Abstract
Our previous studies of Antxr1 knockout mice suggested that fibrotic skin abnormalities in these mice are associated with increased VEGF signaling. Here, based on studies of primary fibroblasts isolated from skin of Antx1+/+ and Antxr1−/− mice at embryonic stage E17.5 and postnatal day P49, we conclude that increased Col1a1 and Fn1 expression in Antxr1-deficient fibroblasts is partly mediated by a cell-autonomous ANTXR1-dependent mechanism. In turn, this may act in parallel with VEGF-dependent regulation of collagen type I and fibronectin production. We demonstrate that shRNA mediated knockdown of VEGF in Antxr1 −/− fibroblasts reduces Col1a1 and Fn1 expression to below control levels, and these are restored by exogenous addition of recombinant VEGF. In addition, the increase in protein levels of collagen type I and fibronectin in mutant cells is blocked by VEGF neutralizing antibody. However, expressing the longest isoform of ANTXR1 (sv1) in mutant fibroblasts decreases levels of Ctgf, Col1a1 and Fn1 transcripts, but has no effect on VEGF expression. Taken together, our data suggest that the increased matrix production in Antxr1- deficient fibroblasts primarily occurs via a CTGF-dependent pathway and that other ANTXR1–associated mechanisms contribute to VEGF-dependent increase of collagen type I and fibronectin expression. Our findings provide a basis for further studies of novel ANTXR1-dependent connective tissue homeostatic control mechanisms in healthy individuals, patients with organ fibrosis, and patients with GAPO syndrome.
Keywords: Extracellular matrix accumulation, skin fibrosis, fibroblasts, ANTXR1/TEM8 GAPO syndrome, VEGF, CTGF, Collagen type I, infantile hemangioma
INTRODUCTION
Previous studies showed that the cell surface receptor ANTXR1/TEM8 has a role in adhesion, spreading and migration of cells through its interaction with extracellular matrix (ECM) components [1]. More recently, it was found that loss-of-function mutations in ANTXR1 cause the GAPO syndrome, a recessive disorder with several connective tissue manifestations and vascular anomalies [2]. GAPO patients have significantly reduced life span and die by the third or fourth decade of life; widespread skin and interstitial fibrosis and atherosclerotic changes have been proposed to be the ultimate causes of death [3, 4]. Our previous studies of Antxr1 knockout mice suggested that fibrotic skin abnormalities in the syndrome are associated with increased VEGF signaling [5].
The anthrax toxin receptor 1, encoded by the ANTXR1 gene, is a type I transmembrane protein that belongs to the ATR family of receptors [6]. The receptor was initially discovered as a product of tumor endothelium [7]. Similar to members of the integrin family of receptors, ANTXR1 binds to extracellular matrix (ECM) components, such as collagen type VI and I and interacts with cytoskeletal proteins [1, 8, 9].
In addition to high expression in tumor angiogenesis [7, 8, 10–12], ANTXR1 is ubiquitously and broadly expressed in various tissues of endothelial and epithelial origin under normal conditions [13]. Homozygous ANTXR1 loss-of-function mutations cause growth retardation, alopecia, pseudo-anodontia and optic atrophy in GAPO patients [2, 14]. Additional GAPO features include prominent scalp veins, hemangioma-like vascular anomalies, and progressive skin fibrosis [3, 15]. The phenotype of Antxr1-deficient mice (Antxr1−/−) resembles that of GAPO patients [5]. We previously showed that progressive skin fibrosis of Antxr1−/− mice is associated with accumulation of extracellular matrix components and leaky blood vessels [5]. In addition, similar to what occurs in hemangioma endothelial cells, cutaneous Antxr1−/− endothelial cells exhibit elevated levels of VEGF-A and activation of VEGFR2 signaling [5, 16].
Given published data indicating that VEGF can induce collagen and fibronectin synthesis in mesangial cells [17, 18] and aggravate fibrosis in models of systemic fibrosis [19], our previously published results raise the question of whether increased matrix protein synthesis in ANTXR1-deficient mice is the result of elevated levels of VEGF in fibroblastic cells. To address this question, in addition to experiments with re-expression of the full-length ANTXR1 splice variant 1 in mutant cells, we treated skin fibroblasts, isolated from 7 weeks-old Antxr1+/+ and Antxr1−/− mice, with Vegf-specific shRNA to knock down expression of Vegf. Our data reveal an indispensable cell autonomous negative role for ANTXR1 splice variant 1 in regulating production of collagen type I and fibronectin via CTGF. These findings, combined with increased activity of Hif1-α and VEGFR2 in ANTXR1-deficient fibroblasts, suggest that other ANTXR1-associated mediators may contribute to VEGF-dependent regulation of collagen type I and fibronectin.
RESULTS AND DISCUSSION
Antxr1-deficient fibroblasts show increased production of extracellular matrix proteins
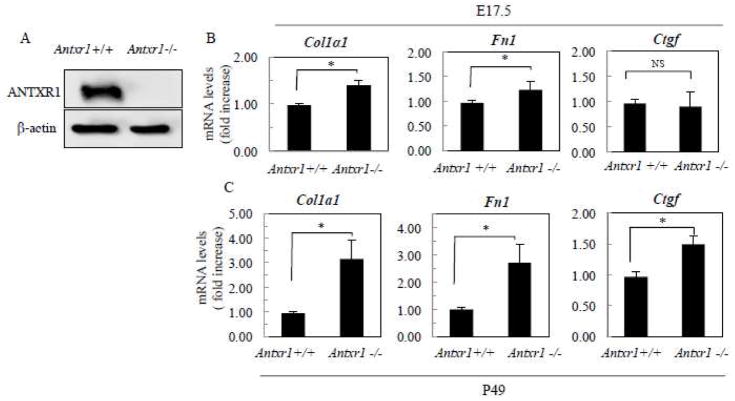
The progressive nature of fibrosis in Antxr1−/− mice suggests that increased collagen production in vivo may, in part, be due to postnatal changes and not necessarily direct effects of loss of ANTXR1 in fibroblasts [5]. In fact, increased numbers of macrophages and mast cells in soft tissues, combined with increased levels of cytokines and growth factors, are consistent with this possibility [5]. Nevertheless, fibroblasts isolated from embryonic (E17.5) Antxr1−/− mice exhibit a significant increase in 14C-proline incorporation into 30% ammonium sulfate collagen precipitates [5]. Here, by immunoblotting we confirmed the expression of ANTXR1 in fibroblasts of Antxr1 +/+ mice (Fig. 1A). Next, we checked whether the dysregulated production of ECM proteins, seen in skin of P49 Antxr1 null mice, also occurs in Antxr1 deficient fibroblasts [5]. Transcript levels for Col1a1 and Fibronectin (Fn1) are slightly, but significantly, higher in embryonic Antxr1 null fibroblasts at E17.5 (Fig. 1B). Larger differences for Col1a1 and Fn1 (3.3- and 2.7-fold increase in mutants, respectively) were found in primary fibroblasts isolated from P49 mutant animals. At P49, an up to 50% increase in Ctgf transcript levels was also observed in Antxr1 −/− fibroblasts (Fig. 1C). As we show below, these changes in transcript levels are associated with up-regulated collagen type I and fibronectin protein levels in primary fibroblasts of P49 Antxr1−/− mice (Fig. 4B). By quantifying immunofluorescence data we were able to demonstrate that the % of areas (normalized to 100 cells) stained with antibodies against collagen α1(I) and Fn1 is increased in ko fibroblasts ~ 5 fold- and ~2 fold, respectively, compared to wt controls (Fig. 4B). Levels of staining for collagen α1(I) are increased from 3 ± 1 % in control fibroblasts to 14 ± 2 % in knockouts. Similar results were seen for Fn1, with levels increasing from 6 ± 2 % in control fibroblasts to 12 ± 1 % in mutants (mean ± s.d.; Fig. 4B). These data suggest that ANTXR1 may act as a negative regulator of fibroblastic synthesis of collagen and other matrix proteins in a cell autonomous manner.
Fig. 1.
Loss of ANTXR1 is associated with increased transcript levels of ECM components. (A) Western blotting of fibroblasts isolated from Antxr1+/+ and Antxr1−/− mice at P49. (B, C) Bar graphs show average transcript levels (fold increase) in lysates of control and mutant cells at E17.5 and P49. (*P<0.05, n = 6 per group).
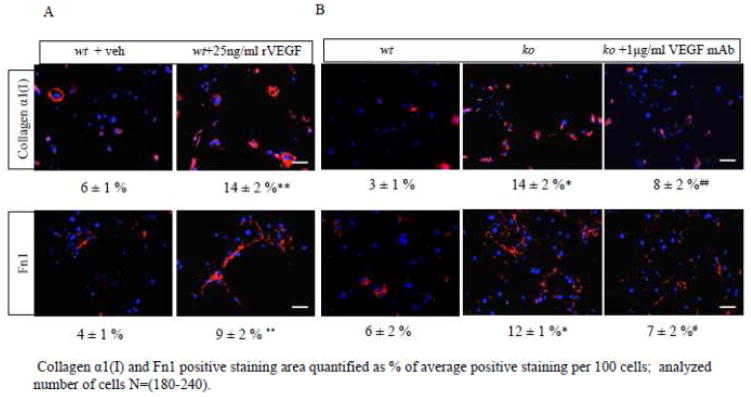
Fig. 4.
VEGF regulates collagen α1(I) and Fn1 protein levels. (A, B) Representative immunofluorescence images and data analyses of cells treated with 1μg/ml of VEGF neutralizing antibody or with 25 ng/ml of rVEGF. (n= 3; *, #, P<0.01; **, ##, P<0.05; where *,** vs. wt (Antxr1+/+) and #, ## vs. ko (Antxr1−/−)). Scale bar is100 μm; veh-vehicle. For each condition, 3–6 fields from duplicate plates were used for analysis, covering a total of 180 to 240 cells. The percentage of areas stained with antibodies against collagen α1(I) or fibronectin, was normalized to 100 cells.
Increased VEGF signaling is associated with loss of ANTXR1 in knockout mice
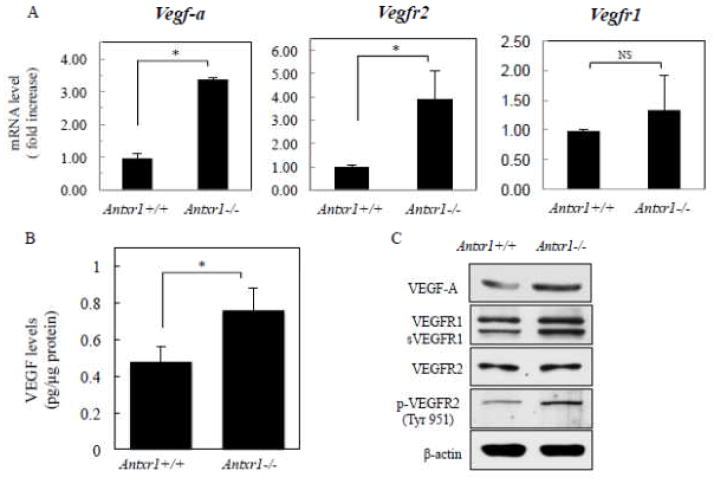
Levels of VEGF in plasma and in skin lysates of Antxr1−/− mice are substantially elevated [5]. High levels of VEGF were shown to aggravate fibrosis in experimental animal models and stimulate collagen production by cultured fibroblasts isolated from the skin in patients with systemic sclerosis [19–22]. To ascertain potential signaling pathways involved in cell autonomous mechanisms of Antrx1-dependent regulation of ECM we asked whether levels of VEGF in fibroblasts are affected by loss of ANTXR1. Total protein and RNA lysates were collected from primary fibroblasts isolated from skin of control and mutant animals at embryonic stage E17.5 and postnatal day P49. The levels of Vegf-a were different between genotypes at P49 (Fig. 2A); a 3-fold increase of Vegf-a expression in mutant cells is consistent with the progressive nature of fibrosis seen in Antxr1 knockout mice [5]. Protein levels assessed by ELISA showed that VEGF levels in fibroblasts of Antxr1 −/− mice, 0.8 ± 0.1 pg/μg, in mutants were about 40 % higher than in cells isolated from controls, 0.5 ± 0.1 pg/μg (Fig. 2B). Immunoblotting with antibody against VEGF corroborated these data (Fig. 2C). Moreover, increased Vegfr2 transcript levels and phosphorylation of VEGFR2, the key transducer of VEGF signaling, indicated an augmented activation of VEGF signaling in Antxr1-deficient fibroblasts (Fig. 2C).
Fig. 2.
Mutant fibroblasts express high VEGF levels. (A) Bar graphs show average transcript levels (fold increase) in control and mutant fibroblasts isolated from P49 mice. (*P<0.05, n = 6 per group). (B) VEGF protein levels were assessed by ELISA (*P<0.05, n = 4 per group). (C) Immunoblotting of lysates from control and mutant fibroblasts of P49 mice.
Data from previous studies of infantile hemangioma suggested that an ANTXR1/TEM8 containing protein complex regulates β1 integrin-dependent adhesion of endothelial cells to fibronectin and collagen I [16]. In addition, control of VEGF-dependent signaling in vascular endothelial cells is critically dependent on interactions between β1 integrin, VEGFR2 and ANTXR1/TEM8 [16]. Moreover, ANTXR1/TEM8 appears to play an essential role in suppressing VEGF signaling in endothelial cells [16]. Similar to hemangioma endothelial cells, endothelial cells isolated from Antxr1 null mice exhibit abnormal activation of VEGFR2 signaling [5]. Activation of VEGFR2 is also detected in Antxr1 −/− fibroblasts (Fig. 2C). Thus, the regulatory mechanism in endothelial cells may also apply to Antxr1-deficient fibroblasts. Increased VEGFR2 activation may further promote VEGF expression in a positive feedback manner [23].
VEGF promotes production of collagen type 1 and fibronectin in fibroblasts
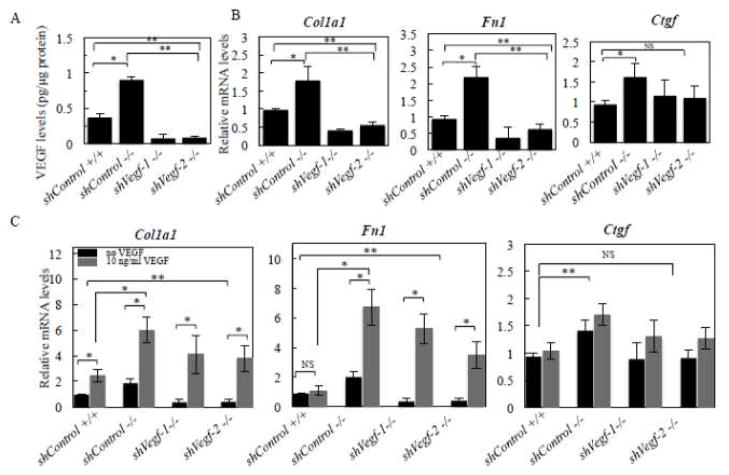
The elevated levels of Vegf and Vegfr2 transcripts, and the high VEGF protein levels and VEGFR2 activities (p-VEGFR2) in Antxr1−/− fibroblasts (Fig. 2) raise the question of whether increased matrix protein synthesis in Antxr1-deficient fibroblasts is mediated by VEGF levels. To address this possibility, we used the following approaches. First, we utilized shRNA-mediated knockdown of VEGF in fibroblastic cells isolated from the skin of mutant animals. The efficiency of shVegf-targeting was evaluated by ELISA assays (Fig. 3A). Two stable cell line clones, shVegf-1 and shVegf-2, demonstrated a significant down regulation of VEGF and were chosen for assessment of transcript levels of ECM components. An 80 % reduction of mRNA levels of Col1a1 and Fn1 in shVegf-1 and -2 lines suggests that VEGF is responsible for their up-regulation in Antxr1 null fibroblasts (Fig. 3B). This was further confirmed when addition of recombinant mouse VEGF164 (10 ng/ml) resulted in a dramatic increase of Col1a1 and Fn1 transcript levels in shControl and shVegf-treated fibroblast cultures (Fig. 3C). Protein levels of collagen α1(I) and fibronectin were also more than 2-fold increased after treatment of wild type fibroblasts with 25 ng/ml of recombinant VEGF for 24 hours (Fig. 4A). In contrast, treatment of Antxr1−/− fibroblasts with 1μg/ml of VEGF monoclonal antibody for 48 hours resulted in ~ 2 fold reduction of collagen α1(I) and Fn1 levels as assessed by immunofluorescence (Fig. 4B). The area stained with antibodies against collagen α1(I) was reduced from 14 ± 2 % in Antxr1 null fibroblasts to 8 ± 2 % after treatment of cells with VEGF monoclonal antibody. Similar results were seen for Fn1; the level of staining for fibronectin dropped from 12 ± 1 % in knockout fibroblasts to 7 ± 2 % in treated cells (mean ± s.d.; Fig. 4B). In contrast, there was no significant elevation of Ctgf transcripts after VEGF treatment (Fig. 3C). These data suggest that increased Ctgf levels in ANTXR1-deficient cells may occur either by a mechanism that is upstream of VEGF or that CTGF and its regulation of extracellular matrix components is independent of VEGF. Restoration of ANTXR1 levels in Antxr1 null cells should help to distinguish between these two possibilities.
Fig. 3.
VEGF induced transcript levels of collagen α1(I) and fibronectin. (A) VEGF protein levels were assessed by ELISA (*P<0.05, n = 4 per group) in shControl and shVegf–treated fibroblasts (*P<0.05, n = 4 per each group). (B) Bar graphs showing average transcript levels (fold increase) of shControl and Vegf knockdown cell lines (*P<0.05, n = 4 per group). (C) RT-PCR data show transcript levels after treatment of cells with 10 ng/ml of recombinant VEGF (rVEGF). (*P<0.05, n = 4 per group).
ANTXR1 regulates Ctgf and fibroblastic synthesis of matrix proteins in a cell–autonomous manner
Alternative splicing of Antxr1 results in five different splice variants (sv1, sv2, sv3, sv4, sv5) [7, 24]. Previous studies provided evidence for direct interaction between ANTXR1 and the actin cytoskeleton and demonstrated a dramatic effect of ANTXR1 loss on cytoskeletal organization [2, 25]. Moreover, a study by Go and colleagues suggested that cytoskeletal dynamics regulates ANTXR1 function and its association with the extracellular matrix [26].
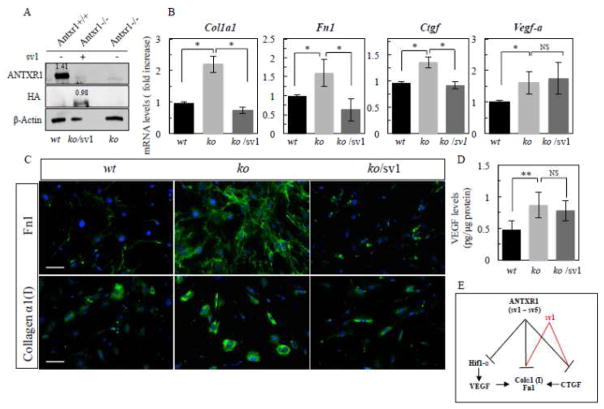
Thus, to dissect role of VEGF in regulation of ECM components, primary P49 Antxr1−/− fibroblasts were transiently transfected for 72 hours with pcDNA3-ANTXR1-sv1-HA plasmid expressing the longest and only ANTXR1 isoform containing an actin-binding site. The generation of pcDNA3-ANTXR1-sv1-HA (sv1) plasmid has been described previously [25]. First, by quantifying western blots we confirmed 70% efficiency of sv1 transfection (normalized to β–actin, relative levels of expression of ANTXR1 and sv1 are 1.41 and 0.98, respectively) (Fig. 5A). Next, we used quantitative assays for Ctgf, Col1a1, Fn1 transcripts and VEGF protein levels. Immunofluorescence for collagen α1(I) and fibronectin was carried out for the assessment of protein expression in lysates obtained from P49 of control (wt) and Antxr1 null (ko) fibroblasts treated with vehicle or sv1- containing expression plasmid (ko/sv1).
Fig. 5.
Re-expression of ANTXR1(sv1) restored levels of Col1a1 and Fn1 transcripts. (A) Assessment of sv1 transfection efficiency by western blotting of lysates from P49 control (wt) and Antxr1 null (ko) fibroblasts treated with vehicle or sv1pcDNA3-ANTXR1-sv1-HA (sv1) expression plasmid (ko/sv1). Numbers above the bands represent relative expression levels of ANTXR1 and ANTXR1-sv1 proteins normalized to β–actin. (B) Bar graphs showing average transcript levels (fold increase) of Ctgf, Col1a1, Fn1 and Vegf-a (*P<0.01; ns-not statistically significant, n = 4 per group). (C) Immunofluorescence staining for collagen α1(I) and fibronectin in cultures of fibroblasts from P49 control (wt) mice and fibroblasts from Antxr1 null (ko) mice treated with vehicle (ko) or sv1- containing expression plasmid (ko/sv1). Scale bar is 50 μm. Representative images are from triplicates of 2 independent experiments. (D) VEGF protein levels were assessed by ELISA in sv1 transfected and untransfected fibroblasts (**P<0.05; ns-not statistically significant, n = 4 per each group). (E) Schematic diagram of ANTXR1-dependent regulation of Col1a1 and Fn1 in fibroblasts. ANTXR1 (including all 5 splice variants sv1- sv5) negatively modulates Ctgf, Col1a1 and Fn1, Vegf-a and Hif1-α/expression (black lines) and sv1 negatively modulates Ctgf, Col1a1 and Fn1 only (red lines). ANTXR1-dependent increase of collagen α1(I) and fibronectin expression in mutant fibroblasts is primarily mediated by sv1/CTGF-dependent mechanism. Other ANTXR1 splice variants or unknown player (s), downstream of ANTXR1 may contribute to Hif1-α/VEGF-dependent matrix production in Antxr1 null fibroblasts.
As shown in Figure 5B, re-expressing ANTXR1 (sv1) in Antxr1 null fibroblasts restored Col1a1 and Fn1 transcripts to wt levels. In addition, protein expression of collagen α1(I) and fibronectin was dramatically reduced in sv1- transfected cells, compared to control ko cells (Fig. 5C). However, re-expression of ANTXR1 sv1 in mutant cells had no effect on transcript and protein levels of VEGF (Fig. 5B and D) and Hif1-α (Supplemental Fig. S1). In contrast, Ctgf transcript levels in ko/sv1 cells were brought back to the levels of wt controls (Fig. 5B). Taken together, these data suggest that CTGF, a target of ANTXR1, primarily contributes to up-regulation of collagen type I and fibronectin in Antxr1 null P49 fibroblasts (Fig. 5B and E). The data also raise the question of why expression of sv1 in mutant fibroblasts does not restore all the consequences of ANTXR1 deficiency. Further studies are clearly needed to address this question, but a potential explanation may be that other splice variants play a role in regulating specific pathways. For example, in a previous paper [16] we demonstrated that the secreted isoform sv3 interferes with ANTXR1 functions and induces elevated levels of Hif1-α and VEGF signaling in vascular endothelial cells.
VEGF is regulated by many factors, including growth factors, such as FGF2, hormones, transcription factors and mechanical stimuli [27]. Among many stimuli, the hypoxia inducible factor-1 (Hif1-α) is considered a major driver of VEGF expression [28, 29]. It has been demonstrated that fibroblasts in the papillary layer are sufficiently hypoxic to have elevated levels of Hif1-α and stimulated VEGF expression, and fibroblasts are known to express the Hif1-α target gene Cxcl12. In equine dermal fibroblasts, hypoxia has been shown to result in increased fibroblast proliferation and up-regulated Col1a1 expression [30]. In addition, recent evidence indicates that VEGF, VEGFR1 and VEGFR2 can be expressed at relatively high levels in ligament and tendon fibroblasts as well [31]. Previously, we demonstrated that skin lysates from Antxr1 knockout mice contained elevated levels of Hif1-α [5]. In addition, Hif1-α/FGF2 signaling is enhanced in Antxr1 null fibroblasts (Supplemental Fig. S1A). Therefore, elevated Hif1-α levels may contribute to the increased VEGF expression, resulting in up-regulation of ECM components in Antxr1 deficient fibroblasts. Such regulatory effects of VEGF on collagen production are consistent with previous reports demonstrating a striking inductive effect of VEGF on collagen expression in cultured fibroblasts and in skin fibrosis [19–22]. It was also shown that VEGF induces fibronectin secretion in human airway smooth muscle cells in an ERK1/2 dependent manner [32]. ERK1/2 activity, a major downstream target of VEGF-dependent signaling is activated in skin of Antrx1 knockout mice [5]. Both CTGF and VEGF are involved in regulation of ECM production and a shift in the balance between CTGF and VEGF is associated with a switch from angiogenesis to fibrosis [19–22, 33]. It has also been reported that overexpression of CTGF has an inhibitory effect on VEGF levels resulting in severe fibrotic scaring and reduced VEGF-dependent angiogenesis [34]. Importantly, the fact that we found transcript levels of Col1a1 and Fn1 to be increased in ANTXR1-deficient E17.5 embryonic fibroblasts, without significant changes in Ctgf and Vegf-a levels (data not shown), suggests that loss of ANTXR1 also has a CTGF/VEGF-independent effect on expression of collagen I and fibronectin (Fig. 5E). The progressive nature of fibrosis in Antxr1−/− mice suggests that increased collagen production in vivo may, in part, be due to a postnatal shift in balance between CTGF and VEGF [5]. In summary, our data demonstrate that ANTXR1 produced by fibroblasts regulates extracellular matrix production at embryonic stages and postnatally. ANTXR1-dependent effects are may primarily mediated by CTGF and partly through indirect VEGF- dependent mechanisms (Fig. 5E). Ongoing studies will further elucidate ANTXR1-CTGF-VEGF relationships in orchestrating regulation of extracellular matrix component expression by fibroblasts.
Altogether, our data suggest that ANTXR1 signaling in fibroblasts play an important role in the synthesis of matrix proteins. The novel cell autonomous mechanism of ANTXR dependent regulation of ECM production described here, potentially contributes to fibrous connective tissue accumulation during tissue reparative or regenerative processes. Given that loss of ANTXR1 function potentiates CTGF and VEGF up-regulation in human and mouse models [5, 16], it is appealing to speculate that dynamic regulation of CTGF and VEGF may be a part of an anti-fibrotic repair program; when ANTXR1 is absent, fibrosis occurs. The regulatory effect of CTGF and VEGF on ECM deposition may help explain accumulation of ECM and skin fibrosis in GAPO syndrome, and provides a potential mechanism for other syndromes with fibrotic skin abnormalities.
METHODS
Mouse line
Antxr1 null mouse line used in current study was described previously [5]. Knockout mice were initially generated using the C57BL6/NTac strain and subsequently crossed into the C57BL6/J background.
Isolation of primary mouse embryonic fibroblasts and fibroblasts from adult mice
P49 fibroblasts and fibroblasts from E17.5 embryos were isolated and characterized as described previously [5]. Cells were isolated from Antxr1+/+ and Antxr1 −/− mice (n=3 independent experiments for genotype). Cells were cultured in DMEM medium supplemented with 15% FBS.
Western blots
The 70–80% confluent cell lysates were collected using M-PER® buffer supplemented with protease and phosphatase inhibitor cocktail (Roche Applied- Science). Protein concentrations were measured by Bradford assay (Thermo Scientific). Lysates containing 10–30 μg of protein were electrophoresed using 10% SDS-bispolyacrylamide gels, and separated proteins in the gels were transferred to nitrocellulose membranes. After 1 hour blocking in 5% milk/TBST, the membranes were incubated overnight at 4 °C with primary antibodies diluted in 4% BSA (Sigma Aldrich)/TBST solution. After washing, the membranes were incubated with secondary antibodies for 1 h at room temperature and immunoreactive bands were visualized by chemiluminescent substrate (Thermo Scientific). Secondary antibodies, anti-mouse HRP (1:1000, #32430, Thermo Scientific), anti-rabbit HRP (1:10,000, #31460, Thermo Scientific) and anti-goat HRP(1:5000, sc-2056, Santa Cruz)were diluted in 5% milk/TBST solution. The following primary antibodies were used for western blotting: ANTXR1 (1:1000, ab21269, Abcam), Flk1 (1:1000, sc-504, Santa Cruz), p-Flk1 (1:1000, sc-101820, Santa Cruz), Flt1 (1:1000, sc-316, Santa Cruz), VEGF-A (1:500, sc-152, Santa Crus), and β-actin (1:5000, A5441, Sigma Aldrich) and HA (1:500, sc-805, Santa Cruz).
ELISA assays
VEGF protein levels in cell lysates of fibroblast were assessed using the Quantikine Mouse VEGF- Immunoassays (R&D Systems) in accordance with the manufacturer’s instructions; VEGF in lysates from confluent fibroblast cultures were normalized to protein levels. The optical density of each well was determined using a microplate reader set to 450 nm.
Generation of stable shVegf cell lines and ANTXR1-sv1 transiently expressing cells
Cultured fibroblasts from mutant mice were used to generate cells with Vegf gene knockdown. The cells were treated with VEGF shRNA Lentiviral Particles (sc-36815-V, Santa Cruz), Control shRNA Lentiviral Particles (sc-108084, SantaCruz), in the presence of 5μg/ml Polybrene (sc-134220, Santa Cruz). To select stable clones expressing shRNA, cells were treated with 2 μg/ml puromycin dihydrochloride (sc-108071, SantaCruz) until resistant colonies were identified and used for subsequent experiments.
The generation of pcDNA3-ANTXR1-sv1-HA plasmid has been described previously [26]. Primary Antxr1-deficient fibroblasts were transfected with pcDNA3-ANTXR1-sv1-HA using Lipofectamine 3000 reagent (Thermo Fisher Scientific) according to manufacture instructions. After 72 hours of treatment, fibroblasts expressing pcDNA3-ANTXR1-sv1-HA and control cells treated with vehicle were analyzed (2 independent experiments in triplicates).
Quantitative real-time PCR
Total RNA was isolated and first strand cDNA was synthesized using iScript (Bio-Rad). Quantitative real-time PCR with a BioRad iCycler® used primers mixed with the iQ™SYBR®Green Supermix (Bio-Rad). Primer sets for Col1a1, Fn1, Ctgf, Vegf, Flt1, Flk1 and Gapdh were purchased from Invitrogen. The sequences of forward and reverse primers are given in the supplemental Table 1 [5]. Specificity of reactions was determined by melting curve analysis. The relative fold changes of gene expression between each gene of interest were calculated by standard curve method. For each gene of interest ≥3 independent experiments were performed and each experiment was run in duplicates or triplicates.
Immunofluorescence
Fibroblasts isolated from Antxr1+/+ and Antxr1−/− mice were seeded on culture slides (BD Falcon). Follow 24 hours of serum withdrawal, exogenous recombinant VEGF (25 ng/ml, R&D) was added to near confluent control and mutant fibroblasts cultured in medium containing 0.5% of serum. After 24 hours of treatment cells were stained and subjected to quantification analysis. For inhibition assays, VEGF neutralizing antibody (1μg/ml, AF-493-NA, R&D) was added to near confluent Antxr1−/− fibroblasts growing in culture medium containing 10% serum. Cells were ready for analysis after 48 hours of incubation. For staining cells were fixed in 4% PFA for 30 min, washed with PBS, blocked with 4% of normal serum and incubated at 4°C overnight in solution containing the following primary antibodies: Collagen α1(I) (1:100, ab21286, Abcam), or fibronectin (1:100, sc-9068, Santa Cruz), followed by Alexa 555, Alexa 594 or Alexa 488 labeled secondary antibody (1:200, Life technologies or 1:500, Thermo Scientific Inc., respectively). The sv1 or vehicle transfected cells were used for the incubation with primary antibodies against collagen α1(I), fibronectin and Hif1-α (1:100, sc-10790, Santa Cruz) after 72 hours of post-transfection. The overnight, at 4°C incubation with primary antibodies followed by staining with Alexa Fluor 488 (1:500, Thermo Scientific Inc.) for 1hr at room temperature. Nuclei were stained with Hoechst 33342 fluorescent dye (Thermo Scientific).
Image J was used for collecting data on the degree of immunofluorescent staining. For each condition, 3–6 fields from duplicate plates were used for analysis, covering a total of 180 to 240 cells. The percentage of areas stained with antibodies against collagen α1(I) or fibronectin, was normalized to 100 cells.
Statistical analysis
Data were analyzed using unpaired 2-tailed Student’s t-test. All results were expressed as mean ± standard deviation or mean ± standard error (data from ≥3 independent experiments). Results were considered significant at P ≤ 0.05.
Study approval of animal use
All animal experiments were done according to the basic protocols approved by the Harvard Medical Area Standing Committee and in an agreement with the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals.
Supplementary Material
Highlights.
We appreciate the thorough comments provided by the reviewers and the editor and have addressed each of the concerns of the reviewers. In revised version of the manuscript entitled “Cell autonomous ANTXR1- mediated regulation of extracellular matrix components in primary fibroblasts” we took advantage of the availability of a pcDNA3-ANTXR1-sv1-HA (sv1) construct that we obtained from Dr. Mogridge. The results of re-expressing the longest ANTXR1 isoform, containing the actin binding site and implicated in ECM interaction/regulation, in Antxr1 null fibroblasts provide deeper mechanistic insights into collagen type I and fibronectin regulation. Quantitative measurements of VEGF, CTGF and matrix components in sv1 – transfected and untransfected cells helped to dissect the role of VEGF in ANTXR1-dependent regulation of matrix expression.
Acknowledgments
We thank the Nikon Imaging Center at Harvard Medical School for providing equipment and technical advices. Authors also thank Yulia Pittel for the secretarial assistance, Sofiya Plotkina for help in mouse husbandry and genotyping analysis. Authors thank Dr, Jeremy Mogridge for providing pcDNA3-ANTXR1-sv1-HA (sv1) plasmid. Authors also thank members of the Olsen laboratory for useful feedback. We are thankful for the service provided by the trans-NIH Knock-Out Mouse Project (KOMP) during generation of the Antxr1 null mouse strain. This work was supported by the NIH Grants AR36819 and AR48564 (B.R.O.) and a Dean’s Scholar Fellowship (T.Y.B.).
Abbreviations
- GAPO
acronym for growth retardation, alopecia, pseudoanodontia and progressive optic atrophy
- ECM
extracellular matrix
- CTGF
connective tissue growth factor
- VEGF
vascular endothelial growth factor A
- TEM8
tumor endothelial marker 8
- ANTXR1
Anthrax toxin receptor 1
- sv1
splice variant 1
Footnotes
AUTHOR CONTRIBUTIONS
T.Y.B. and K.H. designed project. K.H. and T.Y.B. performed experiments, collected data and analyzed the data. K.H., B.R.O. and T.Y.B. wrote the paper. All authors read, contributed to discussion and approved the final manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Werner E, Kowalczyk AP, Faundez V. Anthrax toxin receptor 1/tumor endothelium marker 8 mediates cell spreading by coupling extracellular ligands to the actin cytoskeleton. J Biol Chem. 2006;281:23227–23236. doi: 10.1074/jbc.M603676200. [DOI] [PubMed] [Google Scholar]
- 2.Stranecky V, Hoischen A, Hartmannova H, Zaki MS, Chaudhary A, Zudaire E, Noskova L, Baresova V, Pristoupilova A, Hodanova K, Sovova J, Hulkova H, Piherova L, Hehir-Kwa JY, de Silva D, Senanayake MP, Farrag S, Zeman J, Martasek P, Baxova A, Afifi HH, St Croix B, Brunner HG, Temtamy S, Kmoch S. Mutations in ANTXR1 cause GAPO syndrome. Am J Hum Genet. 2013;92:792–799. doi: 10.1016/j.ajhg.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tipton RE, Gorlin RJ. Growth retardation, alopecia, pseudo-anodontia, and optic atrophy--the GAPO syndrome: report of a patient and review of the literature. Am J Med Genet. 1984;19:209–216. doi: 10.1002/ajmg.1320190202. [DOI] [PubMed] [Google Scholar]
- 4.Wajntal A, Koiffmann CP, Mendonca BB, Epps-Quaglia D, Sotto MN, Rati PB, Opitz JM. GAPO syndrome (McKusick 23074)--a connective tissue disorder: report on two affected sibs and on the pathologic findings in the older. Am J Med Genet. 1990;37:213–223. doi: 10.1002/ajmg.1320370210. [DOI] [PubMed] [Google Scholar]
- 5.Besschetnova TY, Ichimura T, Katebi N, St Croix B, Bonventre JV, Olsen BR. Regulatory mechanisms of anthrax toxin receptor 1-dependent vascular and connective tissue homeostasis. Matrix Biol. 2015;42:56–73. doi: 10.1016/j.matbio.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414:225–229. doi: 10.1038/n35101999. [DOI] [PubMed] [Google Scholar]
- 7.St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, Riggins GJ, Lengauer C, Vogelstein B, Kinzler KW. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 8.Nanda A, Carson-Walter EB, Seaman S, Barber TD, Stampfl J, Singh S, Vogelstein B, Kinzler KW, St Croix B. TEM8 interacts with the cleaved C5 domain of collagen alpha 3(VI) Cancer Res. 2004;64:817–820. doi: 10.1158/0008-5472.can-03-2408. [DOI] [PubMed] [Google Scholar]
- 9.Hotchkiss KA, Basile CM, Spring SC, Bonuccelli G, Lisanti MP, Terman BI. TEM8 expression stimulates endothelial cell adhesion and migration by regulating cell-matrix interactions on collagen. Exp Cell Res. 2005;305:133–144. doi: 10.1016/j.yexcr.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 10.Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- 11.Fernando S, Fletcher BS. Targeting tumor endothelial marker 8 in the tumor vasculature of colorectal carcinomas in mice. Cancer Res. 2009;69:5126–5132. doi: 10.1158/0008-5472.CAN-09-0725. [DOI] [PubMed] [Google Scholar]
- 12.Cullen M, Seaman S, Chaudhary A, Yang MY, Hilton MB, Logsdon D, Haines DC, Tessarollo L, St Croix B. Host-derived tumor endothelial marker 8 promotes the growth of melanoma. Cancer Res. 2009;69:6021–6026. doi: 10.1158/0008-5472.CAN-09-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonuccelli G, Sotgia F, Frank PG, Williams TM, de Almeida CJ, Tanowitz HB, Scherer PE, Hotchkiss KA, Terman BI, Rollman B, Alileche A, Brojatsch J, Lisanti MP. ATR/TEM8 is highly expressed in epithelial cells lining Bacillus anthracis’ three sites of entry: implications for the pathogenesis of anthrax infection. Am J Physiol Cell Physiol. 2005;288:C1402–1410. doi: 10.1152/ajpcell.00582.2004. [DOI] [PubMed] [Google Scholar]
- 14.Bayram Y, Pehlivan D, Karaca E, Gambin T, Jhangiani SN, Erdin S, Gonzaga-Jauregui C, Wiszniewski W, Muzny D, Elcioglu NH, Yildirim MS, Bozkurt B, Zamani AG, Boerwinkle E, Gibbs RA, Lupski JR. Whole exome sequencing identifies three novel mutations in ANTXR1 in families with GAPO syndrome. Am J Med Genet A. 2014;164A:2328–2334. doi: 10.1002/ajmg.a.36678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castrillon-Oberndorfer G, Seeberger R, Bacon C, Engel M, Ebinger F, Thiele OC. GAPO syndrome associated with craniofacial vascular malformation. Am J Med Genet A. 2010;152A:225–227. doi: 10.1002/ajmg.a.33192. [DOI] [PubMed] [Google Scholar]
- 16.Jinnin M, Medici D, Park L, Limaye N, Liu Y, Boscolo E, Bischoff J, Vikkula M, Boye E, Olsen BR. Suppressed NFAT-dependent VEGFR1 expression and constitutive VEGFR2 signaling in infantile hemangioma. Nat Med. 2008;14:1236–1246. doi: 10.1038/nm.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amemiya T, Sasamura H, Mifune M, Kitamura Y, Hirahashi J, Hayashi M, Saruta T. Vascular endothelial growth factor activates MAP kinase and enhances collagen synthesis in human mesangial cells. Kidney Int. 1999;56:2055–2063. doi: 10.1046/j.1523-1755.1999.00796.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu T, Zhang B, Ye F, Xiao Z. A potential role for caveolin-1 in VEGF-induced fibronectin upregulation in mesangial cells: involvement of VEGFR2 and Src. Am J Physiol Renal Physiol. 2013;304:F820–830. doi: 10.1152/ajprenal.00294.2012. [DOI] [PubMed] [Google Scholar]
- 19.Maurer B, Distler A, Suliman YA, Gay RE, Michel BA, Gay S, Distler JH, Distler O. Vascular endothelial growth factor aggravates fibrosis and vasculopathy in experimental models of systemic sclerosis. Ann Rheum Dis. 2014;73:1880–1887. doi: 10.1136/annrheumdis-2013-203535. [DOI] [PubMed] [Google Scholar]
- 20.Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proc Natl Acad Sci U S A. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–1904. doi: 10.1111/j.1538-7836.2005.01365.x. [DOI] [PubMed] [Google Scholar]
- 22.Sack KD, Teran M, Nugent MA. Extracellular Matrix Stiffness Controls VEGF Signaling and Processing in Endothelial Cells. J Cell Physiol. 2016;231:2026–2039. doi: 10.1002/jcp.25312. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S, Heukamp LC, Siobal M, Schottle J, Wieczorek C, Peifer M, Frasca D, Koker M, Konig K, Meder L, Rauh D, Buettner R, Wolf J, Brekken RA, Neumaier B, Christofori G, Thomas RK, Ullrich RT. Tumor VEGF:VEGFR2 autocrine feed-forward loop triggers angiogenesis in lung cancer. J Clin Invest. 2013;123:1732–1740. doi: 10.1172/JCI65385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vargas M, Karamsetty R, Leppla SH, Chaudry GJ. Broad expression analysis of human ANTXR1/TEM8 transcripts reveals differential expression and novel splice variants. PLoS One. 2012;7:e43174. doi: 10.1371/journal.pone.0043174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garlick KM, Batty S, Mogridge J. Binding of filamentous actin to anthrax toxin receptor 1 decreases its association with protective antigen. Biochemistry. 2012;51:1249–1256. doi: 10.1021/bi2016469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go MY, Chow EM, Mogridge J. The cytoplasmic domain of anthrax toxin receptor 1 affects binding of the protective antigen. Infect Immun. 2009;77:52–59. doi: 10.1128/IAI.01073-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clarkin CE, Gerstenfeld LC. VEGF and bone cell signalling: an essential vessel for communication? Cell Biochem Funct. 2013;31:1–11. doi: 10.1002/cbf.2911. [DOI] [PubMed] [Google Scholar]
- 28.Krock BL, Skuli N, Simon MC. Hypoxia-induced angiogenesis: good and evil. Genes Cancer. 2011;2:1117–1133. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, Bouxsein ML, Faugere MC, Guldberg RE, Gerstenfeld LC, Haase VH, Johnson RS, Schipani E, Clemens TL. The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest. 2007;117:1616–1626. doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deschene K, Celeste C, Boerboom D, Theoret CL. Hypoxia regulates the expression of extracellular matrix associated proteins in equine dermal fibroblasts via HIF1. J Dermatol Sci. 2012;65:12–18. doi: 10.1016/j.jdermsci.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 31.Vavken P, Saad FA, Fleming BC, Murray MM. VEGF receptor mRNA expression by ACL fibroblasts is associated with functional healing of the ACL. Knee Surg Sports Traumatol Arthrosc. 2011;19:1675–1682. doi: 10.1007/s00167-011-1443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kazi AS, Lotfi S, Goncharova EA, Tliba O, Amrani Y, Krymskaya VP, Lazaar AL. Vascular endothelial growth factor-induced secretion of fibronectin is ERK dependent. Am J Physiol Lung Cell Mol Physiol. 2004;286:L539–545. doi: 10.1152/ajplung.00130.2003. [DOI] [PubMed] [Google Scholar]
- 33.Kuiper EJ, Van Nieuwenhoven FA, de Smet MD, van Meurs JC, Tanck MW, Oliver N, Klaassen I, Van Noorden CJ, Goldschmeding R, Schlingemann RO. The angio-fibrotic switch of VEGF and CTGF in proliferative diabetic retinopathy. PLoS One. 2008;3:e2675. doi: 10.1371/journal.pone.0002675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang CC, Lin MT, Lin BR, Jeng YM, Chen ST, Chu CY, Chen RJ, Chang KJ, Yang PC, Kuo ML. Effect of connective tissue growth factor on hypoxia-inducible factor 1alpha degradation and tumor angiogenesis. J Natl Cancer Inst. 2006;98:984–995. doi: 10.1093/jnci/djj242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.