Abstract
Objectives:
Allyl nitrile (3-butenenitrile) occurs naturally in the environment, in particular, in cruciferous vegetables, indicating a possible daily intake of the compound. There is no report on actual health effects of allyl nitrile in humans, although it is possible that individuals in the environment are at a risk of exposure to allyl nitrile. However, little is known about its quantitative assessment for the environment and bioactivity in the body. This study provides a review of previous accumulated studies on allyl nitrile.
Methods:
Published literature on allyl nitrile was examined for findings on toxicity, metabolism, risk of various cancers, generation, intake estimates, and low-dose effects in the body.
Results:
High doses of allyl nitrile produce toxicity characterized by behavioral abnormalities, which are considered to be produced by an active metabolite, 3,4-epoxybutyronitrile. Cruciferous vegetables have been shown to have a potential role in reducing various cancers. Hydrolysis of the glucosinolate sinigrin, rich in cruciferous vegetables, results in the generation of allyl nitrile. An intake of allyl nitrile is estimated at 0.12 μmol/kg body weight in Japan. Repeated exposure to low doses of allyl nitrile upregulates antioxidant/phase II enzymes in various tissues; this may contribute to a reduction in neurotoxicity and skin inflammation. These high and low doses are far more than the intake estimate.
Conclusion:
Allyl nitrile in the environment is a compound with diverse bioactivities in the body, characterized by inducing behavioral abnormalities at high doses and some antioxidant/phase II enzymes at low doses.
Keywords: Allyl nitrile, Antioxidants, Cruciferae, Metabolic detoxification, Sinigrin, Toxicity
Introduction
Allyl nitrile (3-butenenitrile) is an organic compound that occurs naturally in the environment. It is one of the nitriles widely used in the manufacture of plastics, solvents, and synthetic intermediates. Thermal degradation of acrylonitrile-based plastics leads to the emission of a large variety of nitriles, including allyl nitrile1). Allyl nitrile is a surgical constituent of smoke generated by surgical lasers and electro-surgical units2). These reports only showed an allyl nitrile detection in the environment with no quantitative data. Furthermore, there is no literature reporting actual health effects in humans to date, although it is possible that individuals in these occupational environment are at a risk of exposure to allyl nitrile. This nitrile is also generated by cruciferous plants3-5), indicating a possible exposure from the consumption of these plants.
To date, numerous epidemiological studies have shown an inverse association between the consumption of cruciferous vegetables and the risk of various cancers. Little is known about the bioactivity of allyl nitrile in the body. This study summarizes what is known about allyl nitrile, including its metabolic transformation and neurotoxic effects. A discussion of cruciferous vegetable consumption and risk of cancers, intake estimate of glucosinolates, degradation of sinigrin, and cascade effects in the body is also included.
Toxicity
Exposure to nitriles by humans and experimental animals can result in neurologic, hepatic, cardiovascular, renal, and gastrointestinal disorders6). The toxicity results largely from the release of cyanide in the body6). Acute toxicity has been shown to vary with nitriles7). A significant correlation has been shown between acute toxicity and the octanol/water partition coefficient for nitriles, including allyl nitrile7). Rodents administered allyl nitrile at high doses exhibited behavioral abnormalities8); this is not known to occur with other mononitriles, with the exception of crotononitrile and 2-pentenenitrile9-12). The dinitrile 3,3'-iminodipropionitrile is also known to induce behavioral abnormalities13,14).
The behavioral abnormalities observed are similar to that of the ECC syndrome (excitement, choreoathetosis, and circling) described by Selye15).
As a review of previous studies on the mechanism underlying allyl nitrile-induced behavioral abnormalities is available in 199916), this study focuses on studies that have appeared since then (Table 1). Balbuena and Llorens (2001) conducted studies on the neurotoxicity and underlying mechanism of allyl nitrile in rats17). Changes below are statistically significant p<0.05, as compared with control. They noted pathological changes, such as corneal opacity (40 and 60 mg/kg/day, for 3 days), increased concentrations of glial fibrillary acidic protein in the retina (60 mg/kg/day, for 3 days) and olfactory bulbs (40 and 60 mg/kg/day, for 3 days), and loss of hair cells in the vestibular sensory epithelia (2-4 and 4-5 vestibular ratings as compared with control (0 ratings) for 40 and 60 mg/kg/day, for 3 days), which resulted in a decreased rearing activity (60 mg/kg/day, for 3 days) and an increased vestibular rating score (40 and 60 mg/kg/day, for 3 days). The vestibular ratings are as follows: 0, no differences from literature descriptions of control adult tissue; 1, presence of hair bundles with abnormal configuration of stereocilia or lack of few hair bundles in the central part of the receptor; 2, loss of hair bundles clearly evident at low magnifications but only in the central region of the receptor; 3, widespread loss of hair bundles, usually complete in the central part of the receptor and evident in more peripheral areas; 4, complete or almost complete loss of hair bundles; 5, complete loss of hair bundles and evident loss of supporting cells. These behavioral changes correlated well with the loss of hair cells, leading to the conclusion that allyl nitrile can induce behavioral abnormalities by the loss of hair cells. In the same manner, Tanii et al. (2000) demonstrated a change in the vestibular system18). Mice administered a single dose of allyl nitrile (84 mg/kg) exhibited a persistent behavioral abnormality 1 to 2 days after dosing. Analysis of the Fos protein in brain structures, an indicator of neuronal activity, showed that Fos-positive structures observed were identical to some Fos-positive structures observed after unilabyrinthectomy. This finding implies that allyl nitrile induces Fos expression by causing a change in the peripheral vestibular system, resulting in behavioral abnormalities. Although changes in the vestibule, such as hair cell loss, appear to contribute to the observed behavioral abnormalities, the full mechanism underlying the abnormalities is not known.
Table 1.
Studies of allyl nitrile-induced behavioral abnormalities.
| Objective of the study | Animal model | Findings | |
|---|---|---|---|
| Balbuena and Lorens, 200117) | Sensory pathology in behavior disturbances | Rats treated with allyl nitrile (0-60 mg/kg/day, for 3 days) | Allyl nitrile induced loss of hair cells |
| Tanii et al., 200018) | Fos induction in the brain of mice after allyl nitrile administration | Mice treated with allyl nitrile (84 mg/kg, 1-2 days postdosing) | The Fos-positive structures observed were identical to some Fos-positive structures after unilabyrinthectomy |
| Tanii et al., 200019) | Neurotransmitters in the brain of mice after allyl nitrile administration | Mice treated with allyl nitrile (84 mg/kg, 0-14 days post- dosing) | Allyl nitrile induced changes in GABAergic systems |
To better understand the mechanism, we examined changes in the neuronal expression of γ-aminobutyric acid (GABA), noradrenaline, dopamine, serotonin, and acetylcholine in the mouse brain following a single dose of allyl nitrile (84 mg/kg)19). In this study, allyl nitrile induced changes in the level of GABA in the medial habenula, interpeduncular nucleus, substantia nigra, dorsal raphe nucleus, and median raphe nucleus. Levels of GABA decreased in all of these brain structures except the medial habenula at 2 days post-dosing, and increased in all of these structures at 14 days post-dosing. Changes in the other neurotransmitters had no apparent bearing on behavioral abnormalities. The GABAergic systems in the medial habenula-interpeduncular nucleus-ascending raphe nuclei relay and in the substantia nigra seem to be involved in the mechanism underlying the abnormalities.
Llorens group advanced their studies with crotononitrile, 2-pentenenitrile, and 3,3'-iminodipropionitrile. The two isomers of crotononitrile have different actions in rodents. While cis-crotononitrile caused behavioral effects and vestibular hair cell loss (1-3, 2-4, and 3-5 vestibular ratings as compared with control (0-1 ratings) in both rats11) (80, 100, and 120 mg/kg/day, for 3 days) and mice20), trans-crotononitrile (250 mg/kg/day, for 3 days) caused rearing deficits with no vestibular dysfunction or hair cell loss11), but caused the same behavioral syndrome and hair cell loss in mice20). Rats receiving 1.5, 1.75, and 2.0 mmol/kg of cis-2-pentenenitrile displayed reduced rearing activity in the open field and increased rating scores on the vestibular dysfunction test battery as well as hair cell loss (1-3, 1-4, and 3-4 vestibular ratings as compared with control (0-1)12). Dose-response studies on allyl nitrile (0, 1.0, 1.25, and 1.50 mmol/kg) and cis-crotononitrile (0, 1.75, 2.25, 2.75, and 3.25 mmol/kg) showed the match between behavioral effects and hair cell loss in mice21). In addition, it is reported that behavioral effects, observed in animals administered 3,3'-iminodipropionitrile (400, 600, and 1000 mg/kg) or crotononitrile (250 mg/kg), are identical to those observed in mutant mice lacking vestibular function and in rodents with bilateral labyrinthectomy13,14). Taken together, these data demonstrate that allyl nitrile-induced behavioral abnormalities are caused by vestibular toxicity. Whether the changes caused by allyl nitrile in the medial habenula and substantia nigra are involved in the behavioral abnormalities could be supported by investigating if similar changes are observed in rodents exposed to other nitriles causing the same behavioral effects.
Dose levels for the behavioral abnormalities and vestibular toxicity are summarized as follows: 40 and more mg/kg (0.6 and more mmol/kg) for 3 days in rats for allyl nitrile, 80 and more mg/kg (1.2 and more mmol/kg) for 3 days in rats for cis-crotononitrile, and 1.5 and more mmol/kg in rats for cis-2-pentenenitrile. The level of 0.6 mmol/kg allyl nitrile is far more than an intake level of 0.12 μmol/kg body weight in Japan, as discussed later.
Metabolism
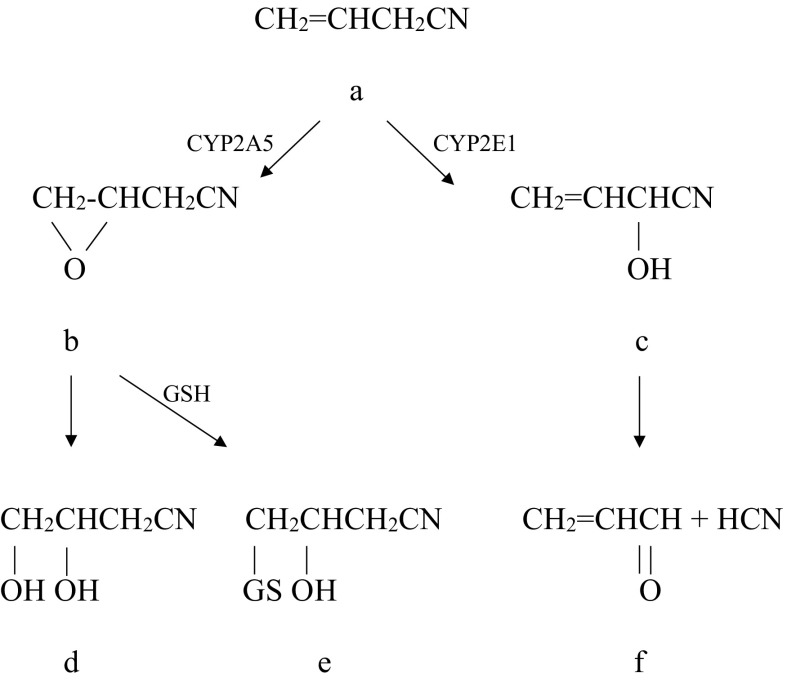
The biological activities of allyl nitrile may be related to its fate in the body (Fig. 1). Allyl nitrile (a) is considered to undergo the alcohol/acetone-inducible isoform of the cytochrome P450 (CYP) 2E1-mediated α-carbon hydroxylation to generate an unstable cyanohydrin (c)22-24), which spontaneously decomposes to 2-propenal (acrolein) and hydrogen cyanide (f). The hydrogen cyanide formed is responsible for the nitrile's acute toxicity7,25). On the other hand, it is considered that allyl nitrile undergoes the epoxidation of the β-γ double bond to form 3,4-epoxybutyronitrile (b). This reaction is mediated in mice by CYP2A5, the ortholog of human CYP2A626). The epoxide (b) is further converted to 3,4-dihydroxybutyronitrile (d) by epoxide hydrolase activity or to a glutathione conjugate (e) by a reaction with glutathione (GSH). The epoxide is reportedly responsible for the vestibulotoxicity of allyl nitrile26). Further studies are needed to explore which metabolite is responsible for bioactivities exhibited by allyl nitrile.
Fig. 1.
Proposed metabolic pathways for allyl nitrile.
Cruciferous Vegetable Consumption and Risk of Various Cancers
Allyl nitrile generation from cruciferous vegetables3-5) raises the question of the relationship between vegetable consumption and disease. Prospective studies on some cancers have revealed no association for total vegetable consumption, but a significant inverse association with cruciferous vegetable consumption27,28). Hence, cruciferous vegetables, such as Brussels sprouts, broccoli, cabbage, cauliflower, and turnip, have been studied with reference to their potential to reduce the risk of cancer. Cruciferous vegetable intake has been observed to be inversely associated with the risk of gastric29), prostate30), bladder31), renal32,33), colon34,35), ovarian36), pancreatic37), breast38), and lung39) cancers, and is associated with a reduced risk of total mortality, as well as mortality from cardiovascular disease40,41). Based on these findings, it would seem that cruciferous vegetables are beneficial to human health. The vegetables contain glucosinolates and it is considered that glucosinolates are responsible for the putative cancer chemoprevention mentioned above42). Allyl nitrile generation from cruciferous vegetables raises the possibility that confers protection, but it is not specific enough to pinpoint allyl nitrile.
Glucosinolate Intake Estimates
The large variety of organic chemicals in cruciferous vegetables makes it difficult to estimate the daily human intake of glucosinolates. However, some studies have reported measuring total glucosinolate intake. Sones et al. (1984) estimated a total glucosinolate intake of 29.4 mg/day from cooked and 46.1 mg/day from fresh vegetables in the United Kingdom, although these quantities possibly vary among regions and seasons43). Steinbrecher and Linseisen (2009) estimated a total intake of glucosinolates from vegetables as 14.2 mg/day for males and 14.8 mg/day for females in Germany44), while studies in Spain, Czechoslovakia, and Japan estimated 6.2 to 6.8 mg/day, 4.7 mg/day, and 37.2 μmol/day, respectively45-47). Epidemiological studies have yet to answer how relevant total glucosinolate intake is to health and disease.
Allyl Nitrile Generation from Glucosinolate Sinigrin
Each cruciferous plant contains a mixture of glucosinolates that varies by species and strain48). Sinigrin is reportedly the predominant glucosinolate in Brussels sprouts, mustard, horseradish, cabbage, cauliflower, and kale44,49). Hydrolysis of sinigrin results in the generation of allyl nitrile as follows. Chewing fresh vegetables or tissue damage produced by bruising during cultivation, harvest, shipping, or handling releases myrosinase, a glycoprotein that coexists with, but is physically separated from, its glucosinolate substrates. In damaged vegetable tissue containing released myrosinase, the glycosinolate sinigrin is converted to hydrolysis products in a manner that depends on the reaction conditions. In addition, myrosinase activity may be present in human colonic microflora, leading to the possibility that sinigrin is hydrolyzed in the gastrointestinal tract during digestion50-52).
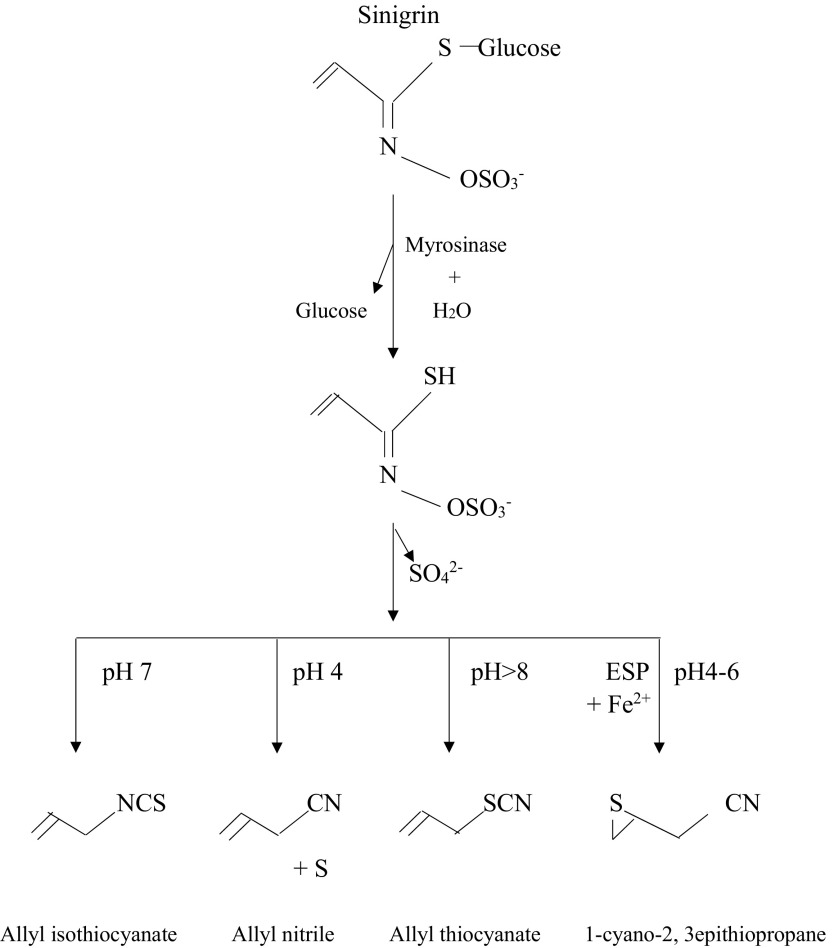
Fig. 2 depicts the hydrolysis of sinigrin. In the presence of myrosinase, sinigrin is converted to thiohydroximate-O-sulfate that then undergoes a Lossen rearrangement, with the elimination of sulfate, to generate multiple products53). Hydrolysis of sinigrin gives rise to allyl isothiocyanate at pH 7, allyl nitrile at pH 4, and allyl thiocyanate at pH >8. At low pH (4-6), the thiohydroximate-O-sulfate may, in the presence of an epithiospecifier protein and ferrous ions, give rise to 1-cyano-2,3-epithiopropane; in this scenario, epithiospecifier protein is known to interact with myrosinase to promote sulfur transfer from the S-glycosyl unit to the alkenyl chain from the aglycon54). To date, several studies on isothiocyanates, such as allyl isothiocyanate, have looked at their biological activity and role in health and disease42), whereas relatively little is known about nitriles, including allyl nitrile.
Fig. 2.
Allyl nitrile generation from sinigrin.
Formation of allyl nitrile has been measured quantitatively in Brussels sprouts. Tanii et al. (2004) reported 1.25 μmol/g tissue for homogenized tissues incubated at 25°C for 8 h5), while Cisca et al. (2015) reported 0.16 μmol/g tissue for boiled tissues treated at 100°C for 30 min55). An intake of 0.12 μmol/kg body weight is estimated5), based on daily dietary consumption data in Japan56).
Effects of Low-dose Allyl Nitrile in the Body
Allyl nitrile at subtoxic levels has been demonstrated to affect redox balance in the body57-60): Exposure to allyl nitrile (up to 700 μmol/kg/day, for 5-8 days) enhanced the activities of glutathione S-transferase, quinone reductase, glutathione, thioredoxin reductase, glutathione peroxidase, and superoxide dismutase, and reduced those of catalase and glutathione reductase in mice (Table 2). The enhancement was observed in the gastrointestinal tract, kidneys, lungs, urinary bladder, and brain, although superoxide dismutase has only been tested in the skin, while the reduction was seen in the colon and skin. Of the tissues that displayed enhanced activities, the gastrointestinal tract, lungs, kidneys, and urinary bladder are associated with a reduced risk of cancer related to the consumption of cruciferous vegetables29,31-35),39). The significance of the reductions in catalase and glutathione reductase activities is unknown. The mechanism by which allyl nitrile exerts the enhancement or reduction of antioxidant/phase II enzyme levels is unknown, but the enhancement activities could be mediated through an activation of nuclear factor erythroid 2-related factor-2 (Nrf2)61). Nrf2 can be activated with electrophilic compounds62). As shown in Fig. 1, allyl nitrile is converted to electrophilic metabolites, such as 3,4-epoxybutyronitrile.
Table 2.
Effects on antioxidant and phase II detoxification enzymes.
| Antioxidant/phase II enzymes | Enhancement/reduction | Tissues |
|---|---|---|
| Glutathione S-transferase57),59) | Enhancement | Stomach, rectum, kidneys, lungs, cortex, hippocampus, striatum and medulla oblongata/pons |
| Quinone reductase57),59) | Enhancement | Stomach, small intestine, urinary bladder, kidneys, lungs, cortex, hippocampus, and medulla oblongata/pons |
| Glutathione57),59) | Enhancement | Stomach, rectum, urinary bladder, and medulla oblongata/pons |
| Thioredoxin reductase58) | Enhancement | Liver, rectum, and kidneys |
| Glutathione peroxidase58),60) | Enhancement | Small intestine, kidneys, and skin |
| Superoxide dismutase60) | Enhancement | Skin |
| Catalase58),60) | Reduction | Colon, and skin |
| Glutathione reductase58) | Reduction | Colon |
The effects of allyl nitrile in the body have been reported in two studies. The first, Tanii et al. (2010), looked at protection against neurotoxicity59). In mice pretreated with allyl nitrile (up to 400 μmol/kg/day, for 5-8 days), elevated activities of antioxidant and phase II enzymes were observed in the brain structures (Table 2). The brain structures in Table 2 were the striatum and hippocampus (dose levels required for upregulation: 100, 200, and 400 μmol/kg/day), medulla oblongata plus pons (400 μmol/kg/day), and cortex (200 and 400 μmol/kg/day) for glutathione S-transferase, the medulla oblongata plus pons (200 and 400 μmol/kg/day), hippocampus (100, 200, and 400 μmol/kg/day), and cortex (400 μmol/kg/day) for quinone reductase, and the medulla oblongata plus pons (100, 200, and 400 μmol/kg/day) for glutathione. Following pretreatment with allyl nitrile, mice were administered a high dose of allyl nitrile (1.2 mmol/kg), leading to a display of behavioral abnormalities. As compared with the group that was not pretreated, animals in the 200 and 400 μmol/kg/day pretreatment groups displayed decreased behavioral abnormalities, and those in the 100, 200, and 400μmol/kg/day groups displayed elevated GABA-positive cell counts in the substantia nigra pars reticulate and the interpeduncular nucleus. Elevated levels of antioxidant and phase II enzymes in the brain owing to repeated exposure to subtoxic levels of allyl nitrile, together with the elevation in other tissues, may contribute to protection against allyl nitrile neurotoxicity.
The other study, Tanii et al. (2016), looked at inflammation60). Skin sensitizers induce allergic reactions (edema) through the induction of reactive oxygen species63). Mice were treated with allyl nitrile (0-200 μmol/kg/day, for 8 days). On days 6, 7, and 8, the animals received a dermal application of one of three sensitizers (formaldehyde, glutaraldehyde, and 2,4-dinitrochlorobenzene) and were examined the following day. Repeated exposure to allyl nitrile reduced edema induced by glutaraldehyde at the level of 50 μmol/kg/day and by 2,4-dinitrochlorobenzene at 100 μmol/kg/day, but not by formaldehyde. Repeated exposure at 50, 100, and 200 μmol/kg/day decreased levels of thiobarbituric acid reactive substances, a marker of oxidative stress, induced by glutaraldehyde and 2,4-dinitrochlorobenzene, but not by formaldehyde. Allyl nitrile enhanced superoxide dismutase levels for the three sensitizers at 200 μmol/kg/day, catalase levels for formaldehyde at 200 μmol/kg/day, and for 2,4-dinitrochlorobenzene at 100 μmol/kg/day, but not for glutaraldehyde. Allyl nitrile increased glutathione peroxidase levels for formaldehyde at 200 μmol/kg/day and for 2,4-dinitrochlorobenzene at 100 μmol/kg/day and decreased for glutaraldehyde at 50 μmol/kg/day. The edema reduction seemed to be associated with oxidative stress and antioxidant enzyme activities60). However, why such a complex dose-response relationship is observed is not clear, and unknown processes or factors could be involved in the dose-response.
Repeated exposure to allyl nitrile appears to decrease the neurotoxicity of allyl nitrile at the levels of 100 to 400 μmol/kg/day and dermal sensitization at 50 to 100 μmol/kg /day probably through upregulation of antioxidant and phase II enzymes. The dose levels of allyl nitrile from animal data are far more than an intake estimate of 0.12 μmol/kg body weight in Japan as mentioned before. Therefore, further studies focusing on a lower dose level of allyl nitrile are needed to evaluate whether it exerts protective effects against occupational chemicals, such as carcinogens, sensitizers, and reproductive toxicants.
Conclusion
Allyl nitrile occurs naturally in the environment, including smoke generated by surgical lasers and electrosurgical units, but no reports are available on quantitative assessment. There is no literature reporting actual health effects in humans, although it is possible that individuals in the environment are at a risk of exposure to allyl nitrile. High doses of allyl nitrile (40-60 mg (0.6-0.9 mmol) /kg for 3 days or 84 mg (1.25 mmol) /kg) in rodents induce behavioral abnormalities, which are probably mediated by changes in the vestibule, medial habenula, and substantia nigra. Loss of hair cells in the vestibule is produced by an active metabolite, 3,4-epoxybutyronitrile. Cruciferous plants may potentially reduce the risk of various cancers, perhaps through glucosinolates acting as chemoprevention agents, although the direct relevance of cruciferous vegetables to allyl nitrile is unknown. Cruciferous vegetables are rich in glucosinolate sinigrin from which allyl nitrile is derived. Low-dose exposure to allyl nitrile (up to 700 μmol/kg/day, for 5-8 days) enhanced the activities of antioxidant/phase II enzymes, including the glutathione S-transferase, quinone reductase, glutathione, thioredoxin reductase, glutathione peroxidase, and superoxide dismutase, and reduced those, including the catalase and glutathione reductase in various tissues. Repeated exposure to low doses of allyl nitrile appears to reduce its inherent neurotoxicity at the level of up to 400 μmol/kg/day for 5 days, as well as mitigating skin inflammations induced by glutaraldehyde and by 2,4-dinitrochlorobenzene at the levels of 50 and 100 μmol/kg/day for 8 days, probably through the enhancement of some antioxidant/phase II enzymes. Both the toxic exposure levels and low-dose repeated exposure levels are far more than an intake of 0.12 μmol allyl nitrile/kg body weight in Japan. Further studies are needed to investigate whether allyl nitrile at about 0.12 μmol/kg has an effect in the body.
Acknowledgments: This work was supported by the Japan Society for the Promotion of Science KAKENHI Grant No. 26460795.
Conflicts of interest: There are no conflicts of interest to declare.
References
- 1). Shapi MM, Hesso A. Gas chromatographic-mass spectrometric analysis of some potential toxicants among volatile compounds emitted during large-scale thermal degradation of poly (acrylonitrile-butadiene-styrene) plastic. J Cromatog 1991; 562: 681-696. [PubMed] [Google Scholar]
- 2). Anon. Surgical smoke. [Online]. 2002; Available from: URL: http://www.surgin.com/clear-flow
- 3). West LG, Bandenhop AF, McLaughlin JL. Allyl isothiocyanate and allyl cyanide production in cell-free cabbage leaf extracts, shredded cabbage, and cole slaw. J Agric Food Chem 1977; 25: 1234-1238. [DOI] [PubMed] [Google Scholar]
- 4). Tolonen M, Taipale M, Viander B, Pihlava JM, Korhonen H, Ryhanen EL. Plant-derived biomolecules in fermented cabbage. J Agric Food Chem 2002; 50: 6798-6803. [DOI] [PubMed] [Google Scholar]
- 5). Tanii H, Takayasu T, Higashi T, Leng S, Saijoh K. Allyl nitrile: generation from cruciferous vegetables and behavioral effects on mice of repeated exposure. Food Chem Toxicol 2004; 42: 453-458. [DOI] [PubMed] [Google Scholar]
- 6). Hartung OR. Cyanide and nitriles. In: Clayton GD, Clayton FE, editors. Patty's industrial hygiene and toxicology. New York: Wiley-Interscience Publication; 1982. p. 4845-4900. [Google Scholar]
- 7). Tanii H, Hashimoto K. Studies on the mechanism of acute toxicity of nitriles in mice. Arch Toxicol 1984; 55: 47-54. [DOI] [PubMed] [Google Scholar]
- 8). Tanii H, Kurosaka Y, Hayashi M, Hashimoto K. Allyl nitrile: a compound which induces long-term dyskinesia in mice following a single administration. Exp Neurol 1989; 103: 64-67. [DOI] [PubMed] [Google Scholar]
- 9). Tanii H, Hayashi M, Hashimoto K. Behavioral syndrome induced by allyl nitrile, crotononitrile or 2-penetenenitrile in rats. Neuropharmacology 1991; 30: 887-892. [DOI] [PubMed] [Google Scholar]
- 10). Tanii H, Hayashi M, Hashimoto K. Nitrile-induced behavioral abnormalities in mice. Neurotoxicology 1989; 10: 157-166. [PubMed] [Google Scholar]
- 11). Balbuena E, Llorens J. Comparison of cis- and trans-crotononitrile effects in the rat reveals specificity in the neurotoxic properties of nitrile isomers. Toxicol Appl Pharmacol 2003; 187: 89-100. [DOI] [PubMed] [Google Scholar]
- 12). Saldaña-Ruíz S, Hernández-Mir G, Sedó-Cabezón L, Cutillas B, Llorens J. Vestibular toxicity of cis-2-pentenenitrile in the rat. Toxicol Lett 2012; 211: 281-288. [DOI] [PubMed] [Google Scholar]
- 13). Llorens J, Demêmes D, Sans A. The behavioral syndrome caused by 3, 3'-iminodipropionitrile and related nitriles in the rat is associated with degeneration of the vestibular sensory hair cells. Toxicol Appl Pharmacol 1993; 123: 199-210. [DOI] [PubMed] [Google Scholar]
- 14). Llorens J, Rodríguez-Farré E. Comparison of behavioral, vestibular, and axonal effects of subchronic IDPN in the rat. Neurotoxicol Teratol 1997; 19: 117-127. [DOI] [PubMed] [Google Scholar]
- 15). Selye H. Lathyrism. Rev Can Biol 1957; 16: 1-73. [PubMed] [Google Scholar]
- 16). Tanii H, Zang XP, Saijoh K. Allyl nitrile-induced behavioral abnormalities and findings êrelating to the mechanism underlying behavioral abnormalities. Jpn J Hyg 1999; 54: 459-466 (in Japanese). [DOI] [PubMed] [Google Scholar]
- 17). Balbuena E, Llorens J. Behavioral disturbances and sensory pathology following allyl nitrile exposure in rats. Brain Res 2001; 904: 298-306. [DOI] [PubMed] [Google Scholar]
- 18). Tanii H, Higashi T, Oka R, Saijoh K. Fos induction in the brain of mice exhibiting behavioral abnormalities following administration of allyl nitrile or crotononitrile. Brain Res 2000; 868: 141-146. [DOI] [PubMed] [Google Scholar]
- 19). Tanii H, Zang XP, Saito N, Saijoh K. Involvement of GABA neurons in allyl nitrile-induced dyskinesia. Brain Res 2000; 887: 454-459. [DOI] [PubMed] [Google Scholar]
- 20). Saldaña-Ruíz S, Soler-Martín C, Llorens J. Role of CYP2E1-mediated metabolism in the acute and vestibular toxicities of nineteen nitriles in the mouse. Toxicol Lett 2012; 208: 125-132. [DOI] [PubMed] [Google Scholar]
- 21). Saldaña-Ruíz S, Boadas-Vaello P, Sedó-Cabezón L, Llorens J. Reduced systemic toxicity and preserved vestibular toxicity following co-treatment with nitriles and CYP2E1 inhibitors: a mouse model for hair cell loss. J Assoc Res Otolaryngol 2013; 14: 661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Ohkawa H, Ohkawa R, Yamamoto I, Casida JE. Enzymatic mechanisms and toxicological significance of hydrogen cyanide liberation from various organothiocyanates and organonitriles in mice and houseflies. Pestic Biochem Physiol 1972; 2: 95-112. [Google Scholar]
- 23). Silver EH, Kuttab SH, Hasan T, Hassan M. Structural considerations in the mechanism of nitriles to cyanide in vivo. Drug Metab Dispos 1982; 10: 495-498. [PubMed] [Google Scholar]
- 24). Boadas-Vaello P, Jover E, Saldana-Ruiz S, et al. . Allyl nitrile metabolism by CYP2E1 and other CYPs leads to distinct lethal and vestibulotoxic effects in the mouse. Toxicol Sci 2009; 107: 461-472. [DOI] [PubMed] [Google Scholar]
- 25). Tanii H, Hashimoto K. Influence of ethanol on the in vivo and in vitro metabolism of nitriles in mice. Arch Toxicol 1986; 58: 171-176. [DOI] [PubMed] [Google Scholar]
- 26). Rúa F, Buffard M, Sedó-Cabezón L, et al. . Vestibulotoxic properties of potential metabolites of allyl nitrile. Toxicol Sci 2013; 135: 182-192. [DOI] [PubMed] [Google Scholar]
- 27). Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, Giovannucci EL. Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst 1999; 91: 605-613. [DOI] [PubMed] [Google Scholar]
- 28). Kirsh VA, Peters U, Mayne ST, et al. . Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst 2007; 99: 1200-1209. [DOI] [PubMed] [Google Scholar]
- 29). Wu QJ, Yang Y, Wang J, Han LH, Xiang YB. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci 2013; 104: 1067-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Liu B, Mao Q, Cao M, Xie L. Cruciferous vegetables intake and risk of prostate cancer: a meta-analysis. Int J Urol 2012; 19: 134-141. [DOI] [PubMed] [Google Scholar]
- 31). Liu B, Mao Q, Lin Y, Zhou F, Xie L. The association of cruciferous vegetables intake and risk of bladder cancer: a meta-analysis. World J Urol 2013; 31: 127-133. [DOI] [PubMed] [Google Scholar]
- 32). Zhao J, Zhao L. Cruciferous vegetables intake is associated with lower risk of renal cell carcinoma: evidence from a meta-analysis of observational studies. Plos One 2013; 8: e75732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Liu B, Mao Q, Wang X, et al. . Cruciferous vegetables consumption and risk of renal cell carcinoma: a meta-analysis. Nutr Cancer 2013; 65: 668-676. [DOI] [PubMed] [Google Scholar]
- 34). Wu QJ, Yang Y, Vogtmann E, et al. . Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol 2013; 24: 1079-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Tse G, Eslick GD. Cruciferous vegetables and risk of colorectal neoplasms: a systematic review and meta-analysis. Nutr Cancer 2014; 66: 128-139. [DOI] [PubMed] [Google Scholar]
- 36). Hu J, Hu Y, Zheng S. Intake of cruciferous vegetables is associated with reduced risk of various cancer: a meta-analysis. Asia Pac J Clin Nutr 2015; 24: 101-109. [DOI] [PubMed] [Google Scholar]
- 37). Li L, Luo Y, Lu M, Xu X, Lin H, Zheng Z. Cruciferous vegetable consumption and the risk of pancreatic cancer: a meta-analysis. World J Surg Oncol 2015; 13: 44 (doi: 10.1186/s12957-015-0454-4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Liu X, Lv K. Cruciferous vegetables intake is inversely associated with risk of breast cancer: a meta-analysis. Breast 2013; 22: 309-313. [DOI] [PubMed] [Google Scholar]
- 39). Lam TK, Gallicchio L, Boyd K, et al. . Cruciferous vegetable consumption and lung cancer risk: a systematic review. Cancer Epidemiol Biomarkers Prev 2009; 18: 184-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40). Zhang X, Shu XO, Xiang YB, et al. . Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr 2011; 94: 240-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Wang X, Quyang Y, Liu J, et al. . Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2014; 349: g4490 (doi: 10.1136/bmj.g4490). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Dinkova-Kostova AT, Kostov RV. Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 2012; 18: 337-347. [DOI] [PubMed] [Google Scholar]
- 43). Sones K, Heaney RK, Fenwick GR. An estimate of the mean daily intake of glucosinolates from cruciferous vegetables in the UK. J Sci Food Agric 1984; 35: 762-766. [Google Scholar]
- 44). Steinbrecher A, Linseisen J. Dietary intake of individual glucosinolates in participants of the EPIC-Heidelberg cohort study. Ann Nutr Metab 2009; 54: 87-96. [DOI] [PubMed] [Google Scholar]
- 45). Agudo A, Ibanez R, Amiano P, et al. . Consumption of cruciferous vegetables and glucosinolates in a Spanish adult population. Eur J Clin Nutr 2008; 62: 324-331. [DOI] [PubMed] [Google Scholar]
- 46). Hrncirik K, Velisek J. Glucosinolate content of common Brassicaceae family vegetables. Potrav Vedy 1977; 15: 161-172. [Google Scholar]
- 47). Kita J, Tada J, Ito M, et al. . Intake of phytochemicals among Japanese, calculated by the new FFF database. Bio Factors 2004; 22: 259-263. [DOI] [PubMed] [Google Scholar]
- 48). Fahey JW, Zalcmann AT, Talalay P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001; 56: 5-51. [DOI] [PubMed] [Google Scholar]
- 49). Kushad MM, Brown AF, Kurilich AC, et al. . Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem 1999; 47: 1541-1548. [DOI] [PubMed] [Google Scholar]
- 50). Cheng DL, Hashimoto K, Uda Y. In vitro digestion of sinigrin and glucotropaeolin by single strains of Bifidobacterium and identification of the digestive products. Food Chem Toxicol 2004; 42: 351-357. [DOI] [PubMed] [Google Scholar]
- 51). Krul C, Humblot C, Philippe C, et al. . Metabolism of sinigrin (2-propenylglucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model. Carcinogenesis 2002; 23: 1009-1016. [DOI] [PubMed] [Google Scholar]
- 52). Elfoul L, Rabot S, Khelifa N, Quinsac A, Duguay A, Rimbault A. Formation of allyl isothiocyanate from sinigrin in the digestive tract of rats monoassociated with a human colonic strain of bacteroidesthetaiotaomicron. FEMS Microbiol Lett 2001; 197: 99-103. [DOI] [PubMed] [Google Scholar]
- 53). Bones AM, Rossiter JT. The enzymic and chemically induced decomposition of glucosinolates. Phytochemistry 2006; 67: 1053-1067. [DOI] [PubMed] [Google Scholar]
- 54). Foo HL, GrØnning LM, Goodenough L, et al. . Purification and characterization of epithiospecifier protein from Brassica napus: enzymic intramolecular sulfur addition within alkenyl thiohydroximates derived from alkenyl glucosinolate hydrolysis. FEBS Lett 2000; 468: 243-246. [DOI] [PubMed] [Google Scholar]
- 55). Cisca E, Drabińska N, Honke J, Narwojsz A. Boiled Brussels sprout: a rich source of glucosinolates and the corresponding nitriles. J Func Foods 2015; 19: 91-99. [Google Scholar]
- 56). Izaki Y, Uchiyama M. Food list for the study of dietary intakes of chemical substances in Japan. J Jpn Soc Nutr Food Sci 1985; 38: 241-258. [Google Scholar]
- 57). Tanii H, Higashi T, Nishimura F, Higuchi Y, Saijoh K. In duction of detoxication enzymes in mice by naturally occurring allyl nitrile. J Agric Food Chem 2005; 53: 8993-8996. [DOI] [PubMed] [Google Scholar]
- 58). Tanii H, Higashi T, Nishimura F, Higuchi Y, Saijoh K. Effects of cruciferous allyl nitrile on phase 2 antioxidant and detoxification enzymes. Med Sci Monit 2008; 14: BR189-192. [PubMed] [Google Scholar]
- 59). Tanii H, Higashi T, Saijoh K. Preconditioning with subneurotoxic allyl nitrile: protection against allyl nitrile neurotoxicity. Food Chem Toxicol 2010; 48: 750-754. [DOI] [PubMed] [Google Scholar]
- 60). Tanii H, Sugitani K, Saijoh K. Anti-inflammatory and antioxidant effects of repeated exposure to cruciferous allyl nitrile in sensitizer-induced ear edema in mice. Med Sci Monit Basic Res 2016; 22: 20-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61). Talalay P. Chemoprotection against cancer by induction of phase 2 enzymes. Bio Factors 2000; 12: 5-11. [DOI] [PubMed] [Google Scholar]
- 62). Itoh K. Disease regulation by Nrf2 antioxidant system. Seikagaku 2009; 81: 447-455 (in Japanese). [PubMed] [Google Scholar]
- 63). Corsini E, Galbiati V, Nikitovic D, Tsatsakisc AM. Role of oxidative stress in chemical allergens induced skin cells activation. Food Chem Toxicol 2013; 61: 74-81. [DOI] [PubMed] [Google Scholar]