Abstract
Objectives:
Evaluating the cost effectiveness and cost utility of an integrated care intervention and participatory workplace intervention for workers with rheumatoid arthritis (RA) to improve their work productivity.
Methods:
Twelve month follow-up economic evaluation alongside a randomized controlled trial (RCT) within specialized rheumatology treatment centers. Adults diagnosed with RA between 18-64 years, in a paid job for at least eight hours per week, experiencing minor difficulties in work functioning were randomized to the intervention (n = 75) or the care-as-usual (CAU) group (n = 75). Effect outcomes were productivity and quality of life (QALYs). Costs associated with healthcare, patient and family, productivity, and intervention were calculated from a societal perspective. Cost effectiveness and cost utility were assessed to indicate the incremental costs and benefits per additional unit of effect. Subgroup and sensitivity analyses evaluated the robustness of the findings.
Results:
At-work productivity loss was about 4.6 hours in the intervention group and 3.5 hours in the care as usual (CAU) group per two weeks. Differences in QALY were negligible; 0.77 for the CAU group and 0.74 for the intervention group. In total, average costs after twelve months follow-up were highest in the intervention group (€7,437.76) compared to the CAU group (€5,758.23). The cost-effectiveness and cost-utility analyses show that the intervention was less effective and (often) more expensive when compared to CAU. Sensitivity analyses supported these findings.
Discussion:
The integrated care intervention and participatory workplace intervention for workers with RA provides gains neither in productivity at the workplace nor in quality of life. These results do not justify the additional costs.
Keywords: Cost effectiveness, Integrated care, Occupational Health, Participatory workplace intervention, Productivity, Rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) patients experience more restrictions in participation in employment and have a higher risk of becoming work-disabled over time1-3). RA is a prevalent condition (between 0.5% and 1% of the population in the developed world) associated with severe impairments4). Furthermore, RA disability-related productivity losses due to time lost from work or during work (respectively, absenteeism and presenteeism) have a substantial socioeconomic impact5-7). Previously conducted research on work disability in RA showed that over a period of almost 13 years, almost 9% of RA patients gave up their paid work and 4% stopped and never resumed paid work8). Work disability occurs in 40% of people in early RA (≤3 years disease duration) and in 60% of people in longstanding RA (>3 years disease duration)9). In previous studies on work participation among RA patients, a large proportion of the patients experienced sickness absence during the early phase of RA, and sick leave prevalence ranged between 53-82% after one year follow-up, resulting in substantial mean annual cost per patient for paid productivity loss (up to about €8,000)4,10).
Absenteeism from paid work represents a major source of productivity costs. In a review combining the results from 26 cost-of-illness studies in RA, mostly conducted in Western Europe, mean annual sick leave costs of €2,770 per patient were shown4). Presenteeism, which refers to at-work productivity loss, is an important cost contributor as well. Furthermore, presenteeism can occur apart from absenteeism, it can precede absenteeism, and it can follow absenteeism4,11). Presenteeism occurs specifically in situations where the employee is not absent from paid work but is struggling with work and health-related disability complaints, as is often the case with RA patients. Costs related to presenteeism are at least as important as absenteeism costs because they are not only an economic loss for society but also a burden for patients. However, studies on at-work productivity loss, which specifically focus on presenteeism, among RA patients are rarely found. Nonetheless, these studies are needed to reduce high costs to society and to reduce the burden for workers with RA regarding their at-work productivity. Previous studies have found that having paid work is associated with better health-related quality of life in patients with RA, and at-work productivity loss is negatively associated with health-related quality of life in patients with RA12,13).
A need emerged to develop (cost) effective worksite interventions to support productive work participation of workers with RA. Therefore, an intervention program consisting of both integrated care and a participatory workplace intervention has been provided to workers with RA. An integrated care approach was already found effective in increasing work participation among chronically ill patients14,15). Especially for patients sick-listed because of chronic low back pain, a substantial economic benefit over CAU was found14). Deducing that integrated care might increase work participation14,15), integrated care programs focused on improving work participation and at-work productivity in chronically ill patients, including RA patients, should be developed. Additionally, a participatory return-to-work (RTW) intervention for sick-listed workers due to musculoskeletal disorders was also found to enhance work resumption and generated a net socioeconomic benefit16). A participatory RTW intervention focusing on improved communication is especially relevant in the Dutch context since the Dutch law on work-related disability requires the occupational physician, employee, and employer to collectively take responsibilities to reduce sickness absence (duration).
The current intervention, thus, includes an integrated care intervention and participatory workplace intervention provided to workers with RA currently at work (no more than three months sick leave at time of inclusion) to improve their work productivity. Before implementing this intervention on a large scale, a broader picture of the total cost and effects of the intervention needs to be evaluated. As (occupational) healthcare budgets are limited, economic evaluations become more important. Cost effectiveness analyses are conducted to estimate the incremental monetary costs and benefits of an intervention per unit of effect gained. Although intervention-related costs to evaluate the cost-effectiveness of a worksite intervention are important to account for, worksite interventions are also associated with healthcare and productivity-related costs.
Economic evaluations are recommended to be conducted from the perspective related to the costs being evaluated17). According to the guidelines for economic evaluations, full economic evaluations are preferred to be conducted from the societal perspective, considering all costs and benefits of interventions no matter on whom they fall18). Adhering to the guidelines, this study includes all relevant costs from the societal perspective (healthcare, patient and family, and costs in other sectors). The costs in other sectors are defined as costs related to productivity and are, henceforth, mentioned as "productivity costs." Although there is still an ongoing debate on how to identify, measure, and value productivity costs in economic evaluations11,19), textbox 1 presents the main productivity cost terms and the different conceptions on how to value productivity costs as presented throughout this study (adapted from Krol et al. 2013)19).
Textbox 1.
Main productivity cost terms
| Absenteeism Not attending work Presenteeism Attending work with diminished functioning (reduced work quantity and quality) Compensation Methods in which lost productivity is compensated for (e.g., extra hours, co-workers took over, etc.) Human Capital approach All potential lost productivity not performed by a person due to work disability Friction Cost approach Lost productivity is limited until the time needed to replace a work-disabled worker and train his/her substitute |
The aim of the present study was to conduct an economic evaluation, including cost-effectiveness and cost-utility analyses, in which the intervention (i.e., an intervention program consisting of both integrated care and a participatory workplace intervention) was compared to care as usual (CAU) for workers with RA. The research questions were as follows:
(i) Is the joint distribution of costs and at-work productivity loss preferable in the intervention group when compared to CAU?
(ii) Is the joint distribution of costs and RA patients' quality of life preferable in the intervention group when compared to CAU?
(iii) How do different methodological considerations under a given set of assumptions impact the results? (Answered with risk-case analysis and sensitivity analyses)
Patients and Methods
The economic evaluation was conducted alongside a randomized controlled trial comparing a workplace intervention, which consists of both integrated care and a participatory workplace intervention, with care as usual among workers with RA. The study was conducted in the Netherlands between 2011 and 2013, and the follow-up period was 12 months. The trial was registered in the Dutch Trial Register (NTR2886). The Medical Ethics Committee of the Slotervaart Hospital and Reade and the VU University Medical Center, Amsterdam, the Netherlands approved the study design, protocols, procedures, and informed consent. Participation was voluntary and all participants signed informed consent.
Eligible RA patients (Table 1) who have visited a rheumatologist of one of the participating hospitals during the last year received an information letter about the project. More details of the study design are described elsewhere20).
Table 1.
Eligible criteria for participation in the trial
| ◦ | Age 18-64 |
| ◦ | Diagnosed with RA |
| ◦ | Having a paid job (paid-employment or self-employed) for at least 8 hours/week |
| ◦ | Experienced at least minor difficulties in functioning at work (obstacles at work) |
| ◦ | No severe comorbidity (as it would hamper compliance to the protocol) |
| ◦ | Being able to read or understand Dutch language |
| ◦ | No more than 3 months sick leave at time of inclusion |
Intervention
The participants in the intervention group were able to take part in the intervention program consisting of two components that complement each other: (i) integrated care and (ii) participatory workplace intervention.
Integrated care was provided based on a case management protocol and delivered by a multidisciplinary team (n = 3), consisting of a trained clinical occupational physician (who acted as care manager), a trained occupational therapist, and the patients' own rheumatologist. The care manager (clinical occupational physician) had an intermediate role between clinical and occupational care and coordinated care. The care manager furthermore communicated with the occupational therapist and the patient's rheumatologist as members of the multidisciplinary team and additionally with the patients' supervisor, occupational physician, and general practitioner. During the first intake, the care manager was responsible for history taking and physical examination to identify functional limitations at work and factors that could influence functioning at work. The care manager proposed a treatment plan at the end of the intake. The patient visited the care manager within 1 week after randomization and again after 6 and 12 weeks to evaluate and, if necessary, adjust the treatment plan. The services provided by the occupational physician are offered by the employer.
The participatory workplace intervention concerned workplace adaptations based on active participation and strong commitment of both the patient and, if relevant, the supervisor. The workplace intervention was coordinated by the occupational therapist. The aim of the workplace intervention was to achieve consensus between patient and supervisor regarding feasible solutions for obstacles for functioning at work. After consensus, the occupational therapist, patient, and supervisor agreed on a plan of action, and the patient and supervisor were responsible for implementing the plan of action. The occupational therapist evaluated the implementation of the plan of action after four weeks20).
All participants received usual rheumatologist-led care as provided in the Netherlands.
Assessments of at-work productivity loss and utilities
The Work Limitations Questionnaire was used to assess at-work productivity loss (WLQ)21). The WLQ consists of four subscales (time management demands, physical demands, mental-interpersonal demands, and output demands), which are calculated into scores ranging from 0 (no limitations) to 100 (highest limitations). Based on high internal reliability and internal consistency, the WLQ concerning RA shows good validity and reliability22-24). The score on all 25 items (which presents the percentage of productivity loss) are multiplied by the number of work hours per two weeks, resulting in an estimation of presenteeism hours (or at-work productivity loss hours) per two weeks.
Secondly, utilities were assessed. Utility is the valuation of the health of the patient. Utilities range from 0 (death) to 1 (full health) and were assessed at baseline and after 6 and 12 months follow-up using questionnaires that were filled in by the patient. Utilities were assessed in two different ways. Patients described their general health status using the EuroQol classification system25). The EQ-5D-5L measures health outcomes on five dimensions (mobility, self-care, daily activities, pain/discomfort, and depression/anxiety) ranging from none to major complaints. The Dutch crosswalk value set was used to estimate the utility of health states as described by the respondent26). This utility measure reflects how the general population values the health status described by the patient.
Utilities were also measured using the RAND-36 questionnaire which measures quality of life. Nine subscales were included and provided information on mental health, pain, physical role limitations, physical functioning, social functioning, vitality, emotional role limitations, general health perception, and perceived health change. From RAND-36 the utility score was calculated by transforming the subscales into a scale score ranging from 0 to 100. Because of the more extensive classification system, RAND-36 could be a more sensitive utility measure than the EQ-5D-5L.
Assessments of costs
As mentioned earlier, adhering to the guidelines, this study included all relevant costs from the societal perspective, including the aggregation of intervention costs27). All costs were indexed to the reference year 2012 (the year in which most participants were included) and reported as annual costs. Discounting of costs was not needed because the follow-up period did not exceed one year.
Healthcare costs
Questionnaires with a six-month recall period were posted to the respondents at baseline and 6 and 12 months after randomization to measure health care utilization (i.e., health service uptake and medication usage). Healthcare costs were monetarily valued by using the standard Dutch unit prices or average tariffs (when standard prices were not available) according to the Dutch Manual for Costing28). For prescribed medication, The Daily Defined Dosages (DDD) were derived from the Dutch Pharmacotherapeutic Compass29), and the price per dosage was based on drug costs in the Netherlands30). Non-prescription drugs were based on their average market prices, including a 6% tax. Medical, personal, and professional aids were based on the aid categories as registered by the Dutch care institute31)or based on their market prices. Healthcare costs were then calculated by multiplying the uptake with the average price.
Patient and Family Costs
Patient and family costs comprised informal care costs and travel and parking costs. Questionnaires with a six-month recall period were posted to the respondents at baseline and 6 and 12 months after randomization. Informal care costs were calculated by multiplying the hours of informal care with the wages that were valued against the shadow price of the wage rate per hour of a housekeeper28). Travel and parking costs were calculated by multiplying the distance to the utilized health care service with the average costs per kilometer as presented in the Dutch guideline for costing research28)and the frequency of used health care services. If applicable, parking costs were added.
Productivity Costs
Productivity costs incorporated both absenteeism and presenteeism from work. Questionnaires with a six-month recall period were posted to the respondents at baseline and 6 and 12 months after randomization. Measuring productivity costs with the modular PROductivity and DISease Questionnaire (PRODISQ) covers all relevant aspects of the relationship between health and productivity, including absence from work, compensation mechanisms that may reduce productivity loss, and reduced productivity at work32). The number of absenteeism days and the quantity and quality of work as an estimate for inefficiency at work were therefore measured by means of the PRODISQ32). As recommended in the Dutch Manual for Costing28), the average day wage, based on age- and gender-specific productivity levels per paid employee, was used to value productivity changes. Absenteeism costs were calculated by using the friction costs method (average wage × friction period). This method assumes production will restore after a fixed amount of time, called the friction period. Once the production is restored, the productivity costs terminate. In the Netherlands, the friction period is based on the average time needed to replace and train new employees. For 2012, the friction period was fixed at 92.68 days33-35). Presenteeism costs were calculated by multiplying the average wage with the estimate of inefficiency28).
Intervention Costs
In order to measure the relevant costs, registers regarding the intervention uptake were used. The intervention providers reported the uptake of each intervention component (for more information on the different components, see Table 3). The intervention costs were calculated by multiplying the average time spent on each intervention component with the average wage of the intervention component provider.
Table 3.
Intervention costs calculation
| Intervention components | Average time spent (hours) | Average wage (€) | Outcome (time*wage) (€) |
|---|---|---|---|
| Wage scale Care Manager/Clinical Occupational Physician based on functional description (36): 11 to 14. Chosen median scale 12 (.8)=€5,033 Hourly wage=Wage per month/hours per week/4.33=5,033/40/4.33 Wage scale occupational therapist based on functional description (36): 6 to 10. Chosen median scale 7 (.9)=€2,743 Hourly wage=Wage per month/hours per week/4.33=2,743/40/4.33 | |||
| Care manager—Integrated care by clinical occupational physician (COP) | |||
| First consultation (including: (i) development of treatment plan, (ii) Contact with rheumatologist and patient's OP concerning treatment plan; (iii) Sending communication form to rheumatologist, OT, and patient's OP; and (iv) Facilitate e-mail contact with OT, rheumatologist, and patient's OP) | 1.50 | 29.06 | 43.59 |
| Second consultation (6 weeks): evaluation with patient | 0.33 | 29.06 | 9.69 |
| Third consultation (12 weeks): evaluation with patient | 0.33 | 29.06 | 9.69 |
| Occupational Therapist (OT)—Workplace visit | |||
| Employment contract | |||
| Including: (i) Organizational preparation of the protocol, (ii) Workplace observation, (iii) Inventory 'problems' worker, (iv) Inventory 'problems' supervisor, (v) Conversations on solutions, (vi) Developing advisory reports, (vii) Meeting and contacts with 'COP' | 4.33 | 15.84 | 68.52 |
| Self-employed | |||
| Including: (i) Organizational preparation of the protocol, (ii) Workplace observation, (iii) Inventory 'problems' worker, (iv) Conversations on solutions, (v) Developing advisory reports, (vi) Meeting and contacts with 'COP' | 3.95 | 15.84 | 62.57 |
| Occupational Therapist (OT)—Advisory reports | |||
| Instructing solutions, Control/evaluation, Adjusting and continuing | 0.74 | 15.84 | 11.72 |
Analysis
The analyses were performed according to the intention-to-treat principle. Missing data were replaced by mean imputation using the mean of the outcome per group to replace the missing values per respondent. On average, 5% of the utility measurements, 4% of the WLQ measurements, and 4.8% of the cost questionnaires were missing. Parametric analyses (i.e., ANOVA) were conducted to compare the baseline characteristics between both groups. Non-parametric analyses of Mann-Whitney U for continuous variables and Pearson χ2 tests for categorical variables were used. Non-parametric bootstrapping yielded the 95% confidence intervals around the mean cost differences. All statistical analyses were performed in IBM SPSS Statistics version 22.
For the economic evaluation, an incremental approach was used by calculating the differences between the intervention and CAU group. The incremental cost effectiveness ratio (ICER) was calculated by dividing the difference in costs (healthcare costs, patient and family costs and intervention costs) by the difference in at-work productivity loss (i.e., hours lost from work due to presenteeism), measured with the WLQ. The incremental cost utility ratio was calculated by dividing the difference in costs (healthcare costs, patient and family costs, productivity costs, and intervention costs) by the difference in QALYs. In the cost effectiveness analysis, the productivity costs are not included to avoid double counting, because presenteeism serves as the outcome measure (i.e., at-work productivity loss). When conducting the cost utility analysis, the productivity costs are included as there is no risk for double counting. A full societal perspective is thereby not applied in the cost effectiveness analysis due to methodological concerns (i.e., double counting). Both the bootstrapped ICER and ICUR pairs were graphically plotted on a cost effectiveness plane (probabilities in bootstrap on the random sampling with replacement based on individual data of the participants). Cost-effectiveness acceptability curves were generated if the ICER or ICUR was located in the northeast quadrant (i.e., the intervention produces superior effects at additional costs relative to CAU) or the south-east quadrant (i.e., the intervention generates superior effects against lower costs). The analyses were performed in IBM SPSS Statistics version 22 and Microsoft Excel 2010.
Risk case- and sensitivity analysis
First, a risk-case analysis followed by two sensitivity analyses were conducted, which aimed to detect whether potential different methodological considerations might impact the results as found in the main analyses.
The risk-case analysis comprised, on an explorative basis, the cost effectiveness analysis (CEA) and the cost utility analysis (CUA) for the subsample in which participants at risk were left out. Due to a systematic error in the minimization procedure, a subgroup of 37 participants was considered at risk to be mistakenly allocated to CAU or intervention group. To determine the potential influence of the allocation error on the study results a sensitivity analysis on a subsample in which the 37 participants at risk were left out was performed.
Firstly, compensation for lost work was accounted for when calculating absenteeism costs. Absenteeism costs were only calculated when the work was taken over in extra hours or when new personnel were hired to compensate for the production loss. Compensation during normal working hours was assumed not to result in productivity costs.
Secondly, as all patients were evaluated according to the intention-to-treat principle, a correction for significant baseline differences were conducted by subtracting from the effectiveness scores (if significantly different) the individual baseline score and adding the overall baseline score within the randomization group.
All analyses were performed in IBM SPSS Statistics version 22 and Microsoft Excel 2010.
Results
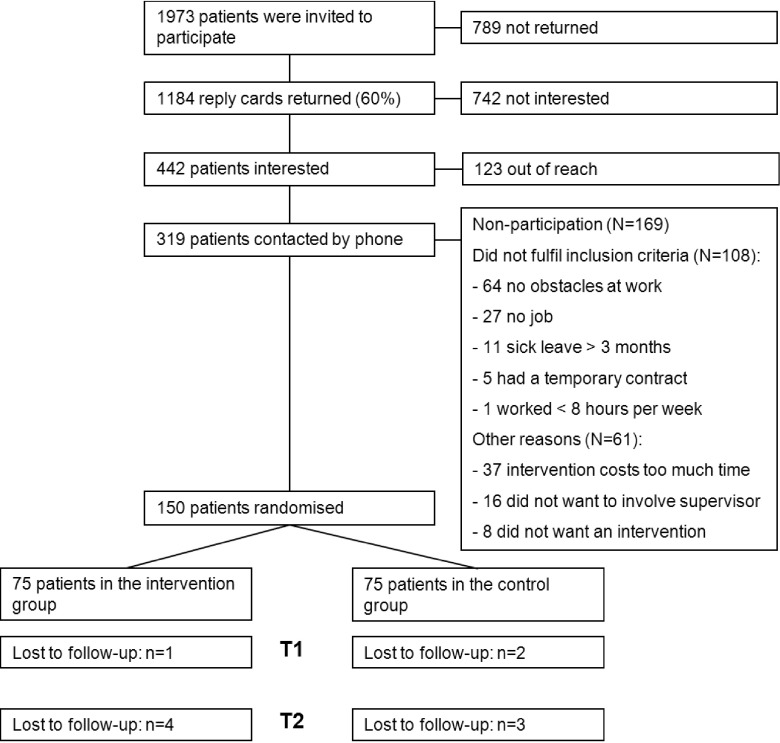
At baseline, 150 participants were included: 75 were randomized into the intervention group and 75 into the CAU group. A more detailed description of the patient flow throughout the study is presented in Fig. 1.
Fig. 1.
Flow-chart of patient inclusion
Table 2 presents the baseline demographic characteristics and mean costs for both groups. A significant difference in quality of work and in presenteeism hours (based on the WLQ) was found between both groups. Informal care costs were also significantly different between the intervention- and CAU group. Managerial positions were more often present in the intervention group (nearly significant) and on average, medical aids were more often used by the CAU group (near to significance).
Table 2.
Baseline characteristics (N=150)
| Intervention Group (n=75) | CAU Group (n=75) | p-value | ||
|---|---|---|---|---|
|
†Low=preschool, primary school; intermediate=lower and upper secondary; high=tertiary school, university, or postgraduate. # Rated on a visual analogue scale from 0 (bad) to 100 (excellent) £ Rated on a visual analogue scale from 0 (very bad/low quality or quantity) to 10 (very good/high quality or quantity) 〒High scores indicate better perceived quality of life outcomes, rated on a scale from (0) bad to 1 (good) (a) Anova (b) Pearson Chi-squared test (c) Fisher's Exact test (d) Non-parametric Mann-Whitney U test * Nearly significant| ** Significant at the 5% level | ||||
| Age, mean (sd) | 49.75 (8.6) | 49.6 (8.7) | 0.93 (a) | |
| Female, N (%) | 63 (84) | 63 (84) | 0.604 (b) | |
| Education, N (%)† | ||||
| Low | 16 (21) | 16 (21) | 0.755 (b) | |
| Intermediate | 22 (30) | 26 (35) | ||
| High | 37 (49) | 33 (44) | ||
| Working hours, mean (sd) | 29.22 (10.18) | 28.21 (9.9) | 0.54 (a) | |
| Managerial position, N (%) | 25 (33) | 16 (21) | 0.064 (c) * | |
| Shift work, N (%) | 13 (17) | 15 (20) | 0.83 (c) | |
| Absenteeism days, mean (sd) | 6.14 (15.69) | 3.79 (8.91) | 0.261 (a) | |
| General Health, mean (sd) # | 70.28 (13.8) | 73.32 (15.7) | 0.236 (a) | |
| Co-morbidity, N (%) | 46 (61) | 51 (68) | 0.125 (c) | |
| Quantity of work, mean (sd) £ | 8.29 (1.7) | 8.74 (1.6) | 0.096 (a) | |
| Quality of work, mean (sd) £ | 8.56 (1.7) | 9.17 (1.3) | 0.014 (a) ** | |
| WLQ based presenteeism hours, mean (sd) | 4.52 (2.41) | 3.46 (2.68) | 0.012 (a) ** | |
| Utilities, mean (sd)〒 | 0.75 (0.14) | 0.78 (0.13) | 0.112 | |
| Costs, mean in € (sd) | ||||
| Prescription medication | 493.9 (1,003.86) | 338.75 (506.93) | 0.925 (d) | |
| Over-the-counter medicines | 40.05 (86.58) | 25.58 (58.36) | 0.147 (d) | |
| Medical aids | 76.61 (209.56) | 134.25 (394.48) | 0.084 (d) * | |
| Health care service utilization | 499.25 (551.77) | 585.2 (724.95) | 0.901 (d) | |
| Total healthcare costs | 1,109.81 (1,353.83) | 1,083.76 (1,088.82) | 0.739 (d) | |
| Informal care costs | 43.76 (88.95) | 23.42 (65.05) | 0.048 (d) ** | |
| Travel and parking costs | 34.66 (35.06) | 39.84 (46.42) | 0.91 (d) | |
| Total patient and family costs | 78.42 (100.84) | 63.26 (86.17) | 0.331 (d) | |
| Absenteeism costs | 1,642.39 (4,401.59) | 988.56 (2,493.78) | 0.74 (d) | |
| Presenteeism costs | 1,198.61 (3,028.9) | 1,132.31 (4,460.36) | 0.4 (d) | |
| Total costs in other sectors | 2,841 (5,390.34) | 2,120.86 (6,689.75) | 0.404 (d) | |
Utility and effectiveness analyses
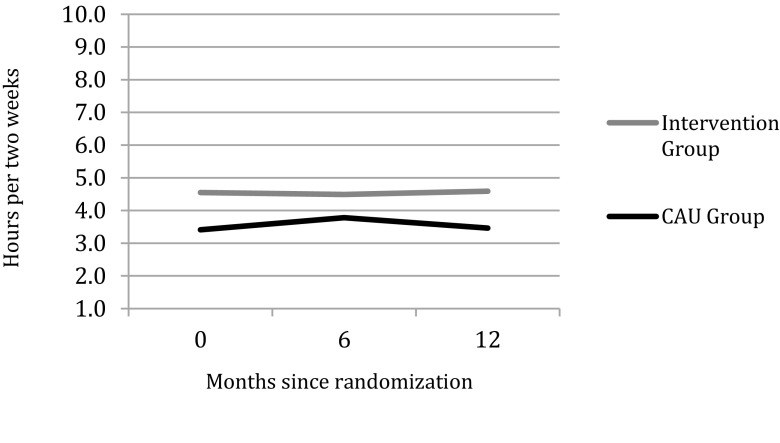
After 12-month follow-up, the mean duration of hours lost from work due to presenteeism were 4.59 hours per two weeks (95% CI 4.04 to 5.15) in the intervention group and 3.46 hours per two weeks (95% CI 2.9 to 4.01) in the CAU group; the difference was significant (p-value 0.05). Overall, there is a constant difference of about 1 hour lost from work due to presenteeism between the CAU group and the intervention group (Fig. 2). The difference over time (T0 and T2) is not significant within the intervention group (p-value 0.772) or within the CAU group (p-value 0.993).
Fig. 2.
Average hours lost from work due to presenteeism per two weeks
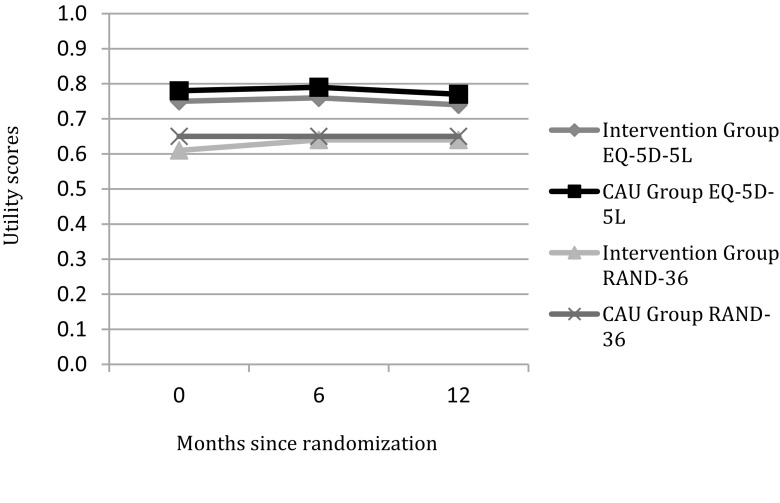
The mean QALY after twelve months follow-up was on average 0.77 (sd 0.016) for the CAU group and 0.74 (sd 0.016) for the intervention groups and was not significantly different. The mean utility score based on the EQ-5D-5L did not significantly change over time (p-value 0.747) for the intervention group or the CAU group (p-value 0.422). Based on the RAND-36, the difference in utilities over time from baseline until twelve months after the intervention did neither change significantly (p-values 0.502 for the intervention group and 0.936 for the CAU group). In both groups, the divergent measurement tools (EQ-5D-5L and RAND-36) did not show any major changes in utility measures over time. The mean RAND-36 and EQ-5D-5L utility scores are illustrated in Fig. 3.
Fig. 3.
Utility scores
Cost analyses
The intervention costs are based on the following: (i) the integrated care provided by a clinical occupational physician, including three consults and (ii) the workplace visit and advisory reports done by the occupational therapist. Details on the components and costs can be found in Table 3. Although the intervention costs depend on the uptake of the different intervention components and the type of employment (employee or self-employed) per patient, the overall costs were estimated at €91.84 per participant in the study (Table 3).
The mean costs related to healthcare, patient and family, intervention, and productivity are presented in Table 4. Regarding healthcare costs, the main cost drivers are medication costs and costs related to hospital care. Overall, the CAU group has the highest total health care costs. The CAU group did use significantly more occupational physician care compared to the intervention group. All other costs did not differ significantly. Patient and family costs and costs related to productivity were higher in the intervention group. Absenteeism and presenteeism accounted for the highest costs categories in both groups, and thereby had the largest impact on the total costs after 12 months follow-up.
Table 4.
Mean component costs
| Mean costs | Mean cost difference (95% CI)¶ | ||
|---|---|---|---|
| Intervention group | CAU group | ||
| ¶mean cost differences and 95% confidence intervals around the mean cost differences were attained by non-parametric bootstrap techniques | ¥Including both prescription and over-the-counter medications | n.a.=not applicable | |||
| Healtdcare costs | |||
| General practitioner | 88.96 | 106.93 | -18 (-46 to 10) |
| Medications¥ | 646.03 | 506.08 | 140 (-137 to 439) |
| Medical specialist | 185.50 | 166.98 | 19 (-19 to 52) |
| Occupational physician | 5.94 | 31.21 | -25 (-45 to -9) |
| Occupational therapist | 129.74 | 206.48 | -131 (-275 to 9) |
| Alternative treatment | 36.00 | 101.07 | -65 (-162 to 5) |
| Professional home care | 22.71 | 19.44 | 3 (-34 to 34) |
| Psychologist | 84.12 | 118.27 | -34 (-157 to 71) |
| Hospital day treatment | 104.60 | 283.95 | -179 (-421 to 23) |
| Hospital admission | 252.68 | 166.95 | 86 (-113 to 293) |
| Total health care costs | 1,551.54 | 1,748.74 | -189 (-768 to 351) |
| Intervention costs | |||
| Intervention care manager | 39.46 (19.98) | n.a. | n.a. |
| Intervention OT | 52.38 (35.43) | n.a. | n.a. |
| Total intervention costs | 91.84 | n.a. | n.a. |
| Patient and Family costs | |||
| Informal care | 74.79 | 41.42 | 33 (-14 to 81) |
| Parking and travel time | 80.56 | 87.66 | -7 (-34 to 22) |
| Total patient and family costs | 156.26 | 128.35 | 28 (-28 to 91) |
| Costs in other sectors | |||
| Absenteeism | 2,854.32 | 2,367.79 | 487 (-859 to 2,117) |
| Presenteeism | 2,827.32 | 1,505.44 | 1,322 (-138 to 3,078) |
| Total costs in other sectors | 5,618.66 | 3,880.70 | 1,738 (-706 to 4,486) |
Cost-utility and cost-effectiveness analyses
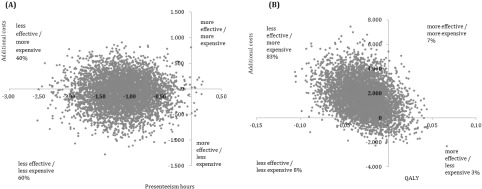
The main cost-effectiveness and cost-utility analyses show ICERs in similar directions: more expensive and less effective (respectively 73.13 and 59,138.30) (Table 4).
The ICER for at-work productivity loss was positive because higher scores indicate more presenteeism hours, and thus worse outcomes when compared with CAU. In the cost-effectiveness plane, almost all bootstrapped cost-effect pairs are located in the west quadrants (Fig. 4 (A)), indicating that the intervention was less effective and thus could not achieve reduced presenteeism hours.
Fig. 4.
Costs effectiveness planes for the incremental cost effectiveness ratio (A) and the incremental cost utility ratio (B)
The incremental cost utility ratio (ICUR) was located mostly (83%) in the northwest quadrant (Fig. 4B), meaning that the intervention was less effective in terms of quality adjusted life years and was more expensive.
Risk-case and sensitivity analyses
The results of the sensitivity analyses can be found in the lower part of Table 5. The overall conclusions did not change when solely analyzing the subgroup of participants not at risk of being mistakenly randomized to one of the groups (n = 113). The results of the sensitivity analyses testing the robustness of the cost utility analysis results by correcting for the compensation of lost work did not differ from the main analysis. The sensitivity analysis with correction for baseline differences (by subtracting from all effectiveness scores the individual baseline score and adding the overall average baseline score) did not influence the cost effectiveness outcomes either.
Table 5.
Total mean effects and costs during 6-months follow-up, incremental cost effectiveness ratios and 95% confidence interval, distribution of the cost-effect pairs on the cost effectiveness plane
| Sample Size | Effect | ΔCosts (€) | ΔEffect | ICER(ΔCosts/Δ Effects) | Distribution CE Plane (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| INT | CAU | NE | SE | SW | NW | |||||
| ¶hours lost from work due to presenteeism, measured with the Work Limitations Questionnaire (WLQ) | ¥ quality adjusted life years calculated using EuroQol-5D-5L | NE=North East quadrant indicating the intervention is more effective and more costly compared with CAU | SE=South East quadrant indicating the intervention is more effective and less costly compared with CAU | SW=South West quadrant indicating the intervention is less effective and less costly compared with CAU | NW=North West quadrant indicating the intervention is less effective and more costly compared with CAU. | ||||||||||
| Main Analyses | ||||||||||
| Cost effectiveness | 75 | 75 | Presenteeism hours¶ | 83.23 | 1.14 | 73.13 | 0 | 0 | 60 | 40 |
| Cost utility | 75 | 75 | QALY¥ | 1,679.53 | 0.03 | 59,138.30 | 7 | 3 | 8 | 83 |
| Sensitivity Analyses | ||||||||||
| Cost effectiveness: without potential risk cases | 55 | 58 | Presenteeism hours¶ | 1.13 | 244.13 | 215.37 | 0 | 0 | 75 | 24 |
| Cost utility: without potential risk cases | 55 | 58 | QALY¥ | 80.85 | 0.01 | 6,037.34 | 8 | 22 | 32 | 38 |
| Cost effectiveness: baseline correction | 75 | 75 | Presenteeism hours¶ | 82.23 | 1,14 | 73.09 | 0 | 0 | 60 | 40 |
| Cost utility: compensation productivity loss | 75 | 75 | QALY¥ | 1,878.24 | 0.03 | 66,135.18 | 0 | 1 | 3 | 88 |
In all sensitivity analyses, the majority of the incremental cost-effect pairs kept indicated that the intervention was less effective in terms of at-work productivity and utility and more costly when compared with CAU.
Discussion
This study presents the results of both the cost effectiveness and cost utility analysis of an integrated care program for workers with rheumatoid arthritis to improve their work productivity. The integrated care program cannot be regarded as cost-effective when compared to usual care in the Netherlands. Although not significant, both costs and effects were more favorable in the CAU group than in the intervention group over the measurement period of twelve months follow-up.
Comparison with other studies
At the moment, cost-effectiveness studies of comparable integrated care interventions in relation to work productivity and quality of life among RA patients are rare and show varying results. Some economic evaluation studies have assessed comparable interventions in a different population such as a previously conducted study that assessed the effectiveness of an integrated care program for patients with chronic low back pain36). The intervention consisted of a workplace intervention based on participatory ergonomics and a graded activity program. The intervention was effective on the outcome measures return to work (HR 1.9) and sustainable return to work (p = 0.003) after a measurement period of 12 months for patients with chronic low back pain36). The same integrated care program was cost effective when compared with usual care for return to work and QALYs gained with 12 months' follow-up in this patient group. Usual care for chronic low back pain consisted of care provided by the general practitioner and occupational physicians14). Another study evaluated the cost effectiveness of integrated, multidisciplinary care for patients with moderate to severe chronic hand dermatitis. The intervention was compared with usual care and was neither effective nor cost effective after 12 months follow-up. These findings contrast the findings after six months follow-up37). The integrated care program as described in the present study showed similar results regarding at-work productivity and utility. The negative findings are in line with the results found for workers with chronic hand dermatitis at twelve months. Both in the intervention and CAU group the at-work productivity and quality of life did not significantly change after twelve months. No effects were found, and the costs within the intervention group were higher when compared to CAU.
Strengths and limitations
The main strengths of the study were the minimal loss to follow-up, the RCT-design, the use of the societal perspective for the economic evaluation, and the detailed cost measurement. Only five participants in each group were lost to follow-up, and only 5% of the utility measurements, 4% of the WLQ measurements, and 4.8% of the cost questionnaires were missing. This small amount of missing data also justifies the usage of mean imputation. Exploring other imputation techniques (e.g., multiple imputation) is not expected to lead to different outcomes. Another strength of the study was its performance alongside a randomized controlled trial. Although a subgroup of the participants risked being mistakenly allocated to one of the groups, the results were reasonably robust. This RCT design allowed for the economic evaluation to be conducted in a real life setting, collecting relevant cost and effect data. Furthermore, this study reflected the societal perspective by including all costs, regardless of who uses the resources or who benefits from them. This is especially advantageous since the data can be disaggregated and further analyses can be done from different perspectives. Additionally, analyses conducted from the societal perspective aid in decision making regarding resource allocation. Another strength is the detailed level of cost measurement including all relevant costs. Healthcare utilization, patient and family costs, productivity costs, and intervention costs were measured. Different methods were used to measure the relevant costs in a detailed manner (i.e., cost questionnaires and registers). The modular PROductivity and DISease Questionnaire (PRODISQ) for the measurement of productivity costs is a validated and extensively used tool. PRODISQ covers all relevant aspects of the relationship between health and productivity, including absence from work, compensation mechanisms that may reduce productivity loss, and reduced productivity at work32). By using the questionnaire this study facilitates valid estimates of productivity costs in this economic evaluation.
Limitations to the study may have influenced the results. The first limitation addresses the study design, which hampered blinding and potentially induced double counting. Due to the character of the intervention, patients, therapists, and researchers could not be blinded for the allocated treatment after randomization, which might increase the risk of bias. Furthermore, the design risked double counting since the primary outcome was work productivity, and the costs included productivity as well. However, all productivity costs were eliminated within the cost effectiveness analysis to avoid double counting. Nonetheless, this might have underestimated the true costs. However, in the cost utility analysis, productivity costs were included, and a clear overview of all costs is presented in this study. A second limitation of the study is the limited generalizability of the results. Generalizability to other work-disabled populations is unknown as the intervention program was tailored to workers with rheumatoid arthritis in a Dutch context. Our specific Dutch setting may differ from other settings regarding labor legislation. In the Netherlands, usual care for work disabled employees or employees at risk for becoming work disabled is already extensively and timely monitored by occupational physicians and occupational therapists, which is hardly seen in other countries. One might argue that the contrast between the intervention and CAU is limited.
Lack of effectiveness
In economic evaluations, it is essential that costs are weighed against an applicable effectiveness measure. The lack in effectiveness for both at-work productivity and quality of life might suggest that this requirement is not satisfied and the outcome measures might not be responsive in this study. The lack of effectiveness might also be explained by the baseline values being already good, leaving less room for improvement. A ceiling effect was potentially already reached. A third reason for the lack of effectiveness in both outcome measurements may be that the results are influenced by some statistically significant baseline difference that we attributed to early dropout among 'worse' patients. It could be assumed that the patients included in this study achieved a 'stable phase' wherein functional abilities no longer influence quality of life or at-work productivity. Due to the strict inclusion criteria (at-work, minor obstacles at work, no severe comorbidities, and less than 3 months on sick leave), this study population had less severe impairments and the included RA patients' needs for an intervention might have been smaller, leaving less opportunities for improvements. True improvements in at-work productivity and quality of life may have been too small to be detected by the available outcome measures. A final potential explanation for the lack of effectiveness, especially regarding presenteeism hours, can be found in the so-called phenomena of 'response-shift'. Hereby, it is hypothesized that patients who are confronted with health-related work disability, by for example actively paying attention to presenteeism as this is being 'intervened', are faced with the necessity to change their internal standards, values, and conceptualization. This might result in more presenteeism hours because of the attention that is being paid to it38).
Lack of cost-effectiveness
A first explanation for the lack of cost-effectiveness might be that the cost data, and especially productivity costs, are highly skewed. Common standards regarding the identification, measurement, and valuation of productivity costs are lacking19,39,40). However, despite of the different methods used in the sensitivity analyses (including compensation costs) to provide crude estimates of productivity costs associated with the intervention, no differences in cost-effect ratios were detected. A second reason for the lack of cost-effectiveness might be that the Dutch labor legislation makes it relatively difficult to detect a contrast with the intervention; especially within a reasonably stable group of RA patients that were still at work. The CAU group used significantly more occupational physician care compared to the intervention group, which suggests that RA patients with work-related complaints are aware of the extensive care as usual. This might have reduced the difference between the randomized groups, and less attention for problems at work in the CAU group might change the economic conclusions. A third explanation might lay in the overall aim of the integrated care program as described in the present study. The main purpose of the integrated care program is to tackle barriers that hamper RTW. When compared to the study of Lambeek et al.14), where major improvements on RTW for chronic low back patients was found, the population in the present study is not yet on sick leave14,36). The chances that a similar effect would be found within a population still at work were perhaps an overestimation. The sense of emergency for an intervention program among RA patients at work was probably less prominent.
Recommendations for the future
As healthcare budgets are shrinking, economic evaluations are increasingly important. This study with a 12 month follow-up illustrates the impact of an integrated care program on at-work productivity and quality of life among working RA patients who experience minor obstacles at work, no severe comorbidities, and have less than three months sick leave. Within this population, the intervention was not promising. However, the effects of the integrated care program on 'severe' RA patients are unknown. Since previous studies within a population with more severe health-related work constraints (i.e., chronic low back pain) found promising results, one could expect that the results of integrated care among severe RA patients who are absent for a longer period of time might be promising as well. Nonetheless, research is needed to confirm the hypothesis. Furthermore, the presented intervention for RA workers in a Dutch context, where extensive CAU is provided, hampers the generalizability of the study results. A replication of this study in another country where CAU is less extensive could lead more towards the conclusion that the combination of a participatory workplace intervention and integrated care for RA patients is cost effective when compared with CAU. The contrast between the intervention and CAU remains an important point of attention.
Conclusion
In conclusion, this study has shown that, compared with care as usual, the integrated care and participatory workplace intervention did not provide greater improvement to at-work productivity nor to quality of life among RA patients. Cautiously, it is concluded that the intervention was not cost effective after twelve months follow-up. At the moment, no evidence to support its implementation based on the results of the economic evaluation is provided.
Conflicts of interest: The authors declare that there are no conflicts of interest.
References
- 1). Verstappen SM, Boonen A, Bijlsma JW, et al. Working status among Dutch patients with rheumatoid arthritis: work disability and working conditions. Rheumatology (Oxford) 2005; 44: 202-206. [DOI] [PubMed] [Google Scholar]
- 2). Zirkzee EJ, Sneep AC, de Buck PD, et al. Sick leave and work disability in patients with early arthritis. Clin Rheumatol 2008; 27: 11-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3). Burton W, Morrison A, Maclean R, Ruderman E. Systematic review of studies of productivity loss due to rheumatoid arthritis. Occup Med (Lond) 2006; 56: 18-27. [DOI] [PubMed] [Google Scholar]
- 4). Boonen A, Severens JL. The burden of illness of rheumatoid arthritis. Clin Rheumatol 2011; 30 Suppl 1: S3-8. [DOI] [PubMed] [Google Scholar]
- 5). Goetzel RZ, Long SR, Ozminkowski RJ, Hawkins K, Wang S, Lynch W. Health, absence, disability, and presenteeism cost estimates of certain physical and mental health conditions affecting U.S. employers. J Occup Environ Med 2004; 46: 398-412. [DOI] [PubMed] [Google Scholar]
- 6). Li X, Gignac MA, Anis AH. The indirect costs of arthritis resulting from unemployment, reduced performance, and occupational changes while at work. Med Care 2006; 44: 304-310. [DOI] [PubMed] [Google Scholar]
- 7). Lundkvist J, Kastang F, Kobelt G. The burden of rheumatoid arthritis and access to treatment: health burden and costs. Eur J Health Econ 2008; 8 Suppl 2: S49-60. [DOI] [PubMed] [Google Scholar]
- 8). Wolfe F, Allaire S, Michaud K. The prevalence and incidence of work disability in rheumatoid arthritis, and the effect of anti-tumor necrosis factor on work disability. J Rheumatol 2007; 34: 2211-2217. [PubMed] [Google Scholar]
- 9). Han C, Smolen J, Kavanaugh A, St Clair EW, Baker D, Bala M. Comparison of employability outcomes among patients with early or long-standing rheumatoid arthritis. Arthritis Rheum 2008; 59: 510-514. [DOI] [PubMed] [Google Scholar]
- 10). Geuskens GA, Burdorf A, Hazes JM. Consequences of rheumatoid arthritis for performance of social roles-a literature review. J Rheumatol 2007; 34: 1248-1260. [PubMed] [Google Scholar]
- 11). Krol M, Brouwer W, Rutten F. Productivity costs in economic evaluations: past, present, future. Pharmacoeconomics 2013; 31: 537-549. [DOI] [PubMed] [Google Scholar]
- 12). Gronning K, Rodevand E, Steinsbekk A. Paid work is associated with improved health-related quality of life in patients with rheumatoid arthritis. Clin Rheumatol 2010; 29: 1317-1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). van Vilsteren M, Boot CR, Knol DL, et al. Productivity at work and quality of life in patients with rheumatoid arthritis. BMC Musculoskelet Disord 2015; 16: 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Lambeek LC, Bosmans JE, Van Royen BJ, Van Tulder MW, Van Mechelen W, Anema JR. Effect of integrated care for sick listed patients with chronic low back pain: economic evaluation alongside a randomised controlled trial. BMJ 2010; 341: c6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Badamgarav E, Croft JD, Hohlbauch A, et al. Effects of disease management programs on functional status of patients with rheumatoid arthritis. Arthritis Rheum 2003; 49: 377-387. [DOI] [PubMed] [Google Scholar]
- 16). Vermeulen SJ, Heymans MW, Anema JR, Schellart AJ, van Mechelen W, van der Beek AJ. Economic evaluation of a participatory return-to-work intervention for temporary agency and unemployed workers sick-listed due to musculoskeletal disorders. Scand J Work Environ Health 2013; 39: 46-56. [DOI] [PubMed] [Google Scholar]
- 17). Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Pharmacoeconomics 2013; 31: 361-367. [DOI] [PubMed] [Google Scholar]
- 18). Drummond M, Manca A, Sculpher M. Increasing the generalizability of economic evaluations: recommendations for the design, analysis, and reporting of studies. Int J Technol Assess Health Care 2005; 21: 165-171. [PubMed] [Google Scholar]
- 19). Krol M, Brouwer W, Rutten F. Productivity Costs in Economic Evaluations: Past, Present, Future. Pharmacoeconomics 2013; 537-549. [DOI] [PubMed] [Google Scholar]
- 20). van Vilsteren M, Boot CR, Steenbeek R, van Schaardenburg D, Voskuyl AE, Anema JR. An intervention program with the aim to improve and maintain work productivity for workers with rheumatoid arthritis: Design of a randomized controlled trial and cost-effectiveness study. BMC Public Health 2012; 12: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Lerner D, Amick BC, Rogers WH, Malspeis S, Bungay K, Cynn D. The Work Limitations Questionnaire. Med Care 2001; 39: 72-85. [DOI] [PubMed] [Google Scholar]
- 22). Walker N, Michaud K, Wolfe F. Work limitations among working persons with rheumatoid arthritis: results, reliability, and validity of the work limitations questionnaire in 836 patients. The J Rheumatol 2005; 32: 1006-1012. [PubMed] [Google Scholar]
- 23). Lerner D, Reed JI, Massarotti E, Wester LM, Burke TA. The Work Limitations Questionnaire's validity and reliability among patients with osteoarthritis. J Clin Epidemiol 2002; 55: 197-208. [DOI] [PubMed] [Google Scholar]
- 24). Allaire S. Measures of adult work disability. Arthritis & Rheumatism 2003; 49: 85-89.12579598 [Google Scholar]
- 25).Group E. EQ-5D User Guide. Rotterdam Erasmus Univeristeit Rotterdam, centrum voor gezondheidsbeleid en recht, 1995
- 26). EuroQol EQ-5D-5L Value Sets. [Online]. [cited 2014 ]; Available from: URL: http://www.euroqol.org/about-eq-5d/valuation-of-eq-5d/eq-5d-5l-value-sets.html
- 27). Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford; 2005. [Google Scholar]
- 28).Hakkaart L, Tan SS, Bouwmans CAM. Handleiding voor kostenonderzoek. Methoden en standaard kostprijzen voor economische evaluaties in de gezondheidszorg. Rotterdam: Instituut voor Medische Technology Assessment, Erasmus Universiteit Rotterdam. CVZ College voor zorgverzekeringen; 2010
- 29). College van Zorgverzekeringen C. Farmacotherapeutisch Kompas: Medisch farmaceutische voorlichting Amstelveen. [Online]. 2012[cited 2012 Sep. 2012]; Available from: URL: http://www.fk.cvz.nl/
- 30). Zorginstituut N. Drug costs in the Netherlands. [Online]. 2014[cited 2014 Aug. 2014]; Available from: URL: http://www.medicijnkosten.nl/
- 31). Zorginstituut N. Medical aids categories. [Online]. 2014[cited 2014 Aug. 2014]; Available from: URL: www.gipdatabank.nl
- 32). Koopmanschap MA. PRODISQ: a modular questionnaire on productivity and disease for economic evaluation studies. Expert Rev Pharmacoecon Outcomes Res 2005; 5: 23-28. [DOI] [PubMed] [Google Scholar]
- 33). Koopmanschap MA, van Ineveld BM. Towards a new approach for estimating indirect costs of disease. Soc Sci Med 1992; 34: 1005-1010. [DOI] [PubMed] [Google Scholar]
- 34). Oostenbrink JB, Koopmanschap MA, Rutten FF. Standardisation of costs: the Dutch Manual for Costing in economic evaluations. Pharmacoeconomics 2002; 20: 443-454. [DOI] [PubMed] [Google Scholar]
- 35). Netherlands S. Jobs (outstanding, new and filled). [Online]. [cited 2014 ]; Available from: URL: http://statline.cbs.nl/StatWeb/publication/?VW=T&DM=SLNL&PA=80857NED&LA=NL
- 36). Lambeek LC, van Mechelen W, Knol DL, Loisel P, Anema JR. Randomised controlled trial of integrated care to reduce disability from chronic low back pain in working and private life. BMJ 2010; 340: c1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37). van Gils RF, Bosmans JE, Boot CR, et al. Economic evaluation of an integrated care programme for patients with hand dermatitis. Contact Dermatitis 2013; 69: 144-152. [DOI] [PubMed] [Google Scholar]
- 38). Sprangers MA, Schwartz CE. Integrating response shift into health-related quality of life research: a theoretical model. Soc Sci Med 1999; 48: 1507-1515. [DOI] [PubMed] [Google Scholar]
- 39). Prasad M, Wahlqvist P, Shikiar R, Shih YC. A review of self-report instruments measuring health-related work productivity: a patient-reported outcomes perspective. Pharmacoeconomics 2004; 22: 225-244. [DOI] [PubMed] [Google Scholar]
- 40). Noben CY, Evers SM, Nijhuis FJ, de Rijk AE. Quality appraisal of generic self-reported instruments measuring health-related productivity changes: a systematic review. BMC Public Health 2014; 14: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]