Abstract
Objective:
The Japanese government established the Pneumoconiosis Law in 1960 to protect health and promote the welfare of workers engaged in dust-exposed works. This article describes Japanese practice in workplace health management as regulated by the Pneumoconiosis Law to reduce pneumoconiosis in Japan.
Methods:
We collected information addressing pneumoconiosis and the health care of dust-exposed workers. We included all types of scientific papers found through a PubMed search as well as official reports, guidelines, and relevant laws published by the Ministry of Health, Labour and Welfare (MHLW) of Japan and other academic institutions.
Results:
In the past, pneumoconiosis has been a major cause of mortality and morbidity for Japanese workers engaged in dust-exposed work. The Pneumoconiosis Law introduced a system of pneumoconiosis health examination and health supervision to protect workers' health. According to the periodic pneumoconiosis health examination reports in Japan, the prevalence of pneumoconiosis fell from the highest reported figure of 17.4% in 1982, where 265,720 examinations were conducted, to 1% in 2013 in which 243,740 workers were examined. The number of new cases of pneumoconiosis dropped from 6,842 cases in 1980 to 227 cases in 2013. One hundred and seventy two workers were diagnosed as having pneumoconiosis complications in 1980; however, the number fell to five in 2013.
Conclusion:
After reaching its peak in the 1980s, pneumoconiosis and its complications fell each year. The achievement of Japanese pneumoconiosis prevention can be credited to a comprehensive provision for worker health, regulated by a thorough legal framework.
Keywords: Dust, Occupational exposure, Occupational health, Workplace
Introduction
Pneumoconiosis, progressive fibrotic lung disease, is caused by occupational exposure to mineral dust and fibers. The prevention of this disease that results from exposure to mineral dust and fibers depends primarily on compliance with workplace exposure management and health management regulated within a relevant legal framework. Japanese workplace exposure management requires employers to establish and implement procedures for recognizing and controlling exposure to hazardous conditions. Workplace health management involves ongoing worker health monitoring to recognize abnormalities early on so as to initiate appropriate measures to prevent disease occurrence and to provide the necessary care to prevent disease progression.
Around the 1940s, the mining industry led to the resurrection of the Japanese war-torn economy. Poor working conditions in mining industries raised the silicosis problem among miners, and it was considered the most important occupational health issue of post-war Japan1). The Ministry of Labour, which merged with the Ministry of Health in 2001 to form the Ministry of Health, Labour and Welfare (MHLW) of today, responded with the establishment of the Special Protection Act for Silicosis in 1955 and initiated health examinations across the country of dust-exposed workers. The passage of Pneumoconiosis Law2) in 1960 prescribed pneumoconiosis health examinations as a legal requirement for all workers engaged in dust-exposed work1). This article describes Japanese workplace health management and relevant law in pneumoconiosis prevention.
Pneumoconiosis Law
The Pneumoconiosis law2) of 1960 was established to protect the health and promote the welfare of workers engaged in dust-exposed work through measures directed against pneumoconiosis. Measures include proper prevention of dust exposure and supervision of workers' health as prescribed by the Pneumoconiosis Law and its related Enforcement Ordinances3), which are revised and released accordingly by the MHLW of Japan. The law did not set any criterion of dust control, which was regulated specifically under different laws4,5) and ordinances6); however, it stated that employers and workers have to exercise every effort to prevent and control the emission of dust, use protective equipment, etc. The law stipulates that employers must provide workers regularly engaged in dust-exposed work with the education necessary for pneumoconiosis prevention. Workers in such occupations should understand the physical and chemical properties of materials they handle, the potential adverse health effects, control measures for the work environment, the use of protective equipment, etc. Moreover, the law mandates employer provision of workers' health, including pneumoconiosis health examination. Employers are required to make records of pneumoconiosis health examinations and keep the records and radiographs for a period of seven years.
The Pneumoconiosis Law established a Japanese radiographic coding system for pneumoconiosis and the accompanying standard chest radiographs2,7). This system provides a tool for pneumoconiosis health examinations in describing and systematically reporting the radiographic abnormalities found in the chest of dust-exposed workers. In addition, the law also introduced a procedure to classify pneumoconiosis for health supervision2,7). Under the Pneumoconiosis Law, the Director General of the Prefectural Labour Bureau determines the class for health supervision in pneumoconiosis (pneumoconiosis supervision class) based on the diagnosis of the prefectural pneumoconiosis physician and the documents submitted. Depending on the determined pneumoconiosis supervision class, Pneumoconiosis Law requires employers to exert every effort so as to enable the worker concerned to receive proper health guidance as well as to take appropriate measures for his or her work. With its enactment in 1960, Pneumoconiosis Law established the legal framework for Japanese workplace health management for pneumoconiosis prevention.
Workplace health management under Pneumoconiosis law
Workplace health management involves ongoing worker health monitoring to recognize abnormalities early and the provision of appropriate care to prevent disease progression. Under the Pneumoconiosis Law, employers are required to provide a pneumoconiosis health examination for workers engaged in dust-exposed work. A pneumoconiosis health examination is administered, in principle, by obtaining the occupational history related to dust exposure, performing a physical examination, administering chest radiographs, pulmonary function tests, and an examination for complications by certified occupational physicians7). Regional pneumoconiosis specialists examine workers with signs of pneumoconiosis, and the results are submitted to the local Prefectural Labour Bureau for further determination of pneumoconiosis supervision class. Based on the pneumoconiosis supervision class, necessary intervention for the care of workers is then implemented as prescribed in the Pneumoconiosis Law and its related ordinances2,3).
Pneumoconiosis health supervision
In compliance with the provisions of Pneumoconiosis Law, workers engaged in dust-exposed work, either currently or in the past, shall be subject to health supervision by being classified within a pneumoconiosis supervision class (Class I to IV)2,3). The law introduced a procedure to determine the pneumoconiosis supervision class based on the diagnosis of a pneumoconiosis specialist, submitted documents such as radiographs, and a written statement certifying the results of a medical examination on pneumoconiosis. At the time of establishment, the pneumoconiosis supervision class was based upon the findings of chest radiographs, pulmonary function assessment, and a positive test for tuberculosis. Cases of pneumoconiosis complicated with pulmonary tuberculosis were classified into supervision Class IV. Following a 1977 amendment to the Pneumoconiosis Law, the supervision class was determined by a combination of the results of the chest radiograph and the extent of pulmonary dysfunction.
To determine the pneumoconiosis supervision class, radiographic findings of the chest are classified into one of seven PR categories (PR 0,1, 2, 3, 4A, 4B, and 4C) in accordance with the Japanese classification system of pneumoconiosis chest radiographs. For a pneumoconiosis chest radiograph, a PR category is determined based on the presence or absence of small and large opacities triggered by inhalation of dust, the number of small opacities, and the size of large opacities in the field of affected lung of dust-exposed workers (detailed under sub-heading "Pneumoconiosis health examination" ). Pulmonary function assessment results also are categorized into F (-), F (+) and F (++), where F (-) means absence of pulmonary impairment, F (+) denotes presence of some pulmonary function impairment, and F (++) indicates a sufferer of severe pulmonary dysfunction (detailed under sub-heading "Pneumoconiosis health examination" ). The Japanese classification for supervision of pneumoconiosis classifies workers affected with pneumoconiosis into Class II or higher (Table 1). Class I supervision, corresponding to chest radiographic appearances of PR0, denotes individuals unaffected by pneumoconiosis. Class II supervision is assigned to individuals whose radiographic appearances correspond to PR1. Class IIIA corresponds to PR2, and class IIIB to PR3, PR4A or PR4B. Class IV is designated for individuals with radiographic appearances of PR4C, or with PR1 to PR4B and having a pulmonary function assessment result of F (++). Based on the determined pneumoconiosis supervision class, necessary measures are then implemented to maintain the health of workers.
Table 1.
Classification of pneumoconiosis health supervision and measures for supervision
| Supervision class | Chest radiographic finding category | Pulmonary function assessment, F (++) | Measure for supervision | |
|---|---|---|---|---|
| Note: PR category is defined by the presence or absence of small and large opacities trigger by inhalation of dust, the number of small opacities and the size of large opacities in the field of affected lung of dust-exposed worker. F (++), severe pulmonary dysfunction is defined by %VC<60% of predicted; FEV1/FVC ratio of <70% and FEV1<50% predicted; PaO2 of <60 Torr; higher than limit in A-aDO2 value. Measures to reduce degree of exposure to dust include change of workplace and a reduction in working hours for dust-exposed work. Pneumoconiosis complications, recognized by Pneumoconiosis law, include pulmonary tuberculosis, tuberculous pleuritis, secondary pneumothorax, secondary bronchitis, secondary bronchiectasis, and primary lung cancer. | ||||
| Class I | PR0 | Absent | No intervention needed | |
| Class II | PR1 | Absent | Reducing degree of exposure to dust | |
| Class III | A | PR2 | Absent | Change of workplace (recommended) |
| B | PR3, PR4 (A, B) | Absent | Change of workplace (mandatory) | |
| Class IV | PR1 to PR4 (A, B) | Present | Rest from work/ medical treatment | |
| PR4C | Not considered | |||
| Complications with underlying Class II, III pneumoconiosis | Rest from work/ medical treatment | |||
In order to maintain the health of workers, Pneumoconiosis Law states that, based on the pneumoconiosis supervision class, employers are responsible to ensure that the workers concerned receive proper health guidance as well as an appropriate job arrangement to reduce the degree of dust exposure2). Pneumoconiosis supervision Class I denotes workers unaffected by pneumoconiosis and considers that no intervention is required. For workers classified as pneumoconiosis supervision Class II or Class IIIA, a workplace change is recommended as well as a reduction in working hours devoted to dust-exposed work in order to reduce the degree of dust exposure. Workers who are classified into supervision Class IIIB are required to transfer from dust-exposed work to maintain their health. Workers from supervision Class IV or sufferers from complications related to pneumoconiosis require medical treatment2). Table 1 summarizes the classification for supervision of pneumoconiosis and measures for health supervision for pneumoconiosis-affected workers.
Pneumoconiosis law requires employers to pay change-of-work allowances to workers who are given a recommendation to change workplace and to those who require a mandatory change of workplace, 30 days worth and 60 days worth of the average daily wage respectively2). The average daily wage mentioned above is calculated, according to the Labour Standard Law of Japan, by dividing the total amount of wages for a period of three months preceding the day on which the calculation of average wage became necessary, by the number of all days during the period. Pneumoconiosis caused by dust-exposed work and diseases recognized as complications of pneumoconiosis by Pneumoconiosis Law are covered by the workmen's accident compensation insurance system8). The Asbestos-Related Health Damage Relief System9,10), effective from 2006, provides relief benefits such as medical care expenses to sufferers of the designated diseases caused by inhaled asbestos and to the bereaved of those who died of the diseases. Officially recognized asbestos-related diseases include mesothelioma, lung cancer (malignant neoplasm of the lung and bronchus), asbestosis with severe pulmonary dysfunction, and diffuse pleural thickening with severe pulmonary dysfunction.
Pneumoconiosis health examination
A pneumoconiosis health examination is performed as part of ongoing worker health monitoring to recognize abnormalities early on and to determine, in combination with other documentation, the pneumoconiosis supervision class of dust-exposed workers. Under the Pneumoconiosis Law, employers are required to provide pneumoconiosis health examinations to workers engaged in dust-exposed work on four occasions: upon employment, periodically, examinations administered on specific occasions, and when workers leave employment2). Specific occasions mentioned above refer to the instances in which a worker currently engaged in dust-exposed work was diagnosed as affected by or suspected of contracting pneumoconiosis during a medical examination prescribed under the Industrial Safety and Health Law4), or a worker returned to work after more than one year of absence from duty to receive medical treatment for complications of pneumoconiosis, or other designated situations. A pneumoconiosis health examination is administered by taking dust exposure history, performing a physical examination, a chest radiograph, pulmonary function tests and an examination for complications by certified occupational physicians2,7). Occupational exposure history, physical examinations and chest radiographs are taken for every health examination; however, the need for pulmonary function tests and clinical examinations for complications is decided by the examining physicians.
Past or current occupational exposure history is collected through questionnaires on an individual's engagement in dust-exposed work. Dust-exposed work is defined, according to Pneumoconiosis law, as the type of work in which engagement by the individual poses a risk of developing pneumoconiosis2), and the scope of dust-exposed work is determined by Ministry of Health, Labour and Welfare Ordinance3,11) (Table 2). In addition to operations where workers are exposed to dust directly, such as digging, drilling in mines and tunnels, stone crushing, welding, and handling asbestos, other occupations such as maintenance inspection of equipment in underground mines are also listed under designated dust-exposed work. Information on the nature of employment, dust content and concentration, exposure duration, and smoking status are important factors in considering work relatedness to fibrotic lung diseases.
Table 2.
Designated dust-exposed works
| * In Japan, the import, manufacture, and use of all forms of asbestos fibres have been prohibited since 2006. In recent years, potential occupational exposure to asbestos arises from demolition activities of asbestos containing materials and building. Note: Table adapted with simplification. English translation of original Japanese is available at http: //www.ilo.org/dyn/natlex/docs/ELECTRONIC/29320/69506/F1079186294/JPN29320.pdf. | |
| 1 | Excavation of soil, sand, rocks or minerals (except wet soil and sand) |
| 2 | Unloading of minerals, etc. by tipping or tipping over the rack of a truck loaded with minerals, etc. (except wet ones) |
| 3 | Cutting, crushing, screening, loading or unloading of minerals, etc., in a pit. (except operation site using water or sprinkler system) |
| 4 | Conveying of minerals, etc. (except wet ones) in a pit. |
| 5 | Filling up of excavations with mineral, etc. (except wet ones) or sprinkling dust in a pit. |
| 5-2 | Removing, evacuating, checking or repairing machines or electric equipment attached or accumulated with dust in a pit. |
| 6 | Cutting, chiseling or finishing of rocks or minerals. (except operation site using sprinkler or oiling system) |
| 7 | Grinding by spraying abrasives or grinding or deflashing of rocks, minerals or metals, or cutting of metal by motive power with abrasives. |
| 8 | Cutting, crushing or screening of carbonic raw materials, or aluminum foil, by motive power. (except operation site using water, sprinkler or oiling system, or work in the open air) |
| 9 | Drying, packing into bags, loading or unloading of cement, pulverized ore, carbonic raw materials or carbonic products. |
| 10 | Packing of pulverized aluminum or titanium oxide into bags. |
| 11 | Mixing, adding or sprinkling of pulverized ore, carbonic raw materials or any other material containing them in the process of manufacturing or processing products. |
| 12 | Mixing raw material, or supplying a melting furnace with raw material or mixture, in the process of manufacturing glass or enamel. (except mixing raw materials in water) |
| 13 | Mixing or shaping raw materials, drying raw materials or unfinished products, loading carts with unfinished products or unloading from the carts, finishing or packing unfinished or manufactured products, or work of entering into a kiln, in the process of manufacturing ceramic ware, fire bricks, diatomaceous earthenware or abrasives. |
| 14 | Mixing or shaping carbonic raw materials, placing unfinished products into kilns, removing unfinished or manufactured products from kilns or finishing those products, in the process of manufacturing carbonic products. (except mixing raw materials in water) |
| 15 | Breaking of sand-molds, cleaning of castings, reclaiming of sand, tempering of sand or scraping off of flashes in the process of manufacturing castings with sand-molds. (except operation site using water, sprinkler or oiling system) |
| 16 | Scraping off minerals, etc. (except wet ones) or scratching them off in the hatch of a ship, which carries minerals, etc. (except wet ones). |
| 17 | Supplying a blast furnace with soil, sand, ores or minerals, reducing of them, steaming or casting in, in the process of smelting or melting metals or other inorganic material. |
| 18 | Scraping off, scratching up, loading or unloading or putting into vessels slag or ash adhering to or accumulating inside a furnace, flue, chimney, etc., or in the process of the combustion of pulverized ores or in the process of smelting or melting metals or other inorganic material. |
| 19 | Building/repairing or dismantling/destroying of kilns, furnaces, etc., built with firebricks. |
| 20 | Cutting metals by means of melting, arc welding indoors, in a pit or inside of the tank, vessel, pipes or vehicles, etc. |
| 21 | Work of metal spraying operations. |
| 22 | Warehousing of rushes covered with soil for dyeing, taking it out from the warehouse, selecting or regulating it, or weaving with it. |
| 23 | Unloading of ballast from hopper cars, or tamping of soil of a road with a multiple-tamper, in a long and large tunnel. |
| 24* | Unraveling, compounding, spinning, weaving, spraying, loading or unloading of asbestos, or placing one asbestos product upon another or stitching up, cutting, grinding, finishing or packing of asbestos products. |
With its enactment in 1960, the Pneumoconiosis Law established the Japanese classification system of pneumoconiosis chest radiographs equipped with its own standard chest films. The Japanese classification7) is almost parallel to "the ILO International Classification of Radiographs of Pneumoconioses" developed by the International Labour Organisation (ILO/ICRP)12), except for coding size of small irregular opacities and description of pleural abnormalities.
The Japanese system was designed to classify chest radiographs with no sign of pneumoconiosis as PR0 and those with pneumoconiotic abnormalities as PR1 to PR4. PR0 of the Japanese system is equivalent to Category 0 of the ILO/ICRP. The Japanese system codes radiographs with small opacities but no large opacities as PR1, PR2, and PR3 depending on increasing number (profusion) of small opacities. This is parallel to the ILO/ICRP's Category 1 to 3 for small opacities. Small opacities in pneumoconiosis chest radiograph refer to parenchymal opacities with size up to 10 mm. The system records small opacities in three increasing size ranges using the letters p, q, and r; however, the ILO/ICRP distinguishes two types of small opacities and labels small rounded opacities with p, q, and r and small irregular opacities with s, t, and u, where the letters p or s represent opacities with size up to 1.5 mm; q or t denote opacities size exceeding 1.5 mm and up to 3 mm; and r or u indicate opacities with size exceeding 3 mm and up to 10 mm. Small opacities profusion is recorded on a 12 points scale from 0/- to 3/+ (presented in Table 3) in both the ILO/ICRP and Japanese classification systems, in which 0/- indicates no trace of abnormality in both lungs and 3/+ signifies the highest concentration of small opacities. The Japanese system classifies radiographs with large opacities as PR4 and sub-categorizes into A, B, and C, depending on increasing size of the lesions. A large opacity in pneumoconiosis chest radiograph is defined as a parenchymal lesion with its longest diameter exceeding 10 mm. Category A defines the size of opacity of up to 50 mm, B indicates a size larger than 50 mm but less than one-third of one side of the lung field, and C specifies a size larger than one-third of one side of the lung field. In practice, the size of a large opacity is determined by measuring the longest diameter of a lesion in radiographs with a single large opacity or by summing the longest diameters of several large opacities and comparing it to the area in the upper third of the right lung field. This is parallel to the ILO/ICRP's categories A, B, and C of large opacities. For pleural abnormalities, the Japanese system has a minimal description of the presence or absence of pleural abnormalities; however, the ILO/ICRP system classifies these into pleural plaques (localized pleural thickening), costophrenic angle obliteration and diffuse pleural thickening12). Pleural plaques are discrete circumscribed areas of hyaline fibrosis of the parietal pleura and are the most common radiographic manifestation of past exposure to asbestos. The ILO/ICRP system records the presence of pleural plaque as in-profile, located at the lateral chest wall or face-on, located at anterior or posterior chest walls, and separately for the right and left sides. A minimum width of about 3 mm is required for an in-profile plaque to be recorded as present. Diffuse pleural thickening refers to thickening of the visceral pleura and is reported as present or absent along the chest wall. Diffuse pleural thickening extending up the lateral chest wall is reported only in the presence of, and in continuity with, an obliterated costophrenic angle. Costophrenic angle obliteration may occur without diffuse pleural thickening and is recorded as either present or absent, separately for the right and left side of lung fields. Table 3 summarizes the classification in the ILO/ICRP system and the Japanese system of small opacities and large opacities triggered by inhalation of dust in the chest of dust-exposed workers. As in the ILO/ICRP system, the Japanese system recognizes additional radiographic features of importance such as cancers, emphysema, pneumothorax, tuberculosis, etc. and reports these with respective symbols, such as 'ca' for cancer, for example.
Table 3.
Classification of pneumoconiosis chest radiograph: Japanese vs. ILO/ICRP
| Japanese classification of pneumoconiosis chest radiograph | ILO/ICRP | |||
|---|---|---|---|---|
| Radiographic appearances of pneumoconiosis | Category | |||
| ILO/ICRP=The ILO International Classification of Radiographs of Pneumoconioses developed by International Labour Organization Note: In Japan, chest radiographs of pneumoconiosis are recorded and reported as PR categories. The ILO/ICRP system classifies small rounded opacities as 'p', 'q', or 'r', and irregular opacities as 's', 't', or 'u' with increasing size of small opacities. Japanese system classifies small rounded opacities as 'p', 'q', or 'r'; however it has no specification for irregular opacities. Where the size of 'p' or 's' is up to 1.5 mm, 'q' or 't' is 1.5 mm - 3 mm, and 'r' or 'u' is 3 mm -10 mm. This table presented only the classification of small and large opacities. | ||||
| No radiographic abnormalities due to pneumoconiosis in both lung fields | PR0 (0/–, 0/0, 0/1) |
Category 0 (0/–, 0/0, 0/1) |
||
| Small opacities profusion in 12-points scale | Small number of rounded or irregular opacities in both lung fields and no large opacities | PR1 (1/0, 1/1, 1/2) |
Category 1 (1/0, 1/1, 1/2) |
|
| Large number of rounded or irregular opacities in both lung fields and no large opacities | PR2 (2/1, 2/2, 2/3) |
Category 2 (2/1, 2/2, 2/3) |
||
| Very large number of rounded or irregular opacities in both lung fields and no large opacities | PR3 (3/2, 3/3, 3/+) |
Category 3 (3/2, 3/3, 3/+) |
||
| Large opacities | Largest diameter of 1-5 cm | PR4 | A | A |
| 5cm<Largest diameter<1/3 of one side of lung field (upper 1/3 of right lung field) | B | B | ||
| Size of large opacity or opacities greater than 1/3 of one side of lung field (upper 1/3 of right lung field) | C | C | ||
The pneumoconiosis examination of chest radiographs is carried out by comparing subject films with standard films of various PR categories in pneumoconiosis. The Japanese standard film set is comprised of chest images with no abnormalities, images representing Classification PR1 to PR3 of small rounded or small irregular opacities, and images for Classification PR4 of large opacities7). Small rounded opacities usually seen in coal dust or silica-exposed workers, and small irregular opacities seen in asbestos or mixed-dust-exposed workers are recorded on a 12 points scale from 0/- to 3/+ in increasing order of profusion. Large opacities of coalescent small opacities or progressive massive fibrosis, usually accompanied with progression of silicosis, are recorded as A, B, and C according to their sizes. The presence of pleural abnormalities with or without pleural calcification is noted with designated symbols 'plc' and 'pl' respectively. The chest radiograph examined is finally reported in the relevant PR category, in accordance with the Japanese classification system of pneumoconiosis chest radiographs, for further determination of class for supervision of pneumoconiosis.
Usually, pneumoconiosis in its early stages has preserved pulmonary function. However, as the disease progresses, pulmonary function will deteriorate. Pulmonary function status in a pneumoconiosis health examination is assessed by questioning the extent of respiratory disability, performing spirometry, obtaining a flow-volume curve, and making a blood gas analysis if necessary7,13). Respiratory disability is quantified by using a breathlessness scale, known in Japan as Hugh-Jones or Fletcher-Hugh-Jones breathlessness scale. This breathlessness scale was originally devised and used by Fletcher and co-workers at the British Medical Research Council Pneumoconiosis Unit and later adopted into the British Medical Research Council (MRC) breathlessness scale14). This breathlessness scale comprises five statements that describe respiratory disability, which can be interpreted in increasing order of severity, from none (Grade 1), slight (Grade 2), moderate (Grade 3), severe (Grade 4) to almost complete incapacity (Grade 5).
In a spirometry assessment, pulmonary function parameters such as forced expiratory volume in one second (FEV1), forced vital capacity (FVC) and vital capacity (VC) are usually expressed as a percentage of predicted values. Predicted values are conventionally calculated from the measurements performed in reference groups, according to height, age, and gender15). Considering significant ethnic differences in normal pulmonary function values exist, and calculations derived from western population are not a good fit for Japanese populations16,17), an amendment to the Pneumoconiosis Law in 2010 introduced a change in prediction of pulmonary function parameters for the Japanese population13). This calculation was proposed by the Japanese Respiratory Society in 2001 and is expressed by the following equations18).
Predictive value of VC for male = 0.045 × height (cm) -0.023 × age -2.258 (L) and for female = 0.032 × height (cm) -0.018 × age -1.178 (L).
Predictive value of FEV1 for male = 0.036 × height (cm) -0.028 × age -1.178 (L) and for female = 0.022 × height (cm) -0.022 × age -0.005 (L).
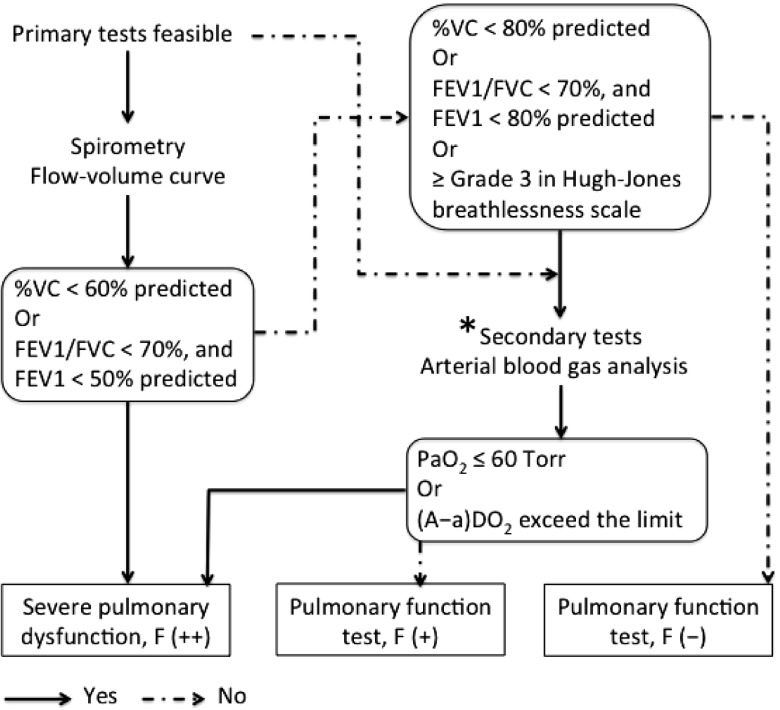
Pulmonary function status in a pneumoconiosis health examination is determined based on %VC (percentage of predicted), FEV1% (FEV1/FVC ratio) and values from arterial blood gas analysis, and reported as F (-), F (+) or F (++) (Fig. 1), where F (-) means absence of pulmonary function impairment; F (+) implies some impairment in pulmonary function which can be defined by %VC less than 80% but higher than 60% predicted or FEV1/FVC ratio less than 70% and FEV1 value between 50% and 80% predicted, or higher than Grade 3 in the Hugh-Jones breathlessness scale; F (++), which indicates a sufferer of severe pulmonary dysfunction, is defined as %VC less than 60% of predicted or FEV1/FVC ratio less than 70% and FEV1 less than 50% predicted. Workers who are incapable of performing spirometry or who have a pulmonary function status of F (+) from a primary assessment are subjected to arterial blood gas analysis for further investigation. Arterial blood gas analysis is conducted to obtain accurate measures of PaO2 (partial pressure of arterial O2), PaCO2 (partial pressure of arterial CO2), and arterial pH. Under normal conditions at sea level, the value for PaO2, irrespective of age, is greater than 80 Torr19). The alveolar-arterial oxygen tension difference (A-aDO2) is defined as the difference in value between the partial pressure of alveolar O2 (PAO2) and PaO2. For a subject at sea level and breathing room air, the A-aDO2 can be simplified as A-aDO2 = 150-PaO2-PaCO2/0.8, where A-aDO2 is normally less than 10 Torr but this value increases with age19). A higher than the limit value in A-aDO2, provided by the MHLW, or hypoxemia with a PaO2 value less than 60 Torr from arterial blood gas analysis in a pneumoconiosis health examination also indicates the presence of severe pulmonary dysfunction13).
Fig. 1.
Schematic flowchart for performing pneumoconiosis pulmonary function assessment
*Decision to perform secondary tests is made by examining physician based on the comprehensive assessment of pulmonary function test, radiographic findings, previous test results and other observations.
Note: Feasibility of primary tests means whether subjects are eligible to perform spirometry or not. The Hugh-Jones breathlessness scale (also known as Fletcher-Hugh-Jones breathlessness scale in Japan) describes respiratory disability from none (Grade 1) to almost complete incapacity (Grade 5), in which Grade 3 represent moderate respiratory disability. The normal value for the PaO2, irrespective of age, is greater than 80 Torr; PaO2 value of less than 60 Torr is generally considered as severe hypoxemia. The A-aDO2 for a subject at sea level and breathing room air is normally less than 10 Torr but the value increases with age. The reference A-aDO2 limit values for Japanese population is provided by the Ministry of Health, Labour and Welfare of Japan.
In compliance with the measures for supervision of health under the Pneumoconiosis Law, workers with complications related to pneumoconiosis shall be subject to medical treatment and mandatory rest from work. Complication of pneumoconiosis is defined, according to Pneumoconiosis Law, as diseases recognized as closely related to pneumoconiosis due to disease progression2). Recognizing the carcinogenicity of crystalline silica, in 2003, primary lung cancer20) was officially stated as a complication of pneumoconiosis, and the current list includes six types of disease, namely, pulmonary tuberculosis, tuberculous pleuritis, secondary pneumothorax, secondary bronchitis, secondary bronchiectasis, and primary lung cancer3). A decision to rule out complications and arrange tests for blood examination, sputum cytology and culture, computed tomography (CT) for the chest, etc. is made by examining physicians. Examination for pulmonary tuberculosis and tuberculous pleuritis includes a tuberculin skin test, chest CT, smear and culture for acid-fast bacilli from sputum, and pleural exudate as necessary. Secondary bronchitis is defined clinically as having daily coughing with sputum that lasts for at least three months in a year, and more than 3 ml of purulent sputum production within 1 hour of getting up. Sputum cytology and chest spiral CT may assist in suspicious primary lung cancer.
Under the Pneumoconiosis Law, a scheme for pneumoconiosis health examination for current and past dust-exposed workers is established and administered through periodic medical examinations based on the pneumoconiosis supervision class (Table 4). For workers who currently and regularly engaged in dust-exposed work, the examination is carried out every 3 years for individuals with no current evidence of pneumoconiosis, and once a year for those classified as supervision Class II or III. For workers who have been exposed to dust in the past but have no current exposure, the arrangement for examinations will be every 3 years for supervision Class II and once a year for Class III2). For present and past workers who handle asbestos as well as indirectly exposed workers, a follow-up examination is arranged twice a year21), and the medical examination is specified under the designated dust-exposed works of Pneumoconiosis Law2) and also by the Ordinance on Prevention of Health Impairment due to Asbestos22) of 2005.
Table 4.
Scheme of periodic pneumoconiosis health examination for current and past dust-exposed workers
| Exposure history | Classification for supervision | Scheme of medical examination |
|---|---|---|
| Regularly engaged in dust-exposed work | Class I | Every 3 years |
| Class II, III | Once a year | |
| Past exposure to dust, but no current exposure | Class II | Every 3 years |
| Class III | Once a year |
Current state of pneumoconiosis in Japan
Pneumoconiosis in Japan is detected primarily through examining chest radiographs of dust-exposed workers in periodic pneumoconiosis health examinations. With recent advances in medical knowledge and technologies, diagnosis methods have changed with the times and the regulations have been revised as well. With the increasing use of CT or high resolution computed tomography (HRCT) for personal diagnosis of pneumoconiosis, a classification system of CT for pneumoconiosis was developed in 1995 and provides a pneumoconiosis classification complement to conventional radiography23). Digital systems, computed radiography (CR) that use a photostimulable phosphor plate for detection of X-rays, and digital radiography using flat panel detectors (FPD DR) to convert X-rays into electrical charges, are rapidly replacing conventional film-based systems in Japanese health facilities. Because of this technology shift, in Japan, CR was officially approved for its use in medico-legal judgments for pneumoconiosis in 200124), and the FPD DR was approved for the same purpose in 200725,26). Accordingly, in 2011 MHLW developed a new set of standard pneumoconiosis chest images27). This new version included hard copy digital images and electronic images (DVD-ROM). Reference CT images were also included in the electronic images.
The Japanese government recommends that workers with pneumoconiosis take an annual spiral CT examination in order to screen for lung cancer, and the Pneumoconiosis Law was revised to include screening for lung cancer as a part of the pneumoconiosis examination in 200320). Based on findings from lung cancer low-dose CT screening studies, such as the US national lung screening trial (NLST)28), internationally, the Helsinki criteria29,30) for diagnosis of asbestos-related diseases recommended screening with low-dose CT for early detection of lung cancer in asbestos-exposed workers with sufficiently high risk of lung cancer. The Helsinki criteria also recommend the use of "International Classification of HRCT for Occupational and Environmental Diseases" (ICOERD)31) for international comparison of studies of asbestos-related non-malignant diseases. The ICOERD has been developed and its reliability has been well validated for the screening, diagnosis and epidemiological reporting of respiratory diseases caused by occupational and environmental factors32). In Japan, use of low-dose CT in lung cancer screening33) was initiated in 1993, and academic societies, such as the Japanese Society of CT Screening, published guidelines34) for pulmonary nodules management in low-dose CT lung cancer screening. However, its use in screening lung cancer is not recognized officially by the Japanese government at the present time. Studies established the superiority of HRCT compared to that of conventional radiography in the identification of early dust-related fibrotic changes in the lung35,36) and pulmonary complications35,37); the associated high technical requirements, financial costs, and higher radiation exposure limits its use in routine pneumoconiosis health examination of dust-exposed workers.
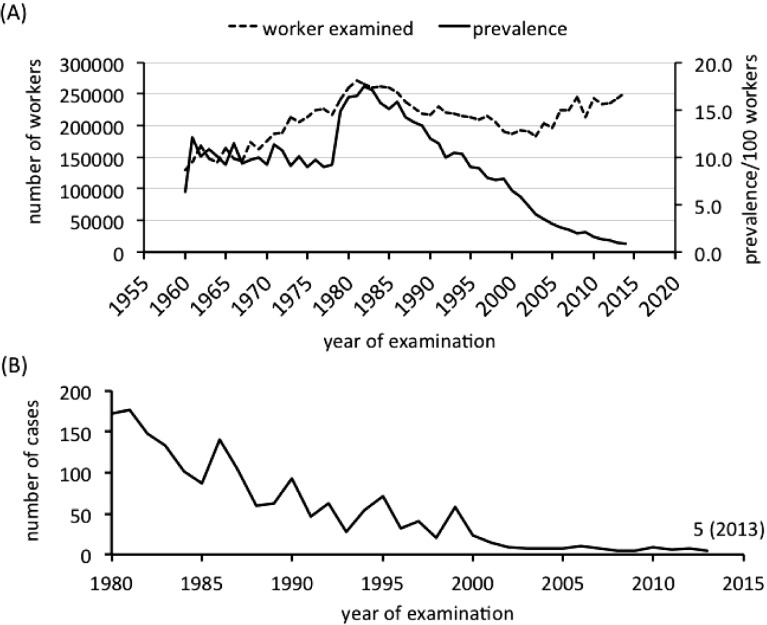
According to the statistics released by the MHLW, following the passage of the Pneumoconiosis Law in 1960, periodic pneumoconiosis health examinations were performed in 129,776 workers, and pneumoconiosis was reported in 8,274 workers, 6.4% of the total. Thereafter, the number of workers examined increased steadily each year, and the number of detected cases of pneumoconiosis increased as well, though with some fluctuation, until a sharp rise occurred in 1979. A large number of workers were identified as having pneumoconiosis around the 1980s. The year 1982 marked the highest number of cases, with pneumoconiosis identified in 46,235 workers, or 17.4% of the 265,720 examinations performed. After reaching its peak in the 1980s, the prevalence of pneumoconiosis showed a downward trend. Although until 1994 the figure remained above 10% of the examinations performed, it fell to 1% in 2013 periodic pneumoconiosis health examination, where 243,740 workers were examined (Fig. 2A). The sudden rise in the number of cases in the 1980s was partly due to changes made to the supervision class through the 1977 amendment to the Pneumoconiosis Law. Japanese classification for pneumoconiosis supervision classifies workers affected with pneumoconiosis into pneumoconiosis supervision Class II or higher, and workers who currently engage in dust-exposed work on a regular basis and have been classified as pneumoconiosis supervision Class II are obliged by the Pneumoconiosis Law to take a pneumoconiosis health examination every year. From 1978, in order to detect disease in its early stages by administering a close follow-up medical examination, subjects with borderline pneumoconiosis, i.e., radiographic appearance between PR0 and PR1, which are classified previously as supervision Class I, are classified into supervision Class II38). Available data showed the number of new cases of pneumoconiosis fell each year. Year 1980 detected 6,842 new cases, and the number dropped to 227 cases in 2013. Cases classified as supervision Class IV, indicating severe pneumoconiosis, and workers detected as having pneumoconiosis complications, have fallen over the years. One hundred seventy two workers were diagnosed as having pneumoconiosis complications in 1980; however, the number fell to five in the 2013 periodic pneumoconiosis health examinations (Fig. 2B). On the other hand, 870 cases were diagnosed as affecting pneumoconiosis from the occasional pneumoconiosis examination, conducted in 2013, for applications from individual workers with a current or past dust exposure history. Among these, 156 cases were classified as pneumoconiosis supervision Class IV, and pneumoconiosis complications were detected in 161 workers. Taking this into consideration, the failure of workers to receive a health examination and the retirement or death of aged workers may also have contributed to the overall reduction in the number of cases detected through recent periodic pneumoconiosis health examinations. One recent study conducted among Japanese ex-tunnel workers showed silicosis progressed at a relatively higher rate and presented an unusual progression pattern in radiographic findings, i.e., directly from PR1 to PR4, even after silica exposure had been discontinued39). In this regard, it is advisable to conduct pneumoconiosis health examinations and regular follow-ups for a long period in workers with past exposure history to relatively high dust concentrations.
Fig. 2.
Changes in the number of workers with pneumoconiosis and pneumoconiosis complications (A) Number of examined workers and pneumoconiosis prevalence between 1960 and 2013 (B) Number of workers with pneumoconiosis complication between 1980 and 2013
Note: Pneumoconiosis cases are classified as pneumoconiosis supervision class II or higher. Pneumoconiosis law was amended in 1977, and from 1978 cases with borderline pneumoconiosis, i.e. radiographic appearance between PR0 and PR1 were classified into supervision class II. Calculation in the number of pneumoconiosis cases before 1980 included cases from occasional pneumoconiosis examination applied by individual engaged in dust-exposed work. Pneumoconiosis complications include pulmonary tuberculosis, tuberculous pleuritis, secondary pneumothorax, secondary bronchitis, secondary bronchiectasis, and primary lung cancer.
Source: Results of periodic pneumoconiosis examination released by Ministry of Health, Labour and Welfare
Although the overall incidence of pneumoconiosis has decreased in recent years, Japanese articles have documented the occurrence of pneumoconiosis among welders40-43) and Takigawa et al.43) noted that welders are at higher risk of contracting pneumoconiosis compare to other occupations. However, the actual picture of welders' pneumoconiosis in the Japanese welding industry cannot be assessed, since most articles are reporting radiographic abnormalities in the chest of an individual welder40-42), and epidemiology studies conducted specific to the Japanese welding business are scarce44). On the other hand, exposure to welding fumes is reported to be a risk factor for chronic respiratory disorders45) and pneumoconiosis46); generally, the respiratory effects of welding are considered reversible if exposure to dust is discontinued. From the standpoint of prevention of welders' pneumoconiosis, early diagnosis is considered essential.
In fact, new cases of lung diseases have emerged from occupations previously not known to be related to a serious health hazard. The first case of interstitial pneumonia triggered by the inhalation of indium compounds (indium tin oxide in this case), known as indium lung, was reported in 200347). As the use of indium compounds increases from their use in optoelectronic technologies, such as in the manufacturing of optical semiconductors, liquid crystal displays, and plasma displays, and from the rapid progress in development and use of nanomaterials, the number of workers engaged in such occupations is expected to increase. The carcinogenicity and chronic toxicity study in small animals48) and causal association between exposure and effects on the human lungs49), human hazards due to indium compounds, such as indium tin oxide, indium oxide, indium hydroxide, and indium chloride exposure has been substantially established. In addition, a recent study conducted on indium metal processing worksites, such as plants for the manufacturing of indium alloy, lead-free solder, dental materials, etc., revealed a dose-effect relationship between serum indium levels (In-S) and Krebs von den Lungen-6 (KL-6) levels in workers currently exposed to indium metal50). However, current studies provide limited evidence on potential health effects of nanomaterials.
Currently, The Japan Society of Occupational Health (JSOH) recommended that workers diagnosed with indium lung submit health checkups once or twice a year, which would include measurement of In-S as an exposure index, and serum KL-6, a sensitive marker of interstitial lung disease, as an adverse pulmonary effect parameter. JSOH further recommended examinations with chest HRCT and spirometry at least once to reveal irreversible effects on the lungs, to be followed-up, if necessary51). In addition, the Japanese government established prevention guidelines52) for workers exposed to indium tin oxide and other indium compounds in 2010, and added indium compounds to the list of substances regulated by the Ordinance on Prevention of Hazards due to Specified Chemical Substances53) in 2013. On the other hand, little is known about the actual exposure status and adverse effects of nanomaterials on human health; as a precautionary approach, the MHLW issued a "Notification on Precautionary Measures for Prevention of Exposure etc. to Nanomaterials" to increase the efficacy of preventive measures against exposure to nanomaterials54).
Conclusion
In order to increase the efficiency of protecting workers' health, it is important to maintain workplace health management in addition to workplace dust exposure management. The Pneumoconiosis Law of 1960 laid a comprehensive health management regimen for workers engaged in dust-exposed works. The law focuses primarily on pneumoconiosis health examinations to detect the disease in its early stage and then to provide proper health supervision. In the past, pneumoconiosis was a major cause of mortality and morbidity in Japanese workers engaged in dust-exposed works. The achievement of Japanese pneumoconiosis prevention can be credited to a comprehensive provision for worker health, regulated by a thoroughgoing legal framework.
Conflicts of interest: None
References
- 1). Takahashi K, Ishii Y. Historical developments of administrative measures for occupational diseases in Japan: A report commissioned by the ILO 10 July 2013. [Google Scholar]
- 2). Ministry of Health, Labour and Welfare Pneumoconiosis law. Law No. 30 of March 31, 1960. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://law.e-gov.go.jp/htmldata/S35/S35HO030.html (in Japanese).
- 3). Ministry of Health, Labour and Welfare Enforcement Ordinance of Pneumoconiosis Law. Ministry of Labour Ordinance No. 6 of March 31, 1960. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://law.e-gov.go.jp/htmldata/S35/S35F04101000006.html (in Japanese).
- 4). Ministry of Health, Labour and Welfare Industrial safety and health law. Law No. 57 of June 8, 1972. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://law.e-gov.go.jp/htmldata/S47/S47HO057.html (in Japanese).
- 5). Ministry of Health, Labour and Welfare Working environment measurement law. Law No. 28 of May 1, 1975. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://law.e-gov.go.jp/htmldata/S50/S50HO028.html (in Japanese).
- 6). Ministry of Health, Labour and Welfare Ordinance on Prevention of hazards due to dust. Ministry of Labour Ordinance No. 18 of April 25, 1979. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://law.e-gov.go.jp/htmldata/S54/S54F04101000018.html (in Japanese).
- 7). Ministry of Labour Handbook of pneumoconiosis health examination. Labour board of health, Industrial safety and health department, Ministry of Labor. Tokyo, Japan: Government of Japan. [Online]. 1979[cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/shingi/2010/04/dl/s0420-5b.pdf (in Japanese).
- 8). Kogi K, Suzuki H. Workers' Accident Compensation in Japan. In: Lesage M, Rey P, International Labor Organization , editors. Chapter 26. Workers' Compensation Systems, Topics in. Geneva: ILO Encyclopedia of Occupational Health and Safety; Jeanne Mager Stellman; 2011. [Google Scholar]
- 9). ERCA Operation of the asbestos-related health damage relief system. Environmental restoration and conservation agency of Japan. Home page (English). [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.erca.go.jp/erca/english/activities/ac_09.html
- 10). Ministry of Health, Labour and Welfare Outline of the act on asbestos health damage relief. Tokyo, Japan: Government of Japan. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/english/wp/wp-hw5/dl/23010405e.pdf
- 11). Dust-exposed work. In: Enforcement Ordinance of Pneumoconiosis Law. Ministry of Labour Ordinance No. 6 of March 31, 1960. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.ilo.org/dyn/natlex/docs/ELECTRONIC/29320/69506/F1079186294/JPN29320.pdf
- 12). ILO Guidelines for the use of the ILO International Classification of Radiographs of Pneumoconioses, Revised Edition 2011. Occupational Safety and Health Series No. 22. Geneva: International Labour Office; 2011. [Google Scholar]
- 13). Ministry of Health, Labour and Welfare Pulmonary function assessment in pneumoconiosis. Labour Standard Bureau. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://anzeninfo.mhlw.go.jp/anzen/hor/hombun/hor1-51/hor1-51-26-1-0.htm (in Japanese).
- 14). Miyamoto K. Confusion about MRC dyspnea scales in Japan-Which MRC dyspnea scale should we employ?-. Nihon Kokyuki Gakkai Zasshi 2008; 46: 593-600 (in Japanese). [PubMed] [Google Scholar]
- 15). Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948-968. [DOI] [PubMed] [Google Scholar]
- 16). Aoki M, Osanai S, Ogasa T, et al. Comparison between predicted equations obtained by standard Japanese values and present predicted equations for vital capacity and forced expiratory volume in one second. Nihon Kokyuki Gakkai Zasshi 2010; 48: 357-363 (in Japanese). [PubMed] [Google Scholar]
- 17). Nakamura M, Takahashi Y, Ohrui T, et al. Criteria of impairment of pulmonary function using Japanese standard values. Nihon Kokyuki Gakkai Zasshi 2002; 40: 925-928 (in Japanese). [PubMed] [Google Scholar]
- 18). The Japanese respiratory society lung physiology expert committee Reference values of spirogram and partial pressure of arterial blood gas in Japanese. [Online]. 2001[cited 2016 Sep. 14]; Available from: URL: http://www.jrs.or.jp/modules/guidelines/index.php?content_id=12 (in Japanese).
- 19). The Japanese respiratory society Guidelines for respiratory function assessment II: Blood gases, pulse oximeter. Tokyo: Medical review; 2006. p. 24. [Google Scholar]
- 20). Ministry of Health, Labour and Welfare Amendment to Pneumoconiosis law and Industrial safety and health law. 2003. Tokyo, Japan: Government of Japan. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/shingi/2002/11/s1118-5d.html (in Japanese).
- 21). Ministry of Health, Labour and Welfare Medical examination scheme for asbestos-exposed workers. Tokyo, Japan: Government of Japan. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/new-info/kobetu/roudou/sekimen/iryo/ (in Japanese).
- 22). Ministry of Health, Labour and Welfare Ordinance on Prevention of Health Impairment due to Asbestos. Ministry of Health, Labour and Welfare Ordinance No. 21 of February 24, 2005. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://law.e-gov.go.jp/htmldata/H17/H17F19001000021.html (in Japanese).
- 23). Suganuma N, Kusaka Y, Hosoda Y, et al. The Japanese classification of computed tomography for pneumoconioses with standard films: Comparison with the ILO international classification of radiographs for pneumoconioses. J Occup Health 2001; 43: 24-31. [Google Scholar]
- 24). Ministry of Health, Labour and Welfare Computed Radiography (CR) chest radiographic image in pneumoconiosis health supervision. Labour Standard Bureau. [Online]. 2001[cited 2016 Sep. 14]; Available from: URL: http://anzeninfo.mhlw.go.jp/anzen/hor/hombun/hor1-42/hor1-42-47-1-0.htm (in Japanese).
- 25). Ministry of Health, Labour and Welfare Flat Panel Detectors Digital Radiography (FPD DR) chest radiographic image in pneumoconiosis health examination and health supervision. Labour Standard Bureau. [Online]. 2007[cited 2016 Sep. 14]; Available from: URL: http://anzeninfo.mhlw.go.jp/anzen/hor/hombun/hor1-48/hor1-48-58-1-0.htm (in Japanese).
- 26). Suganuma N, Murata K, Kusaka Y. CR and FPD DR chest radiographic image parameters for the pneumoconiosis: The Japanese approach and experience. NIOSH Scientific Workshop: Application of the ILO International Classification of Radiographs of Pneumoconiosis to Digital Chest Radiographic Images, March 12-13, 2008. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.cdc.gov/niosh/docs/2008-139/pdfs/manuscript-suganuma-imageparameters.pdf
- 27). Ministry of Health, Labour and Welfare Standard digital radiographs of pneumoconiosis. 2011. Tokyo, Japan. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/stf/shingi/2r9852000001tz0w-att/2r9852000001tz4x.pdf (in Japanese).
- 28). National Lung Screening Trial Research Team The national lung screening trial: Overview and study design. Radiology 2011; 258: 243-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Wolff H, Vehmas T, Oksa P, Rantanen J, Vainio H. Asbestos, asbestosis, and cancer, the Helsinki criteria for diagnosis and attribution 2014: recommendations. Scand J Work Environ Health 2015; 41: 5-15. [DOI] [PubMed] [Google Scholar]
- 30). Finnish Institute of Occupational Health Asbestos, Asbestosis, and Cancer. In: Oksa P, Wolff H, Vehmas T, Pallasaho P, Frilander H, editors. Helsinki criteria for diagnosis and attribution 2014. 2014. [DOI] [PubMed] [Google Scholar]
- 31). Kusaka Y, Hering KG, Parker JE. International classification of HRCT for occupational and environmental respiratory diseases. Tokyo: Springer; 2005. [Google Scholar]
- 32). Suganuma N, Kusaka Y, Hering KG, et al. Reliability of the proposed international classification of high-resolution computed tomography for occupational and environmental respiratory diseases. J Occup Health 2009; 51: 210-222. [DOI] [PubMed] [Google Scholar]
- 33). Nawa T, Nakagawa T, Mizoue T, Endo K. Low-dose computed tomography screening in Japan. J Thorac Imaging 2015; 30: 108-114. [DOI] [PubMed] [Google Scholar]
- 34). The Pulmonary Nodules Management Committee of the Japanese Society of CT Screening Guidelines for the Management of Pulmonary Nodules Detected by Low-dose CT Lung Cancer Screening Version 3. The Japanese Society of CT Screening. [Online]. 2013[cited 2016 Sep. 14]; Available from: URL: http://www.jscts.org/pdf/guideline/gls3rd_english130621.pdf
- 35). Sun J, Weng D, Jin C, et al. The value of high resolution computed tomography in the diagnostics of small opacities and complications of silicosis in mine machinery manufacturing workers, compared to radiography. J Occup Health 2008; 50: 400-405. [DOI] [PubMed] [Google Scholar]
- 36). Savranlar A, Altin R, Mahmutyazicioglu K, et al. Comparison of chest radiography and high-resolution computed tomography findings in early and low-grade coal worker's pneumoconiosis. Eur J Radiol 2004; 51: 175-180. [DOI] [PubMed] [Google Scholar]
- 37). Jun JS, Jung JI, Kim HR, et al. Complications of pneumoconiosis: Radiologic overview. Eur J Radiol 2013; 82: 1819-1830. [DOI] [PubMed] [Google Scholar]
- 38). Ministry of Labour Enforcement of the revised Pneumoconiosis Law. No. 250 of April 28, 1978. Labour Standard Bureau. Ministry of Labour. Tokyo, Japan: Government of Japan. [Online]. [cited 2016 Sep. 14]; Available from: URL: http://www.joshrc.org/~open/kijun/std05-250.htm (in Japanese).
- 39). Dumavibhat N, Matsui T, Hoshino E, et al. Radiographic Progression of Silicosis among Japanese Tunnel Workers in Kochi. J Occup Health 2013; 55: 142-148. [DOI] [PubMed] [Google Scholar]
- 40). Nabe Y, Nakagawa M, Hanagiri T, et al. A case of enlarging welder's lung requiring differential diagnosis from lung cancer. Jpn J Chest Surg 2014; 28: 75-79 (in Japanese). [Google Scholar]
- 41). Yokoyama T, Aoshima M, Kurakawa E, et al. A case of arc welder's lung with ground-glass opacities and progressive massive fibrosis. Nihon Kokyuki Gakkai Zasshi 2005; 43: 302-307 (in Japanese). [PubMed] [Google Scholar]
- 42). Ishida Y, Sera K, Ohta K, Kageshita T. A case of rapid development of arc welder's lung during the course of a year. Nihon Kokyuki Gakkai Zasshi 2003; 41: 351-355 (in Japanese). [PubMed] [Google Scholar]
- 43). Takigawa T, Kishimoto T, Nabe M, et al. The current state of workers' pneumoconiosis in relationship to dusty working environments in Okayama Prefecture, Japan. Acta Med Okayama 2002; 56: 303-308. [DOI] [PubMed] [Google Scholar]
- 44). Saito H, Kubota H, Hisanaga N, et al. Survey on the health impact of welding work: Findings from construction workers. JNIOSH-SRR-No. 41-2-1. [Online]. 2011[cited 2016 Oct. 17]; Available from: URL: http://www.jniosh.go.jp/publication/srr.html (in Japanese).
- 45). Nakadate T, Aizawa Y, Yagami T, Zheg YQ, Kotani M, Ishiwata K. Change in obstructive pulmonary function as a result of cumulative exposure to welding fumes as determined by magnetopneumography in Japanese arc welders. Occup Environ Med 1998; 55: 673-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Yoshii C, Morimoto Y, Kido M. Welder's pneumoconiosis- Pulmonary disease caused by inhalation of welding fumes. J Aerosol Res 2005; 20: 238-242. [Google Scholar]
- 47). Toshiaki H, Ueno T, Sekizawa K, Tanaka A, Hirata M. Interstitial pneumonia developed in a worker dealing with particles containing indium-tin oxide. J Occup Health 2003; 45: 137-139. [DOI] [PubMed] [Google Scholar]
- 48). Nagano K, Nishizawa T, Umeda Y, et al. Inhalation carcinogenicity and chronic toxicity of indium-tin oxide in rats and mice. J Occup Health 2011; 53: 175-187. [DOI] [PubMed] [Google Scholar]
- 49). Nakano M, Omae K, Tanaka A, et al. Causal relationship between indium compound inhalation and effects on the lungs. J Occup Health 2009; 51: 513-521. [DOI] [PubMed] [Google Scholar]
- 50). Nakano M, Tanaka A, Hirata M, Iwasawa S, Omae K. Pulmonary effects in workers exposed to indium metal: A cross-sectional study. J Occup Health 2015; 57: 346-352. [DOI] [PubMed] [Google Scholar]
- 51). Omae K, Nakano M, Tanaka A, Hirata M, Hamaguchi T, Chonan T. Indium lung-case reports and epidemiology. Int Arch Occup Environ Health 2011; 84: 471-477. [DOI] [PubMed] [Google Scholar]
- 52). Ministry of Health, Labour and Welfare Technical guideline for preventing health impairment of workers engaged in the indium tin oxide handling processes. Tokyo, Japan: Government of Japan. [Online]. 2010[cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/bunya/roudoukijun/anzeneisei42/dl/03.pdf
- 53). Ministry of Health, Labour, and Welfare Amendment to Ordinance on Industrial Safety and Health Law and to Ordinance on Prevention of Hazards due to Specified Chemical Substances. Tokyo, Japan: Government of Japan. [Online]. 2013[cited 2016 Sep. 14]; Available from: URL: http://www.mhlw.go.jp/bunya/roudoukijun/anzeneisei48/dl/anzeneisei48-01.pdf (in Japanese).
- 54). Ministry of Health, Labour and Welfare Measures for prevention of exposure etc. to nanomaterials at workplaces. LSB Notification No. 0331013. March 31st, 2009. Tokyo, Japan: Government of Japan. [Online]. [cited 2016 Sep. 14]; Available from: URL: https://www.jniosh.go.jp/publication/doc/houkoku/nano/files/mhlw/Notification_0331013_en.pdf