Abstract
Background
Immune checkpoint inhibitors (ICI) are improving prognosis in advanced stage cancers, but also lead to immune-related adverse events (IRAE). IRAEs targeting many organ systems have been reported, but musculoskeletal and rheumatic IRAE have not been well characterized. We systematically reviewed published literature on musculoskeletal and rheumatic IRAE to better understand prevalence and clinical characteristics.
Methods
Medline and CENTRAL databases were searched for articles reporting rheumatic and musculoskeletal IRAEs secondary to ICI treatment. After screening abstracts and full texts in duplicate, clinical features, prevalence and treatment data were extracted and summarized.
Results
1725 unique abstracts were screened; 231 contained original data and were about ICIs and went to full text screening. Fifty-two of these contained information about musculoskeletal or rheumatic IRAEs or about treatment with ICIs in pre-existing autoimmune disease. Of these, 33 were clinical trials, 3 were observational studies, and 16 were case reports or series. Arthralgia prevalence in clinical trials ranged from 1–43%, and myalgia was reported in 2–20%. Arthritis was reported in 5/33 clinical trials, and vasculitis was reported in only 2. One observational study and 3 case reports described patients with pre-existing autoimmune disease treated with ICIs. Case reports included development of inflammatory arthritis, vasculitis, myositis, and lupus nephritis.
Conclusions
Arthralgia and myalgia have been reported commonly in patients treated with ICIs. The prevalence of rheumatic IRAEs like inflammatory arthritis, vasculitis, and sicca syndrome is less clear from current evidence. There is limited observational and case-level evidence describing ICI use in patients with pre-existing autoimmune disease.
Understanding of the complex relationships between autoimmunity and malignancy continues to evolve. Observations of concurrent or tightly temporally associated autoimmune disease and cancer date back to the early 1900s when cases of myositis associated with gastric and breast cancers were described. Since then, data have emerged suggesting that naturally occurring anti-tumor immune responses may trigger the development of autoimmunity in diseases such as scleroderma and myositis. For instance, scleroderma patients with RNA polymerase III antibodies have an increased risk of cancer around the time of scleroderma onset,1,2 and mechanistic studies suggest that mutations of autoantigens in cancers may trigger immune responses that become cross-reactive in these patients3. In myositis, the myositis-specific antibodies TIF-1 gamma4,5 and NXP-25,6 have been associated with a significantly higher risk of cancer-associated myositis.
In addition to naturally occurring anti-cancer immune responses triggering autoimmunity, it is increasingly recognized that therapies for cancer can trigger similar responses. Immune checkpoint inhibitors (ICIs) block negative costimulation of T-cells leading to an enhanced anti-tumor immune response. Targets of these therapies include cytotoxic T-lymphocyte associated protein-4 (CTLA-4), programmed cell death protein-1 (PD-1), and programmed death-ligand-1 (PD-L1). CTLA-4 and PD-1 are negative regulatory receptors expressed on T-cells. When CTLA-4 and PD-1 engage with their ligands on antigen presenting cells, B7 for CTLA-4 and PD-L1 or PD-L2 for PD-1, T-cell activation is inhibited. Tumors can also express inhibitory ligands like PD-L1 on their cell surfaces, thus downregulating the T-cell response. ICIs block these negative interactions between T-cells, antigen presenting cells and tumors, allowing positive costimulation to occur and T-cells to become activated. Many clinical trials with drugs targeting these and other related immune pathways, including T-cell immunoglobulin and mucin domain-3 (TIM-3), CD137, V-type immunoglobulin domain-containing suppressor of T cell activation (VISTA), and lymphocyte activation gene-3 (LAG-3), are underway for a wide variety of indications7,8.
These immune checkpoints are also relevant in the pathogenesis and treatment of rheumatic diseases8. In cancer therapy, ICIs block negative costimulation; whereas, in the treatment of autoimmunity, negative costimulation is promoted. For example, abatacept, a fusion protein of the extracellular domain of CTLA-4 and the Fc portion of IgG1, is an effective treatment for rheumatoid arthritis. This drug works by blocking binding of the positive costimulatory molecule CD28 to its receptor, CD80/86. Drugs targeting other immune checkpoints are also being investigated to treat autoimmunity.
ICIs have been paradigm shifting in the treatment of advanced malignancies and are currently approved for multiple indications: metastatic melanoma, non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), bladder cancer, head and neck cancer, and Hodgkin’s lymphoma. Ipilimumab, targeting CTLA-4, was the first ICI approved for metastatic melanoma. Ipilimumab has shown survival benefit when compared to chemotherapy9 or peptide vaccine10 controls in phase 3 trials for melanoma. Nivolumab, targeting PD-1, is approved for metastatic NSCLC, RCC, and Hodgkin’s lymphoma in addition to metastatic melanoma, with significant survival benefits when compared to conventional chemotherapy11–13. Pembrolizumab, also targeting PD-1, has shown 26 to 31% response rates in patients with metastatic melanoma refractory to Ipilimumab14. Pembrolizumab is also approved for a subset of lung cancer and metastatic head and neck cancer. Atezolizumab, a PD-L1 inhibitor, is approved for urothelial carcinoma with data showing better tolerance and increased response when compared to traditional treatments15.
ICIs are now being used in combination with even better response rates. For example, a 60% response rate was seen using Nivolumab and Ipilimumab for metastatic melanoma, as compared to an 11% response rate for Ipilimumab alone16.
Although these therapies have been effective, there are significant consequences as a result of activation of the immune system leading to tissue damage, or immune-related adverse events (IRAEs). IRAEs have affected nearly every organ system and range widely in severity. While colitis, hepatitis, pneumonitis and other IRAEs are well documented, IRAEs with rheumatic and musculoskeletal phenotypes are less well described. Events like inflammatory arthritis, arthralgia, myositis, and sicca syndrome induced by ICIs are increasingly being appreciated. In this study, we aimed to review the literature about IRAE with rheumatic and musculoskeletal manifestations as these are events most likely to be referred to rheumatology. We also examined the use of ICIs in those with pre-existing autoimmune conditions.
METHODS
A systematic review of published literature reporting IRAEs secondary to inhibition of PD-1, CTLA-4 or PD-L1 was performed.
Identification of studies
Two search strategies were utilized. A literature search of Medline was performed with a search string that combined the concepts of immune checkpoint inhibitors and their targets with manifestations of immune mediated or inflammatory adverse events. The names of approved drugs at the time of the search, ipilimumab, nivolumab, pembrolizumab and the targets of drugs, PD-1, PD-L1 and CTLA-4 were included. Rheumatic and musculoskeletal manifestations and select other IRAEs were also queried encompassing the terms arthralgia, arthritis, synovitis, xerostomia, xerophthalmia, sicca, Sjogren’s syndrome, SLE, myositis, vasculiitis, colitis, thyroiditis, hypophysitis, endocrinopathy, pneumonitis, vitiligo and hepatitis. The full search string is detailed in Supplement 1.
An additional search for clinical trials in the Cochrane database using the targets of ICIs (PD-1, PD-L1, CTLA-4), the names of approved ICIs at the time of the search (ipilimumab, nivolumab, pembrolizumab), and the clinical trials filter was also performed. Results published prior to February 8th, 2016 were included. Results were de-duplicated with computer software and checked manually.
Screening process
The abstracts were screened for relevance to the topic and inclusion of original data. Abstracts were screened in duplicate (LCC and AKG) and any discrepancies were resolved with discussion. Any results not related to ICIs and IRAEs or not containing original data (e.g. reviews, meta-analyses, letters to the editor) were excluded. References of the meta-analyses were evaluated for studies potentially missed by the two searches.
Full text screening evaluated articles for mention of arthritis, arthralgia, back pain, joint pain, musculoskeletal pain, myositis, myalgia, muscle weakness, vasculitis, polymyalgia rheumatica, Sjogren’s syndrome, sicca syndrome, dry mouth, dry eyes, systemic lupus erythematosus, or other connective tissues diseases as a result of ICIs. This process also evaluated for treatment with ICIs in the context of pre-existing autoimmunity. Full text screening was also performed in duplicate.
Data extraction
Studies were grouped by type of study: case series or reports, observational studies, and clinical trials. For clinical trials, the indication, drug/s studied, dosing regimen and incidence of above events were recorded. For observational studies, the indication for treatment, drug/s used, the type of study (prospective, retrospective, case-control), clinical features of IRAE, and information on underlying autoimmune disease were extracted. For case reports and series, clinical features of the IRAE described including symptoms, examination, laboratory studies, imaging and treatment received were extracted in addition to the type of cancer and type of ICI used and any information on pre-existing autoimmune disease.
Quality assessment
Since the outcome of interest was adverse events, bias related to ascertainment of these events was assessed for the included clinical trials. Categories assessed were sequence generation, allocation concealment, blinding of adverse event assessment, incomplete adverse event data, and selective adverse event reporting using the Cochrane Collaboration’s tool17. The observational studies and case reports were not evaluated with this tool as none were prospective studies, and thus the Cochrane tool did not apply17. Factors potentially contributing to risk of bias in these studies including size and consideration of confounders are discussed in the Results section.
RESULTS
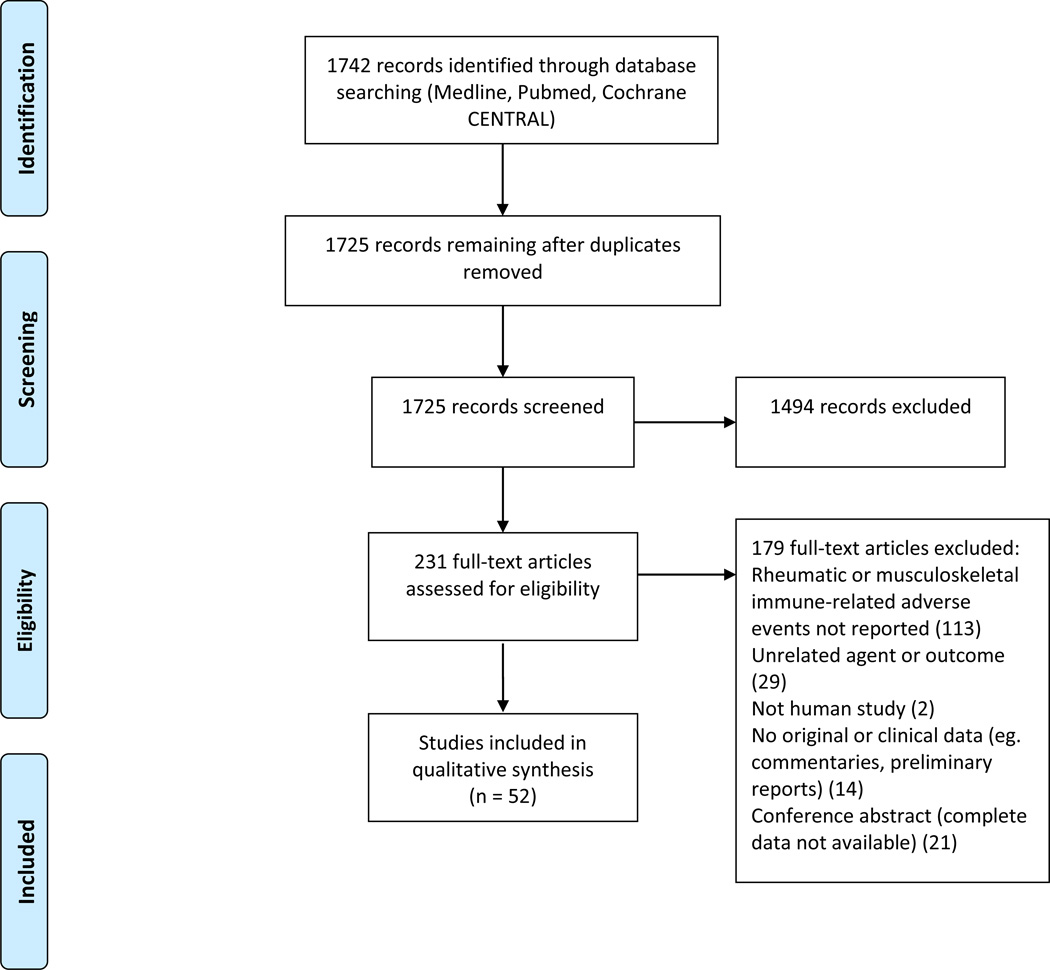
The two searches yielded 1725 unique results after de-duplication (Figure 1). There were 231 abstracts about IRAE that contained original data, which went on to full text screening. Of these full text articles, 52 mentioned a musculoskeletal or rheumatic manifestation of IRAE and were included in the qualitative synthesis. Thirty-three were clinical trials, three were observational studies, and 16 were case series or reports.
Figure 1.
PRISMA flow chart of studies included
The majority of studies were from the United States (N=26, 50%), with France, the Netherlands, Japan, the United Kingdom, Germany, Canada, and Australia also represented. Thirteen of the clinical trials had sites in multiple nations. ICIs administered included ipilimumab (anti-CTLA4), nivolumab (anti-PD-1), pembrolizumab (anti-PD-1), tremelimumab (anti-CTLA4), and one anti-PDL-1 antibody (MDX-1105).
Clinical trials
The 33 clinical trials (full references in Supplement 2) were heterogeneous in a variety of features. There were phase 1 through 3 trials included (Table 1). Some trials combined ICIs with other therapies, including chemotherapy, vaccines, and granulocyte colony stimulating factor (G-CSF). Several trials included only grade 3 or higher adverse events in the report rather than all events (e.g. Hodi JAMA 2014); therefore, many musculoskeletal and rheumatic events may not have been captured. In the grading system used for adverse events in oncology clinical trials, grade 3 events are defined as disabling, requiring prolonged hospitalization, or limiting self-care activities of daily living. Grade 4 events are those that are life threatening and require urgent intervention. The diseases treated were also wide-ranging. Some trials focused on one disease like metastatic melanoma, and others included any advanced solid tumors. The outcomes of interest, IRAEs, were not recorded in a consistent manner throughout the trials. Different trials reported different IRAEs, and no trial reported all IRAEs of interest. Due to the heterogeneity in interventions and ascertainment of adverse events, no meta-analysis for combined incidence estimates was performed.
Table 1.
Characteristics of clinical trials and incidence of reported musculoskeletal and rheumatic IRAE.
| Author, year |
Trial phase |
Indication | Number exposed |
Drug/s | Dose | Arthralgia | Arthritis | Dry eyes | Dry mouth |
Myalgia | Muscle weakness |
Vasculitis | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-CTLA-4 | |||||||||||||
| Bashey 2009 |
1 | Relapse after hematopoetic stem cell transplant |
29 | Ipilimumab | Dose escalation |
NR | 2 (7%) | NR | NR | NR | NR | NR | “bone pain”: 1 (3%); “muscle cramps”: 1 (3%) |
| Downey 2007 |
2 | Metasatic melanoma |
139 | Ipilimumab (+/− peptide vaccine) |
3 mg/kg in one group, dose escalation in other |
10/73 (13.7%) with peptide; 4/66 (without peptide |
NR | NR | NR | NR | NR | NR | NR |
| Hersh 2014 |
2 | Advanced melanoma |
72 | Ipilimumab +/− dacarbazine |
3 mg/kg | NR | NR | NR | NR | NR | NR | 1/35 (3%) in ipi + dacarbazine |
NR |
| Hodi 2014 (1) Cancer Immunol Res |
1 | Metasatic melanoma |
46 | Ipilimumab +/− bevacizumab |
3 mg/kg or 10 mg/kg |
13 (28%) “joint pain” |
NR | 2 (4%) | NR | 6
(13%) “muscle pain” |
NR | 1 (2%) with GCA |
“joint function” problems: 1 (2%) |
| Hodi 2014 (2) JAMA* |
2 | Metasatic melanoma |
245 | Ipilimumab +/− sargramostim |
10 mg/kg | 3/118 (3%) in combination; 1/120 (1%) in ipi alone |
1/118 (1%) in combination |
NR | NR | 2/118
(2%) combinatio n; 4/120 (3%) ipi alone |
1/118 (1%) combinati on; 1/120 (1%) ipi alone |
NR | “autoimmune disease”: 4/120 (3%) in Ipi alone |
| Kwon 2014 |
3 | Metastatic prostate cancer |
399 | Ipilimumab + radiation |
10 mg/kg | 44/393 (11%) | NR | NR | NR | NR | NR | NR | MSK pain: 32 (8%) |
| Le 2013 |
1 | Pancreatic cancer | 30 | Ipilimumab +/− GVAX |
10 mg/kg | Ipi + GVAX: 1/15 (75) |
NR | Ipi: 1/15 (7%) |
NR | NR | NR | NR | NR |
| Lynch 2012 |
3 | NSCLC | 138 | Ipilimumab
+ carboplatin, paclitaxel (concurrent or phased) |
10 mg/kg | Concurrent: 16/71, (22.5%) phased: 12/67 (18%) |
NR | NR | NR | NR | NR | NR | NR |
| Merchant 2015 |
1 | Advanced solid tumors (pediatric) |
33 | Ipilimumab | Dose escalation |
NR | NR | NR | NR | 1/33 (3%) | NR | NR | NR |
| Prieto 2012* |
1/2 | Metastatic melanoma |
177 | Ipi + peptide vaccine; Ipi + IL- 2, Ipi dose escalation + peptide vaccine |
various | NR | 1/36 (3%) in Ipi + IL-2; 1/85 (1%) in Ipi DE + peptide vaccine |
NR | NR | NR | NR | NR | NR |
| Reck 2013 | 2 | Extensive disease small cell lung CA |
85 | Ipilimumab + chemotherapy, concurrent or phased |
10 mg/kg | 24% in concurrent; 46% in phased |
NR | NR | NR | NR | NR | NR | NR |
| Robert 2011 |
Metastatic melanoma |
247 | Ipilimumab + dacarbazine |
NR | NR | NR | NR | NR | NR | NR | NR | Back pain 28/247 (11.3%) |
|
| Sarnaik 2010 |
2 | Stage III/IV melanoma |
75 | Ipilimumab + peptide vaccine |
3 mg/kg, 10 mg/kg |
Arthritis/arthralgia 17/75 (23%) |
2/75 (3%) |
NR | Myositis/ myalgia 16/75 (21%) |
NR | NR | NR | |
| Weber 2008* |
1/2 | Metastatic melanoma |
88 | Ipilimumab | Various | 1/88 (1%) | NR | NR | NR | NR | NR | NR | Pain in extremity: 1/88 (1%) |
| Yamazaki 2015 |
2 | Previously untreated advanced melanoma |
15 | Ipilimumab + dacarbazine |
10 mg/kg | NR | NR | NR | NR | NR | NR | NR | Back pain: 4/15 (27%) |
| Yang 2007 | 2 | Metastatic RCC | 47 | Ipilimumab | Various | 1/47 (2%) | NR | NR | NR | NR | NR | NR | NR |
| Calabro 2014 |
2 | Malignant mesothelioma |
29 | Tremelimumab | Dose escalation |
4 (14%) | NR | NR | NR | NR | NR | NR | NR |
| Ralph 2010 |
2 | Advanced gastric and esophageal adenocarcinoma |
18 | Tremelimumab | 15 mg/kg | 3/18 (17%) | NR | NR | NR | NR | NR | NR | NR |
| Sangro 2013 |
2 | HCC and hepatitis C |
21 | Tremelimumab | 15 mg/kg | NR | 1/21 (5%) | NR | NR | NR | NR | NR | NR |
| Anti-CTLA-4 vs. Anti-PD-1 | |||||||||||||
| Robert 2015 (2) Pembro |
3 | Advanced melanoma |
811 | Ipilimumab vs. Pembrolizumab |
Pembro: 10 mg.kg Ipi: 3 mg/kg |
26/278 (9%) in q2week Pembro, 32/277 (12%) in q3week Pembro, 13/256 (5%) in Ipi |
5/278 (2%) in q2week, 1/277 (.450 in q2week |
MR | 20/278 (8%) in q2week pembro, 11/277 (4%) in q3week pembro, 1/2576 (.4%) in ipi |
19/278 (7%) in q2week, 6/277 (2%) in q3week, 2/256 (2%) in ipi |
NR | NR | Myositis: 2/277 (1%) in q3week, 1/256 (0.5%) in Ipi MSK stiffness: 3/278 (1%) in q2week, 2/277 (1%) in q3week |
| Anti-PD-1 | |||||||||||||
| Borghaei 2015 |
3 | NSLC | 287 | Nivolumab | 3 mg/kg | 46 (16%) | NR | NR | NR | 18 (6%) | NR | NR | MSK pain: 39 (14%) |
| Brahmer 2010 |
1 | Refractory solid tumors |
39 | Nivolumab | Dose escalation |
2 (5%) | NR | NR | NR | NR | NR | NR | MSK “events”: 6 (15%) |
| Brahmer 2015 |
3 | Squamous cell lung CA |
135 | Nivolumab | 3 mg/kg | 7 (5%) | NR | NR | NR | 2 (2%) | NR | NR | NR |
| Gibney 2014 |
1 | Resected metastatic melanoma |
33 | Nivolumab + peptide vaccine |
Dose escalation |
14 (43%) | NR | NR | 8 (24%) | 6 (18%) | 4 (12%) | NR | “eye disorders” (e.g. dry eyes) 8 (24%) |
| Motzer 2015 |
2 | Metastatic renal cell carcinoma |
168 | Nivolumab | 3 doses: 0.3 mg/kg, 2 mg/kg, 10 mg/kg |
1/60 (2%) in 0.3 mg/kg; 4/54 (7%) in 2 mg/kg; 8/54 (15%) in 10 mg/kg |
NR | NR | 0.3mg/kg : 2/60 (3%) in, 2 mg/kg 3/54 (6%), 10 mg/kg 6/54 (11%) |
NR | NR | NR | NR |
| Rizvi 2015 | 2 | Squamous NSCLC | 117 | Nivolumab | 3 mg/kg | NR | NR | NR | 7/117 (6%) |
6/117 (5%) | NR | NR | NR |
| Robert 2015 (1) Nivo |
3 | Previously untreated stage III or IV melanoma |
206 | Nivolumab | 3 mg/kg | 12/206 (6%) | NR | NR | NR | 9/206 (4%) | NR | NR | Pain in extremity: 6/206 (3%) |
| Weber 2015 |
3 | Advanced melanoma progressed after anti-CTLA-4 |
268 | Nivolumab | 3 mg/kg | 14/268 (5%) | NR | NR | NR | NR | NR | NR | NR |
| Ribas 2015 |
2 | Ipilimumab refractory melanoma |
357 | Pembrolizumab | 2 mg/kg, 10 mg/kg |
13/178 (7%) in lower; 11/179 (6%) in higher |
NR | NR | NR | 9/178 (5%) in lower, 7/179 (4%) in higher |
NR | NR | NR |
| Robert 2014* |
1 | Metastatic melnaoma |
173 | Pembrolizumab | 2 mg/kg, 10 mg/kg |
NR | NR | NR | NR | NR | 1/89 (1%) in 2 mg/kg group |
NR | MSK pain: 1/84 (1%) in 10 mg/kg group |
| Anti-PD-L1 | |||||||||||||
| Brahmer 2012 |
1 | Advanced cancers |
207 | MDX-1105 | Dose escalation |
15 (7%) | NR | NR | NR | NR | NR | NR | Sarcoid: 1 (0.5%) |
| Combination Anti-CTLA-4 and Anti-PD-1 | |||||||||||||
| Larkin 2015 |
3 | State III or IV melanoma |
945 | Ipilimumab, Nivolumab or combination |
3 mg/kg ipi, 3 mg/kg nivo, 3 mg/kg ipi + 1 mg/kg nvo |
Nivo: 24/313 (7.7%), Ipi 19/311 (6.1%), both 33/313 (10.5%) |
NR | NR | NR | NR | NR | NR | NR |
| Postow 2015 |
1 | Metastatic melanoma |
142 | Ipilimumab +/− Nivoluamb |
Dose escalation |
10/94 (10%) in combined, 4/46 (9%) in Ipi |
NR | NR | NR | 9/94 (10%) combined; 6/46 (13%) in Ipi |
NR | NR | NR |
NR: Not reported, Ipi: Ipilimumab, DE: dose escalation, Pembro: Pembrolizumab, Nivo: Nivolumab
: Only reported grade 3 or higher adverse events.
Arthralgia was most commonly reported (N=24 trials) musculoskeletal or rheumatic IRAE in clinical trials, with development of arthralgia in 1–43% of participants exposed to ICIs. Arthritis was reported in only five trials with a range of 1–7%. Myalgia was second most commonly reported (N=12 trials) and was present in 2–21% of trial participants. Dry eyes and dry mouth were reported in three and four trials respectively and incidence ranged from 3–24%. Two trials reported vasculitis: giant cell arteritis in one, and no further specification of vasculitis in the other. Additional events of interest reported in a limited number of trials were sarcoidosis, autoimmune disease, back pain, bone pain and musculoskeletal pain (Table 1).
Observational studies
Only three observational studies were included in the present review. One study was a review of radiology on 119 patients treated with CTLA-4 inhibition18. CT scans and PET scans were evaluated, with arthritis noted by imaging in 3.4%. None of these patients were RF or CCP positive. A second observational study reviewed the clinical features of a series of 198 patients treated with ipilimumab plus or minus vaccines for melanoma or RCC.19 In in this report, 2% developed grade 3 or 4 arthritis. Lower grade events were not reported. A third observational study reported on 30 patients treated with ipilimumab who had pre-existing autoimmune disease20, in which 8 patients had an exacerbation of their autoimmune disease and 10 developed another IRAE after treatment with ipilimumab.
Case series and reports
There were 16 case series and reports of patients developing rheumatic phenotypes after treatment with ICIs or of patients with known autoimmune conditions treated with ICIs. The clinical features and treatment of included cases are described in Table 2.
Table 2.
Description of rheumatic and musculoskeletal IRAE from included case reports and series
| Author, Year | Drug | Indication | Clinical Presentation/s | Labs, imaging, and other testing |
Treatment |
|---|---|---|---|---|---|
| Anti-CTLA-4 | |||||
| Bostwick 2015 | Ipilimumab | Metastatic melanoma |
Pre-existing ulcerative colitis. Disease flared while
on ipilimumab initially responding to immunosuppression but ultimately requiring urgent colectomy. |
Colonoscopy after ipilimumab: erosions, ulcerations and pseudo polyps |
Infliximab, Azathioprine, corticosteroids. |
| Conry 2015 | Ipilimumab | Metastatic melanoma |
Arthralgias, myalgias, fevers, progressive
neurologic symptoms (ataxia, aphasia, confusion). |
ANA, anti-dsDNA, RF negative |
Encephalopathy responded to high dose IV corticosteroids |
| Fadel 2009 | Ipilimumab | Metastatic melanoma |
Drug induced lupus nephritis: Nephrotic
range proteinuria after two injections of ipilimumab, also microscopic hematuria. Subsequent venous thrombosis of left kidney. |
Positive ANA (1:100), anti- dsDNA. Biopsy with extramembranous and mesangial deposits of IgG, IgM, C3, C1q. |
Prednisone 1 mg/kg and anticoagulation. |
| Gieliesse 2014 | Ipilimumab | Metastatic melanoma |
Pre-existing Crohn’s disease, monitored with
serial endoscopies before and during treatment. |
Endoscopy before treatment showed no active inflammation; after treatment erosions and small ulcerations. |
None reported. |
| Golstein 2014 | Ipilimumab | Metastatic melanoma |
Two cases of polymyalgia rheumatica/giant cell
arteritis. One with scalp tenderness, headache, jaw claudication. Second patient with arthralgias, morning stiffness in shoulders, left sided facial swelling. |
Both elevated CRP, one with elevated ESR Temporal artery biopsies: one with active arteritis both with intimal proliferation and disruption of elastic lamina. |
Prednisone 50 mg daily and 60 mg daily. |
| Henderson 2015 |
Ipilimumab | Metastatic melanoma |
Presented with conjunctival injection, foreign
body sensation, limited ocular range of motion. Found to have orbital myositis. |
MRI showed inflammation of extraocular muscles. |
Prednisone, dose not stated. |
| Hunter 2009 | Ipilimumab | Metastatic melanoma |
Dysphagia and weakness in facial muscles, neck
flexion, proximal and distal extremities. |
CK > 5000 U/L. EMG showed irritable myopathy. Biopsy showed endomysial inflammatory infiltrate. |
Treated with IVIG and IV methylprednisolone 1 g/day followed by prednisone 1 mg/kg and taper. Improvement of all symptoms to near baseline. |
| Izzedine 2014 | Ipilimumab | Metastatic melanoma |
2 cases of Acute Interstitial
Nephritis. Pre-existing Sjogren’s syndrome in one patient. |
Negative ANA Renal biopsies: interstitial inflammation, tubular injury in one |
Prednisone 1 mg/kg for 4 weeks followed by taper. |
| Minor 2013 | Ipilimumab | Metastatic melanoma |
Uterine lymphocytic vasculitis. Presented with mass
in uterus and pelvic lymphadenopathy. |
Lymphocytic vasculitis involving uterine and ovarian vessels. ANA negative. |
Hysterectomy due to concern for malignancy. No further treatment. |
| Pedersen 2014 |
Ipilimumab | Metastatic melanoma |
Pre-existing ulcerative colitis treated with Ipilimumab.
No flares while treated. |
No evaluation of ulcerative colitis described. |
None. |
| Sheikh 2015 | Ipilimumab | Metastatic melanoma |
Dermatomyositis. Erythematous photodistributed
rash with Gottron’s papules and proximal muscle weakness. |
CK 1854 U/L. Aldolase 23 U/L. ANA 1: 640 speckled, anti-Jo1 negative. |
Prednisone 80 mg daily tapered over 8 weeks + discontinuation of ipilimumab. |
| Anti-PD-1 | |||||
| De Valasco 2015 |
Nivolumab | Metastatic renal cell carcinoma |
Joint pain and stiffness in fingers, uveitis, and
ultimately developed partially reducible swan neck deformities in hands. |
Hand radiographs- no erosions, thought to be consistent with Jaccoud arthropathy. |
None for arthropathy. Intraocular steroids for uveitis. |
| Yoshioka 2015 | Nivolumab | Metastatic melanoma |
Polymyositis with proximal muscle weakness
and respiratory involvement. |
CK 2812 U/L | Prednisone 30 mg daily + discontinuation of Nivolumab. |
| Chan 2015 | Pembrolizumab | Metastatic melanoma |
2 cases of polyarticular inflammatory arthritis.
One involving wrist, knee, ankles. Other involving PIPs, wrist, elbow, knees. |
ANA, RF, anti-CCP negative. Synovitis and tenosynovitis on MRI in both |
NSAIDs in both. Pamidronate in one, hydroxychloroquine in other. |
| Khoja 2016 | Pembrolizumab | Metastatic melanoma |
Patient presented with myalgias and feeling of
heaviness in muscles. Found to have eosinophilic fasciitis and encephalopathy |
Elevated eosinophil count, MRI of arm with fascial edema. |
Corticosteroids 1 gram daily, then taper |
| Manusow 2014 |
Pembrolizumab | Metastatic melanoma |
Retinal vasculitis in the setting of ocular metastasis
after pembrolizumab treatment. Not certain if was due to pembrolizumab or paraneoplastic |
Fluoroscein angiography showed retinal vasculitis with leaking from vessels. |
Improvement with vitrectomy |
CK: creatinine kinase. ANA: antinuclear antibodies. RF: rheumatoid factor. dsDNA: double stranded DNA antibodies. CCP: cyclic citrullinated peptide antibodies. EMG: electromyography. CRP: C reactive protein. ESR: erythrocyte sedimentation rate.
Inflammatory arthritis/arthropathy
Two cases of inflammatory polyarthritis and tenosynovitis were reported after treatment with pembrolizumab for metastatic melanoma21. Both patients were negative for ANA, RF, and anti-CCP antibodies. Another case report described reducible swan neck deformities in the hands that developed along with uveitis after treatment with nivolumab22.
Inflammatory myopathy/eosinophilic fasciitis
Myositis similar to dermatomyositis (one case)23 and polymyositis (two cases)24,25 was described in three separate case reports. Ipilimumab was the inciting agent for the case of dermatomyositis and one of the cases of polymyositis; nivolumab use preceded the development of the other case of polymyositis. All three patients improved with corticosteroids (Table 2). Another patient who presented with myalgias was ultimately found to have eosinophilic fasciitis26.
Vasculitis
Vasculitis has been reported in single organs and in the form of giant cell arteritis. Single organ vasculitis in the retina27 and uterus28 has also been reported, though the case in the retina was not definitively due to pembrolizumab and may have been a paraneoplastic process given the ocular melanoma in this patient. Giant cell arteritis and polymyalgia rheumatica were reported in two patients treated with ipilimumab for metastatic melanoma, with successful treatment using prednisone monotherapy29.
Lupus nephritis
One case report of lupus nephritis after treatment with ipilimumab was included30. The patient had a renal biopsy showing extramembranous and mesangial deposits of IgG, IgM, C3, C1q, and positive antibodies to double stranded DNA. The dsDNA antibodies resolved after treatment with corticosteroids.
Pre-existing autoimmune disease and ICI treatment
Three cases described inflammatory bowel disease (Crohn’s disease or ulcerative colitis) in patients treated with ipilimumab for metastatic melanoma. Two patients did well with no major flare of their inflammatory bowel disease31,32. The third case report by Bostwick described a patient who had a severe flare of ulcerative colitis while on treatment, requiring an urgent colectomy33. Another case reported was the development of acute interstitial nephritis in a patient with pre-existing Sjogren’s syndrome after ipilimumab therapy34.
Others
Orbital myositis35 and encephalopathy with arthralgias36 were also described in case reports as shown in Table 2.
Quality of included studies
For clinical trials, quality assessment is summarized in Table 3. Most of the clinical trials were not blinded in terms of outcome assessment of adverse events, contributing to potential bias in the results. Many were not randomized. Many of the trials had a small sample size, and thus may not be representative of larger populations treated with ICIs, introducing some bias. Since many of the studies were earlier clinical phases, safety, including the development of adverse events, was a main focus so incomplete data was less of an issue. Selective event reporting was present in several studies where only frequent or high grade adverse events were reported.
Table 3.
Risk of bias assessment for included clinical trials
| Author, Year | Sequence generation | Allocation concealment |
Blinding | Incomplete adverse event data |
Selective adverse event reporting |
Other |
|---|---|---|---|---|---|---|
| Bashey 2009 | High | N/A | High | Low | Low | Low |
| Borghaei 2015 | Low | N/A | High | Low | Low | Low |
| Brahmer 2010 | High | N/A | High | Low | High | High |
| Brahmer 2012 | High | N/A | High | Low | Low | Low |
| Brahmer 2015 | Low | Low | High | Low | High | Low |
| Calabro 2014 | N/A | N/A | High | Low | Low | High |
| Downey 2007 | High | N/A | High | Low | Low | High |
| Gibney 2014 | High | N/A | High | Low | Low | High |
| Hersh 2014 | Low | Unclear | High | Low | High | Low |
| Hodi 2014 (1) Cancer Immunol Res |
High | N/A | High | Low | Low | High |
| Hodi 2014 (2) JAMA | Low | Low | High | Low | High | Low |
| Kwon 2014 | Low | Low | Low | Low | Low | Low |
| Larkin 2015 | Low | Low | Low | Low | High | Low |
| Le 2013 | Low | Unclear | High | Low | Low | High |
| Lynch 2012 | Unclear | Unclear | Low | Low | Low | Low |
| Merchant 2015 | High | N/A | High | Low | High | High |
| Motzer 2015 | Unclear | Unclear | Low | Low | Low | Low |
| Postow 2015 | Low | Low | Low | Low | Low | Low |
| Prieto 2012 | High | N/A | High | Low | High | High |
| Ralph 2010 | N/A | N/A | High | Low | Unclear | High |
| Reck 2013 | Unclear | Unclear | Low | Low | High | Low |
| Ribas 2015 | Low | Low | Low | Low | High | Low |
| Rizvi 2015 | N/A | N/A | High | Low | High | High |
| Robert 2011 | Low | Low | Low | Low | High | Low |
| Robert 2014 | Low | Low | High | Low | High | Low |
| Robert 2015 (1) NEJM Nivo |
Low | Low | Low | Low | Low | Low |
| Robert 2015 (2) NEJM Pembro |
Low | Low | Low | Low | Low | Low |
| Sangro 2013 | N/A | N/A | High | Low | Unclear | High |
| Sarnaik 2010 | High | N/A | High | Low | Low | Low |
| Weber 2008 | High | N/A | High | Low | High | Low |
| Weber 2015 | Low | Low | High | Low | High | Low |
| Yamazaki 2015 | N/A | N/A | High | Low | Low | High |
| Yang 2007 | High | N/A | High | Low | High | High |
Nivo: Nivolumab. Pembro: Pembrolizumab.
N/A: not applicable (i.e. allocation concealment is not applicable for trials with no randomization, sequence generation is not applicable in single arm trials with only one dosing regimen). Selective adverse event includes not reporting all grades of events or not reporting all types of events on which information was collected. Other sources of bias: small size of study, stopped early, ascertainment of IRAE changed during the study.
The observational studies were all retrospective in their scope. No studies took into account potentially confounding variables. Two studies had sample sizes greater than 100, while the other had a sample size of only 30 patients. As has been noted, case series and reports represent lower quality evidence, with an inherent risk of reporting bias since only events of interest are described. Nonetheless, when describing a new entity in terms of its clinical manifestations, such spontaneous reports provide important information to describe the range of phenotypes possible as for ICI-induced autoimmune and musculoskeletal manifestations.
DISCUSSION
Several important findings were noted in this first review of rheumatic and musculoskeletal IRAE due to ICIs. We aimed to evaluate the prevalence of these types of IRAEs, their clinical characteristics and treatments to help guide rheumatologists and oncologists in their evaluation of these patients. Rates of arthralgia were most commonly reported in clinical trials and ranged widely, from 1 to 43%. Other musculoskeletal and rheumatologic adverse events were less commonly reported. Case series and reports illustrated a wide variety of rheumatic phenotypes seen after treatment with ICIs, including inflammatory arthritis, inflammatory myopathy, vasculitis, and lupus nephritis. The success and safety of treating those with known autoimmune disease with ICIs varied in the included case reports and observational study. Some patients had flares of their autoimmune disease and/or developed other IRAEs from ICI treatment, while others tolerated ICIs without incident.
This study was unique in being the first to focus on musculoskeletal and rheumatic IRAE as a consequence of treatment with ICIs. Other systematic reviews have focused on case reports of all types of IRAEs37 or IRAEs due to CTLA-438 inhibition, but no study to our knowledge has reviewed the published literature with the same focus as this review. It is also the first to synthesize information from different studies on treating patients with known autoimmune disease with ICIs. The descriptions of the variety of clinical presentations can help oncologists and rheumatologists in recognizing potential IRAEs. Areas for future study were identified by the lack of information found on epidemiology, treatment and evaluation of rheumatic and musculoskeletal IRAEs, and using ICIs in patients with autoimmune disease.
To put our findings in context, the incidence and clinical characteristics of IRAEs with other phenotypes range widely. Colitis and hepatitis, associated with potential mortality, have been reported in up to 2% of patients treated with ICIs39,40. Other IRAEs are more common but less severe. Inflammatory skin conditions, like vitiligo and other rashes, are seen in up to 30% of those treated14, and thyroid dysfunction occurs in as many as 22% of patients treated41. IRAEs affecting the peripheral and central nervous system, kidneys, pancreas and eyes have also been described42. The prevalence of IRAEs differ by type of therapy and indication for treatment. Pneumonitis is more common in those treated with nivolumab for NSCLC and RCC11,12 (4–5%) as compared to melanoma43 (1.5%). Colitis has been reported more commonly from ipilimumab than nivolumab in melanoma, with rates of about 5%10 compared to 1%44,45 respectively. Combination therapy with both ipilimumab and nivolumab has shown the highest rates of colitis46. The time course for IRAEs also differ, with rash and colitis developing early, and pneumonitis and endocrinopathies occurring later47.
In this review, we were not able to comment on the time course for developing musculoskeletal and rheumatic IRAEs, nor the association between particular drugs or tumors and specific events, due to lack of information in the published literature. The lack of complete reporting of the events of interest in the included clinical trials made it difficult to see which drugs were associated with particular rheumatic or musculoskeletal IRAEs. Only two clinical trials included in this report used combination therapy, so it is also unclear if combination therapy will lead to higher rates of events. The association of specific adverse events with particular drug regimens is an important area for investigation. In our limited experience, we have seen persistence of rheumatic IRAEs beyond the cessation of ICI therapy48, which, if confirmed in future studies, has significant implications on the long-term management of these patients.
Since our initial search, several relevant articles have been published. Our group has reported the largest case series of inflammatory arthritis and sicca syndrome secondary to ICIs48. In this series, patients with ICI-induced inflammatory arthritis were seronegative for traditional antibodies associated with rheumatoid arthritis. They required higher doses of steroids for their inflammatory arthritis, and some required additional immunosuppression with methotrexate or TNF-inhibitors. Concomitant colitis, thyroiditis, acute interstitial nephritis, or pneumonitis were present in a subset of patients.
Also after our search was conducted, a case series of 13 patients who had renal biopsies after developing acute kidney injury while being treated with immunotherapy49 was published. Several patients had coexisting IRAEs. One patient treated with nivolumab also had concomitant sicca syndrome with tubulointerstitial nephritis that was lymphocyte predominant on renal biopsy, similar to a patient described in our case series of ICI-induced sicca syndrome48. Both this study and our own study suggests that developing one IRAE is a risk factor for developing musculoskeletal and rheumatic IRAEs.
A limitation of this review is the lack of clinical information on IRAEs provided from clinical trial manuscripts. The classification of the events was likely inconsistent among studies. For example, in a 2010 trial of nivolumab for refractory solid tumors50, two patients were classified as having “arthralgia” but required corticosteroid treatment. It is possible that they actually had inflammatory arthritis rather than just joint pain. The inclusion of only high grade events (grades 3 or higher) in some trials also limited the information on musculoskeletal and rheumatic events that could be extracted from the clinical trial manuscripts. Oncology and rheumatology grading systems for adverse events differ, and musculoskeletal events with substantial functional impact (e.g. limiting instrumental activities of daily living) may be only a grade 2 event by the Common Terminology Criteria for Adverse Events (CTCAE) system used by oncology;51 whereas, they would be a grade 3 event in the Rheumatology Common Toxicity Criteria (RCTC) sytem.
Additionally, our search did not include therapies outside of those targeting PD-1, CTLA-4 and PD-L1 as we wanted to focus on approved targets for therapies at the time. Many other related pathways are being targeted in clinical trials of novel agents, but data is more preliminary for these targets.
In summary, a variety of rheumatic and musculoskeletal IRAE have been described after treatment with ICIs. Rates of arthralgia and myalgia have been widely reported, but other IRAE like arthritis and vasculitis are not consistently described. The case reports highlighted in this review illustrate the wide variety of rheumatic IRAE that rheumatologists are likely to encounter as ICI use expands. Future research should focus on knowledge deficits in epidemiology, evaluation, and treatment of rheumatic and musculoskeletal IRAE. Better understanding of why these musculoskeletal IRAE develop and how to treat them will benefit patients and could give insight into pathogenesis of traditional rheumatic diseases.
Supplementary Material
Significance and Innovation.
-
-
Arthralgia and myalgia were commonly reported in trials of immune checkpoint inhibitors.
-
-
There are no estimates for prevalence or incidence of rheumatic immune-related adverse events in the current literature.
-
-
Case reports highlight a diverse group of rheumatic manifestations after treatment with immune checkpoint inhibitors that may be referred to rheumatologists.
-
-
Immune checkpoint inhibitors have been used in those with pre-existing autoimmune disease and can precipitate flare of the autoimmune disease in some patients.
Acknowledgments
Financial support: NIH P30AR053503, Jerome L. Greene Foundation Discovery Award and Scholar Award; Camille J. Morgan Arthritis Research and Education Fund, NIH/NIAMS K23 AR061439.
Disclosures: Dr. Bingham has served as a consultant for Bristol-Myers Squibb.
REFERENCES
- 1.Shah AA, Rosen A, Hummers L, et al. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 2010;62(9):2787–2795. doi: 10.1002/art.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah AA, Hummers LK, Casciola-Rosen L, et al. Examination of autoantibody status and clinical features associated with cancer risk and cancer-associated scleroderma. Arthritis & rheumatology (Hoboken, NJ) 2015;67(4):1053–1061. doi: 10.1002/art.39022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joseph CG, Darrah E, Shah AA, et al. Association of the autoimmune disease scleroderma with an immunologic response to cancer. Science. 2014;343(6167):152–157. doi: 10.1126/science.1246886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trallero-Araguas E, Rodrigo-Pendas JA, Selva-O'Callaghan A, et al. Usefulness of anti-p155 autoantibody for diagnosing cancer-associated dermatomyositis: a systematic review and meta-analysis. Arthritis Rheum. 2012;64(2):523–532. doi: 10.1002/art.33379. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino DF, Chung LS, Christopher-Stine L, et al. Most patients with cancer-associated dermatomyositis have antibodies to nuclear matrix protein NXP-2 or transcription intermediary factor 1gamma. Arthritis Rheum. 2013;65(11):2954–2962. doi: 10.1002/art.38093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichimura Y, Matsushita T, Hamaguchi Y, et al. Anti-NXP2 autoantibodies in adult patients with idiopathic inflammatory myopathies: possible association with malignancy. Ann Rheum Dis. 2012;71(5):710–713. doi: 10.1136/annrheumdis-2011-200697. [DOI] [PubMed] [Google Scholar]
- 7.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27(4):450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Vlist M, Kuball J, Radstake TR, et al. Immune checkpoints and rheumatic diseases: what can cancer immunotherapy teach us? Nat Rev Rheumatol. 2016;12(10):593–604. doi: 10.1038/nrrheum.2016.131. [DOI] [PubMed] [Google Scholar]
- 9.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 10.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(2):123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015;372(4):311–319. doi: 10.1056/NEJMoa1411087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivashko IN, Kolesar JM. Pembrolizumab and nivolumab: PD-1 inhibitors for advanced melanoma. Am J Health Syst Pharm. 2016;73(4):193–201. doi: 10.2146/ajhp140768. [DOI] [PubMed] [Google Scholar]
- 15.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 16.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 18.Bronstein Y, Ng CS, Hwu P, et al. Radiologic manifestations of immune-related adverse events in patients with metastatic melanoma undergoing anti-CTLA-4 antibody therapy. AJR Am J Roentgenol. 2011;197(6):W992–w1000. doi: 10.2214/AJR.10.6198. [DOI] [PubMed] [Google Scholar]
- 19.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte-associated antigen 4. J Clin Oncol. 2006;24(15):2283–2289. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson DB, Sullivan RJ, Ott PA, et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2015:1–7. doi: 10.1001/jamaoncol.2015.4368. [DOI] [PubMed] [Google Scholar]
- 21.Chan MM, Kefford RF, Carlino M, et al. Arthritis and tenosynovitis associated with the anti-PD1 antibody pembrolizumab in metastatic melanoma. J Immunother. 2015;38(1):37–39. doi: 10.1097/CJI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 22.de Velasco G, Bermas B, Choueiri TK. Auto-immune arthropathy and uveitis as complications from PD-1 inhibitor. Arthritis & rheumatology (Hoboken, NJ) 2015 doi: 10.1002/art.39406. [DOI] [PubMed] [Google Scholar]
- 23.Sheik Ali S, Goddard AL, Luke JJ, et al. Drug-associated dermatomyositis following ipilimumab therapy: a novel immune-mediated adverse event associated with cytotoxic T-lymphocyte antigen 4 blockade. JAMA dermatology. 2015;151(2):195–199. doi: 10.1001/jamadermatol.2014.2233. [DOI] [PubMed] [Google Scholar]
- 24.Hunter G, Voll C, Robinson CA. Autoimmune inflammatory myopathy after treatment with ipilimumab. Can J Neurol Sci. 2009;36(4):518–520. doi: 10.1017/s0317167100007939. [DOI] [PubMed] [Google Scholar]
- 25.Yoshioka M, Kambe N, Yamamoto Y, et al. Case of respiratory discomfort due to myositis after administration of nivolumab. J Dermatol. 2015;42(10):1008–1009. doi: 10.1111/1346-8138.12991. [DOI] [PubMed] [Google Scholar]
- 26.Khoja L, Maurice C, Chappell M, et al. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res. 2016 doi: 10.1158/2326-6066.CIR-15-0186. [DOI] [PubMed] [Google Scholar]
- 27.Manusow JS, Khoja L, Pesin N, et al. Retinal vasculitis and ocular vitreous metastasis following complete response to PD-1 inhibition in a patient with metastatic cutaneous melanoma. Journal for immunotherapy of cancer. 2014;2(1):41. doi: 10.1186/s40425-014-0041-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minor DR, Bunker SR, Doyle J. Lymphocytic vasculitis of the uterus in a patient with melanoma receiving ipilimumab. J Clin Oncol. 2013;31(20):e356. doi: 10.1200/JCO.2012.47.5095. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein BL, Gedmintas L, Todd DJ. Drug-associated polymyalgia rheumatica/giant cell arteritis occurring in two patients after treatment with ipilimumab, an antagonist of ctla-4. Arthritis & rheumatology (Hoboken, NJ) 2014;66(3):768–769. doi: 10.1002/art.38282. [DOI] [PubMed] [Google Scholar]
- 30.Fadel F, El Karoui K, Knebelmann B. Anti-CTLA4 antibody-induced lupus nephritis. N Engl J Med. 2009;361(2):211–212. doi: 10.1056/NEJMc0904283. [DOI] [PubMed] [Google Scholar]
- 31.Gielisse EA, de Boer NK. Ipilimumab in a patient with known Crohn's disease: to give or not to give? J Crohns Colitis. 2014;8(12):1742. doi: 10.1016/j.crohns.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Pedersen M, Andersen R, Norgaard P, et al. Successful treatment with Ipilimumab and Interleukin-2 in two patients with metastatic melanoma and systemic autoimmune disease. Cancer Immunol Immunother. 2014;63(12):1341–1346. doi: 10.1007/s00262-014-1607-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bostwick AD, Salama AK, Hanks BA. Rapid complete response of metastatic melanoma in a patient undergoing ipilimumab immunotherapy in the setting of active ulcerative colitis. Journal for immunotherapy of cancer. 2015;3:19. doi: 10.1186/s40425-015-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Izzedine H, Gueutin V, Gharbi C, et al. Kidney injuries related to ipilimumab. Invest New Drugs. 2014;32(4):769–773. doi: 10.1007/s10637-014-0092-7. [DOI] [PubMed] [Google Scholar]
- 35.Henderson AD, Thomas DA. A case report of orbital inflammatory syndrome secondary to ipilimumab. Ophthal Plast Reconstr Surg. 2015;31(3):e68–e70. doi: 10.1097/IOP.0000000000000081. [DOI] [PubMed] [Google Scholar]
- 36.Conry RM, Sullivan JC, Nabors LB., 3rd Ipilimumab-induced encephalopathy with a reversible splenial lesion. Cancer Immunol Res. 2015;3(6):598–601. doi: 10.1158/2326-6066.CIR-15-0035. [DOI] [PubMed] [Google Scholar]
- 37.Abdel-Wahab N, Shah M, Suarez-Almazor ME. Adverse Events Associated with Immune Checkpoint Blockade in Patients with Cancer: A Systematic Review of Case Reports. PLoS One. 2016;11(7):e0160221. doi: 10.1371/journal.pone.0160221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bertrand A, Kostine M, Barnetche T, et al. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pernot S, Ramtohul T, Taieb J. Checkpoint inhibitors and gastrointestinal immune-related adverse events. Curr Opin Oncol. 2016;28(4):264–268. doi: 10.1097/CCO.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 40.Gettinger SN, Horn L, Gandhi L, et al. Overall Survival and Long-Term Safety of Nivolumab (Anti-Programmed Death 1 Antibody, BMS-936558, ONO-4538) in Patients With Previously Treated Advanced Non-Small-Cell Lung Cancer. J Clin Oncol. 2015;33(18):2004–2012. doi: 10.1200/JCO.2014.58.3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Torino F, Corsello SM, Salvatori R. Endocrinological side-effects of immune checkpoint inhibitors. Curr Opin Oncol. 2016;28(4):278–287. doi: 10.1097/CCO.0000000000000293. [DOI] [PubMed] [Google Scholar]
- 42.Postow MA. Managing immune checkpoint-blocking antibody side effects. Am Soc Clin Oncol Educ Book. 2015:76–83. doi: 10.14694/EdBook_AM.2015.35.76. [DOI] [PubMed] [Google Scholar]
- 43.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:311–319. doi: 10.1056/NEJMoa1412082. (4 // () *Bristol-Myers Squibb*) [DOI] [PubMed] [Google Scholar]
- 44.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373(17):1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 46.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691–2697. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 48.Cappelli LC, Gutierrez AK, Baer AN, et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2016-209595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cortazar FB, Marrone KA, Troxell ML, et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016 doi: 10.1016/j.kint.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woodworth T, Furst DE, Alten R, et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the Rheumatology Common Toxicity Criteria v.2.0. J Rheumatol. 2007;34(6):1401–1414. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.