Abstract
CYP2D6 and CYP2C19 polymorphisms affect the exposure, efficacy and safety of tricyclic antidepressants (TCAs), with some drugs being affected by CYP2D6 only (e.g., nortriptyline and desipramine) and others by both polymorphic enzymes (e.g., amitriptyline, clomipramine, doxepin, imipramine, and trimipramine). Evidence is presented for CYP2D6 and CYP2C19 genotype-directed dosing of TCAs. This document is an update to the 2012 Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Tricyclic Antidepressants.
Keywords: CYP2D6, CYP2C19, pharmacogenetics, tricyclic antidepressants, amitriptyline, clomipramine, desipramine, doxepin, imipramine, nortriptyline, and trimipramine
INTRODUCTION
Observed inter-individual differences in tricyclic antidepressant (TCA) pharmacokinetic parameters and treatment outcomes are associated with CYP2D6 and/or CYP2C19 genetic variation. The purpose of this guideline is to provide information to allow the interpretation of existing CYP2D6 and/or CYP2C19 genotyping results to guide TCA dosing and selection. Other clinical variables that may influence TCA therapy as well as genotyping cost-effectiveness are beyond the scope of this document. CPIC guidelines are periodically updated at https://cpicpgx.org/guidelines/ and http://www.pharmgkb.org.
FOCUSED LITERATURE REVIEW
A systematic literature review focused on CYP2D6 and CYP2C19 genetic variations and their relevance to gene-based dosing of TCAs was conducted (see Supplementary Data online). This guideline was developed based on interpretation of the literature by the authors and by experts in the field.
GENES: CYP2D6 AND CYP2C19
CYP2D6 background
The CYP2D6 gene is highly polymorphic. Over 100 known allelic variants and subvariants have been identified, and there are substantial ethnic differences in observed allele frequencies (CYP2D6 Allele Definition Table and CYP2D6 Frequency Table (1)). The most commonly reported alleles are categorized into functional groups as follows: normal function (e.g., CYP2D6*1 and *2), decreased function (e.g., CYP2D6*9, *10, and *41), and no function (e.g., CYP2D6*3-*6) (2, 3). Because CYP2D6 is subject to deletions or duplications, many clinical laboratories also report copy number. Deletions are indicated by the CYP2D6*5 allele, and gene duplications are denoted by an “xN” following the allele (e.g., CYP2D6*1xN, where xN represents the number of CYP2D6 gene copies).
CYP2C19 background
Similar to CYP2D6, the CYP2C19 gene is highly polymorphic; over 35 known allelic variants and subvariants have been identified (3) (CYP2C19 Allele Definition Table (4)). Although there are ethnic differences in allele frequencies (CYP2C19 Frequency Table (4)), the majority of patients will carry a CYP2C19*1,*2,*3 or *17 allele (5). CYP2C19*1 is the wild-type allele encoding a fully functional enzyme. CYP2C19*2-*8 are no function alleles of which CYP2C19*2 is the most frequently observed, though CYP2C19*3 is more common among individuals of Asian ancestry (3, 5). The CYP2C19*17 allele, defined by a variant in the gene promoter region, causes enhanced gene transcription resulting in greater metabolic capacity (6) and is therefore classified as an increased function allele.
Genetic test interpretation
Clinical laboratories usually interrogate for the more frequently observed CYP2D6 and CYP2C19 genetic variants and translate the results into star-allele (*) nomenclature. Each star-allele, or haplotype, is defined by a specific combination of single-nucleotide polymorphisms and/or other genetic variants within the gene locus of either CYP2D6 or CYP2C19 (5). Genetic test results are reported as the summary of inherited maternal and paternal star-alleles referred to as a diplotype (e.g., CYP2D6*1/*2 and CYP2C19*1/*1). The more frequently observed alleles and their functional status can be found in the CYP2D6 (1) and CYP2C19 Allele Definition Tables (4).
Scoring systems have been developed in an attempt to provide a uniform approach to quantitate the predicted functional status of CYP2D6 alleles as follows: 1 for normal function, 0.5 for decreased function, and 0 for no function alleles (see Supplemental Material; CYP2D6 Allele Definition Table (1)) (2, 7). The activity value for each allele of the diplotype is totaled to provide a CYP2D6 activity score. If CYP2D6 gene duplications are detected, the activity value of the duplicated allele is multiplied by the number of duplications present before calculating the activity score (Table 1, Supplemental Tables S1 and S2). See the Supplement for further explanation.
TABLE 1.
ASSIGNMENT OF LIKELY PHENOTYPES BASED ON DIPLOTYPES
| Assignment of CYP2D6 phenotypes | |||
|---|---|---|---|
| Likely phenotype | Activity Score | Genotypes | Examples of diplotypes |
| Ultrarapid metabolizer (~1–20% of patients)a | > 2.0 | An individual carrying duplications of functional alleles | (*1/*1)xN, (*1/*2)xN, (*2/*2)xNb |
| Normal metabolizer (~72–88% of patients) | 1.0–2.0c | An individual carrying two normal function alleles or two decreased function alleles or one normal and no function allele or one normal function and decreased function allele or combinations of duplicated alleles that result in an activity score of 1.0–2.0. | *1/*1, *1/*2, *2/*2, *1/*9, *1/*41, *41/*41, *1/*5, *1/*4 |
| Intermediate metabolizer (~1–13% of patients) | 0.5 | An individual carrying one decreased function and one no function allele | *4/*41, *5/*9, *4/*10 |
| Poor metabolizer (~1–10% of patients) | 0 | An individual carrying only no function alleles | *4/*4, (*4/*4)xN, *3/*4, *5/*5, *5/*6 |
| Assignment of CYP2C19 phenotypes | ||
|---|---|---|
| Likely phenotype | Genotypes | Examples of diplotypes |
| Ultrarapid metabolizer (~2–5% of patients)a | An individual carrying two increased function alleles | *17/*17 |
| Rapid Metabolizer (~2–30% of patients) | An individual carrying one normal function allele and one increased function allele | *1/*17 |
| Normal metabolizer (~35–50% of patients) | An individual carrying two normal function alleles | *1/*1 |
| Intermediate metabolizer (~18–45% of patients) | An individual carrying one normal function allele and one no function allele or one no function allele and one increased function allele | *1/*2, *1/*3, *2/*17d |
| Poor metabolizer (~2–15% of patients) | An individual carrying two no function alleles | *2/*2, *2/*3, *3/*3 |
CYP2D6 and CYP2C19 metabolizer status frequencies are based on average multi-ethnic frequencies. See the CYP2C19 (4) and CYP2D6 Frequency Tables (1) for population-specific allele and phenotype frequencies.
Where xN represents the number of CYP2D6 gene copies.
Patients with an activity score of 1.0 may be classified as intermediate metabolizers by some reference laboratories.
The predicted metabolizer phenotype for the*2/*17 genotype is a provisional classification. The currently available evidence indicates that the CYP2C19*17 increased function allele is unable to completely compensate for the CYP2C19*2 no function allele. See Supplemental Materials for a more comprehensive list of predicted metabolizer phenotypes.
Patients with two normal function CYP2C19 alleles are categorized as normal metabolizers and individuals carrying one or two no function alleles are considered intermediate and poor metabolizers, respectively (Table 1). Limited data suggest that CYP2C19*17 may not compensate for no function alleles such as the CYP2C19*2 allele (8). Therefore, patients carrying the CYP2C19*17 increased function allele in combination with a no function allele are considered intermediate metabolizers. These phenotype assignments are analogous to the CPIC guideline for selective serotonin reuptake inhibitors (3). See Supplement for discussion regarding CYP2C19 rapid metabolizer phenotype.
Reference laboratories use varying methods to assign phenotypes. Before pharmacotherapy modifications are made based upon this guideline, it is advisable to determine a patient’s phenotype as described above.
Available genetic test options
Commercially available genetic testing options change over time. Additional information about pharmacogenetic testing can be found at the Genetic Testing Registry website (http://www.ncbi.nlm.nih.gov/gtr/).
Incidental findings
Independent of drug metabolism and response, there are currently no diseases or conditions that have been convincingly linked to variants in the CYP2D6 or CYP2C19 genes (5, 7).
DRUGS: AMITRIPTYLINE AND NORTRIPTYLINE
Background
Tricyclic antidepressants (TCAs) are mixed serotonin and norepinephrine reuptake inhibitors used to treat several disease states including depression, obsessive-compulsive disorder, and neuropathic pain in addition to migraine prophylaxis. The TCAs have similar but distinct chemical structures referred to as tertiary and secondary amines. The pharmacological properties of the tertiary and secondary amines differ, with tertiary amines having a more pronounced serotonergic effect and secondary amines having a greater noradrenergic effect (Supplemental Tables S3 and S4) (9, 10). The tertiary amines (e.g., amitriptyline) are mainly metabolized by CYP2C19 to desmethyl-metabolites (Figure 1), also referred to as secondary amines (e.g., nortriptyline). It should be noted that the desmethyl-metabolites nortriptyline as well as desipramine are pharmacologically active with antidepressant properties as well as with distinct clinical features that differ from the parent drugs amitriptyline and imipramine. Both the tertiary and secondary amines are metabolized by CYP2D6 to less active hydroxy-metabolites (Figure 1, Supplemental Table S3). CYP2C19 impacts the ratio of tertiary to secondary amine plasma concentrations, but may have less influence on overall drug clearance than CYP2D6 (11). Although the total concentration of amitriptyline and nortriptyline may be unchanged for a CYP2C19 ultrarapid or poor metabolizer in certain instances, an imbalance between serotonergic and noradrenergic affect could influence clinical response or toxicities. There is limited evidence demonstrating that a serotonergic/noradrenergic imbalance influences outcomes, thus contributing to the optional recommendations in Table 3. Serotonin reuptake inhibition is expected to be more pronounced in CYP2C19 poor metabolizers due to the decreased conversion of parent tertiary amines to their respective metabolites (10).
Figure 1.
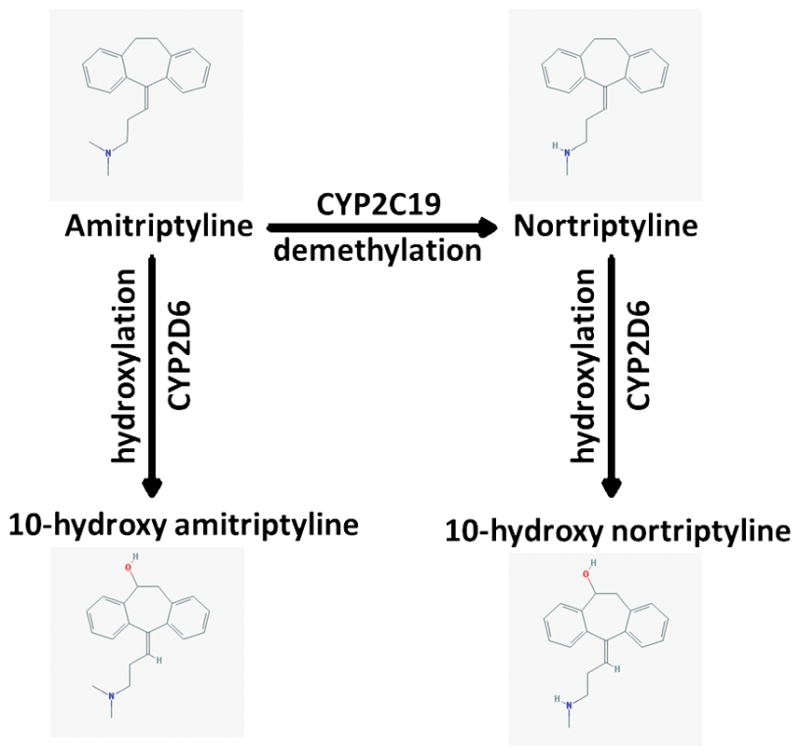
Major metabolic pathway of the tertiary amine amitriptyline and the secondary amine nortriptyline. For the structural representation the following 2D-images from the National Center for Biotechnology Information PubChem Compound Database are used: amitriptyline - CID=2160 (https://pubchem.ncbi.nlm.nih.gov/compound/2160); nortriptyline - CID=4543 (https://pubchem.ncbi.nlm.nih.gov/compound/4543); 10-hydroxyamitriptyline - CID=6420900 (https://pubchem.ncbi.nlm.nih.gov/compound/6420900); 10-hydroxynortriptyline - CID=6420504 (https://pubchem.ncbi.nlm.nih.gov/compound/6420504) (37). All entries were accessed Nov. 8, 2016.
TABLE 3.
DOSING RECOMMENDATIONS FOR THE TERTIARY AMINES AMITRIPTYLINE, CLOMIPRAMINE, DOXEPIN, IMIPRAMINE, AND TRIMIPRAMINE BASED ON CYP2C19 PHENOTYPE
| Phenotype | Implication | Therapeutic Recommendationa,b | Classification of Recommendation for Amitriptylinec | Classification of Recommendation for Other Tertiary Amine TCAsc,d |
|---|---|---|---|---|
| CYP2C19 Ultrarapid metabolizer and CYP2C19 Rapid Metabolizer | Increased metabolism of tertiary amines compared to normal metabolizers Greater conversion of tertiary amines to secondary amines may affect response or side effects |
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. If a tertiary amine is warranted, utilize therapeutic drug monitoring to guide dose adjustmentse. |
Optional | Optional |
| CYP2C19 Normal metabolizer | Normal metabolism of tertiary amines | Initiate therapy with recommended starting dosef. | Strong | Strong |
| CYP2C19 Intermediate metabolizer | Reduced metabolism of tertiary amines compared to normal metabolizers | Initiate therapy with recommended starting dosef. | Strong | Optional |
| CYP2C19 Poor metabolizer | Greatly reduced metabolism of tertiary amines compared to normal metabolizers Decreased conversion of tertiary amines to secondary amines may affect response or side effects |
Avoid tertiary amine use due to potential for sub-optimal response. Consider alternative drug not metabolized by CYP2C19. TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine. For tertiary amines, consider a 50% reduction of the recommended starting dosef. Utilize therapeutic drug monitoring to guide dose adjustmentse. |
Moderate | Optional |
If CYP2D6 genotype results are also available, see Table 2 for CYP2D6-based dosing recommendations and Table 4 for CYP2D6/CYP2C19-based dosing recommendations.
Dosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. See other considerations for dosing recommendations for conditions where lower initial doses are used, such as neuropathic pain.
The rating scheme for the recommendation of classification is described in Supplementary Data.
It may be reasonable to apply this recommendation to other TCAs also metabolized by CYP2C19 including clomipramine, doxepin, imipramine, and trimipramine. There are fewer clinical and pharmacokinetic data supporting dose adjustments for these drugs when compared to amitriptyline or nortriptyline (Supplemental Tables S8–S16).
Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose.
The use of TCAs to treat psychiatric disorders has declined in part due to the occurrence of undesirable side effects along with the growing availability of alternatives with more acceptable side effect profiles. Although TCAs are still used to treat depression (12), they are now more often used in the context of pain management (13, 14). Inter-individual differences in side effects and treatment response have been associated with variability of tricyclic plasma concentrations (15, 16). Patients may be predisposed to treatment failure or adverse effects due to genetic variation in CYP2D6 altering drug clearance or in CYP2C19 altering the ratio of parent drug to metabolites. Common adverse effects include anticholinergic, central nervous system and cardiac effects. Tertiary and secondary amines along with their metabolites each have unique side effect profiles as detailed in Supplemental Table S4.
Both amitriptyline and nortriptyline are used as representative TCAs for this guideline because the majority of pharmacogenomic studies have focused on these two drugs. However, the results of these studies may apply to other TCAs because these drugs have comparable pharmacokinetic properties (15, 17). TCAs are well absorbed from the gastrointestinal tract, and the average extent of first pass metabolism is approximately 50%, although the average first pass metabolism of doxepin may be closer to 70% (15). The clearance of TCAs is mostly a linear process, but saturation of the hydroxylation pathway may occur at higher plasma concentrations for certain TCAs including imipramine and desipramine (15, 18). Additionally, extrapolated dose adjustments based on metabolizer status are similar across the tricyclic class (17). Because some studies investigating the influence of CYP2D6 and/or CYP2C19 genotype/phenotype on the pharmacokinetics of TCAs used a single dose, it should be noted that in this guideline tricyclic metabolism was assumed to be similar after single or multiple dosing, and no differentiation was made between evidence from single dose studies or from multiple dose studies (16).
Linking genetic variability to variability in drug-related phenotypes
There is substantial evidence linking CYP2D6 and CYP2C19 genotypes to phenotypic variability in tricyclic side effects and pharmacokinetic profiles. Modifying pharmacotherapy for patients who have CYP2D6 or CYP2C19 genetic variants that affect drug efficacy and safety could potentially improve clinical outcomes, and reduce the failure rate of initial treatment. The application of a grading system to the evidence linking CYP2D6 and CYP2C19 genotypic variations to phenotypic variability in the response to amitriptyline or nortriptyline indicates a high quality of evidence in the majority of cases (Supplemental Tables S5–S7). This body of evidence, rather than randomized clinical trials, provides the basis for amitriptyline and nortriptyline dosing recommendations in Tables 2 and 3, respectively. Optimal therapeutic plasma concentrations for the TCAs have been defined (19). CYP2D6 and CYP2C19 poor or ultrarapid metabolizers may have tricyclic plasma concentrations outside the recommended therapeutic range, thus increasing the risk of treatment failure or side effects (17, 20–22). TCA plasma concentrations have been shown to be predictive of toxicity and efficacy, with guidelines defining therapeutic ranges for TCAs (19). However, there are less data supporting a direct correlation between genotype and response when compared to the correlation between genotype and plasma concentrations. Some studies describe a relationship between genotype and response (23–25) while other studies do not (26). Therefore, this guideline takes into consideration both clinical outcomes and observed tricyclic plasma concentrations based on genotype/phenotype characteristics.
TABLE 2.
DOSING RECOMMENDATIONS FOR TRICYCLIC ANTIDEPRESSANTS BASED ON CYP2D6 PHENOTYPE
| Phenotype | Implication | Therapeutic Recommendationa,b | Classification of Recommendation for Amitriptyline and Nortripylinec | Classification of Recommendation for Other TCAsc,d |
|---|---|---|---|---|
| CYP2D6 Ultrarapid metabolizer | Increased metabolism of TCAs to less active compounds compared to normal metabolizers Lower plasma concentrations of active drug will increase probability of pharmacotherapy failure |
Avoid tricyclic use due to potential lack of efficacy. Consider alternative drug not metabolized by CYP2D6. If a TCA is warranted, consider titrating to a higher target dose (compared to normal metabolizers)e. Utilize therapeutic drug monitoring to guide dose adjustments. |
Strong | Optional |
| CYP2D6 Normal metabolizer | Normal metabolism of TCAs | Initiate therapy with recommended starting dosef. | Strong | Strong |
| CYP2D6 Intermediate metabolizer | Reduced metabolism of TCAs to less active compounds compared to normal metabolizers Higher plasma concentrations of active drug will increase the probability of side effects |
Consider a 25% reduction of recommended starting dosef. Utilize therapeutic drug monitoring to guide dose adjustmentse. | Moderate | Optional |
| CYP2D6 Poor metabolizer | Greatly reduced metabolism of TCAs to less active compounds compared to normal metabolizers Higher plasma concentrations of active drug will increase the probability of side effects |
Avoid tricyclic use due to potential for side effects. Consider alternative drug not metabolized by CYP2D6. If a TCA is warranted, consider a 50% reduction of recommended starting dosef. Utilize therapeutic drug monitoring to guide dose adjustmentse. |
Strong | Optional |
For tertiary amines (e.g., amitriptyline), if CYP2C19 genotype results are also available, see Table 3 for CYP2C19-based dosing recommendations and Table 4 for CYP2D6/CYP2C19-based dosing recommendations.
Dosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. See other considerations for dosing recommendations for conditions where lower initial doses are used, such as neuropathic pain.
The rating scheme for the recommendation of classification is described in Supplementary Data.
It may be reasonable to apply this recommendation to other TCAs also metabolized by CYP2D6 including clomipramine, desipramine, doxepin, imipramine, and trimipramine. There are fewer clinical and pharmacokinetic data supporting genotype-guided dose adjustments for these drugs when compared to amitriptyline or nortriptyline (Supplemental Tables S8–S16).
Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Patients may receive an initial low dose of a tricyclic, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose.
Therapeutic recommendations
CYP2D6 dosing recommendations
For neuropathic pain treatment, where lower initial doses of TCAs are used, gene-based dosing recommendations are found in the subsection Gene-based dosing recommendations for neuropathic pain treatment. Table 2 summarizes the gene-based dosing recommendations for CYP2D6 and amitriptyline and nortriptyline for situations in which a higher initial dose is warranted, such as depression treatment. The recommended starting dose of amitriptyline or nortriptyline does not need adjustment for those with genotypes predictive of CYP2D6 normal metabolism. A 25% reduction of the recommended dose may be considered for CYP2D6 intermediate metabolizers (27). The strength of this recommendation is classified as “moderate” because patients with a CYP2D6 activity score of 1.0 are inconsistently categorized as intermediate or normal metabolizers in the literature, making these studies difficult to evaluate.
CYP2D6 ultrarapid metabolizers have a higher probability of failing amitriptyline or nortriptyline pharmacotherapy due to subtherapeutic plasma concentrations, and alternate agents are preferred. There are documented cases of CYP2D6 ultrarapid metabolizers receiving large doses of nortriptyline in order to achieve therapeutic concentrations (22). However, very high plasma concentrations of the nortriptyline hydroxy-metabolite were present, which may increase the risk for cardiotoxicity. If a tricyclic is warranted, there are insufficient data in the literature to calculate a starting dose for a patient with CYP2D6 ultrarapid metabolizer status, and therapeutic drug monitoring is strongly recommended. Adverse effects are more likely in CYP2D6 poor metabolizers due to elevated tricyclic plasma concentrations (28); therefore, alternate agents are preferred. If a tricyclic is warranted, consider a 50% reduction of the usual dose, and therapeutic drug monitoring is strongly recommended.
CYP2C19 dosing recommendations
Dosing recommendations for neuropathic pain treatment with amitriptyline are found in the subsection Gene-based dosing recommendations for neuropathic pain treatment. Table 3 summarizes the gene-based dosing recommendations for CYP2C19 and amitriptyline when higher initial starting doses are warranted. The usual starting dose of amitriptyline may be used in CYP2C19 normal and intermediate metabolizers. Although CYP2C19 intermediate metabolizers would be expected to have a modest increase in the ratio of amitriptyline to nortriptyline plasma concentrations, the evidence does not indicate that CYP2C19 intermediate metabolizers should receive an alternate dose.
Patients taking amitriptyline who are CYP2C19 rapid or ultrarapid metabolizers may be at risk for having low plasma concentrations and an imbalance between parent drug and metabolites causing treatment failure and/or adverse events. Although the CYP2C19*17 allele did not alter the sum of amitriptyline plus nortriptyline plasma concentrations, it was associated with higher nortriptyline plasma concentrations, possibly increasing the risk of adverse events (8). For patients taking amitriptyline, extrapolated pharmacokinetic data suggest that CYP2C19 rapid or ultrarapid metabolizers may need a dose increase (17). Due to the need for further studies investigating the clinical importance of CYP2C19*17 regarding tricyclic metabolism and the possibility of altered concentrations, we recommend to consider an alternative tricyclic or other drug not affected by CYP2C19. This recommendation is classified as optional due to limited available data. If amitriptyline is administered to a CYP2C19 rapid or ultrarapid metabolizer, therapeutic drug monitoring is recommended.
CYP2C19 poor metabolizers are expected to have a greater ratio of amitriptyline to nortriptyline plasma concentrations (29). The elevated amitriptyline plasma concentrations may increase the chance of a patient experiencing side effects. Consider a 50% reduction of the usual amitriptyline starting dose along with therapeutic drug monitoring (17).
Other TCAs
Because the TCAs have comparable pharmacokinetic properties, it may be reasonable to extrapolate this guideline to other TCAs including clomipramine, desipramine, doxepin, imipramine, and trimipramine (Tables 2 and 3; Supplemental Tables S8–S16), with the acknowledgement that there are fewer data supporting dose adjustments for these drugs than for amitriptyline or nortriptyline.
CYP2D6 and CYP2C19 combined dosing recommendations
Although specific combinations of CYP2D6 and CYP2C19 alleles are likely to result in additive effects on the pharmacokinetic properties of TCAs, little information is available on how to adjust initial doses based on combined genotype information. Patients carrying at least one CYP2D6 no function allele and two CYP2C19 normal function alleles had an increased risk of experiencing side effects when administered amitriptyline, while patients with at least one CYP2C19 no function allele and two CYP2D6 normal function alleles had a lower risk of experiencing side effects (22, 30). Combinatorial gene-based recommendations are provided in Table 4. Therapeutic drug monitoring may be advised if a tricyclic is prescribed to a patient with CYP2D6 ultrarapid, intermediate or poor metabolism in combination with CYP2C19 ultrarapid, rapid, intermediate or poor metabolism. There are sparse data in patients with a combinatorial CYP2C19 ultrarapid/rapid/intermediate/poor metabolizer phenotype and CYP2D6 ultrarapid/intermediate/poor phenotype. Because there are limited clinical or pharmacokinetic data regarding these combinatorial phenotypes, pharmacotherapy recommendations are classified as optional.
TABLE 4.
| Phenotype | CYP2D6 Ultrarapid metabolizer | CYP2D6 Normal metabolizer | CYP2D6 Intermediate metabolizer | CYP2D6 Poor metabolizer |
|---|---|---|---|---|
| CYP2C19 Ultrarapid or Rapid metabolizer | Avoid amitriptyline usec Classification of recommendationd: Optional |
Consider alternative drug not metabolized by CYP2C19c,e Classification of recommendationd: Optional |
Consider alternative drug not metabolized by CYP2C19 c,e Classification of recommendationd: Optional |
Avoid amitriptyline usec Classification of recommendationd: Optional |
| CYP2C19 Normal metabolizer | Avoid amitriptyline use. If amitriptyline is warranted, consider titrating to a higher target dose (compared to normal metabolizers)f,g Classification of recommendationd: Strong |
Initiate therapy with recommended starting doseh Classification of recommendationd: Strong |
Consider a 25% reduction of recommended starting dosef,h Classification of recommendationd: Moderate |
Avoid amitriptyline use. If amitriptyline is warranted, consider a 50% reduction of recommended starting dosef,h Classification of recommendationd: Strong |
| CYP2C19 Intermediate metabolizer | Avoid amitriptyline usec Classification of recommendationd: Optional |
Initiate therapy with recommended starting doseh Classification of recommendationd: Strong |
Consider a 25% reduction of recommended starting dosef,h Classification of recommendationd: Optional |
Avoid amitriptyline use. If amitriptyline is warranted, consider a 50% reduction of recommended starting dosef,h Classification of recommendationd: Optional |
| CYP2C19 Poor metabolizer | Avoid amitriptyline usec Classification of recommendationd: Optional |
Avoid amitriptyline use. If amitriptyline is warranted, consider a 50% reduction of recommended starting dosef,h Classification of recommendationd: Moderate |
Avoid amitriptyline usec Classification of recommendationd: Optional |
Avoid amitriptyline usec Classification of recommendationd: Optional |
Dosing recommendations only apply to higher initial doses of TCAs for treatment of conditions such as depression. See other considerations for dosing recommendations for conditions where lower initial doses are used, such as neuropathic pain.
The dosing recommendations are based on studies focusing on amitriptyline. Because tricyclic antidepressants have comparable pharmacokinetic properties, it may be reasonable to apply these guidelines to other tertiary amines including clomipramine, doxepin, imipramine and trimipramine (the classification of this recommendation is optional).
If amitriptyline is warranted, utilize therapeutic drug monitoringf to guide dose adjustment.
The rating scheme for the recommendation classification is described in Supplementary Data. See CYP2D6 and CYP2C19 combined dosing recommendations for explanation of classification of recommendations for this table.
TCAs without major CYP2C19 metabolism include the secondary amines nortriptyline and desipramine.
Utilizing therapeutic drug monitoring if a tricyclic is prescribed to a patient with CYP2D6 ultrarapid, intermediate or poor metabolism in combination with CYP2C19 ultrarapid, intermediate or poor metabolism is strongly recommended.
Titrate dose to observed clinical response with symptom improvement and minimal (if any) side effects.
Patients may receive an initial low dose of TCAs, which is then increased over several days to the recommended steady-state dose. The starting dose in this guideline refers to the recommended steady-state dose.
Gene-based dosing recommendations for neuropathic pain treatment
Amitriptyline is often used at lower dosages (e.g., 0.1 mg/kg/day in pediatric patients; initial doses of 25 mg daily may be prescribed to adults) for treatment of neuropathic pain compared to treatment for depressive disorders (13, 14). Because of the lower dosage, it is less likely that CYP2D6 or CYP2C19 poor or intermediate metabolizers will experience adverse effects due to supratherapeutic plasma concentrations of amitriptyline (31). Therefore, we recommend no dose modifications for poor or intermediate metabolizers when prescribed amitriptyline at a lower dose for treatment of neuropathic pain, but these patients should be monitored closely for side effects. If larger doses of amitriptyline are warranted, we recommend following the gene-based dosing guidelines presented in Tables 2–4.
There are limited data to support dose recommendations for CYP2C19*17 carriers who are prescribed amitriptyline at lower doses for neuropathic pain treatment. There are also little data describing the use of amitriptyline for neuropathic pain in CYP2D6 ultrarapid metabolizers. Based on predicted and observed pharmacokinetic data in those with depression, CYP2D6 ultrarapid metabolizers may be at an increased risk of failing amitriptyline therapy for neuropathic pain due to lower than expected drug concentrations, and thus alternative agents should be considered (32). Although there is sparse information on how to adjust initial amitriptyline doses based on combined CYP2D6 and CYP2C19 genetic results when treating neuropathic pain, caution should be used when patients have a combination of poor or ultrarapid phenotypes (e.g., a CYP2D6 poor metabolizer also having CYP2C19 ultrarapid or poor metabolism).
Pediatrics
There are scarce studies focusing solely on CYP2D6 or CYP2C19 genotype and association with pharmacokinetic parameters or treatment outcomes of TCAs in pediatric patients. CYP2D6 activity is fully mature by early childhood, but CYP2C19 activity may be increased in children relative to adults (3). Although further genomic ontogeny studies are needed, there is a lack of evidence suggesting that this guideline cannot be extrapolated to pediatric patients.
Other considerations
Consideration of drug interactions and patient characteristics
Patients treated for psychiatric disorders often require multiple medications, which can influence tricyclic plasma concentrations, side effects, and therapeutic failure (15). Recent data indicate that up to 20% of patients treated for depression may be converted to CYP2D6 poor metabolizer status (33). For example, patients taking amitriptyline in combination with a potent CYP2D6 inhibitor, such as fluoxetine, may have dramatic increases in amitriptyline plasma concentrations (34). It has been suggested that patients taking strong CYP2D6 inhibitors should be treated similarly to CYP2D6 poor metabolizers (7). Additionally, patients with increased age, liver disease, and reduced renal function may require reduced doses of TCAs (15). Drug-drug interactions along with patient characteristics should be considered in addition to the gene-based dosing recommendations presented herein.
Minor metabolic pathways of TCAs
Other cytochrome P450 enzymes including CYP3A4 and CYP1A2 metabolize TCAs to a lesser extent (15, 31, 35, 36). There is currently no strong evidence supporting gene-based dosing recommendations for other CYP enzymes that metabolize TCAs.
Implementation of this guideline
The guideline supplement contains resources that can be used within electronic health records (EHRs) to assist clinicians in applying genetic information to patient care for the purpose of drug therapy optimization (see Resources to incorporate pharmacogenetics into an electronic health record with clinical decision support sections of supplement.).
POTENTIAL BENEFITS AND RISKS FOR THE PATIENT
For patients who have existing CYP2D6 and/or CYP2C19 genotyping test results, the potential benefit is identifying those patients who are at an elevated risk of experiencing side effects or therapeutic failure. For those patients, dose adjustments can be made or an alternative agent selected. A limitation inherent to most commercially available genotyping tests is that rare or de novo variants are not detected. Additionally, some alleles are not well characterized resulting in uncertainty when predicting the phenotype from some genetic test results. Genotyping is reliable when performed in qualified clinical laboratories, but as with any laboratory test, an error can occur. Any errors in genotyping or phenotype prediction, along with the presence of a rare genomic variant not tested for, could potentially have lifelong implications for the patient’s drug therapy.
CAVEATS: APPROPRIATE USE AND/OR POTENTIAL MISUSE OF GENETIC TESTS
The application of genotype-based dosing is most appropriate when initiating therapy with a tricyclic. Obtaining a pharmacogenetic test after months of drug therapy may be less helpful in some instances, as the drug dose may have already been adjusted based on plasma concentrations, response, or side effects. Similar to all diagnostic tests, genetic tests are one of several pieces of clinical information that should be considered before initiating drug therapy.
Supplementary Material
Acknowledgments
We acknowledge the critical input of Dr. M. Relling and members of the Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network, funded by the National Institutes of Health. This work was funded by the National Institutes of Health (NIH) for CPIC (R24GM115264) and PharmGKB (R24GM61374). EDK is supported by R01 GM63674 and R01 DA14211. TCS is supported by R01 GM088076 and the Agency for Healthcare Research and Quality (R01 HS19818-01). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. VLE is supported by the NIMH (R01 MH082784). DJM is supported by a New Investigator Salary Award from the Canadian Institutes of Health Research, a New Investigator Fellowship Award from the Ontario Mental Health Foundation and an Early Researcher Award by the Ministry of Research and Innovation of Ontario. AG is supported by R01 GM088076-05.
Footnotes
DISCLAIMER
CPIC guidelines reflect expert consensus based on clinical evidence and peer-reviewed literature available at the time they are written and are intended only to assist clinicians in decision making and to identify questions for further research. New evidence may have emerged since the time a guideline was submitted for publication. Guidelines are limited in scope and are not applicable to interventions or diseases not specifically identified. Guidelines do not account for all individual variations among patients and cannot be considered inclusive of all proper methods of care or exclusive of other treatments. It remains the responsibility of the health-care provider to determine the best course of treatment for a patient. Adherence to any guidelines is voluntary, with the ultimate determination regarding its application to be made solely by the clinician and the patient. CPIC assumes no responsibility for any injury to persons or damage to persons or property arising out of or related to any use of CPIC’s guidelines, or for any errors or omissions.
CPIC is a registered service mark of the U.S. Department of Health & Human Services (HHS).
CONFLICT OF INTEREST
A.G. is a paid consultant of Millennium Laboratories. T.E.K is a paid scientific advisor to the Rxight™ Pharmacogenetic Program. K.S. has received research support from Shionogi & Co., Ltd., Eli Lilly Japan, K.K., Yoshitomi Pharmaceutical Industries, Ltd., Meiji Seika Pharma Co., Ltd., Eisai Co., Ltd., Pfizer Inc., GlaxoSmithKlein K.K., Otsuka Pharmaceutical Co., Ltd., Daiichi Sankyo Co., and Takeda Pharmaceutical Co., Ltd. and honoraria from Kowa Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Meiji Seika Pharma Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Ono Pharmaceutical Co., Ltd., GlaxoSmithKlein K.K., and Eisai Co., Ltd
All other authors declare no conflict of interest.
References
- 1. [Accessed September 16 2016];PGx Gene-specific Information Tables for CYP2D6. < https://www.pharmgkb.org/page/cyp2d6RefMaterials>.
- 2.Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS. The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clinical pharmacology and therapeutics. 2008;83:234–42. doi: 10.1038/sj.clpt.6100406. [DOI] [PubMed] [Google Scholar]
- 3.Hicks JK, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2D6 and CYP2C19 Genotypes and Dosing of Selective Serotonin Reuptake Inhibitors. Clinical pharmacology and therapeutics. 2015;98:127–34. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [Accessed September 16 2016];PGx Gene-specific Information Tables for CYP2C19. < https://www.pharmgkb.org/page/cyp2c19RefMaterials>.
- 5.Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-2C19 (CYP2C19) genotype and clopidogrel therapy. Clinical pharmacology and therapeutics. 2011;90:328–32. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sim SC, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clinical pharmacology and therapeutics. 2006;79:103–13. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Crews KR, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clinical pharmacology and therapeutics. 2012;91:321–6. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vos A, van der Weide J, Loovers HM. Association between CYP2C19*17 and metabolism of amitriptyline, citalopram and clomipramine in Dutch hospitalized patients. The pharmacogenomics journal. 2011;11:359–67. doi: 10.1038/tpj.2010.39. [DOI] [PubMed] [Google Scholar]
- 9.Gillman PK. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. British journal of pharmacology. 2007;151:737–48. doi: 10.1038/sj.bjp.0707253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimoda K, Jerling M, Bottiger Y, Yasuda S, Morita S, Bertilsson L. Pronounced differences in the dispositon of clomipramine between Japanese and Swedish patients. J Clin Psychopharmacol. 1999;19:393–400. doi: 10.1097/00004714-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Jiang ZP, et al. The role of CYP2C19 in amitriptyline N-demethylation in Chinese subjects. Eur J Clin Pharmacol. 2002;58:109–13. doi: 10.1007/s00228-002-0445-6. [DOI] [PubMed] [Google Scholar]
- 12.Association, A.P. Practice Guideline for the Treatment of Patients With Major Depressive Disorder. 3. American Psychiatric Publishing; Arlington, VA: 2010. [Google Scholar]
- 13.Watson CP. The treatment of neuropathic pain: antidepressants and opioids. The Clinical journal of pain. 2000;16:S49–55. doi: 10.1097/00002508-200006001-00009. [DOI] [PubMed] [Google Scholar]
- 14.Laird B, Colvin L, Fallon M. Management of cancer pain: basic principles and neuropathic cancer pain. European journal of cancer. 2008;44:1078–82. doi: 10.1016/j.ejca.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 15.Rudorfer MV, Potter WZ. Metabolism of tricyclic antidepressants. Cellular and molecular neurobiology. 1999;19:373–409. doi: 10.1023/A:1006949816036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Potter WZ, Zavadil AP, 3rd, Kopin IJ, Goodwin FK. Single-dose kinetics predict steady-state concentrations on imipramine and desipramine. Archives of general psychiatry. 1980;37:314–20. doi: 10.1001/archpsyc.1980.01780160084010. [DOI] [PubMed] [Google Scholar]
- 17.Stingl JC, Brockmoller J, Viviani R. Genetic variability of drug-metabolizing enzymes: the dual impact on psychiatric therapy and regulation of brain function. Molecular psychiatry. 2012 doi: 10.1038/mp.2012.42. [DOI] [PubMed] [Google Scholar]
- 18.Cooke RG, Warsh JJ, Stancer HC, Reed KL, Persad E. The nonlinear kinetics of desipramine and 2-hydroxydesipramine in plasma. Clinical pharmacology and therapeutics. 1984;36:343–9. doi: 10.1038/clpt.1984.185. [DOI] [PubMed] [Google Scholar]
- 19.Hiemke C, et al. AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry: Update 2011. Pharmacopsychiatry. 2011;44:195–235. doi: 10.1055/s-0031-1286287. [DOI] [PubMed] [Google Scholar]
- 20.Dalen P, Dahl ML, Bernal Ruiz ML, Nordin J, Bertilsson L. 10-Hydroxylation of nortriptyline in white persons with 0, 1, 2, 3, and 13 functional CYP2D6 genes. Clinical pharmacology and therapeutics. 1998;63:444–52. doi: 10.1016/S0009-9236(98)90040-6. [DOI] [PubMed] [Google Scholar]
- 21.Kirchheiner J, Seeringer A. Clinical implications of pharmacogenetics of cytochrome P450 drug metabolizing enzymes. Biochimica et biophysica acta. 2007;1770:489–94. doi: 10.1016/j.bbagen.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Bertilsson L, Aberg-Wistedt A, Gustafsson LL, Nordin C. Extremely rapid hydroxylation of debrisoquine: a case report with implication for treatment with nortriptyline and other tricyclic antidepressants. Therapeutic drug monitoring. 1985;7:478–80. [PubMed] [Google Scholar]
- 23.Bertilsson L, et al. Molecular basis for rational megaprescribing in ultrarapid hydroxylators of debrisoquine. Lancet. 1993;341:63. doi: 10.1016/0140-6736(93)92546-6. [DOI] [PubMed] [Google Scholar]
- 24.Kawanishi C, Lundgren S, Agren H, Bertilsson L. Increased incidence of CYP2D6 gene duplication in patients with persistent mood disorders: ultrarapid metabolism of antidepressants as a cause of nonresponse. A pilot study. Eur J Clin Pharmacol. 2004;59:803–7. doi: 10.1007/s00228-003-0701-4. [DOI] [PubMed] [Google Scholar]
- 25.Penas-Lledo EM, et al. CYP2D6 ultrarapid metabolism and early dropout from fluoxetine or amitriptyline monotherapy treatment in major depressive patients. Molecular psychiatry. 2013;18:8–9. doi: 10.1038/mp.2012.91. [DOI] [PubMed] [Google Scholar]
- 26.Hodgson K, et al. Genetic differences in cytochrome P450 enzymes and antidepressant treatment response. J Psychopharmacol. 2014;28:133–41. doi: 10.1177/0269881113512041. [DOI] [PubMed] [Google Scholar]
- 27.Swen JJ, et al. Pharmacogenetics: from bench to byte--an update of guidelines. Clinical pharmacology and therapeutics. 2011;89:662–73. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 28.Bertilsson L, Mellstrom B, Sjokvist F, Martenson B, Asberg M. Slow hydroxylation of nortriptyline and concomitant poor debrisoquine hydroxylation: clinical implications. Lancet. 1981;1:560–1. doi: 10.1016/s0140-6736(81)92894-4. [DOI] [PubMed] [Google Scholar]
- 29.Shimoda K, et al. The impact of CYP2C19 and CYP2D6 genotypes on metabolism of amitriptyline in Japanese psychiatric patients. J Clin Psychopharmacol. 2002;22:371–8. doi: 10.1097/00004714-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 30.Steimer W, et al. Amitriptyline or not, that is the question: pharmacogenetic testing of CYP2D6 and CYP2C19 identifies patients with low or high risk for side effects in amitriptyline therapy. Clinical chemistry. 2005;51:376–85. doi: 10.1373/clinchem.2004.041327. [DOI] [PubMed] [Google Scholar]
- 31.Halling J, Weihe P, Brosen K. The CYP2D6 polymorphism in relation to the metabolism of amitriptyline and nortriptyline in the Faroese population. British journal of clinical pharmacology. 2008;65:134–8. doi: 10.1111/j.1365-2125.2007.02969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dworkin RH, et al. Pharmacologic management of neuropathic pain: evidence-based recommendations. Pain. 2007;132:237–51. doi: 10.1016/j.pain.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Preskorn SH, et al. Cytochrome P450 2D6 phenoconversion is common in patients being treated for depression: implications for personalized medicine. J Clin Psychiatry. 2013;74:614–21. doi: 10.4088/JCP.12m07807. [DOI] [PubMed] [Google Scholar]
- 34.el-Yazigi A, Chaleby K, Gad A, Raines DA. Steady-state kinetics of fluoxetine and amitriptyline in patients treated with a combination of these drugs as compared with those treated with amitriptyline alone. Journal of clinical pharmacology. 1995;35:17–21. doi: 10.1002/j.1552-4604.1995.tb04740.x. [DOI] [PubMed] [Google Scholar]
- 35.Ketter TA, et al. The emerging role of cytochrome P450 3A in psychopharmacology. J Clin Psychopharmacol. 1995;15:387–98. doi: 10.1097/00004714-199512000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Shen WW. Cytochrome P450 monooxygenases and interactions of psychotropic drugs: a five-year update. International journal of psychiatry in medicine. 1995;25:277–90. doi: 10.2190/29NP-2XPN-X0ME-MQWU. [DOI] [PubMed] [Google Scholar]
- 37.Kim S, et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016;44:D1202–13. doi: 10.1093/nar/gkv951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


