Abstract
Cell membranes isolated from brain tissues, obtained surgically from six patients afflicted with drug-resistant temporal lobe epilepsy and from one nonepileptic patient afflicted with a cerebral oligodendroglioma, were injected into frog oocytes. By using this approach, the oocytes acquire human GABAA receptors, and we have shown previously that the “epileptic receptors” (receptors transplanted from epileptic brains) display a marked run-down during repetitive applications of GABA. It was found that exposure to the neurotrophin BDNF increased the amplitude of the “GABA currents” (currents elicited by GABA) generated by the epileptic receptors and decreased their run-down; both events being blocked by K252A, a neurotrophin tyrosine kinase receptor B inhibitor. These effects of BDNF were not mimicked by nerve growth factor. In contrast, the GABAA receptors transplanted from the nonepileptic human hippocampal uncus (obtained during surgical resection as part of the nontumoral tissue from the oligodendroglioma margins) or receptors expressed by injecting rat recombinant α1β2γ2 GABAA receptor subunit cDNAs generated GABA currents whose time-course and run-down were not altered by BDNF. Loading the oocytes with the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester (BAPTA-AM), or treating them with Rp-8-Br-cAMP, an inhibitor of the cAMP-dependent PKA, did not alter the GABA currents. However, staurosporine (a broad spectrum PK inhibitor), bisindolylmaleimide I (a PKC inhibitor), and U73122 (a phospholipase C inhibitor) blocked the BDNF-induced effects on the epileptic GABA currents. Our results indicate that BDNF potentiates the epileptic GABAA currents and antagonizes their use-dependent run-down, thus strengthening GABAergic inhibition, probably by means of activation of tyrosine kinase receptor B receptors and of both PLC and PKC.
Keywords: microtransplantation into Xenopus oocytes, temporal lobe epilepsy
Recently, much attention has been directed to the neurotrophin BDNF because it is highly expressed in many areas of the CNS and is critically involved in synaptic plasticity and neurotransmission by mechanisms that are still poorly understood (1–6). Moreover, BDNF regulates both fast synaptic inhibition and excitation (mediated by GABAA and glutamate receptors, respectively) by means of activation of tyrosine kinase receptor B (TrkB) receptors (7–13).
Epileptic seizures in epileptic patients and animal models have been linked to BDNF and TrkB receptors, based on the findings that BDNF can increase excitation and decrease inhibition in the brain and that seizures induce up-regulation of both BDNF and TrkB receptors (9, 14–17). However, other studies have reported (12, 18, 19) complex mechanisms by which BDNF acts on nerve cells, contradicting the view that BDNF is proexcitatory in the brain. To explore some of these issues, we have studied the effects of BDNF on the epileptic GABAA receptors microtransplanted to Xenopus oocytes by injecting them with membranes isolated from surgically resected brain tissues of patients afflicted with medically intractable temporal lobe epilepsy (TLE). This form of epilepsy is poorly controlled by medical treatment and, for that reason, the preferred therapy in some patients is to remove the regions identified as epileptogenic foci. Here, we report that BDNF positively modulates and “stabilizes” the GABAA receptors, probably by means of the activation of TrkB receptors, with both human receptors having been transplanted from the epileptic brains into the oocytes. Also, we report that the effects of BDNF on “GABA currents” (currents elicited by GABA) probably are mediated by means of PKC activity, in agreement with the view that BDNF modulates GABAA receptors through mechanisms involving protein phosphorylation (12, 20, 21).
Materials and Methods
Patients. Surgical specimens were obtained from the hippocampus and temporal neocortex of six patients with cryptogenic drug-resistant TLE (see Table 1, which is published as supporting information on the PNAS web site) and from the hippocampal, nonepileptic, tumoral uncus of a 27-year-old (female) patient who was afflicted with a left-temporal-lobe oligodendroglioma and did not suffer epileptic episodes [nonepileptic TLG, grade II; all performed at the Neuromed Neurosurgery Center for Epilepsy (Venafro, Italy)]. Informed consent was obtained from all of the patients to use part of the biopsy material for our experiments; and the Ethics Committees of Neuromed and the University of Rome “La Sapienza” approved the selection processes and procedures. The histopathology of all specimens showed the typical neuropathological features of Ammon's horn sclerosis and did not show obvious sclerosis in the temporal lobe.
Membrane Preparation and Injection Procedures. Membranes from human nervous tissue (temporal lobe, hippocampus, or hippocampal uncus), were prepared and injected into oocytes by using procedures described in detail in refs. 22–24 (see Supporting Methods, which is published as supporting information on the PNAS web site). Intranuclear injections and neurotransmitter receptor expression were performed as described (25). Rat α1β2γ2L subunit cDNAs (plasmid PGW1) were a generous gift from Enrico Cherubini (Scuola Internazionale Superiore di Studi Avanzati, Trieste, Italy).
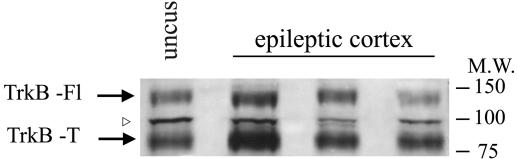
Immunoblot Analysis of Epileptic and Nonepileptic Membrane Preparations. Neocortical tissues resected from three epileptic patients, and the hippocampal uncus tissue from the nonepileptic patient were used for membrane preparation as described (26). For each patient, 5 μl of membranes (1–2 mg of protein per ml) were run on SDS/PAGE and analyzed by Western blotting with Abs specific for human TrkB (H-181; Santa Cruz Biotechnology). We were able to detect TrkB isoforms: the full-length 145-kDa isoform and two 95-kDa truncated isoforms. Immunoreactivity was detected by chemiluminescence.
Electrophysiological Recordings from Oocytes. At 12–48 h after injection, membrane currents were recorded from voltage-clamped oocytes by using two microelectrodes filled with 3 M KCl. The oocytes were placed in a recording chamber (volume, 0.1 ml) and perfused continuously (10–11 ml/min) with oocyte Ringer's solution (82.5 mM NaCl/2.5 mM KCl/2.5 mM CaCl2/1 mM MgCl2/5 mM Hepes, adjusted to pH 7.4 with NaOH) at room temperature (20–22°C). GABA responsiveness was tested by applying GABA (1 mM) every 7–8 min. Dose–response curves were constructed as described (25). GABA-current run-down, after repetitive neurotransmitter applications, was defined as the decrease (in percentage) of the GABA-current amplitude after six GABA applications (1 mM; 10-s duration at 40-s intervals). BDNF was dissolved in H20, stored as frozen stock solution (50 μg/ml) and, unless otherwise indicated, diluted to the working concentration of 500 ng/ml before each recording session. The BDNF was applied to oocytes for 1–2 h. The same procedure was used for nerve growth factor (NGF) (Calbiochem, Darmstadt, Germany). When necessary, inhibitors of PKs and PLC as well as the Ca2+ chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate-acetoxymethyl ester (BAPTA-AM) were coapplied with BDNF. BAPTA-AM and all of the inhibitors that were used [except the PKA inhibitor adenosine 3′,5′-cyclic monophosphorothionate, 8Br-Rp-isomer (Rp-8-Br-cAMP; Calbiochem), which was dissolved in H2O] were dissolved in DMSO (1:200 to 1:500 dilutions) at concentrations one order of magnitude higher that the final concentration and stored as frozen stock solutions. The experimental protocol was as follows. As soon as they were voltage-clamped and tested for GABA currents, the oocytes were unclamped, kept separately in multiwell dishes, and treated with the drugs, as indicated. Subsequently, the identified oocytes were voltage-clamped again and exposed to GABA. Desensitization of GABA currents was estimated as the time for the current to decay to half of its peak to value (T0.5). K252A, U73122, and bisindolylmaleimide I were also obtained from Calbiochem. All other drugs were purchased from Sigma Italia.
Binding Assay. Oocytes that had been injected with human neocortex membranes from four patients were electrophysiologically tested with GABA (1 mM) and then assayed for plasma membrane GABA binding according to a method described in ref. 27. Some oocytes were also tested electrophysiologically before and 1 h after BDNF treatment to determine whether BDNF altered the GABA-current amplitude in sister oocytes. Briefly, to measure binding, control and injected oocytes were incubated in 1 ml of oocyte Ringer's solution containing [3H]GABA 1 μM ([3H]GABA, specific activity 92 Ci/mmol; 1 Ci = 37 GBq) for 30 s at room temperature. After incubation, the labeled medium was removed and the oocytes were rinsed for 5 s in 1 ml Ringer's solution at 0°C (to remove unbound 3H-ligand). Last, oocytes were placed in 500 μl of Ringer's solution at room temperature for 85 s to let the bound 3H-ligand dissociate. The labeled medium was mixed with 4 ml of scintillation fluid and counted on a β-counter (Beckman Coulter), and the cpm provided a measure of the total binding. The nonspecific binding was determined by incubating the oocytes with the same concentration of [3H]GABA plus 200 μM of the antagonist bicuculline and the cpm released were subtracted from the total binding.
Results
TRkB Expression in Epileptic Membranes. The neurotrophin receptor TrkB is expressed as a full-length receptor containing an intracellular tyrosine kinase domain that mediates the effects of BDNF, as well as two truncated receptor variants (28). To determine whether the full-length isoform and its truncated variants are present in the membrane preparations used for oocyte injections, a Western blot analysis was performed. It was found that the full-length and the truncated isoforms were present in the neocortex of all three examined patients, as well as in the nonepileptic hippocampal uncus (Fig. 1).
Fig. 1.
Expression of TrkB full (Fl) and truncated (T) isoforms in the epileptic neocortex and nonepileptic uncus. Membrane preparations from the neocortex of three different epileptic patients and from the hippocampal uncus of a nonepileptic patient were analyzed by Western blotting using an anti-human TrkB Ab. The arrowhead indicates a nonspecific reactive band. A molecular weight (M.W.) ladder is indicated on the right.
Positive Modulation of GABA Currents Induced by BDNF. Injection of membranes from the TLE brain into oocytes leads to the incorporation of GABAA receptors whose activation by the neurotransmitter GABA generates inward currents of variable amplitudes (23). In this study, oocytes injected with membranes isolated from the TLE neocortex exhibited GABA currents that ranged from –20 to –400 nA (–159 ± 18 nA; 41 cells/seven frogs; six TLE patients), and were blocked by bicuculline (100 μM; 5/2; data not shown). Coapplication of BDNF with GABA (1 mM) induced a small and prompt decrease of GABA current (to 85 ± 3%; 6/2; data not shown). This effect was rapidly reversible and was probably not mediated through the BDNF receptor TrkB, because it was not blocked by its inhibitor, K252A (10 μM) (1, 3, 6, 8, 10). Note that AMPA-elicited currents (AMPA, 100 μM) were similarly inhibited by BDNF and this effect was again insensitive to K252A (4/1; one patient). Therefore, this effect of BDNF was not specific to GABAA receptors, was not mediated through TrkB receptors, and it was not investigated further.
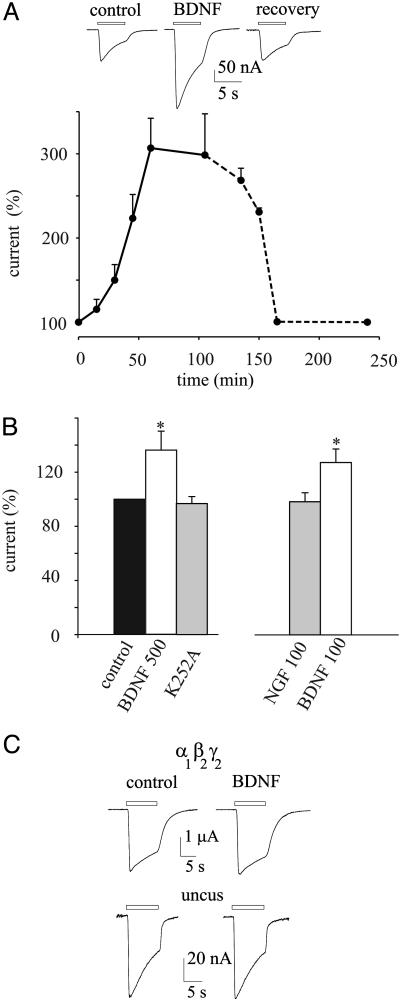
However, during continuous exposure to BDNF the GABA-current amplitude increased progressively, reached a plateau after ≈1 h and recovered slowly after the BDNF was washed out (Fig. 2A). This 1.4- to 3.1-fold increase in GABA-current amplitude was not accompanied by changes in current desensitization [T0.5 = 4.47 ± 1.0 s (control) vs. T0.5 = 4.6 ± 1.31 s (BDNF); 41/7; six patients], and it was prevented by 1 h of cotreatment with 10 μM K252A (Fig. 2B, 10/2; two patients), suggesting that the effect of BDNF is mediated through TrkB receptors (11, 12).
Fig. 2.
Increase of GABA currents by BDNF in oocytes injected with TLE neocortex membranes. (A Upper) Sample currents from one oocyte. Bars indicate the timing of GABA applications, BDNF refers to 1 h of BDNF pretreatment, and recovery is after a 100-min washout. (A Lower) Time course of GABA current amplitude increase during a 100-min exposure to BDNF (500 ng/ml, solid line) and its recovery after washout (dashed line). Points represent means ± SEM of six oocytes (one frog, one patient) normalized to Icontrol = –143 ± 10 nA. (B) GABA current amplitudes upon indicated treatments in another patient. Currents were normalized to Icontrol = –93 ± 14 nA. Columns represent values (in percentage) after 1 h of drug treatments. *, P < 0.001 (Student's t test). (C) Current traces (representative of 10), control and after 1 h treatment with BDNF, recorded from oocytes injected with cloned rat α1β2γ2L GABAA receptor subunit cDNAs (Upper) or with membranes from nonepileptic hippocampal uncus (Lower).
The potentiation of GABA currents by BDNF did not extend to other neurotrophins, because applications of NGF, at the same concentration as BDNF, were ineffective in influencing GABA currents (8/2; two patients; Fig. 2B). Moreover, BDNF applied acutely to oocytes expressing either cloned α1β2γ2L-GABAA receptors or GABAA receptors microtransplanted from nonepileptic hippocampal uncus caused only a small and reversibly inhibition of the GABA current and this effect was, again, not prevented by K252A (data not shown). Furthermore, prolonged treatment with BDNF did not cause the GABA-current amplitude to increase (Fig. 2C; 10/3). Thus, these findings indicate that only the GABA currents generated by activation of epileptic GABAA receptors are positively modulated by a GABA receptor–BDNF interaction mediated through TrkB receptors.
It has been reported recently that the neuron-specific KCl cotransporter, KCC2, is down-regulated by BDNF through TrkB receptors, impairing neuronal Cl– extrusion and modifying the GABA-current reversal potential (EGABA) (29). Because the BDNF-induced increase of the GABA current could be caused indirectly by a shift in EGABA, experiments were made to determine whether EGABA was modified by BDNF. It was found that the increase in GABA-current amplitude was not accompanied by a change in EGABA (EGABA = –23.6 ± 0.5 mV for control vs. EGABA = –23.6 ± 0.6 mV after BDNF; 9/2; two patients; data not shown).
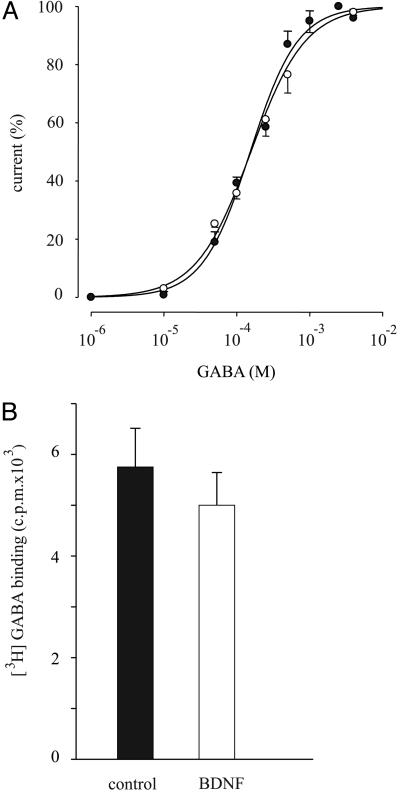
To investigate further the mechanism by which BDNF potentiates the GABA currents generated by activation of the epileptic GABAA receptors, experiments were addressed to determine whether the receptor affinity was modified by BDNF treatment. Dose–response relationships show that the receptor affinity for GABA is not altered by the BDNF treatment (Fig. 3A), indicating that the BDNF-induced positive modulation of GABA currents is not due to a shift in the receptor affinity.
Fig. 3.
Lack of effect of BDNF on GABA receptor activation and receptor numbers. (A) GABA dose–current response relationships in control (•) and BDNF-treated oocytes (○). BDNF refers to ≈1 h of treatment. EC50 = 154 ± 13 μM; nH = 1.2 ± 0.1; Imax = –200 ± 47 nA (•), and EC50 = 150 ± 16 μM; nH = 1.4 ± 0.2 Imax = –287 ± 30 nA (○). Points represent mean ± SEM from 8/2 oocytes (two patients). (B) Specific binding of GABA to oocytes injected with temporal neocortex membranes, control and treated for 1 h with BDNF. Values were significantly similar at P > 0.05. Oocytes were incubated as described in Materials and Methods. For control and BDNF, 100 oocytes were collected from the same frog. Data are representative of six experiments from four patients.
It is known that BDNF can increase the surface expression of GABAA receptors of nerve cells from the rat visual cortex (11). To investigate whether BDNF enhances the epileptic GABA currents by increasing the number of GABAA receptors present in the oocyte plasma membrane, we measured the binding of [3H]GABA (27) in oocytes expressing epileptic GABAA receptors before and after BDNF treatment. In all six experiments (four patients) the amount of [3H]GABA bound was not significantly affected by BDNF treatment (Fig. 3B). Therefore, it is unlikely that BDNF increases the GABA currents, or the currents elicited by GABA, by inducing an ex novo incorporation of GABAA receptors into the plasma membrane.
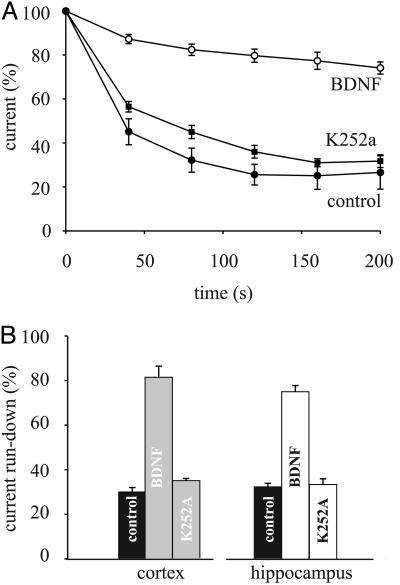
Reduction of GABAA-Current Run-Down by BDNF. We have reported (26) that the GABA currents generated by GABAA receptors microtransplanted from TLE tissues exhibit a marked run-down during repetitive activation by the neurotransmitter, a run-down that probably depends on the phosphorylation state of the GABAA receptors. Because BDNF regulates GABAA receptor phosphorylation (12), it was pertinent to examine the effects of BDNF on the GABA-current run-down of Xenopus oocytes that had incorporated cell membranes isolated from the neocortex of TLE patients. In all of the examined six patients, the GABA currents fell, to 26.2 ± 3% (range, 17–33%; 47/18), after the repetitive GABA applications (Fig. 4A and see Materials and Methods). This run-down was reversible and, after a 10-min wash, the GABA current recovered to 60 ± 5% (6/2). Whereas there was no obvious effect on the GABA-current run-down when BDNF was acutely applied, preincubation of the oocytes with BDNF strongly reduced the GABA-current run-down (Fig. 4A), reaching a plateau after a 2-h incubation. This effect was weakly dose-dependent, because with 50 ng/ml BDNF, the repetitively elicited GABA currents fell to 52 ± 8% (range: 35–58%; 9/2; two patients), whereas with 500 ng/ml BDNF the currents fell to 79.7 ± 6% (range, 74–92%; 50/18; six patients; P < 0.01; Fig. 4A). Moreover, the BDNF effect was reversible after a 2-h washout (data not shown), was abolished by 1 h of cotreatment with K252A (10 μM; Fig. 4), and was not induced by NGF (100 ng/ml; data not shown).
Fig. 4.
GABA-current run-down reduction by BDNF and block of BDNF action by K252A. (A) Time course of GABA-current run-down in patient 5. Points represent mean current amplitudes ± SEM from 12 oocytes collected from two frogs. Data were normalized to Imax = –120 ± 23 nA (○); –187 ± 14 nA (▪); 110 ± 19 nA (•). (B) Current run-down values in oocytes were treated as indicated (36 oocytes/three frogs) injected with membranes isolated from the indicated regions (three patients).
We have shown (26) that cloned rat GABAA (α1β2γ2L) receptors are very stable and show only a weak GABA-current run-down after repetitive GABA applications. Here, it was found that oocytes expressing these cloned GABAA receptors showed a small and reversible GABA-current run-down (fall to 86.9 ± 3%; 10/2) that was not influenced by BDNF (fall to 85.4 ± 4%; 10/2; data not shown), in agreement with the finding that Xenopus oocytes do not express endogenous TrkB receptors activated by human BDNF (30).
Experiments were carried out also with membranes isolated from the hippocampus of the same six TLE patients, and we found that BDNF significantly reduced the current run-down of the microtransplanted GABAA receptors and this effect was again blocked by K252A (Fig. 4B). Note that the GABA-current run-down of oocytes injected with membranes from the nonepileptic hippocampal uncus was smaller and was not influenced by BDNF, compared with TLE tissues. Actually, the GABA currents fell to 73.3 ± 2% or to 76.6 ± 3%, before and after BDNF, respectively (12/2; data not shown). All of these findings indicate that BDNF influences the GABA-current run-down of GABAA receptors derived from the epileptic tissue, independently of the brain areas from which the membranes derive, whereas it is ineffective on GABAA receptors from nonepileptic tissue.
Signaling Cascade Triggered by BDNF. Because EGABA, surface receptor number, or receptor affinity were apparently not responsible for the BDNF-induced enhancement of GABA currents, further experiments were aimed at identifying the mechanisms underlying the effect of BDNF. It is well established that the BDNF-induced phosphorylation regulates the function of GABAA receptors (12, 20, 21, 31) and that the BDNF-induced TrkB receptor activation triggers two downstream events: PLC-γ activation and intracellular Ca2+ elevation (32, 33). To explore the signaling pathways through which the epileptic GABAA receptors are regulated by BDNF, we examined the effects of BDNF in the presence of U73122, a membrane-permeant inhibitor of PLC activity (11, 33, 34). After U73122 pretreatment (40 min, 5 μM; 11/3, three patients) of oocytes expressing GABAA receptors from the TLE neocortex, BDNF failed to potentiate the GABA currents and also failed to reduce the GABA-current run-down, indicating that activation of PLC-γ is probably involved in the effects of BDNF on epileptic GABAA receptors.
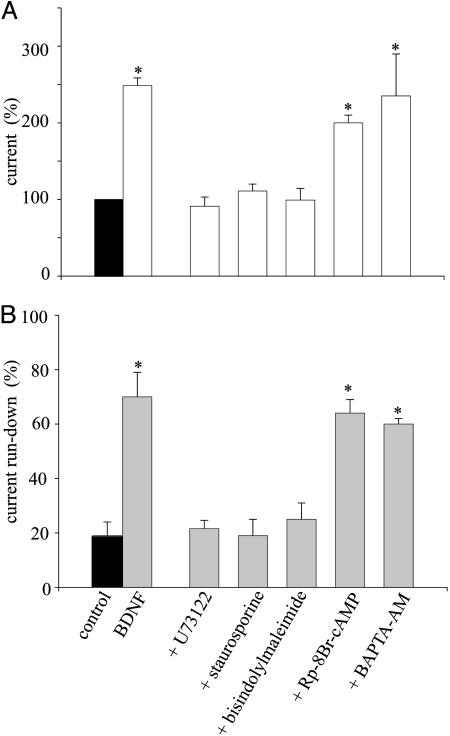
Furthermore, it is known that the PLC signaling activates PKC. Accordingly, treatment with the PK inhibitor staurosporine (1 μM; 1 h) suppressed both the increase of GABA current and the decrease of run-down induced by BDNF (8/2, two patients). In a similar way, bisindolylmaleimide I (1 μM for 2 h), a broad spectrum PKC inhibitor (34), prevented the effects of BDNF on GABA-current amplitude and run-down (12/2, two patients). In contrast, Rp-8-Br-cAMP, an inhibitor of PKA (200 μM; 20 min) (12), failed to block the BDNF-induced effects (6/2, two patients), indicating that PKA is not involved in the regulation of the epileptic GABA currents. Last, loading the oocytes with the membrane-permeant Ca2+ chelator BAPTA-AM (50 μM; 2h) (34), did not abolish the BDNF effects (6/2; two patients), whereas it abolished the oscillatory currents caused by the rise in intracellular calcium that follows serum activation of the phosphatidyl inositol system (35) and greatly reduced the Tout current caused by Ca2+ entering through voltage-gated Ca-channels (36). This finding suggests that intracellular Ca2+ is not involved in the development of the effects of BDNF on epileptic GABAA receptors. Fig. 5 shows a summary of the values for both current amplitude and run-down determined from all these experiments. Thus, these findings indicate that the BDNF signaling affects the functional properties of the GABAA receptors through activation of both PLC (likely PLC-γ) and PKC.
Fig. 5.
Effects of agents that alter cellular signaling on the action of BDNF. (A) Effects on the GABA-current increase induced by BDNF. Icontrol = –107 ± 10 nA (16/3; one patient representative of three). (B) Effects on the GABA-current run-down after the indicated treatments. Data are given as means ± SEM. *, P < 0.001. See Results and Materials and Methods for doses and protocols.
Discussion
GABAA receptors play a pivotal role in all brain activities and their dysfunction is implicated in epilepsy (37). Thus, to pave the way toward new treatments for medically intractable TLE, it is very important to discover any epileptogenic GABAA receptor dysfunction. We have reported (26) that epileptic GABAA receptors, present in temporal lobe pyramidal neurons in human TLE slices and in oocytes injected with membranes obtained from the same human tissue, display an abnormal GABA-current run-down, and this putative epileptogenic dysfunction can be reverted by inhibiting phosphatase activity. This finding suggests that phosphorylation of GABAA receptors and/or associated proteins (38) are linked to the run-down of the epileptic GABAA receptors.
BDNF, an endogenous growth factor present in the CNS and over-expressed in the epileptic brain (14, 16), exerts its effects by activating TrkB receptors, which in turn trigger many downstream signals. Moreover, BDNF is implicated in neuronal survival and differentiation as well as in synapse formation (32), and it regulates phosphorylation of GABAA receptors (12, 20, 21, 31). For these reasons, it was of interest to investigate whether BDNF could prevent the use-dependent GABAA receptor run-down, similar to the action exerted by phosphatase inhibitors (26).
We report that, in oocytes transplanted with human TLE GABAA receptors, BDNF causes a large enhancement of GABA currents and prevents most of their run-down, thus leading to an increased inhibitory action. Both effects of BDNF are specific to GABAA receptors transplanted from an epileptic brain because they fail to occur after receptor transplantation from a nonepileptic tissue. Moreover, both effects are specifically mediated through TrkB receptors because they are suppressed by the TrkB receptor inhibitor K252A, and NGF does not mimic the effects of BDNF. It is very likely that the putative TrkB receptors, mediating the effects of BDNF, are transplanted from the TLE brain to the oocytes together with the GABA receptors, because we found that TrkB receptors are abundantly present in the membrane preparations and because human BDNF is ineffective in stimulating any TrkB receptor activity in noninjected Xenopus oocytes (30) or in altering the functional properties of GABAA receptors expressed in oocytes injected with α1β2γ2L subunit cDNAs (see above). Last, on exploring the downstream signaling events underlying the effects of BDNF, we found that both PLC and PKC, but not PKA or intracellular Ca2+ activities are necessary for the increased GABA currents. Together, our findings indicate that BDNF corrects the GABAA receptor malfunction through PKC-mediated signaling, in agreement with the hypothesis that the abnormal GABA current run-down is coupled to phosphorylation of epileptic GABAA receptors and/or associated proteins (26).
A large body of evidence suggests that BDNF is involved in TLE, although it is not entirely clear whether its action is proepileptogenic or antiepileptogenic (17, 18). Accordingly, a large effort has been placed toward understanding the modulation of inhibitory GABAergic neurotransmission by BDNF. For example, if the inhibitory GABA system is strengthened by BDNF, this finding may be interpreted as an antiepileptogenic action of BDNF on nerve cell excitability. However, it has not been fully ascertained whether the BDNF target is a presynaptic or postsynaptic site (4). For example, in human TLE hippocampal dentate granule cells, BDNF impairs the evoked inhibitory postsynaptic currents but does not alter the spontaneous synaptic currents (9), suggesting that BDNF acts presynaptically. However, the BDNF target mediating GABAA-receptor-negative modulation is postsynaptic in rat cerebellar granule cells (10). Actually, an unequivocal action of BDNF on GABAA receptors has not yet been established: BDNF treatment increases (13, 39) or decreases (7, 10, 21), or it exerts biphasic effects on GABAergic synapses (12, 18, 40). Our main contribution to this issue is the finding that BDNF increases the GABA currents and has a stabilizing action on the repetitively activated epileptic GABAA receptors, an action mediated through a TrkB signaling pathway. However, because the membranes used to transplant the human GABAA receptors derived from both glial and nerve cells, more investigations are needed to detail how BDNF is involved in epileptogenesis. If confirmed in the original neurons, our findings would favor an antiepileptogenic action of BDNF in the human TLE brain and, thus, suggest paths for its treatment.
Supplementary Material
Acknowledgments
We thank the epileptic patients, C.L., C.L.M., P.E., I.A., G.F., and S.L., and the nonepileptic patient, P.C., whose generous contributions have made this work possible. This work was supported by Ministero Università e Ricerca and Fondo per gli Investimenti della Ricerca di Base Grant RBNE01NR34-003 (to F.E.) and Universidad Nacional Autónoma de México Programa de Apoyo a Proyectos de Innovación Tecnológica Grant IN212702 (to R.M.).
Abbreviations: TLE, temporal lobe epilepsy; NGF, nerve growth factor; EGABA, GABA-current reversal potential; TrkB, tyrosine kinase receptor B.
References
- 1.Levine, E. S., Dreyfus, C. F., Black, I. B. & Plummer, M. R. (1995) Proc. Natl. Acad. Sci. USA 92, 8074–8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yan, Q., Resenfeld, R. D., Hawkins, N., Lopez, O. T., Bennett, L. & Welcher, A. A. (1997) Neuroscience 78, 431–448. [DOI] [PubMed] [Google Scholar]
- 3.Li, Y. X., Zhang, H. A., Schuman, E. M. & Davidson, N. (1998) J. Neurosci. 18, 10231–10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manabe, T. (2002) Science 295, 1651–1653. [DOI] [PubMed] [Google Scholar]
- 5.Kovalchuk, Y., Hanse, E., Kafitz, K. W. & Konnerth, A. (2002) Science 295, 1729–1734. [DOI] [PubMed] [Google Scholar]
- 6.Koyama, R., Yamada, M. K., Fujisawa, S., Katoh-Semba, R., Matsuki, N. & Ikegaya, Y. (2004) J. Neurosci. 24, 9215–9224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frerking, M., Malenka, R. C. & Nicoll, R. A. (1998) J. Neurophysiol. 80, 3383–3386. [DOI] [PubMed] [Google Scholar]
- 8.Brunig, I., Penschuck, S., Berninger, B., Benson, J. & Fritschy, J. M. (2001) Eur. J. Neurosci. 13, 1320–1328. [DOI] [PubMed] [Google Scholar]
- 9.Zhu, W. J. & Roper, S. N. (2001) Ann. Neurol. 50, 188–194. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, Q. & Yeh, H. H. (2003) J. Physiol. 548.3, 711–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mizoguchi, Y., Kanematsu, T., Hitrata, M. & Nabekura, J. (2003) J. Biol. Chem. 278, 44097–44102. [DOI] [PubMed] [Google Scholar]
- 12.Jovanovic, J. N., Thomas, P., Kittler, J. T., Smart, T. G. & Moss, S. J. (2004) J. Neurosci. 24(2), 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palizvan, M. R., Sohya, K., Kohara, K., Maruyama, A., Yasuda, H., Kimura, F. & Tsumoto, T. (2004) Neuroscience 126, 955–966. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi, M., Hayashi, S., Kakita, A., Wakabayashi, K., Fukuda, M., Kameyama, S., Tanaka, R., Takahashi, H. & Nawa, H. (1999) Brain Res. 818, 579–582. [DOI] [PubMed] [Google Scholar]
- 15.Murray, K. D., Isackson, P. J., Eskin, T. A., Montesinos, S. P., Abraham, L. A. & Roper, S. N. (2000) J. Comp. Neurol. 418, 411–422. [DOI] [PubMed] [Google Scholar]
- 16.Binder, D. K., Croll, S. D., Gall, C. M. & Scharfman, H. E. (2001) Trends Neurosci. 24, 47–53. [DOI] [PubMed] [Google Scholar]
- 17.Binder, D. K. (2004) Adv. Exp. Med. Biol. 548, 34–56. [DOI] [PubMed] [Google Scholar]
- 18.Reibel, S. Larmet Y., Le, B. T., Carnahan, J., Marescaux, C. & Depaulis, A. (2000) Neuroscience 100, 777–788. [DOI] [PubMed] [Google Scholar]
- 19.Xu, B., Michalski, B., Racine, R. J. & Fahnestock, M. (2004) J. Neurosci. 126, 521–531. [DOI] [PubMed] [Google Scholar]
- 20.Brandon, N. J., Delmas, P., Kittler, J. T., McDonald, B. J., Sieghart, W., Brown, D. A., Smart, T. G. & Moss, S. J. (2000) J. Biol. Chem. 275, 38856–38862. [DOI] [PubMed] [Google Scholar]
- 21.Brandon, N. J., Jovanovic, J. N., Smart, T. G. & Moss, S. J. (2002) J. Neurosci. 22, 6353–6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marsal, J., Tigyi, G. & Miledi, R. (1995) Proc. Natl. Acad. Sci. USA 92, 5224–5228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miledi, R., Eusebi, F., Martinez-Torres, A., Palma, E. & Trettel, F. (2002) Proc. Natl. Acad. Sci. USA 99, 13238–13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miledi, R., Duenas, Z., Martinez-Torres, A., Kawas, C. H. & Eusebi, F. (2004) Proc. Natl. Acad. Sci. USA 101, 1760–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palma, E., Mileo, A. M., Eusebi, F. & Miledi, R. (1996) Proc. Natl. Acad. Sci. USA 93, 11231–11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palma, E., Ragozzino, D. A., Di Angelantonio, S., Spinelli, G., Trettel, F., Martinez-Torres, A., Torchia, G., Arcella, A., Di Gennaro, G., Quarato, P. P., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 10183–10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang, Y. C., Ghansah, E., Chen, Y., Ye, J. & Weiss, D. S. (2002) J. Neurosci. 15, 7982–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patapoutian, A. & Reichardt, L. F. (2001) Curr. Opin. Neurobiol. 11, 272–280. [DOI] [PubMed] [Google Scholar]
- 29.Rivera, C., Li, H., Thomas-Crusells, J., Lahatinen, H., Vitanen T., Nanobashvili, A., Kokaia, Z., Airaksinen, M. S., Voipio, J., Kaila, K., et al. (2002) J. Cell. Biol. 159, 747–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eide, F. F., Vining, E. R., Eide, B. L., Zang, K., Wang, X.-Y. & Reichard, L. F. (1996) J. Neurosci. 16, 3123–3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang, R. & Dillon, G. H. (1998) J. Pharmacol. Exp. Ther. 286, 243–255. [PubMed] [Google Scholar]
- 32.Huang, E. J. & Reichardt, L. F. (2003) Annu. Rev. Biochem. 72, 609–642. [DOI] [PubMed] [Google Scholar]
- 33.Du, J. L. & Poo, M. M. (2004) Nature 429, 878–883. [DOI] [PubMed] [Google Scholar]
- 34.Vial C., Tobin A. B. & Evans, R. J. (2004) Biochem. J. 382, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tigyi, G., Dyer, D., Matute, C. & Miledi, R. (1990) Proc. Natl. Acad. Sci. USA 87, 1521–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miledi, R. (1982) Proc. R. Soc. London Ser. B 215, 491–497. [DOI] [PubMed] [Google Scholar]
- 37.Jones-Davis, D. M & Macdonald, R. L. (2003) Curr. Opin. Pharmacol. 3, 12–18. [DOI] [PubMed] [Google Scholar]
- 38.Kneussel, M. & Betz, H. (2000) J. Physiol. 525.1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizoguchi, Y., Ishibashi, H. & Nabekura, J. (2003) J. Physiol. 548.3, 703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Danzer, S. C. & McNamara, J. O. (2004) J. Neurosci. 24, 11346–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.