Abstract
Osteoarthritis is a slowly progressive disease which includes the intervention of several cytokines, macrophage metalleinoproteinases reaction, leading to the degradation of the local cartilage but also having an impact on the serum acute phase proteins (APPs). Subsequently, biomarkers seem to be essential to estimate its progression and the need for any surgical intervention such as total arthroplasty, but also can be used as therapeutic agents. Recently, among APPs, fetuin A drew attention regarding its possible anti-inflammatory role in animal models but also as a therapeutic agent in the inflammatory joint disease in clinical trials. In contrast with other APPs such as C-reactive protein, fetuin A appears to be lower in the serum of patients with degenerative joint disease in comparison with the healthy ones, and also acts as an antagonist of the anti-proliferative potential of transforming growth factor-β (TGF-β) cytokines. Because of its lower serum levels in arthritis, an unregulated binding of TGF-β and bone morphogenetic proteins takes place leading to further arthritic lesions. The purpose of the present review is to assess the current evidence regarding the multipotent role of the alpha-2-HS-glycoprotein or as also known Fetuin-a in animal models but also as a biomarker of the degenerative joint arthritis in clinical trials.
Keywords: Fetuin A, Arthritis, Alpha-2-HS-glycoprotein, Bone morphogenetic protein, Inflammation, Glycoprotein, Treatment
Core tip: Fetuin A, an acute phase glycoprotein, recently drew scientific attention regarding its anti-inflammatory role. In the case of arthritis, clinical studies have shown its therapeutic potential as well as its anti-inflammatory role as it has been indicated by animal models. In this manuscript, we intend to review the current evidence concerning its anti-inflammatory and therapeutic role in degenerative joint disease.
INTRODUCTION
Osteoarthritis, which is also known as degenerative arthritis, is a deteriorating musculoskeletal condition that includes the decrease in the articular cartilage but also leads to a progressive subchondral erosion of the bone[1]. It is assumed that the joint arthritis consists of an inflammatory response which is introduced by several cytokines which are produced by the local reaction of macrophages, such as interleukin (IL)-1a, IL-8, IL-10 and matrix metalleinoproteinases (MMPs). These agents are measurable in the serum but also in the joint synovial fluid and all lead to cartilage breakdown[2]. Additional to the imbalance of the cytokines driven inflammatory process, the collagen of type 1 is transformed to collagen of type 2, while at the same time a decrease in the joint chondrocytes also takes place. It is widely accepted that the activity of IL-1 has a leading role in the arthritis inflammatory process leading to the production of MMPs which further leads to cartilage degradation. Acute phase proteins (APPs), such as C-reactive protein, are also elevated in patients with severe osteoarthritis[2,3].
Regarding APPs, fetuin A has recently draw attention regarding its possible anti-inflammatory role[4]. The complete structure of the complex oligosaccharides of fetuin A has been established[5]. Thanks to ion exchange chromatography following pronase digestion, identical molar ratios of sialic acid, mannose and N-acetylglycosamine of 3:3:3:5 was revealed (Figure 1).
Figure 1.
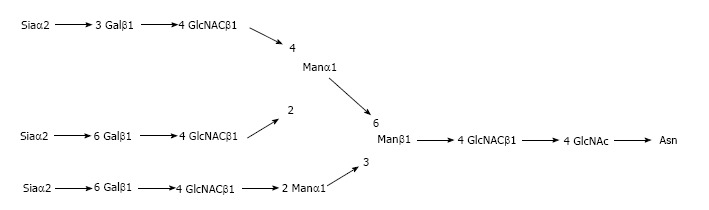
Structure of fetuin A.
Fetuin A is also known as alpha-2-HS-glycoprotein (AHSG) for the human homologue and was first declared as a fetus major plasma protein. During the fetus growth, it is expressed in the liver, kidney, gastrointestinal tract, skin and brain[6]. Concerning the adults, among APPs, fetuin A is produced by the liver and has recently drawn attention regarding its possible anti- inflammatory role in injury or infection, classifying it as a negative APP due to a regulation of pre-inflammatory cytokines such as tumor necrosis factor (TNF), IL-1, IL-6 and interferon (IFN)-γ, but also as a positive APP thanks to the mediation of HMGB1[7].
The aim of this mini review is to investigate the possible role of fetuin A in the inflammatory response, in processes such as the joint osteoarthritis.
ETIOPATHOLOGY OF ARTHRITIS
The joint homeostasis seems to take place due to a balance between catabolic factors of the adult joint cartilage (IL-1 and TNF) as well as the anabolic ones [insulin like growth factor (IGF), bone morphogenetic protein (BMP) morphogens such as BMP-7 and cartilage-derived morphogenetic proteins (CDMPs)], transforming growth factor-β (TGF-β) and fibroblast growth factors (FGFs). CDMP-1 is expressed in the deeper damaged areas of the cartilage of osteoarthritic joints where it leads to an increase of the local chondrocytes but also promotes the local production of proteoglycans[8,9].
Among the morphogens above, BMP-7 leads an important role for the maintenance of the joint homeostasis. Normally, BMP-7 emerges in the upper matrix of articular cartilage adhering to the expression of BMP receptors (BMPR-IA, IB, and II). BMP-7 has many roles in the inflammatory disease of the joint, including the preservation of surfaces of the articular cartilage by promoting the expression of the chondrocyte phenotype of dedifferentiated cells, increasing synthesis of tissue inhibitor of metalloproteinase (TIMP). Moreover, leads to the expression of IGFI, and cytoskeletal proteins of the chondrocytes[10]. It is also known that multipotent mesenchymal stem cells (MSCs) that express BMPs and BMPRs have been isolated from adult human synovial membrane[11]. So, it is assumed that morphogens from the TGF-β family seem to be involved in the remodeling of the arthritic cartilage. Components of synovial joints, such as the bone marrow, the synovium and the periosteum, contain MSCs that are capable of inducing chondrogenesis. It is suggested that the unregulation and likely the up regulation of the activity of TGF-β and BMP are likely to make MSCs in numerous joint sites to form excessive amounts of tissues of cartilage, bone and fibre, leading to fibrosis and osteophyte formation, characteristics of joint osteoarthritis. So, any imbalance between the factors above is likely to establish the degenerative joint disease (DJD) which with further progression may lead to the need of a total joint replacement[12]. However, the bone itself has a leading role in the pathogenesis of osteoarthritis. The level of bone remodeling plays a critical role under mechanical loading conditions, as demonstrated by consistent experimental studies. Yet, new clinical biomarkers have being developed to assess the bone phenotype of osteoarthritic patients. This stratification strategy is likely to better identify groups of patients who would benefit from bone-acting drugs to decrease disease progression and improve pain and disability[13].
FETUIN-A (AHSG) IN DJD
As it was stated above, APPs such as C reactive protein is mentioned to be elevated due to arthritis, depending on the severity of the disease[14]. Fetuin A protein is also mentioned to be influenced by the inflammation as an APP[15,16], in addition to other biomarkers which have been recently studied. For example, serum cartilage oligometric matrix protein was also found elevated in cases of knee joint osteoarthritis[17,18]. Interestingly, recent research has shown lower serum levels of AHSG in patients with DJD, accompanied with lower BMP levels than the healthy ones[12].
Studies have shown that AHSG is a negative acute phase reactant and its level is correlated with CRP circulation in the serum inversely[19]. Further research has also shown a close association between fetuin A and metabolic syndrome[20], obesity and dyslipidemia[21], or even blood pressure regulation[22]. Additionally, it has been linked closely with type 2 diabetes mellitus and many authors have suggested that it may be an independent risk factor for the expression of the disease. Specifically, patients with high levels of serum AHSG may have increased risk of incident diabetes[23,24]. Mixed results have been drawn regarding the role of fetuin A in the cardiovascular disease. However, all studies have shown a positive or negative effect of the circulating levels and the presence of the disease[19,25,26]. With no exaggeration, one can admit that AHSG is indeed a multifunctional protein[16]!
AHSG is an antagonist of the TGF-β/BMP, as it is mentioned that it antagonizes the osteogenic as well as the anti-proliferative actions of TGF-β cytokines in vitro. More specifically, the 18-19 amino acid region in the AHSG molecule is identical to the TGF-β receptor type II (TBRII) and appears to be the BMP antagonist site. This domain is also designated the TGF-β receptor II homology 1 domain (TRH1), which is essential for the cytokine binding and as a result leads to the non binding with the TBRII[27]. So, inflammatory diseases characterized by low serum AHSG, such as DJD, leads to an unregulated binding of TGF-β and BMP, having further fibrosis, osteophyte and ectopic bone formation as a result[28].
During the progression of the arthritis, a down regulation of the liver production of AHSG leads to it lower serum levels[12]. Another explanation for that is likely to be the elevated figures of MMPs which are produced by the inflamed tissues, Especially, MMP-1, 3 and 9 are increased in inflamed joints. However, recent studies suggest that AHSG can be eliminated by MMPs, either systematically or locally in the joint[29]. As a result, this could lead to increased activity of the BMPs that would also lead to further progression of the arthritis.
Regarding the prevention of DJD, it is important to state that the therapeutic interventions and goals should take place during the early stages of the arthritis, as chondrocytes are still able to respond to the anabolic factors[9]. The existence of a therapeutic window should be established regarding the concentration and the exposure of BMP-7. Moreover, in order to maintain the joint homeostasis it is important that other proteins such as AHSG that are down-regulators of the BMPs, should be available in order to enhance the protective role of the cytokines above.
As for the therapeutic potential of AHSG in the DJD, Rittenberg et al[30] described in 2005 the regulated release of intrarticular injections on experimental level of BMP7 by co-injection of its regulatory molecules such as AHSG in clinical trials. Moreover, besides to the regulation of BMPs, therapeutic potential of AHSG is also based on its ability to eliminate the inflammation and the local tissue destruction. In 1998, Wang et al[31] proved through examination of murine cell cultures that AHSG can be used by the local macrophages as an opsonin for macrophage deactivating molecules. Furthermore, Wang et al[32] in 2010 established the protective role of AHSG in the ischemic cerebral inflammation in animal models of rats, as well as the the suppression of sepsis mediators in late stages of sepsis in the same animal model. Also, TNF increase from lipopolysaccharide stimulated macrophages was inhibited significantly in an animal model of inflammation which was carried out by Wang et al[33] in 1997. Consequently, the co-administration of AHSG and BMPs is able to set a therapeutic intervention for the degenerative bone disease, taking into account the anti-inflammatory role of the agents above.
CONCLUSION
In conclusion, joint arthritis’ diagnosis and treatment as well as its pathophysiology have been studied during the years. However, further research seems to be essential for more effective prevention. The protein Fetuin A has the potential to be used as a biomarker of the disease, as well as a therapeutic agent for the DJD. As a result, physicians should be aware of the fetuin A as a marker of activity and also to be informed in order to have a correct approach to the patients disease and treatment, but also for additional inflammatory diseases. Besides, additional clinical studies are likely to validate the measurement of BMPs as well as AHSG serum levels as a diagnostic means in the clinical entity of DJD. The identification of AHSG levels in combination with the clinical evaluation of the patients, are not only likely to diagnose the disease in even subclinical stage but also to reduce the need for any further joint salvage procedures, such as total arthroplasty.
Footnotes
Conflict-of-interest statement: There is no conflict of interest associated with any of the senior author or other co-authors contributed their efforts in this manuscript.
Manuscript source: Invited manuscript
Specialty type: Orthopedics
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
Peer-review started: January 21, 2017
First decision: March 8, 2017
Article in press: May 5, 2017
P- Reviewer: Garip Y, Saviola G, Song J, Saviola G S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
References
- 1.Goldring SR, Goldring MB. Clinical aspects, pathology and pathophysiology of osteoarthritis. J Musculoskelet Neuronal Interact. 2006;6:376–378. [PubMed] [Google Scholar]
- 2.Hulejová H, Baresová V, Klézl Z, Polanská M, Adam M, Senolt L. Increased level of cytokines and matrix metalloproteinases in osteoarthritic subchondral bone. Cytokine. 2007;38:151–156. doi: 10.1016/j.cyto.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Attur M, Krasnokutsky-Samuels S, Samuels J, Abramson SB. Prognostic biomarkers in osteoarthritis. Curr Opin Rheumatol. 2013;25:136–144. doi: 10.1097/BOR.0b013e32835a9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao J, Wang XR, Hu KZ, Li MQ, Chen JW, Ma T, Li ZC. Serum fetuin-A levels are inversely associated with clinical severity in patients with primary knee osteoarthritis. Biomarkers. 2013;18:51–54. doi: 10.3109/1354750X.2012.730551. [DOI] [PubMed] [Google Scholar]
- 5.Baenziger JU, Fiete D. Structure of the complex oligosaccharides of fetuin. J Biol Chem. 1979;254:789–795. [PubMed] [Google Scholar]
- 6.Pedersen K. Fetuin, a new globin isolated from serum. Nature. 1944:575–570. [Google Scholar]
- 7.Wang H, Sama AE. Anti-inflammatory role of fetuin-A in injury and infection. Curr Mol Med. 2012;12:625–633. doi: 10.2174/156652412800620039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki T, Bessho K, Segami N, Nojima T, Iizuka T. Bone morphogenetic protein-2 in temporomandibular joints with internal derangement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;88:670–673. doi: 10.1016/s1079-2104(99)70007-7. [DOI] [PubMed] [Google Scholar]
- 9.Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol. 2006;20:1003–1025. doi: 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luyten FP. Mesenchymal stem cells in osteoarthritis. Curr Opin Rheumatol. 2004;16:599–603. doi: 10.1097/01.bor.0000130284.64686.63. [DOI] [PubMed] [Google Scholar]
- 12.Albilia JB, Tenenbaum HC, Clokie CM, Walt DR, Baker GI, Psutka DJ, Backstein D, Peel SA. Serum levels of BMP-2, 4, 7 and AHSG in patients with degenerative joint disease requiring total arthroplasty of the hip and temporomandibular joints. J Orthop Res. 2013;31:44–52. doi: 10.1002/jor.22182. [DOI] [PubMed] [Google Scholar]
- 13.Funck-Brentano T, Cohen-Solal M. Subchondral bone and osteoarthritis. Curr Opin Rheumatol. 2015;27:420–426. doi: 10.1097/BOR.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 14.Przepiera-Będzak H, Fischer K, Brzosko M. Serum Interleukin-18, Fetuin-A, Soluble Intercellular Adhesion Molecule-1, and Endothelin-1 in Ankylosing Spondylitis, Psoriatic Arthritis, and SAPHO Syndrome. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17081255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dabrowska AM, Tarach JS, Wojtysiak-Duma B, Duma D. Fetuin-A (AHSG) and its usefulness in clinical practice. Review of the literature. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:352–359. doi: 10.5507/bp.2015.018. [DOI] [PubMed] [Google Scholar]
- 16.Mori K, Emoto M, Inaba M. Fetuin-A: a multifunctional protein. Recent Pat Endocr Metab Immune Drug Discov. 2011;5:124–146. doi: 10.2174/187221411799015372. [DOI] [PubMed] [Google Scholar]
- 17.Verma P, Dalal K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarker. J Orthop Res. 2013;31:999–1006. doi: 10.1002/jor.22324. [DOI] [PubMed] [Google Scholar]
- 18.Tseng S, Reddi AH, Di Cesare PE. Cartilage Oligomeric Matrix Protein (COMP): A Biomarker of Arthritis. Biomark Insights. 2009;4:33–44. doi: 10.4137/bmi.s645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vörös K, Gráf L, Prohászka Z, Gráf L, Szenthe P, Kaszás E, Böröcz Z, Cseh K, Kalabay L. Serum fetuin-A in metabolic and inflammatory pathways in patients with myocardial infarction. Eur J Clin Invest. 2011;41:703–709. doi: 10.1111/j.1365-2362.2010.02456.x. [DOI] [PubMed] [Google Scholar]
- 20.Ix JH, Shlipak MG, Brandenburg VM, Ali S, Ketteler M, Whooley MA. Association between human fetuin-A and the metabolic syndrome: data from the Heart and Soul Study. Circulation. 2006;113:1760–1767. doi: 10.1161/CIRCULATIONAHA.105.588723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen HY, Chiu YL, Hsu SP, Pai MF, Lai CF, Peng YS, Kao TW, Hung KY, Tsai TJ, Wu KD. Association of serum fetuin A with truncal obesity and dyslipidemia in non-diabetic hemodialysis patients. Eur J Endocrinol. 2009;160:777–783. doi: 10.1530/EJE-08-0813. [DOI] [PubMed] [Google Scholar]
- 22.Lavebratt C, Wahlqvist S, Nordfors L, Hoffstedt J, Arner P. AHSG gene variant is associated with leanness among Swedish men. Hum Genet. 2005;117:54–60. doi: 10.1007/s00439-005-1286-z. [DOI] [PubMed] [Google Scholar]
- 23.Laughlin GA, Barrett-Connor E, Cummins KM, Daniels LB, Wassel CL, Ix JH. Sex-specific association of fetuin-A with type 2 diabetes in older community-dwelling adults: the Rancho Bernardo study. Diabetes Care. 2013;36:1994–2000. doi: 10.2337/dc12-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ix JH, Wassel CL, Kanaya AM, Vittinghoff E, Johnson KC, Koster A, Cauley JA, Harris TB, Cummings SR, Shlipak MG. Fetuin-A and incident diabetes mellitus in older persons. JAMA. 2008;300:182–188. doi: 10.1001/jama.300.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Häring HU, Boeing H, Fritsche A. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–2562. doi: 10.1161/CIRCULATIONAHA.108.814418. [DOI] [PubMed] [Google Scholar]
- 26.Zhao ZW, Lin CG, Wu LZ, Luo YK, Fan L, Dong XF, Zheng H. Serum fetuin-A levels are associated with the presence and severity of coronary artery disease in patients with type 2 diabetes. Biomarkers. 2013;18:160–164. doi: 10.3109/1354750X.2012.762806. [DOI] [PubMed] [Google Scholar]
- 27.Demetriou M, Binkert C, Sukhu B, Tenenbaum HC, Dennis JW. Fetuin/alpha2-HS glycoprotein is a transforming growth factor-beta type II receptor mimic and cytokine antagonist. J Biol Chem. 1996;271:12755–12761. doi: 10.1074/jbc.271.22.12755. [DOI] [PubMed] [Google Scholar]
- 28.van der Kraan PM, van den Berg WB. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007;15:237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 29.So A, Chamot AM, Péclat V, Gerster JC. Serum MMP-3 in rheumatoid arthritis: correlation with systemic inflammation but not with erosive status. Rheumatology (Oxford) 1999;38:407–410. doi: 10.1093/rheumatology/38.5.407. [DOI] [PubMed] [Google Scholar]
- 30.Rittenberg B, Partridge E, Baker G, Clokie C, Zohar R, Dennis JW, Tenenbaum HC. Regulation of BMP-induced ectopic bone formation by Ahsg. J Orthop Res. 2005;23:653–662. doi: 10.1016/j.orthres.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhang M, Bianchi M, Sherry B, Sama A, Tracey KJ. Fetuin (alpha2-HS-glycoprotein) opsonizes cationic macrophagedeactivating molecules. Proc Natl Acad Sci USA. 1998;95:14429–14434. doi: 10.1073/pnas.95.24.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang H, Li W, Zhu S, Li J, Ward MF, Huang Y, Yang H, Tracey KJ, Wang P, Sama AE. Fetuin protects mice against lethal sepsis by modulating bacterial endotoxin induced hmgb1 release and autophagy. Shock. 2010;33(Suppl 1):1–13. [Google Scholar]
- 33.Wang H, Zhang M, Soda K, Sama A, Tracey KJ. Fetuin protects the fetus from TNF. Lancet. 1997;350:861–862. doi: 10.1016/S0140-6736(05)62030-2. [DOI] [PubMed] [Google Scholar]


