brain areas important for basic physiological functions are certainly mature at birth, but continue to mature and develop more complex connections with other brain areas and within themselves (Rinaman et al., 2011). Regions involved in complex behaviors and higher-order functioning, including the amygdala, hippocampus, and prefrontal cortex (PFC), are more delayed in maturation, although recent evidence suggests specific functions of each of these brain regions have their own developmental trajectories. For instance, hippocampus-dependent contextual fear learning emerges around postnatal (PN) day 23 in rodents (Raineki et al., 2010a), while other hippocampal dependent learning behaviors emerge either before or after this age (Moye and Rudy, 1987, Stanton, 2000). Traditional measures of neural maturation, such as long-term potentiation (LTP), a presumed measure of synaptic plasticity, emerge over a week earlier than contextual fear learning (Ainge and Langston, 2012, Bekenstein and Lothman, 1991, Harris and Teyler, 1983, Swann et al., 1990, Wilson, 1984) and do not highlight the distinct behavioral trajectories of specific hippocampal-dependent behaviors. Furthermore, some brain areas likely encode information at one age but affect behavioral expression at another (Moye and Rudy, 1987, Pattwell et al., 2012, Poulos et al., 2014).
Emerging evidence also suggests brain areas can have unique function in early life, such as the important role of locus coeruleus (LC) norepinephrine (NE) in attachment that is described below (Landers and Sullivan, 2012). Neurochemicals, too, can have age-specific effects, such as the switch between excitation and inhibition by GABA at birth (Ben-Ari, 2014). The changing roles of other molecules across development are less clear: while oxytocin has a demonstrable role in prosocial behavior and maternal care in adults (Calcagnoli et al., 2015, Frijling et al., 2016, Johnson and Young, 2015, Lee et al., 2007, Marlin and Froemke, 2017, Nelson and Panksepp, 1998, Shamay-Tsoory and Abu-Akel, 2016), its role in very early life and the sensitive period for attachment remains unclear (Nelson and Panksepp, 1998, Parr et al., 2016, Sannino et al., 2017). Finally, the numerous connections among brain areas can be further delayed in maturation so that feedback systems that form complex loops of bidirectional information processing become functional at a later age. Thus, as we consider how early life trauma can impact a child, it is important to consider when brain areas implicated in adult trauma processing are functionally mature and functionally connected to other brain areas. As will be shown below, processing of trauma in early life is different from processing such information in adulthood (Gunnar et al., 2007, Opendak and Sullivan, 2016, Teicher et al., 2003).
The amygdala-hippocampus-PFC circuit is crucial for threat detection and many forms of social behavior, but the maturation and connectivity between these brain regions develops slowly over early life in humans (Casey et al., 2005, Gee et al., 2013, Graham et al., 1999, Malter Cohen et al., 2013, Skuse et al., 2003, Tottenham, 2012, Tottenham and Sheridan, 2009), nonhuman primates (Bachevalier and Loveland, 2006, Chareyron et al., 2012a, Chareyron et al., 2012b, Lavenex and Banta Lavenex, 2013, Sanchez et al., 2001, Skuse et al., 2003) and rodents (Brummelte and Teuchert-Noodt, 2006, Holland and Gallagher, 2004). The amygdala is considered to be the critical structure involved in the formation and storage of conditioned fear associations (Davis et al., 1994, Phelps and LeDoux, 2005), but it has a role in many emotional functions, including those unrelated to fear, such as social odor processing and assessing hedonic value of stimuli (Holland and Gallagher, 2004, Maren and Fanselow, 1996, Phelps and LeDoux, 2005, Royet et al., 2000). It has been suggested that neonatal amygdala connectivity correlates with fear responses at six months of age in human infants (Graham et al., 2016). Furthermore, volumetric analysis indicates that amygdala volume peaks in pre-adolescence (Uematsu et al., 2012). The PFC works with the amygdala to regulate complex decision-making, particularly in functions relevant to threat processing and social behavior, but the developmental trajectory of this region is not fully understood. The PFC subarea orbitofrontal cortex (OFC), which plays an important role in assessing the hedonic value of odors (Anderson et al., 2003, Gottfried et al., 2002, Rolls, 2015, Zald and Pardo, 1997), is postulated as functional by the time a child is two or three years old, while the anterior cingulate (ACC) and medial PFC (mPFC) are thought to possibly become functional around four months and as early as four years, respectively (Allman et al., 2001, Gee et al., 2013, Graham et al., 2015).
The hippocampus is a region with diverse functions, including the ability to remember specific information about events, such as where and when events occurred. These functions appear to develop around two years old in children, but show great improvement over the next four years (Gomez and Edgin, 2015, Lavenex and Banta Lavenex, 2013). Since the child’s hippocampus is difficult to image using brain scanning techniques, data on hippocampal functional emergence does not exist, although hippocampal growth rates appear to peak around 9–11 years of age (Uematsu et al., 2012). Evidence from rodent studies on development of the amygdala and hippocampus show a similar developmental time course; both regions demonstrate considerable growth during the initial postnatal period and become functionally mature in normal rearing conditions at PN10 and PN23, respectively (Chareyron et al., 2012b, Raineki et al., 2010a, Raineki et al., 2010c). These ages reflect significant developmental milestones in rats: although no consensus exists mapping rodent age onto human age, weaning around PN23 is considered a marker of early or peri-adolescence in infant rats, while PN0-PN9 is thought to represent infancy (Andersen, 2003, Sullivan and Holman, 2010). As will be discussed further, these first ten days represent a sensitive period for forming attachments. Although the amygdala, hippocampus, and PFC are robustly involved in trauma processing during adulthood, their involvement in processing early-life trauma is complex, due to their limited functional unavailability and/or immaturity at this age.
3. Infant social interaction: importance of the caregiver as regulator of brain and behavior
Understanding the role of a caregiver relies on a rich historical literature that has demonstrated the importance of the relationship between a child and a caregiver across a variety of species – a relationship primarily understood in terms of attachment. Animal research has provided clear evidence of the critical role of early life attachment in programming cognitive and emotional health. Specifically, seminal works by Niko Tinbergen, Konrad Lorenz, and John Hinde characterized how the newly hatched chick attached (imprinted) to the parent (Hess, 1962) within a temporally limited sensitive period. Around the same time, Harry Harlow and his colleagues were working with rhesus monkeys and assessing the effects of being reared without a mother but providing basic food, water and warmth (Harlow and Harlow, 1965). This work clearly highlighted the importance of the infant’s social interactions with the mother during a sensitive period in development since, without the caregiver, infants showed emotional and cognitive disabilities that were reminiscent of human children reared in inadequate orphanages without an attachment figure (Gunnar et al., 2015, Humphreys and Zeanah, 2015, Levin et al., 2015, Teicher et al., 2016).
Attachment to a caregiver during a sensitive window is of paramount importance to altricial infant survival due to the infant’s greatly reduced ability to acquire food, protection, and warmth. Furthermore, during early life, the infant relies on the caregiver for regulation of basic physiology, ranging from vital functions, such as heart rate and respiration, to emotional regulation. Caregiver regulation of the infant's emotional state is seen during perfunctory caregiving, such as by soothing a crying infant or by smiling at or tickling an infant to increase arousal. In turn, this stimulation of the infant's sensory systems changes physiology; for example, soothing a stressed infant can lower stress hormone levels (Gunnar and Quevedo, 2007, Gunnar et al., 2007, Hofer, 1994, Sarro et al., 2014a, Sarro et al., 2014b). In typically developing children, this caregiver regulation of infant physiology has been shown to be critical for a child’s interaction with the world, including reduction of fear, response to novelty, and learning (Humphreys and Zeanah, 2015, Gee et al., 2013, Nachmias et al., 1996). In turn, this system appears to be compromised in children with early life trauma. Specifically, neglectful and/or abusive caregiving has been associated with poor regulation of infant physiology and is correlated with disrupted developmental trajectories related to social behavior and threat processing (Gunnar et al., 2007, Mikics et al., 2008, Nemeroff, 2004, Rincon-Cortes and Sullivan, 2014). These effects have little to do with the level of sensory stimulation of the infant, as this varies across cultures without producing deficits in caregiver regulation (Choi, 1995, Welles-Nystrom et al., 1994).
The effects of inadequate and abusive care on the etiology of neurobehavioral deficits remain poorly understood, although animal models have attempted to add some insight into this complex issue (Gunnar et al., 2015, Hennessy et al., 2006, Hennessy et al., 2009, Hostinar et al., 2014, Opendak and Sullivan, 2016, Raineki et al., 2010b, Roth et al., 2013, Sarro et al., 2014a, Sarro et al., 2014b, Sullivan et al., 2011, Sullivan and Holman, 2010). When drawing parallels between work in humans and animal models, it is important to clarify that animal studies typically model this attachment–trauma within the framework of either harmful or species-atypical input (e.g. abusive caregiving) or absence of expected input (e.g. maternal deprivation, decreased maternal care) (Champagne et al., 2008, Francis et al., 2002, Tottenham, 2012, Tottenham and Sheridan, 2009). Similarly, trauma studies in children distinguish between the neurobehavioral effects of abuse, neglect, and trauma that is not associated with a caregiver (Bowlby, 1984, Bremner, 2003, Maestripieri and Carroll, 1998, Neigh et al., 2009). For instance, data from children raised in institutional care typically reflects the consequences of neglect, rather than abuse (Humphreys and Zeanah, 2015). Although these various subtypes of early-life trauma produce divergent adult outcomes, research using animal models and clinical populations highlights the amygdala’s involvement in the etiology of psychopathology (Raineki et al., 2012, Sitko et al., 2014, Teicher et al., 2016, Tzanoulinou and Sandi, 2017). By modeling attachment in normal and abusive circumstances, we can explore some of the neural mechanisms by which early life attachment programs social and threat processing throughout the lifespan.
4. The infant attachment circuit
The infant learns about the smell, sight, touch, sound and taste of the caregiver during social interactions. Once learned, these sensory cues from the caregiver are preferred and help regulate infant behavior and physiology. How the infant learns about the caregiver relies upon the unique neurobiology of the infant brain for attachment learning, which is describe below as identified in infant rodents. A useful framework in which to understand the changing learning circuitry of the young brain is to understand that the brain must continuously morph to accommodate the specific behavioral niche at each stage of development. For example, the young infant does not need a brain that supports learning behaviors to gather food from the environment or procure a receptive mate; rather, the brain is designed to learn about the caregiver and show prosocial behaviors toward the caregiver that will engage the caregiver to provide those resources needed for survival (Bowlby, 1978, Hess, 1962). As the child matures, he or she gains adult-like functioning for different tasks at different ages and this is presumably supported by transitions in brain morphology and function.
Given that an altricial infant’s attachment to a caregiver is critical for survival, the attachment circuit was likely shaped by evolutionary pressures to ensure attachment formation occurred, regardless of the quality of caregiving received. Indeed, John Bowlby’s Attachment Theory described the infant brain of altricial species as designed to support attachment to the caregiver, even when the quality of care is compromised or abusive, during a temporally defined sensitive period (Bowlby, 1965, Bowlby, 1978). This feature of attachment formation is supported by clinical and epidemiological literature indicating that abused children frequently long to be reunited with their abusive attachment figure after separation and placement in a safe home (Ainsworth, 1969, Perry and Sullivan, 2014). This has been modeled in a variety of species including birds, non-human primates, dogs, and rodents (Cirulli et al., 2009, Goursaud and Bachevalier, 2007, Harlow and Harlow, 1965, Malkova et al., 2010, O’Connor and Cameron, 2006, Raper et al., 2014, Sanchez, 2006, Sanchez et al., 2015, Suomi, 2003).
A rich literature has identified a unique learning circuit involved in the formation of attachment across a variety of species, although the neurobiology of attachment has mostly been described using rodents. Infant rodents, called pups, can neither hear nor see until the third week of life, and olfaction is the main sensory system used for interactions with the caregiver (in contrast to newborn humans, who use all of their sensory systems) (Ehret, 1976, Weber and Olsson, 2008). Specifically, maternal odor is of paramount importance to pups’ survival, as they rely on this odor cue for nipple attachment, proximity seeking, and social behavior. Without these, pups cannot access nourishment, thermoregulation, or maternal care. Indeed, pups without the ability to smell rarely survive as they frequently fail to nipple attach and can become malnourished (Landers and Sullivan, 2012).
The incredibly complex process of caregiver-infant interaction was long considered to be innate and guided by a pheromone (Blass and Teicher, 1980, Distel and Hudson, 1985, Leon, 1983). However, research has indicated that learning is of major importance for activating behavioral systems that are age-relevant and biologically predisposed towards preference for maternal odor. In rat pups, this learning process begins in the prenatal environment, where amniotic odors can guide nipple attachment as soon as pups are born. Even a neutral odor can acquire the valence of a maternal odor if placed into the amniotic fluid a few days before birth, suggesting the odor itself is arbitrary for both rats and mice (Hepper and Cleland, 1998, Leon, 1992, Logan et al., 2012, Pedersen and Blass, 1982, Smotherman and Robinson, 1987, Sullivan and Leon, 1986, Sullivan and Wilson, 1991). Once pups are born, a new maternal odor can be rapidly learned; a novel odor (e.g. peppermint) placed either on the mother or in the air surrounding her will readily take on the properties of maternal odor (Cheslock et al., 2000, Roth and Sullivan, 2005, Sullivan et al., 1990). Outside the nest, if a neutral odor is paired with warmth, milk, or stroking – stimuli designed to mimic maternal behavior– this odor acquires the value of a new maternal odor that is not only preferred, but can support nipple attachment and prosocial behavior in the absence of a natural maternal odor (Roth et al., 2013, Roth and Sullivan, 2005, Roth and Sullivan, 2006, Sullivan et al., 1986). This is especially important to ensure a robust attachment, given the fact that a dam’s odor can change with her diet and is dependent on gut bacteria (Leon, 1983, Leon, 1992).
During the first ten days of life, the learning process for new maternal odors in rat pups occurs through a relatively simple neurobiological substrate. At this early age, learning-associated plasticity occurs within the olfactory bulb, the first relay station for olfactory processing. This process requires that an odor is paired with copious amounts of NE (Sullivan et al., 2000b, Sullivan et al., 1992, Yuan et al., 2000). The sole source of the NE to the olfactory bulb is the LC, and this structure’s unique physiology during early life is essential for neonatal odor approach learning. In particular, the large amounts of NE required for this attachment-related plasticity results from the failure of the infant LC to show habituation or to turn itself off via auto-inhibition (as occurs in older pups and adults) (Nakamura et al., 1987, Winzer-Serhan et al., 1996). In addition, the olfactory bulb undergoes a host of anatomical and physiological changes reflecting enhanced responding to the learned maternal odor (Raineki et al., 2009, Roth and Sullivan, 2006, Sullivan et al., 1990, Yuan et al., 2002). It is important to note that both natural maternal odor and a learned artificial maternal odor generate the same responses from the olfactory bulb (Raineki et al., 2010c, Roth and Sullivan, 2005). The olfactory bulb axons of mitral cells project directly to the piriform cortex (Haberly, 2001, Schwob and Price, 1984, Swanson and Petrovich, 1998, Wilson and Stevenson, 2003); this region plays a key role in assigning the hedonic value to a learned odor stimuli in a region-specific manner. In particular, the anterior piriform is activated by odors learned during this sensitive period, while the posterior piriform is engaged in response to learned odor in older pups and adults (Moriceau and Sullivan, 2006, Moriceau et al., 2006, Roth and Sullivan, 2005). The sensitive period terminates when pups are around 10 days old, as the LC becomes more adult-like: NE release is greatly restricted due to the development of recurrent collaterals that quickly self-inhibit the LC’s response. After the sensitive period, NE takes on a modulatory role in odor learning that is more similar to what has been described in adult rats (Ferry and McGaugh, 2000).
Human infants also show learning of caregiver cues across multiple sensory modalities (DeCasper and Fifer, 1980, Sullivan et al., 2011), which enables them to form attachments to adoptive parents and caregivers of either sex. Although it remains unclear whether learning in human infants is the same as the rodent, NE plays a critical role in bond formation in numerous species (Nelson and Panksepp, 1998, Numan and Young, 2016), suggesting it is a phylogenetically conserved system. Notably, NE levels are very high in humans at birth and over the first two years of life (Lagercrantz and Bistoletti, 1977). Further work will be necessary to determine whether humans engage the same neural circuitry as rodents in forming attachments to a caregiver and whether the child’s brain is predisposed towards forming odor preferences at this age.
5. Maternal control over stress hormones: social buffering
Once the attachment figure’s smell, sight, sound, touch and taste are learned, these cues take on the role of regulating the brain and behavior. A crucial factor mediating the effects of early-life attachment on adult outcomes is how well the caregiver can regulate stress reactivity in the infant. As noted above, maternal cues regulate a wide variety of neurobehavioral functions (Hofer, 1994). Social buffering is a phenomenon that has been observed in myriad species and throughout the lifespan and describes the reduction of both the stress response and release of stress hormones (Ditzen and Heinrichs, 2014, Hennessy et al., 2009, Hostinar et al., 2015, Kikusui et al., 2006, Sanchez et al., 2015, Sullivan and Perry, 2015, Takahashi et al., 2013). This has been demonstrated in children, for whom maternal presence dampens cortisol reactivity to threats even when they behaviorally exhibit fear (Nachmias et al., 1996).
Social buffering of the infant is a dynamic process that wanes as individuals across species mature and become independent (Gee et al., 2014, Levine, 2001, McCormack et al., 2009, Sanchez, 2006, Stanton and Levine, 1990, Suchecki et al., 1995, van Oers et al., 1998) (Fig. 1). For example, at birth, rat pups have a functional HPA axis, although it soon becomes hypo-responsive and not activated by most painful stimuli, a period of life termed the stress hypo-responsive period (SHRP) (Dallman, 2000, Stanton and Levine, 1985). Since no stress response occurs, we have traditionally viewed social buffering as nonfunctional or irrelevant in infants.Although the specific time-course is unknown, there appears to be a similar stress hypo-responsive period in human children. Studies show that infants begin to exhibit dampened cortisol reactivity during the first year of life (6–12 months) (Gunnar and Donzella, 2002, Gunnar et al., 2015). Although the duration of this period is unknown, basal cortisol remains at low levels through the preschool period (Grunau et al., 2004, Watamura et al., 2004).
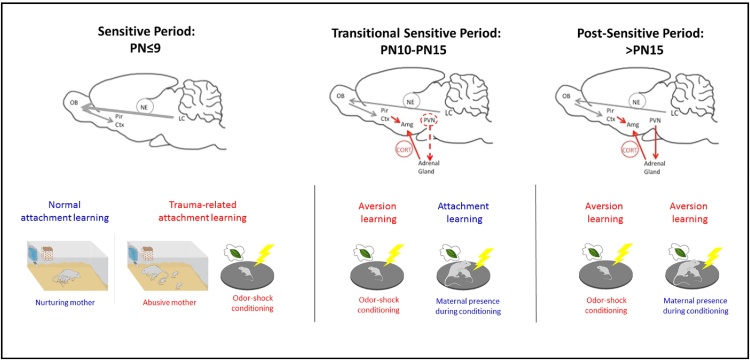
Fig. 1.
Transitions in learning across early development. Using a fear conditioning paradigm of odor-shock presentations has enabled us to uncover a developmentally unique learning system in pups that typically supports attachment learning. Data indicate that during the sensitive period for attachment learning (PN < 9), low CORT levels block amygdala plasticity to prevent pups from learning amygdala-dependent fear/threat. Instead, this learning paradigm activates the attachment learning neural circuit involving elevated NE (thick gray arrow) to produce approach responses to the odor (Moriceau et al., 2006). The odor also takes on qualities of the maternal odor to support nipple attachment and enhance prosocial behaviors to the mother. In pups older than PN9, this fear conditioning paradigm accesses the amygdala to support fear/threat learning if the pup is alone. A critical feature of this learning is that shock induces activation of the HPA axis and CORT release, which is necessary for the young amygdala to learn. However, if the mother is present, she socially buffers the pup’s stress response, and pups revert to sensitive period learning and learn an odor preference (red dashed line). This mother-controlled switch between fear and attachment learning is mediated through the mother’s ability to control pups CORT (Sullivan, in press). A more adult-like fear learning system, which cannot be switched on/off by CORT develops by PN15. Environmental variables that control pups’ CORT level, such as receiving CORT from a stressed mother via milk, environmental manipulations that increase pups’ CORT (abusive rearing) or the mother’s ability to socially buffer the pups (compromised in abusive mothers), have the potential to modify the age of these transitions and whether a pup learns fear or attachment (Moriceau et al., 2006, Perry and Sullivan, 2014, Raineki et al., 2012, Shionoya et al., 2007, Sullivan and Holman, 2010). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At PN10 in rodents, the SHRP begins to wane and we begin to see increases in CORT release in response to shock and other stressful stimuli in pups (Sullivan and Holman, 2010). It is also the age at which pups transition from crawling to walking and begin to leave the nest and nibble solid foods (Galef, 1981). As mentioned above, the LC takes on more adult-like functioning at this age (Moriceau and Sullivan, 2004, Nakamura and Sakaguchi, 1990, Sullivan and Wilson, 1994). The mother also begins to socially buffer the stress response, in a manner similar to that seen in adults. Specifically, at PN10, stressful stimuli begin to produce a more immediate increase in pups’ CORT levels, and the presence of the mother completely blocks its release (Moriceau et al., 2006, Stanton and Levine, 1990, Suchecki et al., 1993). Research has begun to explore the specific neural mechanisms involved in social buffering (Hennessy et al., 2006, Hennessy et al., 2009, Hennessy et al., 2015, Moriceau et al., 2006, Shionoya et al., 2007). It has been shown that in infant rodents, social buffering by the mother greatly attenuates stress hormone release by the HPA axis at the level of the hypothalamic paraventricular nucleus (PVN), through suppression of NE afferents from medullary A1/A2 noradrenergic neurons (Shionoya et al., 2007)–a system identified in adults (Ziegler and Herman, 2002). This social buffering by the mother has profound effects on whether pups have access to the attachment learning neural circuitry: the maternal social buffering can reopen pups sensitive period for attachment between the ages of PN10-15. In particular, during this transitional sensitive period, maternal presence can modulate whether pups learn to avoid or prefer an odor paired with shock; this process depends on amygdala serotonin and CORT levels (Moriceau et al., 2006) (Fig. 2).
Fig. 2.
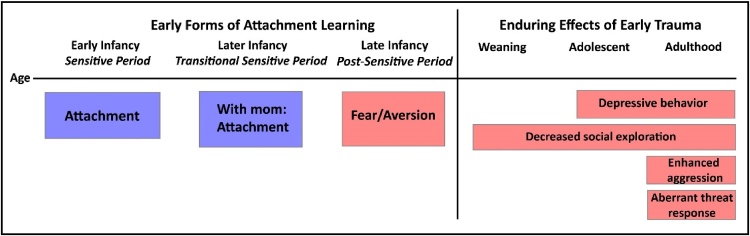
Timeline of attachment learning and the effects of early life maltreatment on later-life social and emotional behavior in the rat model of trauma associated with attachment. Early infants will learn attachment regardless of the quality of care, while slightly older infants (PN10-PN15) will either learn to fear a traumatic associated stimulus when away from the mother or learn an attachment if acquisition takes place with the mother. Testing later in life shows that only the early life trauma associated with attachment will lead to lifelong amygdala-dependent behavioral deficits, such as poor social behavior (onset prior to weaning) and depressive-like behaviors (onset post- weaning (Raineki et al., 2012, Raineki et al., 2010b, Sevelinges et al., 2011, Sullivan et al., 2000a, Sullivan et al., 2000b). In adulthood, early trauma produces enhanced aggression (Marquez et al., 2013) and impaired threat response (Perry, in press).
After PN15, pups show a rapid transition to independence and by weaning age (∼PN23), they show stress-induced activation of the HPA axis similar to adult-like levels. At this age, the relative ability of the mother to decrease the pups’ adult level stress hormone response is greatly reduced and leaves pups with significant CORT levels (Levine et al., 1988, Stanton and Levine, 1990, Suchecki et al., 1993, Upton and Sullivan, 2010). The human literature also suggests that, with further maturation, maternal presence loses some value to socially buffer children beginning to enter adolescence (Gee et al., 2014, Hostinar et al., 2015, Sanchez et al., 2015, Sandi and Haller, 2015) which is consistent with the animal literature (Barr et al., 2009, Ditzen and Heinrichs, 2014, Hennessy et al., 2015, Kiyokawa et al., 2004, Sanchez et al., 2015, Shionoya et al., 2007, Sullivan and Perry, 2015, Takahashi et al., 2013).
6. Attachments formed to abusive caregivers
The quality of care an infant receives from the caregiver, while preserving attachment, does alter how well the caregiver can regulate the infant’s physiology. For instance, highly stressed caregivers have a reduced capacity to socially buffer children (Ainsworth and Bell, 1970, Gunnar et al., 2007, Gunnar et al., 1996, Nachmias et al., 1996). In spite of this, throughout the animal kingdom, young, including humans, form attachments to abusive caregivers (Harlow and Harlow, 1965, Hess, 1962, Rajecki et al., 1978, Salzen, 1970, Stanley, 1962). This abuse-related attachment appears phylogenetically conserved across species, including chicks that form attachments after being shocked during imprinting (Hess, 1962, Rajecki et al., 1978, Salzen, 1970), dogs (Stanley, 1962), and monkeys raised with a wire surrogate that inflict pain (Harlow and Harlow, 1965). More recent work has modeled abusive caregiving in nonhuman primates and again shows that infants retain strong preferences for the abusive caregiver (Maestripieri et al., 1999, O’Connor and Cameron, 2006, Sanchez et al., 2001, Suomi, 2003). Attachments to an abusive or negligent caregiver may have short-term advantages, e.g. there is neonatal access to care, but long-term consequences associated with compromised threat processing and emotion expression.
Due to the unique neurobiology of the infant brain that is biased towards forming attachments, traumatic cues during the sensitive period for attachment are processed within the attachment circuitry rather than the threat processing circuitry. Again, the rodent literature provides some clues to understanding why this occurs. Our lab employs an abuse paradigm in which the mother rat is provided insufficient bedding to build a nest for the pups. Under these circumstances, the mother becomes highly agitated and frequently builds and re-builds her nest. In the process, she mistreats the pups, behaviors that include stepping on pups, dragging them across the cage floor, and transporting them inappropriately, inducing pain-related vocalizations in pups (Roth and Sullivan, 2005, Moriceau et al., 2009). Pairing this painful maternal care with a novel peppermint odor does not activate the important survival circuit within the brain to support aversion learning. Rather, pups not only learn to approach this odor, but this odor takes on the qualities of maternal odor to support nipple attachment and prosocial behaviors (Roth and Sullivan, 2005, Sullivan et al., 1986, Sullivan et al., 2000a). This learning can occur rapidly, within as little as 10–30 min. While the behavioral output of attachment formation with pain appears similar to typical attachment within the nest, as noted below, stress can uncover infant neurobehavioral problems consistent with disordered attachment (Raineki et al., 2010b).
Although this naturalistic maternal abuse paradigm is highly informative, its complexity makes it difficult to assess the mechanisms linking low resources and neurobehavioral pathology. Therefore, we complement this model with a classical conditioning paradigm to mimic abusive attachment. Just as new maternal odors can be learned when a previously neutral odor is paired with stimuli evoking maternal care, such as milk or stroking, we paired an odor with a moderately painful foot-shock (0.5 mA) or tail-pinch; this produced an odor preference in young pups (Camp and Rudy, 1988, Haroutunian and Campbell, 1979, Spear, 1978, Sullivan et al., 1986); we later showed this procedure also produces a new maternal odor that pups prefer (Raineki et al., 2012). The inability of the paired odor-pain procedure to produce fear learning is not due to pups’ inability to detect the aversive stimulus or feel pain. Noxious stimuli readily elicit <PN9 pup escape responses and the pain threshold does not appear to change as shock switches from supporting preference to supporting aversion learning (Barr, 1995, Collier and Bolles, 1980, Emerich et al., 1985, Stehouwer and Campbell, 1978). As described above, the pup’s olfactory bulb, anterior piriform cortex, and a hyper-functioning LC work together to generate enhanced odor preference learning despite adversity. Critically, shock presentations that are not paired with an attachment odor fail to produce the same neurobehavioral sequelae as either abusive care or paired odor-shock treatment (Raineki et al., 2012).
As mentioned above, termination of the sensitive period for attachment at PN10 in typical rearing conditions is primarily due to increasing levels of CORT and functional emergence of the amygdala (Sullivan and Holman, 2010). While CORT levels naturally increase at PN10, the environment readily changes pups CORT levels, providing environmental control of the sensitive period for attachment termination. Specifically, increasing CORT during the sensitive period, either via rearing with an abusive mother, or through pharmaceutical manipulations (systemic injections or by intra-amygdala microinfusions), can prematurely end sensitive period learning. Indeed, the amygdala is mature enough to support threat learning in pups as young as PN6, provided sufficient levels of corticosterone are available within the amygdala (Debiec and Sullivan, 2014, Moriceau et al., 2004, Moriceau et al., 2009, Moriceau and Sullivan, 2004, Moriceau and Sullivan, 2006). Pups reared by an abusive mother during the SHRP receive CORT through her milk and her ability to socially buffer this CORT elevation is compromised; when these pups are trained on a peppermint odor-shock conditioning paradigm outside the nest at PN7, they will learn an aversion through amygdala-dependent mechanisms (Moriceau et al., 2004, Raineki et al., 2010b). Similarly, pups reared with a normal nurturing mother but trained on a 5-day odor-shock conditioning procedure during the SHRP will learn an aversion to the odor if they receive CORT injections before each training session (Raineki et al., 2010b, Raineki et al., 2010c). It is important to note that, although abused pups or pups injected with CORT can learn arbitrary odor aversions (eg. peppermint) via premature amygdala engagement, they will nevertheless always show a preference for the maternal odor. As will be discussed further, this preference accompanies a disordered, rather than typical, attachment.
Although abuse and repeated odor-shock fails to produce an aversion to maternal odor, these manipulations generate latent changes in amygdala function that emerge around weaning age. Importantly, direct abuse from the caregiver and pairing of the maternal odor with shock produce indistinguishable neurobehavioral outcomes. Amygdala-dependent deficits are expressed in a task-specific manner: amygdala hyperactivity underlies decreased social exploration in adolescence and depressive and anxiety-like behavior in adulthood (Fig. 2), in parallel with impaired ability to learn aversions to threat and blunted amygdala activation during fear conditioning (Raineki et al., 2012, Sevelinges et al., 2007, Sevelinges et al., 2011, Sevelinges et al., 2008). These results parallel clinical studies showing a role for amygdala dysfunction in psychiatric sequelae in adults with a history of attachment trauma, resulting from abuse and/or neglect, during childhood (Callaghan et al., 2014, Teicher et al., 2003).
7. Stress uncovers latent consequences of early-life trauma
Across many species, many of the effects of infant abuse remain latent until peri-adolescence (Adriani and Laviola, 2004, Amaral, 2003, Andersen and Teicher, 2008, Bauman et al., 2006, Costello et al., 2003). For this reason, it can be difficult to identify abused children in the absence of physical evidence. In parallel with observations of latent abuse-related deficits in rodents and non-human primates, many of the effects of early life trauma on children’s mental health appear to be delayed until they begin to transition into adolescence (Bachevalier and Loveland, 2006, Bachevalier et al., 2001, Callaghan et al., 2014, Goursaud and Bachevalier, 2007, Goursaud et al., 2014, Machado and Bachevalier, 2003, Raper et al., 2014, Tottenham and Sheridan, 2009). However, stress can uncover deficits associated with trauma that may otherwise be hidden, as demonstrated by Mary Ainsworth’s Strange Situation Test. In this procedure, repeatedly removing children’s caregiver and introducing a stranger was necessary to reveal behavioral impairments marking disordered attachment (Ainsworth, 1969, Crittenden, 1992, Nachmias et al., 1996). This can also be modeled in rodents: pups that were exposed to abuse in the low bedding procedure and repeated odor-shock pairings form a disordered attachment to the mother, expressed in decreased approach behavior towards the maternal odor, less nipple attachment, and amygdala hyperactivity if given CORT injections before testing (Raineki et al., 2012). The CORT injection in pups models a high-stress environment, which appears necessary to uncover neurobehavioral deficits before weaning. Importantly, this CORT injection did not disrupt social interaction or activate the amygdala in pups reared with a nurturing mother, nor was there evidence of disordered attachment in pups that received only shock trauma or unpaired odor-shock training. Taken together, these findings suggest that repeatedly experiencing trauma associated with caregiver cues during the sensitive period for attachment has a unique neural signature, producing latent changes in amygdala function to program emotionality and social behavior throughout the lifespan.
8. Amygdala involvement in social behavior throughout the lifespan
In humans, the amygdala is implicated in social behavior in adults (Thomas et al., 2001), as well as during development (Skuse et al., 2003, Tottenham and Sheridan, 2009). Furthermore, research has shown that humans with disorders associated with social behavior deficits, such as Autism and Williams Syndrome, show amygdala abnormalities (Baron-Cohen et al., 2000, Bachevalier et al., 2000, Critchley et al., 2000, Howard et al., 2000, Pierce et al., 2001, Haas et al., 2009, Paul et al., 2009). Moreover, studies in nonhuman primates demonstrate that adult monkeys without amygdalae display inappropriate social behavior (Amaral, 2003, Baron-Cohen et al., 2000, Bliss-Moreau et al., 2011, Brothers et al., 1990, Emery et al., 2001, Malkova et al., 2010).
While the amygdala is also implicated in social behavior in children (Tottenham and Sheridan, 2009), its role is less clear. Studies on abused and neglected children, as well as children with PTSD, indicates changes in the response to threatening faces (Pine et al., 2005, Pollak et al., 2000). The most dramatic developmental differences were first observed in non-human primates, where infant amygdala lesions lead to decreased fear response to normally threatening stimuli and an enhanced response to novel social situations (Amaral, 2002, Amaral, 2003). This parallels work showing impaired threat assessment in abused rats with amygdala dysfunction (Perry, Santiago, & Sullivan, in press) and rats that were stressed during peripuberty (Marquez et al., 2013).
The rodent literature allows for more precise manipulations of specific amygdala nuclei. Social behavior in adult rodents appears to rely on the medial amygdala (Rasia-Filho et al., 2000). It has been shown that c-Fos expression, an indirect marker of neural activation, increases in the medial amygdala following social encounters and maternal behavior in rodent models (Fleming et al., 1994, Kirkpatrick et al., 1994). Medial amygdala activation is also associated with rodent parental behavior, which is blocked by lesioning this nucleus (Ferguson et al., 2002, Gobrogge et al., 2007, Kirkpatrick et al., 1994). While the medial amygdala has a prominent role in social behavior, the basolateral, central and cortical amygdala nuclei have also been implicated (Katayama et al., 2009). As will be discussed below, these nuclei form part of a functional circuit with the hippocampus and vmPFC that is critically engaged in complex forms of social behavior.
It is important to note that while social behavior in adult rats involves the amygdala and can be a behavioral measure used to reveal those adults with early life trauma, in rat pups, it is only during periods of heightened stress (eg. heightened CORT levels) combined with early life trauma that social behavior deficits and heightened amygdala neural activity can be uncovered. This suggests that the amygdala is not involved with social behavior in infancy and its activation can, in fact, impair social behavior at this age.
9. Implications for complex social behavior
Aberrant processing of social and threatening cues stemming from amygdala dysfunction is a hallmark of psychiatric sequelae following early-life abuse and neglect (Levin et al., 2015, Teicher et al., 2003, Troller-Renfree et al., 2016, Zeanah and Gleason, 2015). As individuals mature, increasingly complex social demands may multiply the consequences of impaired social behavior, amplifying stress and even compromising welfare. Exploring these complex social arrangements in animal models can provide insight into the mechanisms underlying long-term effects of social deficits and identify therapeutic targets for attachment trauma in early life.
Dominance hierarchies provide one example of a complex social arrangement determined by individual differences that can result from early life experience. In the wild, rats are among a wide variety of species that naturally form dominance hierarchies as a result of competition for limited resources (Sapolsky, 2005). This can be simulated in the laboratory using a visible burrow system, a semi-naturalistic enclosure that replicates many of the challenges and opportunities of group-living in nature; in this setting, rats rapidly form stable dominance hierarchies (Fig. 3) (Blanchard et al., 1995, Blanchard et al., 1988, Opendak et al., 2016). A rich literature has described the effects of life in a dominance hierarchy on its members, including measures of behavior, hormones and physiology (Blanchard et al., 1995, Hardy et al., 2002). These studies have focused on differences between dominants and subordinates within the aggressive Long Evans (LE) rat strain. The factors that contribute to social position are complex, but the consequences of stratification can be dramatic for an animal’s quality of life. When LE rats form a dominance hierarchy within a laboratory enclosure, it has been shown that subordinate rats have elevated levels of CORT compared to dominants and can be prone to illness and weight loss due to chronic stress (Blanchard et al., 1995, Hardy et al., 2002). Subordinate rats show a decrease in overall activity and social behaviors, including aggression and sexual advances. In addition, they show an increase in a range of defensive responses to the dominant male (Blanchard et al., 1995, Blanchard et al., 2001). Furthermore, subordinates exhibit increases in the relative sizes of adrenal glands and spleen and decreases in the sizes of the thymus and testes than dominants. In contrast, dominants enjoy preferential access to resources, as well as increases in markers of adult-brain plasticity. Specifically, dominant rats show enhanced adult neurogenesis in the ventral dentate gyrus of the hippocampal formation, an effect that also has been shown in baboons (Kozorovitskiy and Gould, 2004, Peragine et al., 2014, Wu et al., 2014).
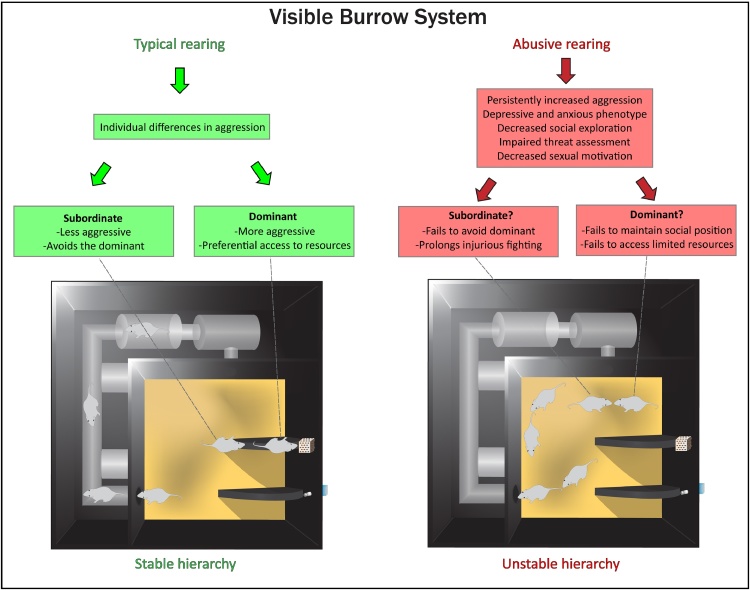
Fig. 3.
Schematic of a visible burrow system for use in rodents and putative effects of early-life abuse on dominance hierarchy formation. This enclosure, adapted from Blanchard et al. (1995), allows for a semi-naturalistic setting in which rodents can be group-housed with limited access to resources including food, water, and receptive females. Social position can emerge from individual differences in aggression and stress reactivity. These factors may be compromised in cases of caregiver abuse in early life, which produces enhanced aggression (Marquez et al., 2013), impaired threat response (Perry, in press), depressive and anxiety-like behavior (Raineki et al., 2012, Raineki et al., 2015 and decreased social exploration (Raineki et al., 2015). As a result, abuse may affect formation and maintenance of a stable dominance hierarchy.
When animal size and previous agonistic experience are equivalent, social stratification emerges from small individual differences in several factors, including aggression, stress reactivity, social behavior, and “home-field advantage”, or benefits for residents versus intruders (Barnett, 1958, Sapolsky, 2005, So et al., 2015). Although the neurobiology of forming a stable hierarchy is incredibly complex and involves myriad brain regions, several brain areas have been identified as key mediators of social dominance, such as the vmPFC, ventromedial hypothalamus, ventral hippocampus, and amygdala (Bauman et al., 2006, Rosvold et al., 1954, So et al., 2015, Watanabe and Yamamoto, 2015, Wong et al., 2016. These regions have been implicated in aggression-seeking behavior, social recognition, and memory for social position. Importantly, the basolateral amygdala inhibits the ventral hippocampus during social interaction (Felix-Ortiz et al., 2016, Felix-Ortiz and Tye, 2014, Gunaydin et al., 2014). Interestingly, dominance has been linked with levels of corticotropin response factor (CRF) mRNA in the medial amygdala in mice (So et al., 2015).
Compromised amygdala function in animals with a history of caregiver abuse during the sensitive period for attachment may have highly maladaptive consequences with respect to social position (Fig. 3). For instance, global impairments in social behavior may pre-dispose abused rats to a lifetime of subordination, resulting in not only a lack of rewards afforded to dominants, but also increased stress and illness in some types of hierarchies (Blanchard et al., 1995, Sapolsky, 2005). On the other hand, increased aggression may lead to dominance in some abused animals. However, heightened anxiety and/or depressive-like behavior may prevent these same animals from maintaining their social position, resulting in a chronically unstable hierarchy that can produce profound changes in adult brain plasticity for all members (Green et al., 2013, Opendak and Gould, 2015, Opendak et al., 2016, Weathington et al., 2012). Furthermore, caregiver abuse decreases sexual motivation in adult rodents (Raineki et al., 2015), suggesting that these animals may fail to engage receptive females, a benefit of dominance in typically-reared individuals (Blanchard et al., 1995, Blanchard et al., 1988, White et al., 1986).
Rats with a history of caregiver abuse show impaired response to a salient predator odor threat and amygdala dysfunction, suggesting that maintenance of a social hierarchy may also be compromised in these animals (Perry et al., in press) (Fig. 3). Specifically, these abused rats may fail to show subordination to an established dominant, leading to prolonged injurious fighting and precluding their timely access to limited resources (Blanchard et al., 1995, Blanchard and Blanchard, 1989). Indeed, rats that were stressed during adolescence show persistent aggression towards larger intruders in the resident-intruder paradigm, against whom a more optimal strategy may be to show submission (Marquez et al., 2013). Avoiding the dominant involves multiple learned cues, including social odor, and has been linked with adult-born neurons in the hippocampus (Lagace et al., 2010) and oxytocin receptor mRNA in the medial amygdala (Timmer et al., 2011). A role for the amygdala in this process is further supported by data in non-human primates showing impaired responses to fearful stimuli following infant amygdala lesions (Amaral, 2003, Bauman et al., 2006, Marquez et al., 2013).
Abuse-related impairments in amygdala function may have implications for other social behaviors as well. As mentioned previously, rats that were abused during the sensitive period for attachment show decreased social exploration in adulthood (Raineki et al., 2015). Specifically, they exhibit decreased preference for interacting with a conspecific over an empty container in a classic three-chamber test (Crawley, 2004). A variation on this test involves comparison between a novel conspecific and a familiar conspecific; at baseline, rats tend to prefer to interact with the novel rat. This behavior has been shown to be affected by specific social experiences within a dominance hierarchy. In particular, destabilization of a stable dominance hierarchy of rats by removal of the dominant reverses the social preference for novelty in all community members: in the three-chamber test, rats spend more time with a familiar conspecific. This behavior has been linked with changes in the number of adult-born neurons (Opendak et al., 2016). Work showing changes in novelty-seeking following early-life amygdala lesions in non-human primates (Amaral, 2003) suggests that social novelty preference may also be affected in animals with a history of abuse. Given the extensive connections between the amygdala and ventral hippocampus in social interaction and novelty-seeking (Felix-Ortiz and Tye, 2014, Gunaydin et al., 2014, Zeamer and Bachevalier, 2013), exploring these interactions in a model of amygdala dysfunction presents an exciting opportunity for further research.
10. Concluding remarks
Early life provides a sensitive window for programming lifelong emotional and cognitive processing, which has profound influence over threat-processing and social behaviors. In altricial species, this period of brain development favors forming attachments to a caregiver, regardless of the quality of care, in order to ensure the infant receives access to warmth, protection, and food. However, attachments formed in threatening or traumatic contexts have unique consequences for the development of social behavior and learning about threat. In the clinical literature, this has been demonstrated in individuals with a history of abuse or disordered attachment who exhibit compromised fear and social behavior, as well as vulnerability to later-life mental health disorders (Bowlby, 1984, Bremner, 2003, Famularo et al., 1992, Graham et al., 1999, Nemeroff and Vale, 2005, Pechtel et al., 2014).
Animal models across a variety of species have allowed us to assess the mechanisms by which caregiver abuse engenders neurobehavioral deficits, with programming of the stress system as a point of convergence in adult outcomes (Andersen and Teicher, 2008, Denenberg, 1963, Famularo et al., 1992, Harlow and Harlow, 1965, Hofer, 1994, Levine, 1957, Levine et al., 1985, Teicher et al., 2003). Here we have focused on two very selective models of caregiver abuse in rodents, namely, rough handling by the mother given low nesting resources, and repeated presentations of paired odor-shock during the sensitive period for attachment. Both of these manipulations produces latent changes in amygdala function and depressive-like behavior, aberrant fear expression, and social behavior deficits (Perry et al., in press; Raineki et al., 2010b, Raineki et al., 2010c, Raineki et al., 2015, Roth et al., 2014, Sevelinges et al., 2007, Sevelinges et al., 2011, Sevelinges et al., 2008). Importantly, these effects are not produced when a pup experiences shock without the mother (Sarro et al., 2014a, Tyler et al., 2007). These results suggest that caregiver abuse during the sensitive period for attachment produces a unique outcome, with the social dimension of early abuse, rather than pain alone, predisposing amygdala development towards an impaired phenotype.
In the clinical population, the amygdala has also been implicated in the pathogenesis of psychiatric sequelae. For instance, patients with depression also show alterations in amygdala function and its connectivity with other brain areas (Elzinga et al., 2003, Frijling et al., 2016, Jedd et al., 2015, McEwen, 2003, Ressler and Mayberg, 2007, Savitz and Drevets, 2009, Sibille et al., 2009, Teicher et al., 2003). In light of the relationship between attachment, the amygdala, and long-term mental health outcomes, we may predict that early life is not only a sensitive period for rodent amygdala development, but also for humans. The amygdala undergoes major development progress throughout the first seven years of life in children but continues to develop into adolescence (Giedd et al., 1996, Humphrey, 1968, Letcher et al., 2009, Lupien et al., 2009, Tottenham and Sheridan, 2009, Uematsu et al., 2012, Ulfig et al., 2003). This suggests that early life may be a period of rapid change and, likewise, heightened vulnerability of the amygdala to environmental influence, as well as multiple interconnected brain regions, including the hippocampus and PFC (Ehrlich and Josselyn, 2016, Ehrlich et al., 2012, Lupien et al., 2009). Future work will be necessary to fully uncover the unique role of the caregiver in programming brain regions relevant for adaptive threat processing and social behavior.
Author contributions
MO, EG and RMS wrote the review.
Conflict of interest
None.
Acknowledgments
This work was supported by training grant T32MH019524 (supports MO), and NIH DC009910, MH091451, HD083217 (supports RMS)
References
- Adriani W., Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav. Pharmacol. 2004;15(5–6):341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Ainge J.A., Langston R.F. Ontogeny of neural circuits underlying spatial memory in the rat. Front. Neural Circuits. 2012;6:8. doi: 10.3389/fncir.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth M.D., Bell S.M. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970;41(1):49–67. [PubMed] [Google Scholar]
- Ainsworth M.D. Object relations, dependency, and attachment: a theoretical review of the infant-mother relationship. Child Dev. 1969;40(4):969–1025. [PubMed] [Google Scholar]
- Allman J.M., Hakeem A., Erwin J.M., Nimchinsky E., Hof P. The anterior cingulate cortex. Ann. N. Y. Acad. Sci. 2001;935(1):107–117. [PubMed] [Google Scholar]
- Amaral D.G. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biol. Psychiatry. 2002;51(1):11–17. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral D.G. The amygdala, social behavior, and danger detection. Ann. N. Y. Acad. Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- Andersen S.L., Teicher M.H. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31(4):183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen S.L. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27(1–2):3–18. doi: 10.1016/s0149-7634(03)00005-8. S0149763403000058 [pii] [DOI] [PubMed] [Google Scholar]
- Anderson A.K., Christoff K., Stappen I., Panitz D., Ghahremani D.G., Glover G. Dissociated neural representations of intensity and valence in human olfaction. Nat. Neurosci. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Loveland K.A. The orbitofrontal-amygdala circuit and self-regulation of social-emotional behavior in autism. Neurosci. Biobehav. Rev. 2006;30(1):97–117. doi: 10.1016/j.neubiorev.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bachevalier J., Malkova L., Mishkin M. The amygdala, social cognition, and autism. In: Aggleton J., editor. The amygdala. Oxford University Press; New York: 2000. pp. 509–543. [Google Scholar]
- Bachevalier J., Malkova L., Mishkin M. Effects of selective neonatal temporal lobe lesions on socioemotional behavior in infant rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2001;115(3):545–559. doi: 10.1037//0735-7044.115.3.545. [DOI] [PubMed] [Google Scholar]
- Bagot R.C., van Hasselt F.N., Champagne D.L., Meaney M.J., Krugers H.J., Joels M. Maternal care determines rapid effects of stress mediators on synaptic plasticity in adult rat hippocampal dentate gyrus. Neurobiol. Learn. Mem. 2009;92(3):292–300. doi: 10.1016/j.nlm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Bale T.L. Epigenetic and transgenerational reprogramming of brain development. Nat. Rev. Neurosci. 2015;16(6):332–344. doi: 10.1038/nrn3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett S.A. An analysis of social behavior in wild rats. Proc. Zool. Soc. Lond. 1958;130(1):107–152. [Google Scholar]
- Baron-Cohen S., Ring H.A., Bullmore E.T., Wheelwright S., Ashwin C., Williams S.C. The amygdala theory of autism. Neurosci. Biobehav. Rev. 2000;24(3):355–364. doi: 10.1016/s0149-7634(00)00011-7. [DOI] [PubMed] [Google Scholar]
- Barr G.A., Moriceau S., Shionoya K., Muzny K., Gao P., Wang S., Sullivan R.M. Transitions in infant learning are modulated by dopamine in the amygdala. Nat. Neurosci. 2009;12(11):1367–1369. doi: 10.1038/nn.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr G. Ontogeny of nociception and antinociception. NIDA Res. Monogr. 1995;158:172–201. [PubMed] [Google Scholar]
- Bauman M.D., Toscano J.E., Mason W.A., Lavenex P., Amaral D.G. The expression of social dominance following neonatal lesions of the amygdala or hippocampus in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2006;120(4):749–760. doi: 10.1037/0735-7044.120.4.749. [DOI] [PubMed] [Google Scholar]
- Bekenstein J.W., Lothman E.W. An in vivo study of the ontogeny of long-term potentiation (LTP) in the CA1 region and in the dentate gyrus of the rat hippocampal formation. Brain Res. Dev. Brain Res. 1991;63(1–2):245–251. doi: 10.1016/0165-3806(91)90084-v. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y. The GABA excitatory/inhibitory developmental sequence: a personal journey. Neuroscience. 2014;279:187–219. doi: 10.1016/j.neuroscience.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Berdel B., Morys J., Maciejewska B. Neuronal changes in the basolateral complex during development of the amygdala of the rat. Int. J. Dev. Neurosci. 1997;15(6):755–765. doi: 10.1016/s0736-5748(97)00022-1. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Blanchard R.J., Blanchard D.C. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol. 1989;103(1):70–82. doi: 10.1037/0735-7036.103.1.70. [DOI] [PubMed] [Google Scholar]
- Blanchard R.J., Flannelly K.J., Blanchard D.C. Life-span studies of dominance and aggression in established colonies of laboratory rats. Physiol. Behav. 1988;43(1):1–7. doi: 10.1016/0031-9384(88)90089-3. [DOI] [PubMed] [Google Scholar]
- Blanchard D.C., Spencer R.L., Weiss S.M., Blanchard R.J., McEwen B., Sakai R.R. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- Blanchard R.J., Yudko E., Dulloog L., Blanchard D.C. Defense changes in stress nonresponsive subordinate males in a visible burrow system. Physiol. Behav. 2001;72(5):635–642. doi: 10.1016/s0031-9384(00)00449-2. [DOI] [PubMed] [Google Scholar]
- Blass E.M., Teicher M.H. Suckling. Science. 1980;210(4465):15–22. doi: 10.1126/science.6997992. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E., Bauman M.D., Amaral D.G. Neonatal amygdala lesions result in globally blunted affect in adult rhesus macaques. Behav. Neurosci. 2011;125(6):848–858. doi: 10.1037/a0025757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bock J., Rether K., Groger N., Xie L., Braun K. Perinatal programming of emotional brain circuits: an integrative view from systems to molecules. Front. Neurosci. 2014;8:11. doi: 10.3389/fnins.2014.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby J. Basic Books; New York: 1965. Attachment. [Google Scholar]
- Bowlby J. Attachment theory and its therapeutic implications. Adolesc. Psychiatry. 1978;6:5–33. [PubMed] [Google Scholar]
- Bowlby J. Violence in the family as a disorder of the attachment and caregiving systems. Am. J. Psychoanal. 1984;44(1):9–27. doi: 10.1007/BF01255416. 29–31. [DOI] [PubMed] [Google Scholar]
- Bremne J.D., Vermetten E. Stress and development: behavioral and biological consequences. Dev. Psychopathol. 2001;13(3):473–489. doi: 10.1017/s0954579401003042. [DOI] [PubMed] [Google Scholar]
- Bremner J.D. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc. Psychiatr. Clin. N. Am. 2003;12(2):271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Brothers L., Ring B., Kling A. Response of neurons in the macaque amygdala to complex social stimuli. Behav. Brain Res. 1990;41(3):199–213. doi: 10.1016/0166-4328(90)90108-q. [DOI] [PubMed] [Google Scholar]
- Brummelte S., Teuchert-Noodt G. Postnatal development of dopamine innervation in the amygdala and the entorhinal cortex of the gerbil (Meriones unguiculatus) Brain Res. 2006;1125(1):9–16. doi: 10.1016/j.brainres.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Calcagnoli F., Stubbendorff C., Meyer N., de Boer S.F., Althaus M., Koolhaas J.M. Oxytocin microinjected into the central amygdaloid nuclei exerts anti-aggressive effects in male rats. Neuropharmacology. 2015;90:74–81. doi: 10.1016/j.neuropharm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Caldji C., Tannenbaum B., Sharma S., Francis D., Plotsky P.M., Meaney M.J. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl. Acad. Sci. U. S. A. 1998;95(9):5335–5340. doi: 10.1073/pnas.95.9.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Sullivan R.M., Howell B., Tottenham N. The international society for developmental psychobiology Sackler symposium: early adversity and the maturation of emotion circuits-a cross-species analysis. Dev. Psychobiol. 2014;56(8):1635–1650. doi: 10.1002/dev.21260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaghan B.L., Cowan C.S., Richardson R. Treating generational stress: effect of paternal stress on development of memory and extinction in offspring is reversed by probiotic treatment. Psychol. Sci. 2016;27(9):1171–1180. doi: 10.1177/0956797616653103. [DOI] [PubMed] [Google Scholar]
- Camp L.L., Rudy J.W. Changes in the categorization of appetitive and aversive events during postnatal development of the rat. Dev. Psychobiol. 1988;21(1):25–42. doi: 10.1002/dev.420210103. [DOI] [PubMed] [Google Scholar]
- Caron E.B., Weston-Lee P., Haggerty D., Dozier M. Community implementation outcomes of attachment and biobehavioral catch-up. Child Abuse Negl. 2015;53:128–137. doi: 10.1016/j.chiabu.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Tottenham N., Liston C., Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends Cogn. Sci. 2005;9(3):104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Champagne D.L., Bagot R.C., van Hasselt F., Ramakers G., Meaney M.J., de Kloet E.R. Maternal care and hippocampal plasticity: evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. J. Neurosci. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L.J., Lavenex P.B., Amaral D.G., Lavenex P. Postnatal development of the amygdala: a stereological study in macaque monkeys. J. Comp. Neurol. 2012;520(9):1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron L.J., Lavenex P.B., Lavenex P. Postnatal development of the amygdala: a stereological study in rats. J. Comp. Neurol. 2012;520(16):3745–3763. doi: 10.1002/cne.23132. [DOI] [PubMed] [Google Scholar]
- Cheslock S.J., Varlinskaya E.I., Petrov E.S., Spear N.E. Rapid and robust olfactory conditioning with milk before suckling experience: promotion of nipple attachment in the newborn rat. Behav. Neurosci. 2000;114(3):484–495. [PubMed] [Google Scholar]
- Choi E.C. A contrast of mothering behaviors in women from Korea and the United States. J. Obstet. Gynecol. Neonatal Nurs. 1995;24(4):363–369. doi: 10.1111/j.1552-6909.1995.tb02488.x. [DOI] [PubMed] [Google Scholar]
- Cirulli F., Francia N., Berry A., Aloe L., Alleva E., Suomi S.J. Early life stress as a risk factor for mental health: role of neurotrophins from rodents to non-human primates. Neurosci. Biobehav. Rev. 2009;33(4):573–585. doi: 10.1016/j.neubiorev.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A., Bolles R. The ontogensis of defensive reactions to shock in preweanling rats. Dev. Psychobiol. 1980;13:141–150. doi: 10.1002/dev.420130206. [DOI] [PubMed] [Google Scholar]
- Costello E.J., Mustillo S., Erkanli A., Keeler G., Angold A. Prevalence and development of psychiatric disorders in childhood and adolescence. Arch. Gen. Psychiatry. 2003;60(8):837–844. doi: 10.1001/archpsyc.60.8.837. [DOI] [PubMed] [Google Scholar]
- Crawley J.N. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disabil. Res. Rev. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- Critchley H., Daly E., Bullmore E., Williams S., Van Amelsvoort T., Robertson T., Rowe A., Phillips M., McAlonan G., Howlin P., Murphy D. The functional neuroanatomy of social behaviour: changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–2212. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Crittenden P.M. Children’s strategies for coping with adverse home environments: an interpretation using attachment theory. Child Abuse Negl. 1992;16(3):329–343. doi: 10.1016/0145-2134(92)90043-q. [DOI] [PubMed] [Google Scholar]
- Cunningham M.G., Bhattacharyya S., Benes F.M. Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J. Comp. Neurol. 2002;453(2):116–130. doi: 10.1002/cne.10376. [DOI] [PubMed] [Google Scholar]
- Dallman M.F. Moments in time-the neonatal rat hypothalamo-pituitary-adrenal axis. Endocrinology. 2000;141(5):1590–1592. doi: 10.1210/endo.141.5.7527. [DOI] [PubMed] [Google Scholar]
- Davis M., Rainnie D., Cassell M. Neurotransmission in the rat amygdala related to fear and anxiety. Trends Neurosci. 1994;17 doi: 10.1016/0166-2236(94)90106-6. [DOI] [PubMed] [Google Scholar]
- De Bellis M.D., Thomas L.A. Biologic findings of post-traumatic stress disorder and child maltreatment. Curr. Psychiatr. Rep. 2003;5:108–117. doi: 10.1007/s11920-003-0027-z. [DOI] [PubMed] [Google Scholar]
- DeCasper A.J., Fifer W.P. Of human bonding: newborns prefer their mothers’ voices. Science. 1980;208(4448):1174–1176. doi: 10.1126/science.7375928. [DOI] [PubMed] [Google Scholar]
- Debiec J., Sullivan R.M. Intergenerational transmission of emotional trauma through amygdala-dependent mother-to-infant transfer of specific fear. Proc. Natl. Acad. Sci. U. S. A. 2014;111(33):12222–12227. doi: 10.1073/pnas.1316740111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denenberg V. Early experience and emotional development. Sci. Am. 1963;208:138–146. doi: 10.1038/scientificamerican0663-138. [DOI] [PubMed] [Google Scholar]
- Distel H., Hudson R. The contribution of the olfactory and tactile modalities to the nipple-search behaviour of newborn rabbits. J. Comp. Physiol. A: Sens. Neural Behav. Physiol. 1985;157(5):599–605. doi: 10.1007/BF01351354. [DOI] [PubMed] [Google Scholar]
- Ditzen B., Heinrichs M. Psychobiology of social support: the social dimension of stress buffering. Restor. Neurol. Neurosci. 2014;32(1):149–162. doi: 10.3233/RNN-139008. [DOI] [PubMed] [Google Scholar]
- Drury S.S., Theall K., Gleason M.M., Smyke A.T., De Vivo I., Wong J.Y. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol. Psychiatry. 2012;17(7):719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury S.S., Sanchez M.M., Gonzalez A. When mothering goes awry: challenges and opportunities for utilizing evidence across rodent, nonhuman primate and human studies to better define the biological consequences of negative early caregiving. Horm. Behav. 2015;77:182–192. doi: 10.1016/j.yhbeh.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G. Development of absolute auditory thresholds in the house mouse (Mus musculus) J. Am. Audiol. Soc. 1976;1(5):179–184. [PubMed] [Google Scholar]
- Ehrlich D.E., Josselyn S.A. Plasticity-related genes in brain development and amygdala-dependent learning. Genes Brain Behav. 2016;15(1):125–143. doi: 10.1111/gbb.12255. [DOI] [PubMed] [Google Scholar]
- Ehrlich D.E., Ryan S.J., Rainnie D.G. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J. Physiol. 2012;590(Pt. 19):4819–4838. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga B.M., Schmahl C.G., Vermetten E., van Dyck R., Bremner J.D. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28(9):1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Emerich D., Scalzo F., Enters E., Spear N., Spear L. Effects of 6-hydroxydopamine-induced catecholamine depletion on shock-precipitated wall climbing of infant rat pups. Dev. Psychobiol. 1985;18(3):215–227. doi: 10.1002/dev.420180303. [DOI] [PubMed] [Google Scholar]
- Emery N.J., Capitanio J.P., Mason W.A., Machado C.J., Mendoza S.P., Amaral D.G. The effects of bilateral lesions of the amygdala on dyadic social interactions in rhesus monkeys (Macaca mulatta) Behav. Neurosci. 2001;115(3):515–544. [PubMed] [Google Scholar]
- Famularo R., Kinscherff R., Fenton T. Psychiatric diagnoses of maltreated children: preliminary findings. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31(5):863–867. doi: 10.1097/00004583-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz A.C., Tye K.M. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J. Neurosci. 2014;34(2):586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz A.C., Burgos-Robles A., Bhagat N.D., Leppla C.A., Tye K.M. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience. 2016;321:197–209. doi: 10.1016/j.neuroscience.2015.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson J.N., Young L.J., Insel T.R. The neuroendocrine basis of social recognition. Front. Neuroendocrinol. 2002;23(2):200–224. doi: 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Ferry B., McGaugh J.L. Role of amygdala norepinephrine in mediating stress hormone regulation of memory storage. Acta Pharmacol. Sin. 2000;21(6):481–493. [PubMed] [Google Scholar]
- Fisher A.E. 1955. The Effects of Differential Early Treatment on the Social and Exploratory Behavior of Puppies. Ph.D. [Google Scholar]
- Fleming A.S., Suh E.J., Korsmit M., Rusak B. Activation of Fos-like immunoreactivity in the medial preoptic area and limbic structures by maternal and social interactions in rats. Behav. Neurosci. 1994;108(4):724–734. doi: 10.1037//0735-7044.108.4.724. [DOI] [PubMed] [Google Scholar]
- Francis D.D., Diorio J., Plotsky P.M., Meaney M.J. Environmental enrichment reverses the effects of maternal separation on stress reactivity. J. Neurosci. 2002;22(18):7840–7843. doi: 10.1523/JNEUROSCI.22-18-07840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling J.L., van Zuiden M., Koch S.B., Nawijn L., Veltman D.J., Olff M. Effects of intranasal oxytocin on amygdala reactivity to emotional faces in recently trauma-exposed individuals. Soc. Cogn. Affect. Neurosci. 2016;11(2):327–336. doi: 10.1093/scan/nsv116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef B.G. The ecology of weaning: parasitism and the achievement of independenceby altricial mammals. In: Gubernick D.J., Klopfer P.H., editors. Parental Care in Mammals. Plenum Publishing Corporation; 1981. pp. 211–241. [Google Scholar]
- Gee D.G., Gabard-Durnam L.J., Flannery J., Goff B., Humphreys K.L., Telzer E.H., Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc. Natl. Acad. Sci. U. S. A. 2013;110(39):15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee D.G., Gabard-Durnam L., Telzer E.H., Humphreys K.L., Goff B., Shapiro M. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 2014;25(11):2067–2078. doi: 10.1177/0956797614550878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Vaituzis A.C., Hamburger S.D., Lange N., Rajapakse J.C., Kaysen D. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. J. Comp. Neurol. 1996;366(2):223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gobrogge K.L., Liu Y., Jia X., Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J. Comp. Neurol. 2007;502(6):1109–1122. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gold A.L., Sheridan M.A., Peverill M., Busso D.S., Lambert H.K., Alves S. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J. Child Psychol. Psychiatry. 2016;57(10):1154–1164. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R.L., Edgin J.O. The extended trajectory of hippocampal development: implications for early memory development and disorder. Dev. Cogn. Neurosci. 2015;18:57–69. doi: 10.1016/j.dcn.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried J.A., Deichmann R., Winston J.S., Dolan R.J. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J. Neurosci. 2002;22(24):10819–10828. doi: 10.1523/JNEUROSCI.22-24-10819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud A.P., Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behav. Brain Res. 2007;176(1):75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Goursaud A.P., Wallen K., Bachevalier J. Mother recognition and preference after neonatal amygdala lesions in rhesus macaques (Macaca mulatta) raised in a semi-naturalistic environment. Dev. Psychobiol. 2014;56(8):1723–1734. doi: 10.1002/dev.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham Y.P., Heim C., Goodman S.H., Miller A.H., Nemeroff C.B. The effects of neonatal stress on brain development: implications for psychopathology. Dev. Psychopathol. 1999;11(3):545–565. doi: 10.1017/s0954579499002205. [DOI] [PubMed] [Google Scholar]
- Graham A.M., Pfeifer J.H., Fisher P.A., Lin W., Gao W., Fair D.A. The potential of infant fMRI research and the study of early life stress as a promising exemplar. Dev. Cogn. Neurosci. 2015;12:12–39. doi: 10.1016/j.dcn.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A.M., Buss C., Rasmussen J.M., Rudolph M.D., Demeter D.V., Gilmore J.H., Styner M., Entringer S., Wadhwa P.D., Fair D.A. Implications of newborn amygdala connectivity for fear and cognitive development at 6-months-of-age. Dev. Cogn. Neurosci. 2016;18:12–25. doi: 10.1016/j.dcn.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.R., Barnes B., McCormick C.M. Social instability stress in adolescence increases anxiety and reduces social interactions in adulthood in male Long-Evans rats. Dev. Psychobiol. 2013;55(8):849–859. doi: 10.1002/dev.21077. [DOI] [PubMed] [Google Scholar]
- Green S.A., Goff B., Gee D.G., Gabard-Durnam L., Flannery J., Telzer E.H. Discrimination of amygdala response predicts future separation anxiety in youth with early deprivation. J. Child Psychol. Psychiatry. 2016;57(10):1135–1144. doi: 10.1111/jcpp.12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunau R.E., Weinberg J., Whitfield M.F. Neonatal procedural pain and preterm infant cortisol response to novelty at 8 months. Pediatrics. 2004;114(1):e77–84. doi: 10.1542/peds.114.1.e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaydin L.A., Grosenick L., Finkelstein J.C., Kauvar I.V., Fenno L.E., Adhikari A. Natural neural projection dynamics underlying social behavior. Cell. 2014;157(7):1535–1551. doi: 10.1016/j.cell.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27(1–2):199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Gunnar M., Quevedo K. The neurobiology of stress and development. Annu. Rev. Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar M.R., Brodersen L., Nachmias M., Buss K., Rigatuso J. Stress reactivity and attachment security. Dev. Psychobiol. 1996;29(3):191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar M., Quevedo K.M., De Kloet E.R., Oitzl M.S., Vermetten E. Early care experiences and HPA axis regulation in children: a mechanism for later trauma vulnerability. Prog. Brain Res. 2007;167:137–149. doi: 10.1016/S0079-6123(07)67010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar M.R., Hostinar C.E., Sanchez M.M., Tottenham N., Sullivan R.M. Parental buffering of fear and stress neurobiology: reviewing parallels across rodent, monkey, and human models. Soc. Neurosci. 2015;10(5):474–478. doi: 10.1080/17470919.2015.1070198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas B., Mills D., Yam A., Hoeft F., Bellugi U., Reiss A. Genetic influences on sociability: heightened amygdala reactivity and event-related responses to positive social stimuli in williams syndrome. J. Neurosci. 2009;29:1132–1139. doi: 10.1523/JNEUROSCI.5324-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberly L.B. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem. Senses. 2001;26(5):551–576. doi: 10.1093/chemse/26.5.551. [DOI] [PubMed] [Google Scholar]
- Halevi G., Djalovski A., Vengrober A., Feldman R. Risk and resilience trajectories in war-exposed children across the first decade of life. J. Child Psychol. Psychiatry. 2016;57(10):1183–1193. doi: 10.1111/jcpp.12622. [DOI] [PubMed] [Google Scholar]
- Hardy M.P., Sottas C.M., Ge R., McKittrick C.R., Tamashiro K.L., McEwen B.S., Sakai R.R. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol. Reprod. 2002;67(6):1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- Harlow H., Harlow M. The affectional systems. In: Schrier A., Harlow H., Stollnitz F., editors. vol. 2. Academic Press; New York: 1965. pp. 287–334. (Behavior of Nonhuman Primates). [Google Scholar]
- Haroutunian V., Campbell B.A. Emergence of interoceptive and exteroceptive control of behavior in rats. Science. 1979;205(4409):927–929. doi: 10.1126/science.472715. [DOI] [PubMed] [Google Scholar]
- Harris K.M., Teyler T.J. Age differences in a circadian influence on hippocampal LTP. Brain Res. 1983;261(1):69–73. doi: 10.1016/0006-8993(83)91284-2. [DOI] [PubMed] [Google Scholar]
- Hartley C.A., Lee F.S. Sensitive periods in affective development: nonlinear maturation of fear learning. Neuropsychopharmacology. 2015;40(1):50–60. doi: 10.1038/npp.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C., Binder E.B. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp. Neurol. 2012;233(1):102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Hornschuh G., Kaiser S., Sachser N. Cortisol responses and social buffering: a study throughout the life span. Horm. Behav. 2006;49(3):383–390. doi: 10.1016/j.yhbeh.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Kaiser S., Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrinol. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hennessy M.B., Schiml P.A., Willen R., Watanasriyakul W., Johnson J., Garrett T. Selective social buffering of behavioral and endocrine responses and Fos induction in the prelimbic cortex of infants exposed to a novel environment. Dev. Psychobiol. 2015;57(1):50–62. doi: 10.1002/dev.21256. [DOI] [PubMed] [Google Scholar]
- Hepper P.G., Cleland J. Developmental aspects of kin recognition. Genetica. 1998;104(3):199–205. doi: 10.1023/a:1026477724836. [DOI] [PubMed] [Google Scholar]
- Hess E. Ethology: an approach to the complete analysis of behavior. In: Brown R., Galanter E., Hess E., Mendler G., editors. New Directions in Psychology. Holt, Rinehart and Winston; New York: 1962. pp. 159–199. [Google Scholar]
- Hofer M.A. Early relationships as regulators of infant physiology and behavior. Acta Paediatr. Suppl. 1994;397:9–18. doi: 10.1111/j.1651-2227.1994.tb13260.x. [DOI] [PubMed] [Google Scholar]
- Holland P.C., Gallagher M. Amygdala-frontal interactions and reward expectancy. Curr. Opin. Neurobiol. 2004;14(2):148–155. doi: 10.1016/j.conb.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Hostinar C.E., Sullivan R.M., Gunnar M.R. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: a review of animal models and human studies across development. Psychol. Bull. 2014;140(1):256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar C.E., Johnson A.E., Gunnar M.R. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci. 2015;18(2):281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston S.M., Herting M.M., Sowell E.R. The neurobiology of childhood structural brain development: conception through adulthood. Curr. Top. Behav. Neurosci. 2014;16:3–17. doi: 10.1007/7854_2013_265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Cowell P., Boucher J., Broks Mayes A., Farrant A., Roberts N. Convergent neuroanatomical and behavioural evidence of an amygdala hypothesis of autism. Neuroreport. 2000;11:2931–2935. doi: 10.1097/00001756-200009110-00020. [DOI] [PubMed] [Google Scholar]
- Humphrey T. The development of the human amygdala during early embryonic life. J. Comp. Neurol. 1968;132(1):135–165. doi: 10.1002/cne.901320108. [DOI] [PubMed] [Google Scholar]
- Humphreys K.L., Zeanah C.H. Deviations from the expectable environment in early childhood and emerging psychopathology. Neuropsychopharmacology. 2015;40(1):154–170. doi: 10.1038/npp.2014.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys K.L., Kircanski K., Colich N.L., Gotlib I.H. Attentional avoidance of fearful facial expressions following early life stress is associated with impaired social functioning. J. Child Psychol. Psychiatry. 2016;57(10):1174–1182. doi: 10.1111/jcpp.12607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy A.S., Brunson K.L., Sandman C., Baram T.Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience. 2008;154(3):1132–1142. doi: 10.1016/j.neuroscience.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedd K., Hunt R.H., Cicchetti D., Hunt E., Cowell R.A., Rogosch F.A. Long-term consequences of childhood maltreatment: altered amygdala functional connectivity. Dev. Psychopathol. 2015;27(4 Pt. 2):1577–1589. doi: 10.1017/S0954579415000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Z.V., Young L.J. Neurobiological mechanisms of social attachment and pair bonding. Curr. Opin. Behav. Sci. 2015;3:38–44. doi: 10.1016/j.cobeha.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J.C., Murray L.K., Cohen J., Dorsey S., Skavenski van Wyk S., Galloway Henderson J. Moderators of treatment response to trauma-focused cognitive behavioral therapy among youth in Zambia. J. Child Psychol. Psychiatry. 2016;57(10):1194–1202. doi: 10.1111/jcpp.12623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T., Jodo E., Suzuki Y., Hoshino K.Y., Takeuchi S., Kayama Y. Phencyclidine affects firing activity of basolateral amygdala neurons related to social behavior in rats. Neuroscience. 2009;159(1):335–343. doi: 10.1016/j.neuroscience.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Kennedy M., Kreppner J., Knights N., Kumsta R., Maughan B., Golm D. Early severe institutional deprivation is associated with a persistent variant of adult attention-deficit/hyperactivity disorder: clinical presentation, developmental continuities and life circumstances in the English and Romanian Adoptees study. J. Child Psychol. Psychiatry. 2016;57(10):1113–1125. doi: 10.1111/jcpp.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikusui T., Winslow J.T., Mori Y. Social buffering: relief from stress and anxiety. Philos. Trans. R. Soc. Lond. B: Biol. Sci. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B., Carter C.S., Newman S.W., Insel T.R. Axon-sparing lesions of the medial nucleus of the amygdala decrease affiliative behaviors in the prairie vole (Microtus ochrogaster): behavioral and anatomical specificity. Behav. Neurosci. 1994;108(3):501–513. doi: 10.1037//0735-7044.108.3.501. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y., Kikusui T., Takeuchi Y., Mori Y. Partner’s stress status influences social buffering effects in rats. Behav. Neurosci. 2004;118(4):798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Knudsen E.I. Sensitive periods in the development of the brain and behavior. J. Cogn. Neurosci. 2004;16(8):1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Kozorovitskiy Y., Gould E. Dominance hierarchy influences adult neurogenesis in the dentate gyrus. J. Neurosci. 2004;24(30):6755–6759. doi: 10.1523/JNEUROSCI.0345-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace D.C., Donovan M.H., DeCarolis N.A., Farnbauch L.A., Malhotra S., Berton O. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. U. S. A. 2010;107(9):4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagercrantz H., Bistoletti P. Catecholamine release in the newborn infant at birth. Pediatr. Res. 1977;11(8):889–893. doi: 10.1203/00006450-197708000-00007. [DOI] [PubMed] [Google Scholar]
- Landers M.S., Sullivan R.M. The development and neurobiology of infant attachment and fear. Dev. Neurosci. 2012;34(2–3):101–114. doi: 10.1159/000336732. 000336732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P., Banta Lavenex P. Building hippocampal circuits to learn and remember: insights into the development of human memory. Behav. Brain Res. 2013;254:8–21. doi: 10.1016/j.bbr.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Lawler J.M., Koss K.J., Doyle C.M., Gunnar M.R. The course of early disinhibited social engagement among post-institutionalized adopted children. J. Child Psychol. Psychiatry. 2016;57(10):1126–1134. doi: 10.1111/jcpp.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P.R., Brady D.L., Shapiro R.A., Dorsa D.M., Koenig J.I. Prenatal stress generates deficits in rat social behavior: reversal by oxytocin. Brain Res. 2007;1156:152–167. doi: 10.1016/j.brainres.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon M. Chemical communication in mother-young interactions. In: Vandebergh J.G., editor. Pheromones and Reproduction in Mammals. Academic Press; New York: 1983. (pp. 39) [Google Scholar]
- Leon M. The neurobiology of filial learning. Annu. Rev. Psychol. 1992;43:377–398. doi: 10.1146/annurev.ps.43.020192.002113. [DOI] [PubMed] [Google Scholar]
- Letcher P., Smart D., Sanson A., Toumbourou J.W. Psychosocial precursors and correlates of differing internalizing trajectories from 3 to 15 years1. Soc. Dev. 2009;18(3):618–646. [Google Scholar]
- Levin A.R., Fox N.A., Zeanah C.H., Jr., Nelson C.A. Social communication difficulties and autism in previously institutionalized children. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(2):108–115. doi: 10.1016/j.jaac.2014.11.011. (e1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S., Johnson D.F., Gonzalez C.A. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behav. Neurosci. 1985;99(3):399–410. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Levine S., Stanton M.E., Gutierrez Y.R. Maternal modulation of pituitary-adrenal activity during ontogeny. Adv. Exp. Med. Biol. 1988;245:295–310. doi: 10.1007/978-1-4899-2064-5_24. [DOI] [PubMed] [Google Scholar]
- Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- Levine S. Primary social relationships influence the development of the hypothalamic–pituitary-adrenal axis in the rat. Physiol. Behav. 2001;73(3):255–260. doi: 10.1016/s0031-9384(01)00496-6. [DOI] [PubMed] [Google Scholar]
- Levine S. Developmental determinants of sensitivity and resistance to stress. Psychoneuroendocrinology. 2005;30(10):939–946. doi: 10.1016/j.psyneuen.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Logan D.W., Brunet L.J., Webb W.R., Cutforth T., Ngai J., Stowers L. Learned recognition of maternal signature odors mediates the first suckling episode in mice. Curr. Biol. 2012;22(21):1998–2007. doi: 10.1016/j.cub.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Machado C.J., Bachevalier J. Non-human primate models of childhood psychopathology: the promise and the limitations. J. Child Psychol. Psychiatry. 2003;44(1):64–87. doi: 10.1111/1469-7610.00103. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Carroll K.A. Child abuse and neglect: usefulness of the animal data. Psychol. Bull. 1998;123(3):211–223. doi: 10.1037/0033-2909.123.3.211. [DOI] [PubMed] [Google Scholar]
- Maestripieri D., Tomaszycki M., Carroll K.A. Consistency and change in the behavior of rhesus macaque abusive mothers with successive infants. Dev. Psychobiol. 1999;34(1):29–35. [PubMed] [Google Scholar]
- Mainardi D., Marsan M., Pasquali A. Causation of sexual preferences of the house mouse. The behaviour of mice reared by parents whose odour was artificially altered. Atti della Societa Italiana di scienze nationali e del museo civico di storia naturale di Milano. 1965;104:325–338. [Google Scholar]
- Malkova L., Mishkin M., Suomi S.J., Bachevalier J. Long-term effects of neonatal medial temporal ablations on socioemotional behavior in monkeys (Macaca mulatta) Behav. Neurosci. 2010;124(6):742–760. doi: 10.1037/a0021622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M., Jing D., Yang R.R., Tottenham N., Lee F.S., Casey B.J. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl. Acad. Sci. U. S. A. 2013;110(45):18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S., Fanselow M.S. The amygdala and fear conditioning: has the nut been cracked? Neuron. 1996;16(2):237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- Marlin B.J., Froemke R.C. Oxytocin modulation of neural circuits for social behavior. Dev. Neurobiol. 2017;77(2):169–189. doi: 10.1002/dneu.22452. [DOI] [PubMed] [Google Scholar]
- Marquez C., Poirier G.L., Cordero M.I., Larsen M.H., Groner A., Marquis J. Peripuberty stress leads to abnormal aggression, altered amygdala and orbitofrontal reactivity and increased prefrontal MAOA gene expression. Transl. Psychiatry. 2013;3:e216. doi: 10.1038/tp.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack K., Newman T.K., Higley J.D., Maestripieri D., Sanchez M.M. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm. Behav. 2009;55(4):538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. Early life influences on life-long patterns of behavior and health. Ment. Retard. Dev. Disabil. Res. Rev. 2003;9(3):149–154. doi: 10.1002/mrdd.10074. [DOI] [PubMed] [Google Scholar]
- Mikics E., Toth M., Varju P., Gereben B., Liposits Z., Ashaber M. Lasting changes in social behavior and amygdala function following traumatic experience induced by a single series of foot-shocks. Psychoneuroendocrinology. 2008;33(9):1198–1210. doi: 10.1016/j.psyneuen.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Unique neural circuitry for neonatal olfactory learning. J. Neurosci. 2004;24(5):1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Sullivan R.M. Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci. 2006;9(8):1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Roth T.L., Okotoghaide T., Sullivan R.M. Corticosterone controls the developmental emergence of fear and amygdala function to predator odors in infant rat pups. Int. J. Dev. Neurosci. 2004;22(5–6):415–422. doi: 10.1016/j.ijdevneu.2004.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Wilson D.A., Levine S., Sullivan R.M. Dual circuitry for odor-shock conditioning during infancy: corticosterone switches between fear and attraction via amygdala. J. Neurosci. 2006;26(25):6737–6748. doi: 10.1523/JNEUROSCI.0499-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriceau S., Shionoya K., Jakubs K., Sullivan R.M. Early-life stress disrupts attachment learning: the role of amygdala corticosterone, locus ceruleus corticotropin releasing hormone, and olfactory bulb norepinephrine. J. Neurosci. 2009;29(50):15745–15755. doi: 10.1523/JNEUROSCI.4106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye T.B., Rudy J.W. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev. Psychobiol. 1987;20(4):405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- Nachmias M., Gunnar M., Mangelsdorf S., Parritz R.H., Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 1996;67(2):508–522. [PubMed] [Google Scholar]
- Nakamura S., Sakaguchi T. Development and plasticity of the locus coeruleus: a review of recent physiological and pharmacological experimentation. Prog. Neurobiol. 1990;34(6):505–526. doi: 10.1016/0301-0082(90)90018-c. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kimura F., Sakaguchi T. Postnatal development of electrical activity in the locus ceruleus. J. Neurophysiol. 1987;58(3):510–524. doi: 10.1152/jn.1987.58.3.510. [DOI] [PubMed] [Google Scholar]
- Neigh G.N., Gillespie C.F., Nemeroff C.B. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse. 2009;10(4):389–410. doi: 10.1177/1524838009339758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E.E., Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci. Biobehav. Rev. 1998;22(3):437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Lau J.Y., Jarcho J.M. Growing pains and pleasures: how emotional learning guides development. Trends Cogn. Sci. 2014;18(2):99–108. doi: 10.1016/j.tics.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff C.B., Vale W.W. The neurobiology of depression: inroads to treatment and new drug discovery. J. Clin. Psychiatry. 2005;66(Suppl. 7):5–13. [PubMed] [Google Scholar]
- Nemeroff C.B. Neurobiological consequences of childhood trauma. J. Clin. Psychiatry. 2004;65(Suppl. 1):18–28. [PubMed] [Google Scholar]
- Numan M., Young L.J. Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm. Behav. 2016;77:98–112. doi: 10.1016/j.yhbeh.2015.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor T.G., Cameron J.L. Translating research findings on early experience to prevention: animal and human evidence on early attachment relationships. Am. J. Prev. Med. 2006;31(6 Suppl. 1):S175–S181. doi: 10.1016/j.amepre.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Opendak M., Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn. Sci. 2015;19(3):151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Opendak M., Sullivan R.M. Unique neurobiology during the sensitive period for attachment produces distinctive infant trauma processing. Eur. J. Psychotraumatol. 2016;7(31279) doi: 10.3402/ejpt.v7.31276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opendak M., Offit L., Monari P., Schoenfeld T.J., Sonti A.N., Cameron H.A., Gould E. Lasting adaptations in social behavior produced by social disruption and inhibition of adult neurogenesis. J. Neurosci. 2016;36(26):7027–7038. doi: 10.1523/JNEUROSCI.4435-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr L.A., Brooks J.M., Jonesteller T., Moss S., Jordano J.O., Heitz T.R. Effects of chronic oxytocin on attention to dynamic facial expressions in infant macaques. Psychoneuroendocrinology. 2016;74:149–157. doi: 10.1016/j.psyneuen.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell S.S., Duhoux S., Hartley C.A., Johnson D.C., Jing D., Elliott M.D. Altered fear learning across development in both mouse and human. Proc. Natl. Acad. Sci. U. S. A. 2012;109(40):16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul B., Snyder A., Haist F., Raichle M., Bellugi M., Stiles J. Amygdala response to faces parallels social behavior in Williams syndrome. Cogn. Affect. Neurosci. 2009;4:278–285. doi: 10.1093/scan/nsp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Lyons-Ruth K., Anderson C.M., Teicher M.H. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen P.E., Blass E.M. Prenatal and postnatal determinants of the 1st suckling episode in albino rats. Dev. Psychobiol. 1982;15(4):349–355. doi: 10.1002/dev.420150407. [DOI] [PubMed] [Google Scholar]
- Peragine D.E., Simpson J.A., Mooney S.J., Lovern M.B., Holmes M.M. Social regulation of adult neurogenesis in a eusocial mammal. Neuroscience. 2014;268:10–20. doi: 10.1016/j.neuroscience.2014.02.044. [DOI] [PubMed] [Google Scholar]
- Perry R., Sullivan R.M. Neurobiology of attachment to an abusive caregiver: short-term benefits and long-term costs. Dev. Psychobiol. 2014;56(8):1626–1634. doi: 10.1002/dev.21219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.E., Santiago A.N., Sullivan R.M. 2017. From Trauma to Safety: Rescuing Adults from Neurobehavioral Impacts of Infant Abuse. (in press) [Google Scholar]
- Phelps E.A., LeDoux J.E. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron. 2005;48(2):175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- Pierce K., Muller R., Ambrose J., Allen G., Courchesne E. Face processing occurs outside the fusiform ‘face area’ in autism: evidence from functional MRI. Brain. 2001;124:2059–2073. doi: 10.1093/brain/124.10.2059. [DOI] [PubMed] [Google Scholar]
- Pine D.S., Mogg K., Bradley B.P., Montgomery L., Monk C.S., McClure E. Attention bias to threat in maltreated children: implications for vulnerability to stress-related psychopathology. Am. J. Psychiatry. 2005;162(2):291–296. doi: 10.1176/appi.ajp.162.2.291. [DOI] [PubMed] [Google Scholar]
- Pollak S.D., Cicchetti D., Hornung K., Reed A. Recognizing emotion in faces: developmental effects of child abuse and neglect. Dev. Psychol. 2000;36(5):679–688. doi: 10.1037/0012-1649.36.5.679. [DOI] [PubMed] [Google Scholar]
- Poulos A.M., Reger M., Mehta N., Zhuravka I., Sterlace S.S., Gannam C. Amnesia for early life stress does not preclude the adult development of posttraumatic stress disorder symptoms in rats. Biol. Psychiatry. 2014;76(4):306–314. doi: 10.1016/j.biopsych.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puetz V.B., Viding E., Palmer A., Kelly P.A., Lickley R., Koutoufa I. Altered neural response to rejection-related words in children exposed to maltreatment. J. Child Psychol. Psychiatry. 2016;57(10):1165–1173. doi: 10.1111/jcpp.12595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Shionoya K., Sander K., Sullivan R.M. Ontogeny of odor-LiCl vs. odor-shock learning: similar behaviors but divergent ages of functional amygdala emergence. Learn. Mem. 2009;16(2):114–121. doi: 10.1101/lm.977909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Holman P.J., Debiec J., Bugg M., Beasley A., Sullivan R.M. Functional emergence of the hippocampus in context fear learning in infant rats. Hippocampus. 2010;20(9):1037–1046. doi: 10.1002/hipo.20702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Moriceau S., Sullivan R.M. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol. Psychiatry. 2010;67(12):1137–1145. doi: 10.1016/j.biopsych.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Pickenhagen A., Roth T.L., Babstock D.M., McLean J.H., Harley C.W. The neurobiology of infant maternal odor learning. Braz. J. Med. Biol. Res. 2010;43(10):914–919. doi: 10.1590/s0100-879x2010007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Cortes M.R., Belnoue L., Sullivan R.M. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J. Neurosci. 2012;32(22):7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C., Sarro E., Rincon-Cortes M., Perry R., Boggs J., Holman C.J. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology. 2015;40(4):906–914. doi: 10.1038/npp.2014.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki D., Lamb M., Obmascher P. Towards a general theory of infantile attachment: a comparative review of aspects of the social bond. Behav. Brain Sci. 1978;3:417–464. [Google Scholar]
- Raper J., Stephens S.B., Sanchez M., Bachevalier J., Wallen K. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Dev. Psychobiol. 2014;56(8):1711–1722. doi: 10.1002/dev.21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho A.A., Londero R.G., Achaval M. Functional activities of the amygdala: an overview. J. Psychiatry Neurosci. 2000;25(1):14–23. [PMC free article] [PubMed] [Google Scholar]
- Ressler K.J., Mayberg H.S. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat. Neurosci. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A., Moffitt T.E., Caspi A., Belsky D.W., Harrington H., Schroeder F. Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J. Child Psychol. Psychiatry. 2016;57(10):1103–1112. doi: 10.1111/jcpp.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L., Banihashemi L., Koehnle T.J. Early life experience shapes the functional organization of stress-responsive visceral circuits. Physiol. Behav. 2011;104(4):632–640. doi: 10.1016/j.physbeh.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M., Sullivan R.M. Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front. Endocrinol. (Lausanne) 2014;5:33. doi: 10.3389/fendo.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls E.T. Taste, olfactory, and food reward value processing in the brain. Prog. Neurobiol. 2015;127–128:64–90. doi: 10.1016/j.pneurobio.2015.03.002. [DOI] [PubMed] [Google Scholar]
- Rosvold H.E., Mirsky A.F., Pribram K.H. Influence of amygdalectomy on social behavior in monkeys. J. Comp. Physiol. Psychol. 1954;47(3):173–178. doi: 10.1037/h0058870. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Sullivan R.M. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol. Psychiatry. 2005;57(8):823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Roth T.L., Sullivan R.M. Examining the role of endogenous opioids in learned odor-stroke associations in infant rats. Dev. Psychobiol. 2006;48(1):71–78. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.L., Raineki C., Salstein L., Perry R., Sullivan-Wilson T.A., Sloan A. Neurobiology of secure infant attachment and attachment despite adversity: a mouse model. Genes Brain Behav. 2013;12(7):673–680. doi: 10.1111/gbb.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T.L., Matt S., Chen K., Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev. Psychobiol. 2014;56(8):1755–1763. doi: 10.1002/dev.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royet J.P., Zald D., Versace R., Costes N., Lavenne F., Koenig O., Gervais R. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. J. Neurosci. 2000;20(20):7752–7759. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzen E. Imprinting and environmental learning. In: Aronson L., Tobach E., Lehrman D., Rosenblatt J., editors. Development and Evolution of Behavior. W.H Freeman; San Francisco: 1970. [Google Scholar]
- Sanchez M., Ladd C., Plotsky P. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sanchez M.M., McCormack K.M., Howell B.R. Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soc. Neurosci. 2015;10(5):512–526. doi: 10.1080/17470919.2015.1087426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M.M. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm. Behav. 2006;50(4):623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sandi C., Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat. Rev. Neurosci. 2015;16(5):290–304. doi: 10.1038/nrn3918. [DOI] [PubMed] [Google Scholar]
- Sannino S., Chini B., Grinevich V. Lifespan oxytocin signaling: maturation, flexibility and stability in newborn, adolescent and aged brain. Dev. Neurobiol. 2017;77(2):158–168. doi: 10.1002/dneu.22450. [DOI] [PubMed] [Google Scholar]
- Sapolsky R.M. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- Sarro E.C., Sullivan R.M., Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. 2014;258:147–161. doi: 10.1016/j.neuroscience.2013.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarro E.C., Wilson D.A., Sullivan R.M. Maternal regulation of infant brain state. Curr. Biol. 2014;24(14):1664–1669. doi: 10.1016/j.cub.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J., Drevets W.C. Bipolar and major depressive disorder: neuroimaging the developmental-degenerative divide. Neurosci. Biobehav. Rev. 2009;33(5):699–771. doi: 10.1016/j.neubiorev.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob J.E., Price J.L. The development of axonal connections in the central olfactory system of rats. J. Comp. Neurol. 1984;223(2):177–202. doi: 10.1002/cne.902230204. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Moriceau S., Holman P., Miner C., Muzny K., Gervais R. Enduring effects of infant memories: infant odor-shock conditioning attenuates amygdala activity and adult fear conditioning. Biol. Psychiatry. 2007;62(10):1070–1079. doi: 10.1016/j.biopsych.2007.04.025. [DOI] [PubMed] [Google Scholar]
- Sevelinges Y., Sullivan R.M., Messaoudi B., Mouly A.M. Neonatal odor-shock conditioning alters the neural network involved in odor fear learning at adulthood. Learn. Mem. 2008;15(9):649–656. doi: 10.1101/lm.998508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y., Mouly A.M., Raineki C., Moriceau S., Forest C., Sullivan R.M. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infant’s sensitive period attachment learning. Dev. Cogn. Neurosci. 2011;1(1):77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Abu-Akel A. The social salience hypothesis of oxytocin. Biol. Psychiatry. 2016;79(3):194–202. doi: 10.1016/j.biopsych.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Shionoya K., Moriceau S., Bradstock P., Sullivan R.M. Maternal attenuation of hypothalamic paraventricular nucleus norepinephrine switches avoidance learning to preference learning in preweanling rat pups. Horm. Behav. 2007;52(3):391–400. doi: 10.1016/j.yhbeh.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E., Wang Y., Joeyen-Waldorf J., Gaiteri C., Surget A., Oh S. A molecular signature of depression in the amygdala. Am. J. Psychiatry. 2009;166(9):1011–1024. doi: 10.1176/appi.ajp.2009.08121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitko K., Bentall R.P., Shevlin M., O’Sullivan N., Sellwood W. Associations between specific psychotic symptoms and specific childhood adversities are mediated by attachment styles: an analysis of the National Comorbidity Survey. Psychiatry Res. 2014;217(3):202–209. doi: 10.1016/j.psychres.2014.03.019. [DOI] [PubMed] [Google Scholar]
- Skuse D., Morris J., Lawrence K. The amygdala and development of the social brain. Ann. N. Y. Acad. Sci. 2003;1008:91–101. doi: 10.1196/annals.1301.010. [DOI] [PubMed] [Google Scholar]
- Smotherman W.P., Robinson S.R. Prenatal expression of species-typical action patterns in the rat fetus (Rattus norvegicus) J. Comp. Psychol. 1987;101(2):190–196. [PubMed] [Google Scholar]
- So N., Franks B., Lim S., Curley J.P. A social network approach reveals associations between mouse social dominance and brain gene expression. PLoS One. 2015;10(7):e0134509. doi: 10.1371/journal.pone.0134509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear N. Erlbaum; Hillsdale, New Jersey: 1978. Processing Memories: Forgetting and Retention. [Google Scholar]
- Stanley W. Differential human handling as reinforcing events and as treatments influencing later social behavior in basenji puppies. Psychol. Rep. 1962;10:775–788. [Google Scholar]
- Stanton M.E. Multiple memory systems, development and conditioning. Behav. Brain Res. 2000;110(1–2):25–37. doi: 10.1016/s0166-4328(99)00182-5. [DOI] [PubMed] [Google Scholar]
- Stanton M.E., Levine S. Brief separation elevates cortisol in mother and infant squirrel monkeys. Physiol. Behav. 1985;34(6):1007–1008. doi: 10.1016/0031-9384(85)90029-0. [DOI] [PubMed] [Google Scholar]
- Stanton M., Levine S. Inhibition of infant glucocorticoid stress response: specific role of maternal cues. Dev. Psychobiol. 1990;23(5):411–426. doi: 10.1002/dev.420230504. [DOI] [PubMed] [Google Scholar]
- Stehouwer D., Campbell B. Habituation of the forelimb-withdrawal response in neonatal rats. J. Exp. Psychol. Anim. Behav. Process. 1978;4(2):104–119. doi: 10.1037//0097-7403.4.2.104. [DOI] [PubMed] [Google Scholar]
- Suchecki D., Rosenfeld P., Levine S. Maternal regulation of the hypothalamic-pituitary-adrenal axis in the infant rat: the roles of feeding and stroking. Brain Res. Dev. Brain Res. 1993;75(2):185–192. doi: 10.1016/0165-3806(93)90022-3. [DOI] [PubMed] [Google Scholar]
- Suchecki D., Nelson D.Y., Van Oers H., Levine S. Activation and inhibition of the hypothalamic-pituitary-adrenal axis of the neonatal rat: effects of maternal deprivation. Psychoneuroendocrinology. 1995;20(2):169–182. doi: 10.1016/0306-4530(94)00051-b. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Holman P.J. Transitions in sensitive period attachment learning in infancy: the role of corticosterone. Neurosci. Biobehav. Rev. 2010;34(6):835–844. doi: 10.1016/j.neubiorev.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Leon M. Early olfactory learning induces an enhanced olfactory bulb response in young rats. Brain Res. 1986;392(1–2):278–282. doi: 10.1016/0165-3806(86)90256-7. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Perry R.E. Mechanisms and functional implications of social buffering in infants: lessons from animal models. Soc. Neurosci. 2015;10(5):500–511. doi: 10.1080/17470919.2015.1087425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. The role of norepinephrine in the expression of learned olfactory neurobehavioral responses in infant rats. Psychobiol. (Austin, Tex) 1991;19(4):308–312. doi: 10.3758/bf03332084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A. The locus coeruleus, norepinephrine, and memory in newborns. Brain Res. Bull. 1994;35(5–6):467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Hofer M.A., Brake S.C. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev. Psychobiol. 1986;19(6):615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Wilson D.A., Wong R., Correa A., Leon M. Modified behavioral and olfactory bulb responses to maternal odors in preweanling rats. Brain Res. Dev. Brain Res. 1990;53(2):243–247. doi: 10.1016/0165-3806(90)90013-o. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Zyzak D., Skierkowski P., Wilson D.A. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res. Dev. Brain Res. 1992;70(2):279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- Sullivan R.M., Landers M., Yeaman B., Wilson D.A. Good memories of bad events in infancy. Nature. 2000;407(6800):38–39. doi: 10.1038/35024156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R.M., Stackenwalt G., Nasr F., Lemon C., Wilson D.A. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav. Neurosci. 2000;114(5):957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R., Perry R., Sloan A., Kleinhaus K., Burtchen N. Infant bonding and attachment to the caregiver: insights from basic and clinical science. Clin. Perinatol. 2011;38(4):643–655. doi: 10.1016/j.clp.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi S.J. Gene-environment interactions and the neurobiology of social conflict. Ann. N. Y. Acad. Sci. 2003;1008(1):132–139. doi: 10.1196/annals.1301.014. [DOI] [PubMed] [Google Scholar]
- Swann J.W., Smith K.L., Brady R.J. Neural networks and synaptic transmission in immature hippocampus. Adv. Exp. Med. Biol. 1990;268:161–171. doi: 10.1007/978-1-4684-5769-8_19. [DOI] [PubMed] [Google Scholar]
- Swanson L.W., Petrovich G.D. What is the amygdala? Trends Neurosci. 1998;21(8):323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Takahashi Y., Kiyokawa Y., Kodama Y., Arata S., Takeuchi Y., Mori Y. Olfactory signals mediate social buffering of conditioned fear responses in male rats. Behav. Brain Res. 2013;240:46–51. doi: 10.1016/j.bbr.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Tang A.C., Reeb-Sutherland B.C., Romeo R.D., McEwen B.S. On the causes of early life experience effects: evaluating the role of mom. Front. Neuroendocrinol. 2014;35(2):245–251. doi: 10.1016/j.yfrne.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Andersen S.L., Polcari A., Anderson C.M., Navalta C.P., Kim D.M. The neurobiological consequences of early stress and childhood maltreatment. Neurosci. Biobehav. Rev. 2003;27(1–2):33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A., Anderson C.M., Ohashi K. The effects of childhood maltreatment on brain structure, function and connectivity. Nat. Rev. Neurosci. 2016;17(10):652–666. doi: 10.1038/nrn.2016.111. [DOI] [PubMed] [Google Scholar]
- Thomas K.M., Drevets W.C., Whalen P.J., Eccard C.H., Dahl R.E., Ryan N.D., Casey B.J. Amygdala response to facial expressions in children and adults. Biol. Psychiatry. 2001;49(4):309–316. doi: 10.1016/s0006-3223(00)01066-0. [DOI] [PubMed] [Google Scholar]
- Timmer M., Cordero M.I., Sevelinges Y., Sandi C. Evidence for a role of oxytocin receptors in the long-term establishment of dominance hierarchies. Neuropsychopharmacology. 2011;36(11):2349–2356. doi: 10.1038/npp.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Sheridan M.A. A review of adversity, the amygdala and the hippocampus: a consideration of developmental timing. Front. Hum. Neurosci. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-expected caregiving. Dev. Psychobiol. 2012;54(6):598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troller-Renfree S., Nelson C.A., Zeanah C.H., Fox N.A. Deficits in error monitoring are associated with externalizing but not internalizing behaviors among children with a history of institutionalization. J. Child Psychol. Psychiatry. 2016;57(10):1145–1153. doi: 10.1111/jcpp.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K., Moriceau S., Sullivan R.M., Greenwood-van Meerveld B. Long-term colonic hypersensitivity in adult rats induced by neonatal unpredictable vs predictable shock. Neurogastroenterol. Motil. 2007;19(9):761–768. doi: 10.1111/j.1365-2982.2007.00955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzanoulinou S., Sandi C. The programming of the social brain by stress during childhood and adolescence: from rodents to humans. Curr. Top. Behav. Neurosci. 2017;30:411–429. doi: 10.1007/7854_2015_430. [DOI] [PubMed] [Google Scholar]
- Uematsu A., Matsui M., Tanaka C., Takahashi T., Noguchi K., Suzuki M., Nishijo H. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulfig N., Setzer M., Bohl J. Ontogeny of the human amygdala. Ann. N. Y. Acad. Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- Umemori J., Winkel F., Castren E., Karpova N.N. Distinct effects of perinatal exposure to fluoxetine or methylmercury on parvalbumin and perineuronal nets, the markers of critical periods in brain development. Int. J. Dev. Neurosci. 2015;44:55–64. doi: 10.1016/j.ijdevneu.2015.05.006. [DOI] [PubMed] [Google Scholar]
- Upton K.J., Sullivan R.M. Defining age limits of the sensitive period for attachment learning in rat pups. Dev. Psychobiol. 2010;52(5):453–464. doi: 10.1002/dev.20448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden C., Uylings H. Postnatal volumetric development of the prefrontal cortex in the rat. J. Comp. Neurol. 2004;241(3):268–274. doi: 10.1002/cne.902410303. [DOI] [PubMed] [Google Scholar]
- Wakefield C.L., Levine M.S. Early postnatal development of basolateral amygdala in kitten: a Golgi morphometric analysis. Brain Res. Bull. 1985;14(2):159–167. doi: 10.1016/0361-9230(85)90075-9. [DOI] [PubMed] [Google Scholar]
- Watamura S.E., Donzella B., Kertes D.A., Gunnar M.R. Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev. Psychobiol. 2004;45(3):125–133. doi: 10.1002/dev.20026. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Yamamoto M.1. Neural mechanisms of social dominance. Front. Neurosci. 2015;9:154. doi: 10.3389/fnins.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathington J.M., Arnold A.R., Cooke B.M. Juvenile social subjugation induces a sex-specific pattern of anxiety and depression-like behaviors in adult rats. Horm. Behav. 2012;61(1):91–99. doi: 10.1016/j.yhbeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Weber E.M., Olsson I.A.S. Maternal behaviour in Mus musculus sp.: An ethological review. Appl. Anim. Behav. Sci. 2008;114(1–2):1–22. [Google Scholar]
- Welles-Nystrom B., New R., Richman A. The ‘good mother’—a comparative study of Swedish, Italian and American maternal behavior and goals. Scand. J. Caring Sci. 1994;8(2):81–86. doi: 10.1111/j.1471-6712.1994.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Werker J.F., Hensch T.K. Critical periods in speech perception: new directions. Annu. Rev. Psychol. 2015;66:173–196. doi: 10.1146/annurev-psych-010814-015104. [DOI] [PubMed] [Google Scholar]
- White P.J., Fischer R.B., Meunier G.F. Female discrimination of male dominance by urine odor cues in hamsters. Physiol. Behav. 1986;37(2):273–277. doi: 10.1016/0031-9384(86)90232-5. [DOI] [PubMed] [Google Scholar]
- Wilson D.A., Stevenson R.J. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci. Biobehav. Rev. 2003;27(4):307–328. doi: 10.1016/s0149-7634(03)00050-2. [DOI] [PubMed] [Google Scholar]
- Wilson D.A. A comparison of the postnatal development of post-activation potentiation in the neocortex and dentate gyrus of the rat. Brain Res. 1984;318(1):61–68. doi: 10.1016/0165-3806(84)90063-4. [DOI] [PubMed] [Google Scholar]
- Winzer-Serhan U.H., Raymon H.K., Broide R.S., Chen Y., Leslie F.M. Expression of α2 adrenoceptors during rat brain development—II. α2C messenger RNA expression and [3H]rauwolscine binding. Neuroscience. 1996;76(1):261–272. doi: 10.1016/s0306-4522(96)00369-7. [DOI] [PubMed] [Google Scholar]
- Wong L.C., Wang L., D'Amour J.A., Yumita T., Chen G., Yamaguchi T. Effective modulation of male aggression through lateral septum to medial hypothalamus projection. Curr. Biol. 2016;26(5):593–604. doi: 10.1016/j.cub.2015.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.V., Shamy J.L., Bedi G., Choi C.W., Wall M.M., Arango V. Impact of social status and antidepressant treatment on neurogenesis in the baboon hippocampus. Neuropsychopharmacology. 2014;39(8):1861–1871. doi: 10.1038/npp.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Harley C.W., Bruce J.C., Darby-King A., McLean J.H. Isoproterenol increases CREB phosphorylation and olfactory nerve-evoked potentials in normal and 5-HT-depleted olfactory bulbs in rat pups only at doses that produce odor prference learning. Learn. Mem. 2000;7(6):413–421. doi: 10.1101/lm.35900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q., Harley C.W., McLean J.H., Knöpfel T. Optical imaging of odor preference memory in the rat olfactory bulb. J. Neurophysiol. 2002;87(6):3156–3159. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]
- Zald D.H., Pardo J.V. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc. Natl. Acad. Sci. U. S. A. 1997;94(8):4119–4124. doi: 10.1073/pnas.94.8.4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas A.S., Binder E.B. Gene-environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13(1):25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zeamer A., Bachevalier J. Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus. 2013;23(9):745–750. doi: 10.1002/hipo.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C.H., Gleason M.M. Annual research review: attachment disorders in early childhood-clinical presentation, causes, correlates, and treatment. J. Child Psychol. Psychiatry. 2015;56(3):207–222. doi: 10.1111/jcpp.12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeanah C.H., Sonuga-Barke E.J.S. Editorial: the effects of early trauma and deprivation on human development—from measuring cumulative risk to characterizing specific mechanisms. J. Child Psychol. Psychiatry. 2016;57(10):1099–1102. doi: 10.1111/jcpp.12642. [DOI] [PubMed] [Google Scholar]
- Zeanah C.H., Keyes A., Settles L. Attachment relationship experiences and childhood psychopathology. Ann. N. Y. Acad. Sci. 2003;1008:22–30. doi: 10.1196/annals.1301.003. [DOI] [PubMed] [Google Scholar]
- van Oers H.J., de Kloet E.R., Whelan T., Levine S. Maternal deprivation effect on the infant's neural stress markers is reversed by tactile stimulation and feeding but not by suppressing corticosterone. J. Neurosci. 1998;18(23):10171–10179. doi: 10.1523/JNEUROSCI.18-23-10171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D.R., Herman J.P. Neurocircuitry of stress integration: anatomical pathways regulating the hypothalamo-pituitary-adrenocortical axis of the rat. Integr. Comp. Biol. 2002;42(3):541–551. doi: 10.1093/icb/42.3.541. [DOI] [PubMed] [Google Scholar]