Abstract
Objectives:
In this study, we focused on the qualitative and quantitative differences of the lung lesions induced by single or multiple intratracheal administration of nickel oxide nanoparticles (NiO).
Methods:
Male rats were randomized into groups receiving intratracheal administrations in a single dose or two to four divided doses of 2 mg/kg/bw. Bronchoalveolar lavage fluid (BALF) analyses were performed at 3 and 28 d post-dose. Histopathological analyses were performed at 28 and 91 d post-dose.
Results:
BALF analyses revealed pulmonary injury, inflammation, and increases in the parameters indicating processing the foreign material in all the NiO-treated groups. Histopathological analyses showed the phagocytosis of NiO by alveolar macrophages, degeneration and necrosis of alveolar macrophages, and inflammatory responses. In the comparison between single and multiple administrations, the trend for stronger toxicity effects was observed after multiple application at 3 d post-dose, while the obvious toxicity effects were also seen in case of single administration. No particular differences of lung lesions depending on the frequency of administration at 28 and 91 d post-dose were evident.
Conclusion:
Intratracheal NiO administration induced strong toxic response thoroughly even by single administration. Therefore, single administration was concluded to be applicable to assess the inhalation toxicity of nanomaterials and can be used in the screening test.
Keywords: BALF, Intratracheal administration, Lung toxicity, Nanomaterial, Nickel oxide, Rat
Introduction
At present, the Organisation for Economic Cooperation and Development (OECD) has been gathering toxicological information about 11 types of nanomaterials as dossiers1). Individual nanomaterials are likely to have different toxicities. Therefore, it is essential to assess the toxicity, especially inhalation toxicity of each nanomaterial, because workers are mainly exposed to them through inhalation. However, it is extremely difficult to conduct inhalation exposure tests to assess the toxicity of all industrial nanomaterials, which have developed rapidly at present, due to the cost, inhalation technique, institution, etc. Therefore, the development of an easy and convenient screening method to detect the toxicity of nanomaterials is highly desired.
The intratracheal administration method readily offers a result of toxicity assessment in a brief and convenient manner, while the dose has been established conveniently, as compared with the inhalation exposure test. Therefore, this method has been frequently used for toxicity assessment of nanomaterials2-16). However, this method should be standardized to apply it for toxicity screening of industrial nanomaterials. Thus far, only few reports have been published, which report the existing differences in toxicity depending on the frequency of intratracheal administration. Therefore, it is important to investigate those differences as a basic point of risk assessment to apply intratracheal administration for screening2). In addition, NiO nanoparticles were reported to induce inflammation in the lung of rats by a single intratracheal administration5-16).
In this study, we focus on the differences of the lung lesions and their number induced by single and multiple NiO administrations in rats, as well as their implication in toxicity assessment.
Materials and Methods
Test substance
In this experiment, nickel oxide nanoparticles (NiO, US3352, US Research Nanomaterials, Inc. Texas, USA) were used as nanomaterial samples. NiO was ≥99.98% in purity, 20 nm in average primary particle size, spherical in shape, and hydrophilic. NiO samples were prepared by dispersing NiO in ultrapure water by Nanosystem Research Institute, The National Institute of Advanced Industrial Science and Technology (AIST), Tsukuba, Japan. NiO was dispersed using ultrasonic wave (3 h) to a concentration of 10 mg/ml. Then, the dispersion was centrifuged (×1000, 25 min) and the supernatant was diluted to predetermined concentrations. The NiO particle was well dispersed and the secondary particle size was 37 to 68 nm (Number-based average, analyzed by dynamic light scattering). The concentrations of NiO particles were analyzed by the gravimetric measurement. The established concentrations were 0.50, 0.67, 1.0, and 2.0 mg/ml and the actual concentrations were 0.49, 0.67, 1.0, and 2.0 mg/ml, respectively.
Animals and husbandry
Male F344/DuCrlCrlj rats (SPF) (Chares River Laboratories Japan, Inc., Atsugi, Japan) were used (total, 245 rats). The animals, 11-week-old at arrival, were quarantined and acclimated for 1 week. Breeding conditions of the animal room were 23±2°C in temperature and 55±15% in humidity. The animals were fed CRF-1 pellet diet (sterilized by gamma-ray irradiation, Oriental Yeast Co., Ltd., Tokyo, Japan) and given filtered and ultraviolet-irradiated municipal water ad libitum. The present studies were conducted in compliance with the basic policy on the conduct of animal experiments in institutions under the control of the Japanese Ministry of Health, Labour and Welfare (Notification by Director of Health Sciences Division, Minister's Secretariat, MHLW) and the policies on animal experiments (established by the Japan Bioassay Research Center; JBRC). In addition, this study was approved by the Animal Experiment Committee of JBRC. The animals were taken care of in accordance with the Guide for Animal Experimentation (Japanese Association for Laboratory Animal Science).
Experimental design
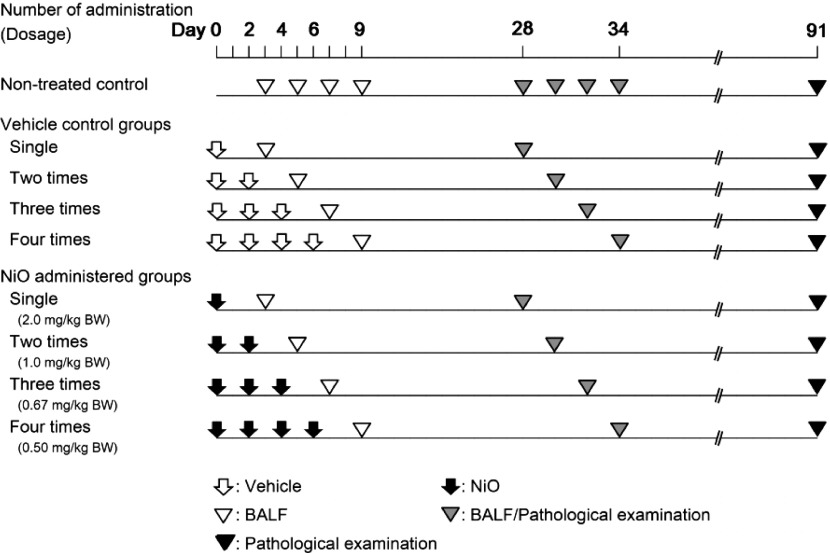
The study design is presented in Fig. 1. The male rats (12-week-old, mean body weight of 250 g) were divided into four NiO-treated groups and four vehicle-control groups, which corresponded to the respective NiO-treated groups. In addition, four untreated control groups were established. The NiO-treated animals were divided into four groups: A group receiving intratracheal administration of a single dose of 2 mg/kg/bw, a group receiving 2 doses of 1 mg/kg/bw, a group receiving 3 doses of 0.67 mg/kg/bw, and a group receiving 4 doses of 0.5 mg/kg/bw. The total NiO doses of each group were equal. The dispersed NiO were administered within 1 minute after ultrasonication. The multiple-administration groups were treated with NiO samples every other day. The animals in the vehicle-control groups were administered ultrapure water intratracheally at the same frequency and timing as those of the NiO-treated groups. Intratracheal administration was not performed in the untreated control groups. Bronchoalveolar lavage fluid (BALF) analyses were performed at 3 and 28 d post-dose. Histopathological examinations were performed at 28 and 91 d post-dose (Fig. 1).
Fig. 1.
Study design
Multiple intratracheal administrations were performed every other day. Bronchoalveolar lavage fluid (BALF) test at 3 d post-dose, BALF analysis and pathological examination at 28 d post-dose, and pathological examination at 91 d post-dose were performed. The control groups (untreated-control and vehicle-control groups) corresponding to each autopsy period were established.
Intratracheal administration
NiO was administered intratracheally under isoflurane (2-3%) inhalation anesthesia using a 1-ml disposable syringe and MicroSprayer (Penn-Century, Inc: IA-1B-R). The administration volume per animal was 1 ml/kg/bw.
Clinical observations, body weights, and hematological and blood biochemical analyses
Clinical signs were evaluated every day in all rats. Hematological and blood biochemistry analyses were performed at 28 and 91 d post-dose. The number of rats at each time-point was five animals per group; however, at 28 d post-dose, the number of rats was ten animals per group. Hematology included parameters of RBC, hemoglobin, hematocrit, MCV, MCH, MCHC, platelet, reticulocytes, white blood cells, and differential leukocytes count. Blood biochemistry included parameters of total protein, albumin, A/G ratio, total bilirubin, glucose, total cholesterol, triglyceride, phospholipid, Aspartate transaminase (AST), Alanine transaminase (ALT), Lactate Dehydrogenase (LDH), Alkaline Phosphatase (ALP), γ-glutamyltransferase (γ-GTP), creatine kinase, urea nitrogen, creatinine, sodium, potassium, chloride, calcium, inorganic phosphorus, and total bile acid17,18).
Cytological and biochemical analyses of bronchoalveolar lavage fluid (BALF)
BALF analyses were performed in all lung lobes of five rats in each group at 3 and 28 d post-dose. The animals were anesthetized by the intraperitoneal injection of pentobarbital sodium (Somnopentyl, Kyoritsuseiyaku Corporation, Tokyo, Japan) and euthanized by exsanguination from the abdominal aorta under anesthesia. BALF was collected using the following procedure. After injecting 7 ml of saline to the lung from a height of 30 cm, BALF was collected by spontaneous dripping. This procedure was repeated two times. In all the groups, the BALF collector rate was about 80%. With regard to the cytological examination of BALF, total cells were counted with an automatic cell analyzer, and smear preparations were made for the determination of cell classification (neutrophil, lymphocyte, alveolar macrophage, eosinophil, and basophil). With regard to biochemistry, BALF was centrifuged and the total protein, albumin, LDH, ALP and γ-GTP levels were examined17,18).
Organ weights and histopathological examinations
Organ weights of the liver, kidneys, lungs, spleen, and brain were measured. Furthermore, histopathological analyses were performed at 28 and 91 d post-dose in the liver, kidneys, lungs, spleen, brain, and pulmonary-related lymph nodes (posterior mediastinal lymph node and parathymic lymph node). The number of rats was the same as that in hematological analyses.
Results
In the untreated control and vehicle-control groups, no particular differences in any of the laboratory parameters were found. Therefore, the results are presented below by comparing the data obtained for the vehicle-control and NiO-treated groups.
Clinical observations, body weights, hematology, and blood biochemistry
With regard to the clinical signs, no substantial changes were found in any of the NiO-treated groups. In the vehicle-control and the NiO-treated groups, a temporary body weight reduction was observed on the day after intratracheal administration; however, no significant difference was found in the body weight between the vehicle-control and the NiO-treated groups (data not shown). The hematology and blood biochemistry results showed no toxicologically significant changes (data not shown).
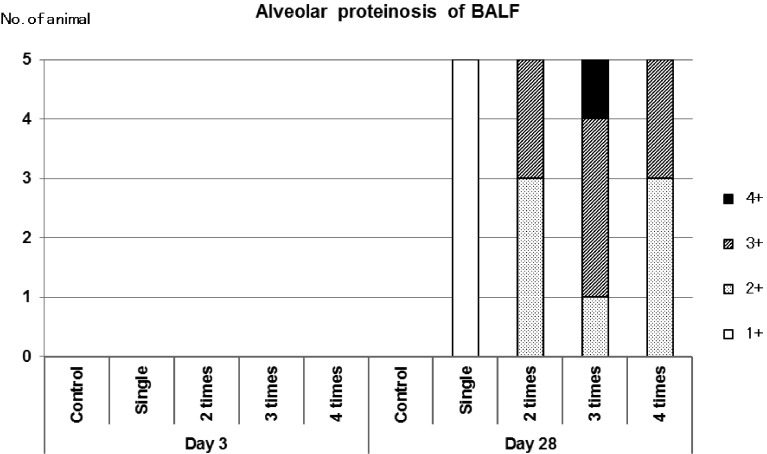
Properties of BALF (Fig. 2)
Fig. 2.
Cytological analysis of BALF
Smear preparations of BALF were stained with PAS and examined by microscopy and alveolar proteinosis was graded according to 1+ to 4+.
BALF collected at 3 d post-dose was transparent in all the groups. However, in the NiO-treated groups, BALF collected at 28 d post-dose produced white turbidity both in the single- and multiple-dose groups. BALF with white turbidity showed a positive periodic acid-schiff (PAS) reaction revealing that the white turbidity was a protein-like substance (lipoprotein), which led to a diagnosis of alveolar proteinosis. The level of alveolar proteinosis was slight in all the animals receiving a single dose of NiO and moderate to severe in all the animals receiving multiple doses of NiO (Fig. 2). At 3 d post-dose, alveolar proteinosis was not detected in any of the groups and it was neither observed in the vehicle-control group.
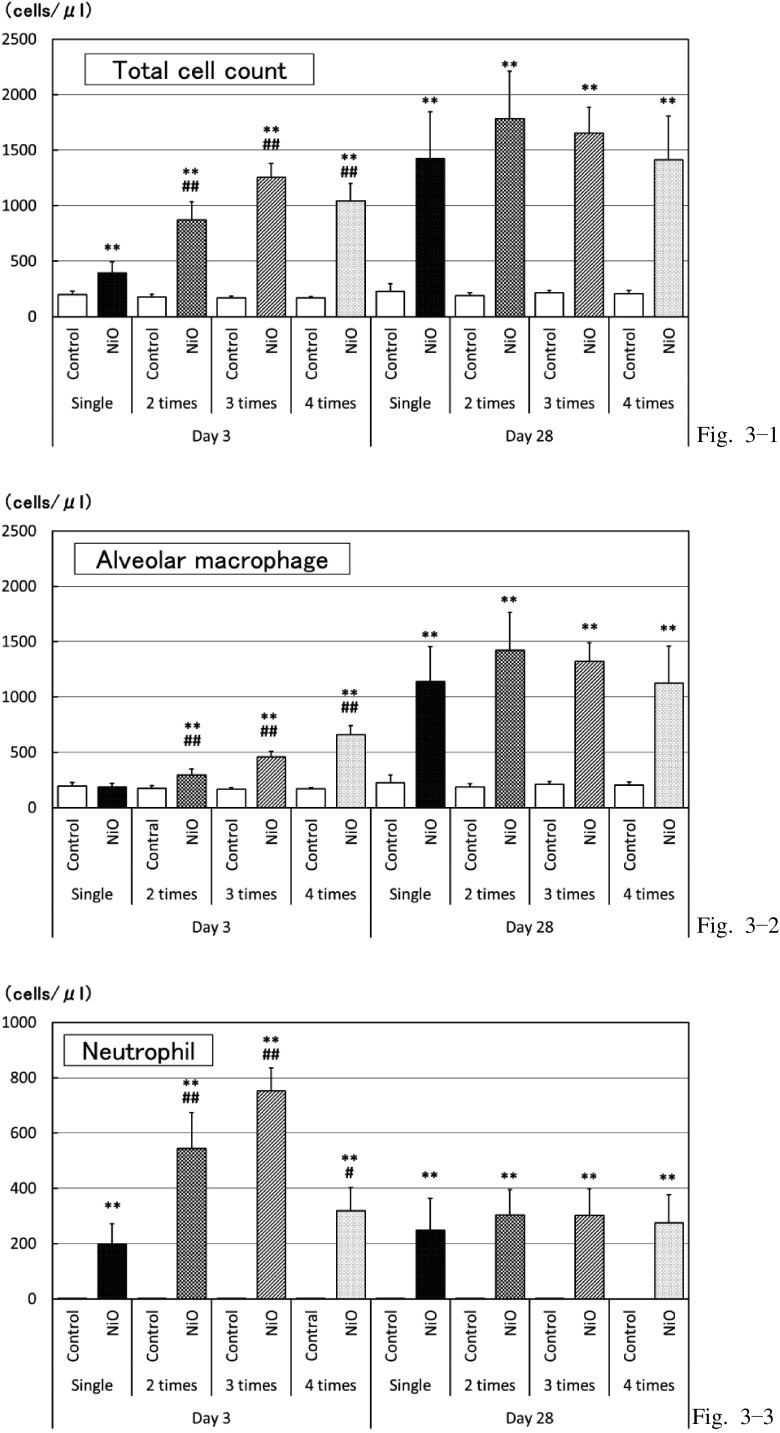
Cytological analysis of BALF (Fig. 3)
Fig. 3.
Cytological examination of BALF
Total cell count, alveolar macrophage, and neutrophil counts at 3 and 28 d post-dose.
*: p<0.05 **: p<0.01: Significantly different as compared to respective control groups (vehicle-control groups)
#: p<0.05 ##: p<0.01: Significantly different in multiple administration test as compared to single administration.
Due to the comparison between the vehicle-control and NiO-treated groups at 3 d post-dose, the total cell, neutrophil, and eosinophil counts were found to have increased in all the NiO-treated groups (Fig. 3-1, 3-3). Furthermore, the macrophage and lymphocyte counts were elevated in the NiO-treated groups receiving multiple doses of NiO (Fig. 3-2). When compared between single and multiple doses, all measured parameters were higher in the animals receiving multiple doses and tended to be higher by increasing the frequency of doses.
At 28 d post-dose, the total cell, macrophage, neutrophil, and lymphocyte counts were high (Fig. 3-1, 3-2, 3-3); however, eosinophil was rarely detected in the NiO-treated group. No particular differences of cytological parameters between the single- and multiple-dose groups were obvious. At 28 d post-dose, the total cell and macrophage counts were higher and the neutrophil and eosinophil counts were lower than that of the 3 d post-dose group (Fig. 3-1, 3-2, 3-3).
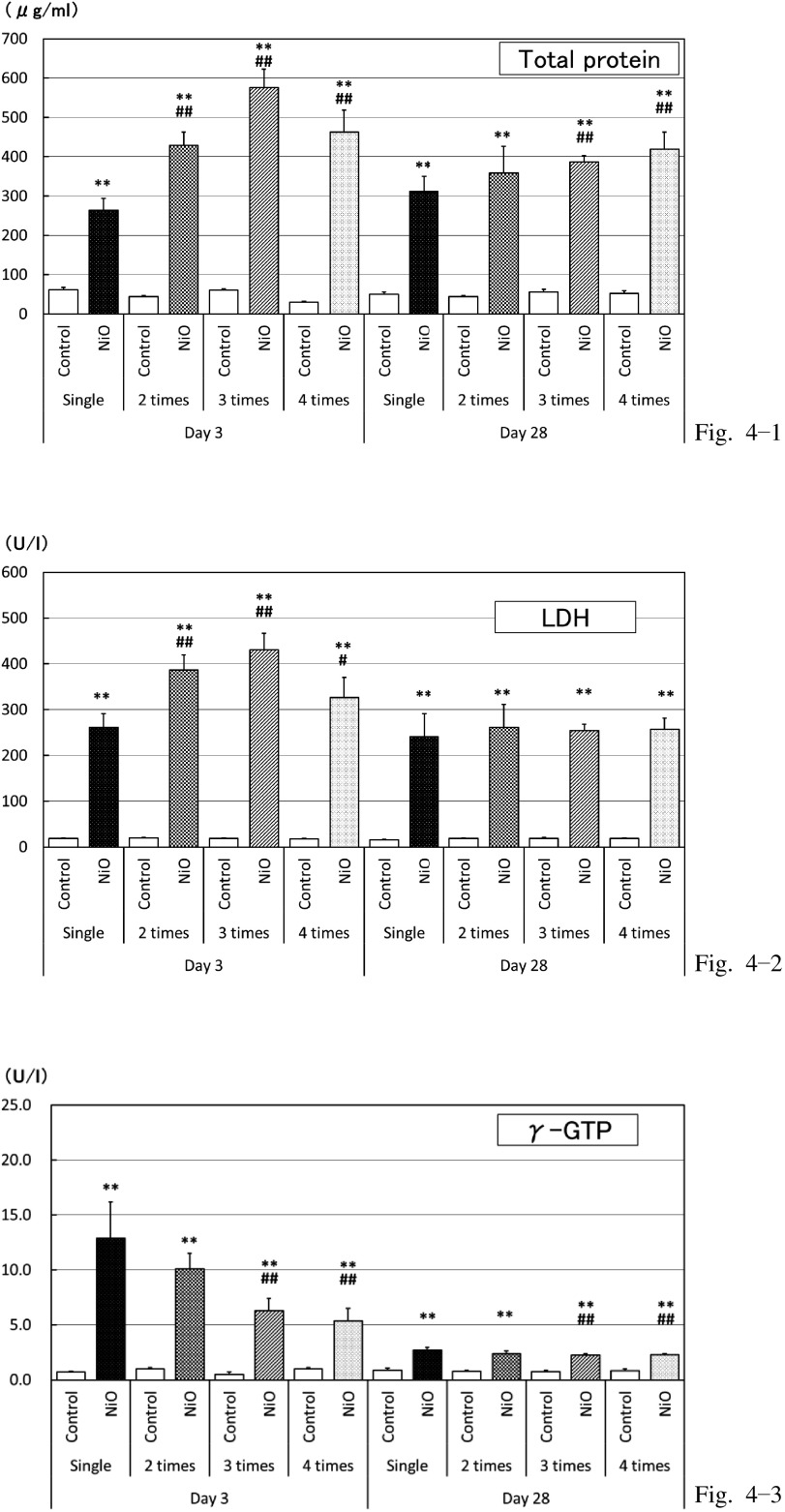
Biochemical analysis of BALF (Fig. 4)
Fig. 4.
Biochemistry of BALF
Total protein, Lactate Dehydrogenase (LDH), and γ-glutamyltransferase (γ-GTP) levels at 3 and 28 d post-dose.
*: p<0.05 **: p<0.01: Significantly different as compared to respective control groups (vehicle-control groups).
#: p<0.05 ##: p<0.01: Significantly different in multiple administration test as compared to single administration.
The levels of TP, albumin, LDH, and γ-GTP were high in the NiO-treated group at both 3 d and 28 d post-dose (Fig. 4-1, 4-2, 4-3). At 3 d post-dose, the trends for an increase were detected in the TP, albumin, and LDH levels in the animals receiving multiple doses (Fig. 4-1, 4-2); however, the trends for a decrease in the γ-GTP level were observed in the animals receiving multiple doses (Fig. 4-3). At 28 d post-dose, the TP and albumin levels showed a trend for an increase in the animals receiving multiple doses (Fig. 4-1). In addition, no particular differences existed in the LDH and γ-GTP levels depending on the frequency of administration (Fig. 4-2, 4-3). Most parameters were higher in the NiO-treated group as compared to that in the vehicle-control group, although slightly lower responses were observed at 3 d post-dose as compared to those at 28 d post-dose. The ALP level, however, did not increase after NiO administration and decreased at 28 d post-dose (data not shown).
Organ weights
The lung weight was higher in all the NiO-treated groups than in the respective vehicle-control groups. At 28 d post-dose, the lung weight was significantly lower in the animals receiving 3 or 4 doses than in those receiving a single dose; however, there was only a slight difference. At 91 d post-dose, no particular differences were found in the lung weights among the NiO-treated groups (data not shown).
Histopathological findings (Table 1, Fig. 5)
Table 1.
Histopathological findings of the lung
| Table 1-1. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 28 after administration | |||||||||||
| Control | NiO administered | ||||||||||
| All grades are slight. | |||||||||||
| Number of times administered | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| Number of rats examined | 5 | 5 | 5 | 5 | 10 | 10 | 10 | 10 | |||
| Lung | |||||||||||
| inflammatory cell infiltration (perivascular) | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 10 | |||
| alveolar space | |||||||||||
| increase of macrophage (NiO phagocytosed) | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 10 | |||
| degeneration / necrosis of macrophage | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 10 | |||
| alveolar proteinosis | 0 | 0 | 0 | 0 | 10 | 10 | 10 | 10 | |||
| alveolar wall | |||||||||||
| fibrosis | 0 | 0 | 0 | 0 | 6 | 8 | 10 | 7 | |||
| hyperplasia of type Ⅱ pneumocyte | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | |||
| bronchus-associated lymphoid tissue (BALT) | |||||||||||
| NiO particle | 0 | 0 | 0 | 0 | 4 | 6 | 7 | 5 | |||
| Lymph node (peritracheal) | |||||||||||
| NiO particle | 0 | 0 | 0 | 0 | 9 | 10 | 10 | 8 | |||
| Table 1-2. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 91 after administration | |||||||||||
| Control | NiO administered | ||||||||||
| All grades are slight. | |||||||||||
| Number of times administered | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |||
| Number of rats examined | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | |||
| Lung | |||||||||||
| inflammatory cell infiltration (perivascular) | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | |||
| alveolar space | |||||||||||
| increase of macrophage (NiO phagocytosed) | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | |||
| degeneration / necrosis of macrophage | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | |||
| alveolar proteinosis | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | |||
| alveolar wall | |||||||||||
| fibrosis | 0 | 0 | 0 | 0 | 5 | 3 | 3 | 3 | |||
| hyperplasia of type Ⅱ pneumocyte | 0 | 0 | 0 | 0 | 2 | 2 | 1 | 3 | |||
| bronchus-associated lymphoid tissue (BALT) | |||||||||||
| NiO particle | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | |||
| Lymph node (peritracheal) | |||||||||||
| NiO particle | 0 | 0 | 0 | 0 | 5 | 5 | 5 | 5 | |||
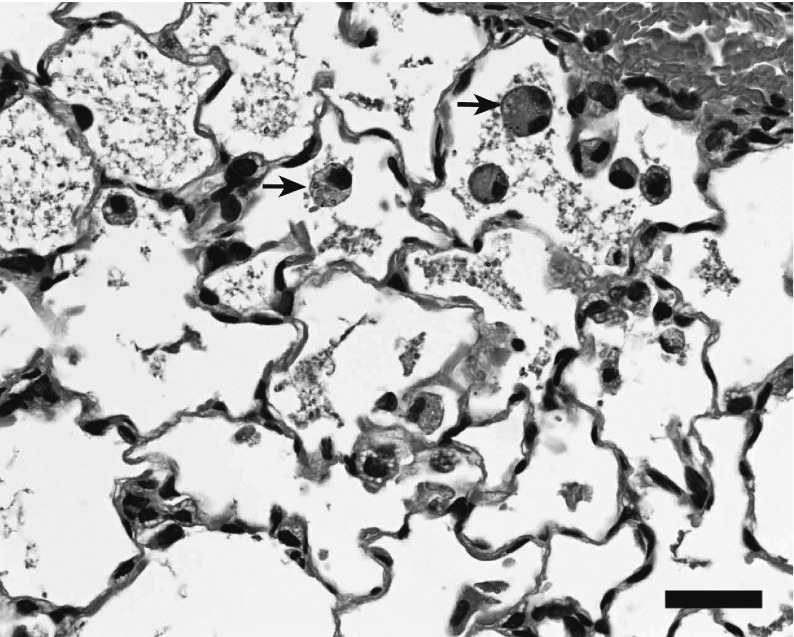
Fig. 5.
Histopathological findings of the lung
Increased alveolar macrophages and alveolar proteinosis (28 d post-dose), single administration of NiO (2 mg/kg), arrows: protein and particle phagocytosed macrophages. Bar, 50 μm
Histological examinations at 28 and 91 d post-dose revealed the following findings as effects on the lungs in the NiO-treated group; however, no particular differences were found between single and multiple administrations at any time point. In the pulmonary alveoli, an increase of macrophages, NiO-phagocytized macrophages, and degeneration and necrosis of macrophages were observed. Perivascular inflammatory cell infiltration in the alveolar space and alveolar wall was detected. Furthermore, type II alveolar epithelial hyperplasia, alveolar wall fibrosis, and alveolar proteinosis were evident. NiO particles were found in the alveolar space and bronchus-associated lymphoid tissue. In the comparison between 28 and 91 d post-dose, a slight increase of the incidence of type II alveolar epithelial hyperplasia at 91 d post-dose was observed; however, no particular differences were found with regard to the incidence or grade of other findings. NiO deposition was observed in the pulmonary mediastinal lymph node, but it was not identified in other lymph nodes. In other organs, except the lung and lung-associated lymph nodes, NiO particles were not detected and no pathological changes were observed.
Discussion
The toxic effects of intratracheal NiO administration in the lungs of F344 rats were observed irrespective of single or multiple treatment. The toxicity was detected from the observation of the lung weight changes, BALF analyses, and histopathological examinations. The cytological examination of BALF revealed increases in the total cell, neutrophil, eosinophil, and lymphocyte counts indicating an inflammatory response, while the increase of macrophages indicated the phagocytosis of particles in all the NiO-treated groups. The BALF biochemistry results showed changes in parameters that indicate pulmonary injury and inflammatory response. All these parameters were substantially higher in the NiO-treated group than in the vehicle-control group at 3 d post-dose and were higher even at the 28 d post-dose time point. PAS staining of BALF revealed alveolar proteinosis at 28 d post-dose. The lung weights were higher in the NiO-treated groups than in the corresponding vehicle-control groups. Histopathological examination of the lung demonstrated the phagocytosis of NiO, degeneration and necrosis of macrophages, and inflammation in the NiO-treated group at 28 d post-dose, and these changes persisted up to 91 d post-dose. Other studies on the effects of intratracheal NiO administration revealed that NiO induced pulmonary inflammation, elevation of total protein, and LDH levels in the BALF13), and that this inflammatory response was prolonged6,14). NiO applied in this experiment was different from that used in the reports, and experimental conditions were also different. Previous reports demonstrated similar inflammatory responses and alveolar proteinosis to those observed in this experiment6,14,18), while the inflammation in the lung was found at 6 mo post-dose6).
The results of the comparison between the effects of single and multiple intratracheal NiO administration on BALF at 3 d post-dose showed that multiple administration had stronger effects on all parameters of the cytological examination, total protein, albumin and LDH levels than single administration. With regard to the effects of the frequency of administration, multiple administration had the tendency of stronger influence on the BALF biochemical parameters except γ-GTP as compared to single administration. In contrast to these results, the data obtained from BALF analysis conducted at 28 d post-dose showed no particular differences between the parameters of the cytological examination and biochemistry depending on the frequency of administration. Moreover, the results of histopathological examinations of the lung at 28 and 91 d post-dose demonstrated no particular changes depending on the frequency of administration. Comparing the effects of single (15 mg/rat) and multiple (3 mg/rat/d ×5 d) intratracheal administration of crystalline silica nanoparticles to rats on BALF demonstrated the existence of minor differences dependent on the frequency of administration, and therefore, no advantages for multiple administration to assess the toxic effects of crystalline silica on the lung2). The experiment that we conducted previously to compare the effects of single and multiple intratracheal administrations of TiO2 demonstrated that single NiO administration produced stronger effects than multiple administration (unpublished data). However, the present experiment showed that multiple administration of NiO induced more severe inflammation. This could be dependent on the special characteristics of the substances that caused the difference in the effects by the frequency of administration.
In this experiment, BALF with white turbidity was observed at 28 d post administration. Furthermore, BALF showed a positive PAS reaction, while the retention of the protein-like substance was detected in alveolar spaces. Histopathological examination revealed alveolar proteinosis. Cho et al.19) also reported the development of alveolar proteinosis following intratracheal NiO administration, and these data were in line with those of this experiment. Alveolar proteinosis develops due to the overproduction of pulmonary surfactant in the type II alveolar epithelial cells and impaired pulmonary surfactant clearance by macrophages. We demonstrated that NiO administration induced the degeneration and necrosis of macrophages, indicating that alveolar proteinosis was caused by impaired pulmonary surfactant clearance. The lungs were diagnosed as alveolar proteinosis in 28 d post-dose; in contrast, the lungs in 3 d post-dose were negative for alveolar proteinosis. However, TP of BALF in 28 d post-dose was not increased in comparison to that in 3 d post-dose. Therefore, TP of BALF did not seem to be related to alveolar proteinosis. Thus, it does not indicate alveolar proteinosis.
At 3 d post-dose, macrophage and lymphocyte counts did not show any significant differences among the parameters of BALF analyses following single administration. Macrophages are involved in processing foreign materials, while lymphocytes are related to chronic inflammation. Therefore, from this viewpoint, macrophage and lymphocyte counts are likely not to increase only 3 d post-dose after single NiO administration. The differences between single and multiple administrations might be caused by the changes that occurred in the period between the start of administration and BALF analyses.
In this study, many parameters of BALF analyses showed higher values in NiO-treated rats as compared to the vehicle-control rats; on the contrary, the ALP level was decreased. This reduction was also observed after intratracheal NiSO4 administration20), indicating that it may be a specific reaction to nickel. The significance of the observed phenomena has not yet been elucidated; however, the reduction of the ALP level could be considered as a functional change.
The comparison of toxicity effects caused by single and multiple intratracheal administrations of NiO showed that both of them induced inflammation of the lungs. At 3 d post-dose, a tendency for stronger toxicity was observed in case of multiple administration. At 28 and 91 d post-dose, toxicity effects were obvious, but no particular differences were found depending on the frequency of administration. Furthermore, no particular differences existed in the types of lesions induced by intratracheal NiO administration, while both single and multiple administrations induced sufficiently strong toxic responses. Thus, no particular differences in toxicity assessment are expected to occur depending on the frequency of NiO administration, even if intratracheal administration is applied for screening. Therefore, single administration is sufficient to assess the inhalation toxicity in the screening assay of nanomaterials. However, in some cases, for example, a test material of high viscosity is unsuitable for intratracheal administration. In that case, multiple administrations would be more effective by adjusting the test material to a lower concentration. Furthermore, when a test material is thoroughly dispersed only at a low concentration, multiple administration would be more suitable for toxicity assessment. Compared with inhalation exposure, intratracheal administration is effective to confirm the toxicity in a short period, because intratracheal administration can make high pulmonary concentrations. Moreover, single or multiple intratracheal administration is an effective method for maintaining lung concentrations.
Acknowledgments: This work was supported by the grant "Development of innovative methodology for safety assessment of industrial nanomaterials" from the Ministry of Economy, Trade and Industry (METI) of Japan. We would like to thank Kenji Kawaguchi (AIST) for the preparation of samples, Tadao Toya for the valuable technical advice, and Anna Kakehashi for the valuable advice. We also acknowledge the work of members of the JBRC.
Conflicts of interest: The authors declare that there are no conflicts of interest.
References
- 1). OECD Testing Programme of Manufactured Nanomaterials. [Online]. 2016[cited 2016 Jul. 7]; Available from: URL: http://www.oecd.org/chemicalsafety/nanosafety/testing-programme-manufactured-nanomaterials.htm
- 2). Reasor MJ, Antonini JM. Pulmonary responses to single versus multiple intratracheal instillations of silica in rats. J Toxicol Environ Health A 2000; 62: 9-21. [DOI] [PubMed] [Google Scholar]
- 3). Toya T, Takata A, Otaki N, et al. Pulmonary toxicity induced by intratracheal instillation of coarse and fine particles of cerium dioxide in male rats. Ind Health 2010; 48: 3-11. [DOI] [PubMed] [Google Scholar]
- 4). Toya T, Serita F, Sawatari K, Fukuda K. Lung lesions induced by intratracheal instillation of nickel fumes and nickel oxide powder in rats. Ind Health 1997; 35: 69-77. [DOI] [PubMed] [Google Scholar]
- 5). Nishi K, Morimoto Y, Ogami A, et al. Expression of cytokine-induced neutrophil chemoattractant in rat lungs by intratracheal instillation of nickel oxide nanoparticles. Inhal Toxicol 2009; 21: 1030-1039. [DOI] [PubMed] [Google Scholar]
- 6). Ogami A, Morimoto Y, Myojo T, et al. Pathological features of different sizes of nickel oxide following intratracheal instillation in rats. Inhal Toxicol 2009; 21: 812-818. [DOI] [PubMed] [Google Scholar]
- 7). Jones JG, Warner CG. Chronic exposure to iron oxide, chromium oxide, and nickel oxide fumes of metal dressers in a steelworks. Brit J Ind Med 1972; 29: 169-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Morimoto Y, Hirohashi M, Ogami A, et al. Pulmonary toxicity following an intratracheal instillation of nickel oxide nanoparticle agglomerates. J Occup Health 2011; 53: 293-295. [DOI] [PubMed] [Google Scholar]
- 9). Morimoto Y, Ogami A, Todoroki M, et al. Expression of inflammation-related cytokines following intratracheal instillation of nickel oxide nanoparticles. Nanotoxicology 2010; 4: 161-176. [DOI] [PubMed] [Google Scholar]
- 10). Morimoto Y, Izumi H, Yoshiura Y, et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicol 2016; 10: 607-618. [DOI] [PubMed] [Google Scholar]
- 11). Horie M, Fukui H, Nishio K, et al. Evaluation of acute oxidative stress induced by NiO nanoparticles in vivo and in vitro. J Occup Health 2011; 53: 64-74. [DOI] [PubMed] [Google Scholar]
- 12). Mizuguchi Y, Myojo T, Oyabu T, et al. Comparison of dose-response relations between 4-week inhalation and intratracheal instillation of NiO nanoparticles using polimorphonuclear neutrophils in bronchoalveolar lavage fluid as a biomarker of pulmonary inflammation. Inhal Toxicol 2013; 25: 29-36. [DOI] [PubMed] [Google Scholar]
- 13). Zhang Q, Kusaka Y, Zhu X, et al. Comparative toxicity of standard nickel and ultrafine nickel in lung after intratracheal instillation. J Occup Health 2003; 45: 23-30. [DOI] [PubMed] [Google Scholar]
- 14). Kadoya C, Ogami A, Morimoto Y, et al. Analysis of bronchoalveolar lavage fluid adhering to lung surfactant -Experiment on intratracheal instillation of nickel oxide with different diameters-. Industrial Health 2012; 50: 31-36. [DOI] [PubMed] [Google Scholar]
- 15). Hirano S, Shimada T, Osugi J. Pulmonary clearance and inflammatory potency of intratracheally instilled or acutely inhaled nickel sulfate in rats. Arch Toxicol 1994; 68: 548-554. [DOI] [PubMed] [Google Scholar]
- 16). English JC, Parker RD, Sharma RP, Oberg SG. Toxicokinetics of nickel in rats after intratracheal administration of a soluble and insoluble form. Am Ind Hyg Assoc J 1981; 42: 486-492. [DOI] [PubMed] [Google Scholar]
- 17). Kasai T, Gotoh K, Nishizawa T, et al. Development of a new multi-walled carbon nanotube (MWCNT) aerosol generation and exposure system and confirmation of suitability for conducting a single-exposure inhalation study of MWCNT in rats. Nanotoxicology 2014; 8: 169-178. [DOI] [PubMed] [Google Scholar]
- 18). Umeda Y, Kasai T, Saoto M, et al. Two-week toxicity of multi-walled carbon nanotubes by whole-body inhalation exposure in rats. J Toxicol Pathol 2013; 26: 131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Cho WS, Duffin R, Bradley M, et al. NiO and Co3O4 nanoparticles induce lung DTH-like responses and alveolar lipoproteinosis. Eur Respir J 2012; 39: 546-557. [DOI] [PubMed] [Google Scholar]
- 20). Benson JM, Henderson RF, McClellan RO, Hanson RL, Rebar AH. Comparative acute toxicity of four nickel compounds to F344 rat lung. Fundam Appl Toxicol 1986; 7: 340-347. [DOI] [PubMed] [Google Scholar]