Abstract
The CD1 family of proteins binds self and foreign glycolipids for presentation to CD1-restricted T cells. To identify previously uncharacterized active CD1 ligands, especially those of microbial origin, numerous glycolipids were synthesized and tested for their ability to stimulate mouse and human natural killer T (NKT) cells. They included analogs of the well known NKT cell agonist α-galactosyl ceramide (α-GalCer), bacterial glycolipids, and variations of the self-glycolipid, sulfatide. Bacterial glycolipids, α-galacturonosyl-ceramides from Sphingomonas wittichii, although structurally similar to α-GalCer, have significant differences in the sugar head group as well as the ceramide portion. The Sphingomonas glycosphingolipids (GSLs) and sulfatide variants were shown to activate human NKT cells as measured by IL-4 and IFN-γ secretion. Moreover, CD1d-dimer staining revealed human NKT cell reactivity toward these GSLs and to the sulfatides in a fashion comparable with α-GalCer. Because α-GalCer is a marine-sponge-derived ligand, our study here shows that bacterium-derived antigens are also able to stimulate mouse and human NKT cells.
CD1 molecules are a family of highly conserved antigen-presenting proteins that are similar in function to classic MHC molecules (1). Whereas classic MHC molecules present peptides, CD1 proteins bind and display a variety of lipids and glycolipids to T lymphocytes (2). The five known isoforms are classified into two groups, group I (CD1a, CD1b, CD1c, and CD1e in humans) and group II (CD1d in humans and mice), based on similarities between nucleotide and amino acid sequences (3).
A great diversity of lipids and glycolipids have been shown to bind specifically to each of the four isoforms. The first crystal structure of murine CD1d (mCD1d) revealed that it adopts a folded conformation closely related to major histocompatibility complex (MHC) class I proteins (4). Also, it revealed that mCD1d could accommodate long lipid tails in two hydrophobic pockets, designated A′ and F′, located in the binding groove. Two additional structures of hCD1b and hCD1a (5–7) confirmed this model by demonstrating that CD1, when loaded with antigenic glycolipids, binds the lipid portion in a hydrophobic groove while making the hydrophilic sugar moiety available to make contact with the T cell receptor.
Mammalian and mycobacterial lipids are known to be presented by human CD1a, CD1b, CD1c, and CD1d (1). α-Galactosyl ceramide (α-GalCer), a lipid found in the marine sponge Agelas mauritianus (8), has been the most extensively studied ligand for CD1d. α-GalCer, when bound to CD1d, stimulates rapid Th1 and Th2 cytokine production by Vα14i natural killer T (Vα14i NKT) cells in mice and the human homolog Vα24i NKT cells (9–13) (Fig. 1). However, its physiological significance in mammals remains unclear because it is enigmatic why an α-galactosyl ceramide of marine origin is such a potent agonist. Other known ligands, which stimulate only relatively small populations of CD1d-restricted Vα14i NKT cells, include a bacterial phospholipid (PIM4) and a tumor-derived ganglioside (GD3) (14, 15). A phosphoethanolamine, found in human tumor extracts, was shown to be recognized by 24.8.A invariant NKT cell hybridomas (16). The C-linked analog of α-GalCer (17) and α-GalCer analogs with different lipid chain lengths (18) have also been reported to have significant activities toward NKT cells.
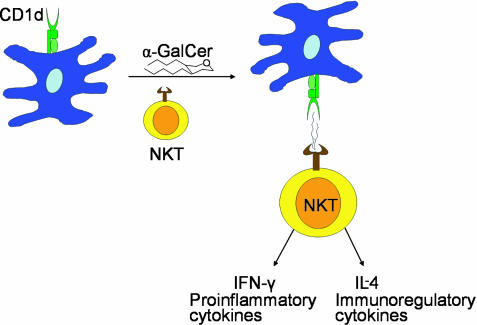
Fig. 1.
NKT cells are reactive to CD1d-bound α-GalCer. This stimulation results in the production of large amounts of cytokines such as IL-4 and IFN-γ.
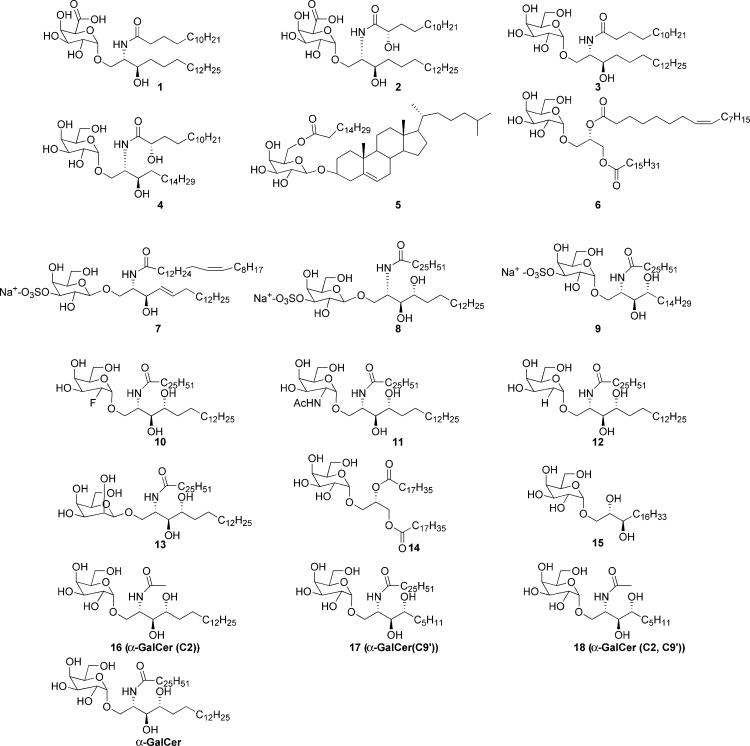
As a part of our efforts to further understand the mechanism of CD1d-restricted T cell activation and the discovery of new glycolipid antigens, we have synthesized a number of glycolipids and tested them for NKT cell activation (Fig. 2). These included glycolipids of bacterial origin (compounds 1, 2, 5, and 6), α-GalCer analogs modified on the galactose moiety and acyl group (compounds 10–18), and variations of sulfatide (compounds 7–9), the only known promiscuous ligand for CD1 (19). The bacterial glycolipids included those isolated from the outer membrane of Sphingomonas wittichii (20) and glycolipids from Borrelia burgdorferi, the Lyme disease spirochete. CD1d-deficient (CD1d-/-) mice were shown to have impaired host resistance to infection by B. burgdorferi, making its glycolipids attractive compounds for further study as possible natural CD1d antigens (21). The structures of its two major glycolipids were recently elucidated as cholesteryl 6-O-acyl-β-d-galactopyranoside, 5 (B. burgdorferi glycolipid 1, BbGL-I), and 1,2-di-O-acyl-3-O-α-d-galactopyranosyl-sn-glycerol, 6 (BbGL-II) (22). The Sphingomonas glycolipids, two previously uncharacterized α-linked glycosphingolipids (GSLs), 1 and 2 (GSL-1 and GSL-2, respectively), differ most significantly from α-GalCer in the carbohydrate moiety because they contain galactosyluronic acids as the polar head group (23). However, they are more physiologically relevant as natural ligands for CD1d-mediated NKT cell activation because they originate from bacteria. Biological experiments further show that galactouronic sphingolipids stimulate IL-2 secretion in 1.2 (Vα14 Vβ8.2 DN3A4) NKT cell hybridomas. An α-GalCer analog, 9, 3″-O-sulfo-galactosylceramide (3″-O-sulfo-GalCer), also caused significant IL-2 secretion, demonstrating that Vα14i NKT cell response is less sensitive to modification at the 3″-OH position of galactose. By contrast, any modification made at the 2″-OH position of galactose abolished all biological activity. Most other synthetic analogs, however, were active. In addition, we report the conservation of reactivity of human Vα24i NKT cells to GSL-1 and GSL-2 and sulfatides 7–9.
Fig. 2.
Structures of glycolipids and analogs.
Materials and Methods
The synthesis of α-GalCer has been reported by this laboratory (24). Compounds 16–18 were synthesized by the same procedure with slight modifications. Intermediates 19, 26, and 30 are known compounds (24–26). Experimental details for 1, 2, 7, 20, 23, 27, and 31 are described. The synthesis of the remaining compounds, except 8 and 9, and their intermediates are reported separately in the supporting information, which is published on the PNAS web site.
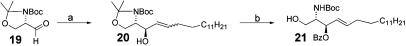
Sphingosine Intermediate Compound 20. As shown in Fig. 3, compound 19 (3.31 g, 13.5 mmol) (25) was dissolved in 70 ml of dry tetrahydrofuran (THF). The solution was cooled to -40°C, and vinyl Grignard solution (31 ml ofa1M solution in THF) was added via a dropping funnel over a period of 1 h. The temperature was kept between -20°C and -40°C. The reaction mixture was allowed to warm to room temperature and stirred for another hour. The reaction was quenched by addition of 60 ml of saturated (NH4)2SO4 solution and evaporated to dryness. The residue was diluted with water and extracted with ethyl acetate (3×). The combined organic layer was extracted with brine, dried over MgSO4, and evaporated to give a yellow oil. Column chromatography (Hex:EtOAc 3:1) yielded the syn diastereomer (2.11 g, 8.2 mmol, anti/syn = 3.5:1) in 61% yield. Then the syn diastereomer (300 mg, 1.16 mmol) was dissolved in 1 ml of dry dichloromethane in a two-necked flask equipped with a reflux condenser under argon, and 486 mg (3.48 mmol) of pentadecene was added by syringe. A solution of 20 mg (2 mol %) of Grubb's second generation catalyst (purchased from Strem Chemicals) in 1 ml of dichloromethane was added, and the solution was heated under rapid reflux for 40 h. The reaction mixture was evaporated and then directly chromatographed (Hex:EtOAc 6:1), yielding 0.82 mmol (71%) of the desired product.
Fig. 3.
Synthesis of sphingosine. Reagents and conditions are as follows. a, (i)C2H3MgBr, THF, Anti:Syn 3.5:1 61%; (ii) Grubbs catalyst second generation, CH2Cl2, pentadecene, 71%. b, (i) BzCl, pyridine, 90%; (ii) amberlyst 15 H+ form, MeOH, 70%.
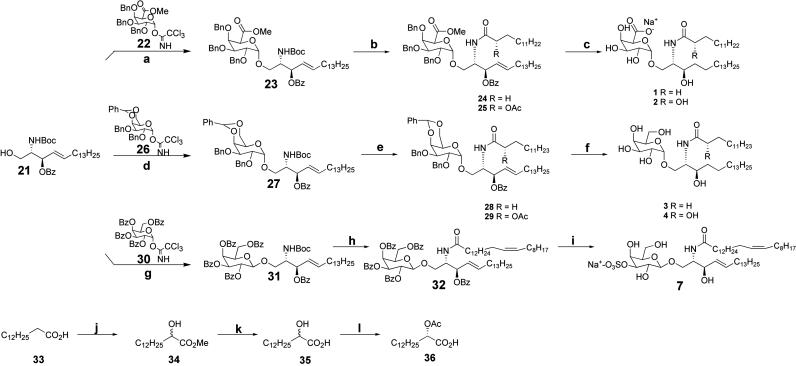
Synthesis of Glycolipids. As shown in Fig. 4, a solution of trichloroacetimidate 22 (160.4 mg, 0.258 mmol) and sphingosine acceptor 21 (100 mg, 0.198 mmol) in 4 ml of anhydrous Et2O and 2 ml of anhydrous THF was added over freshly dried 4-Å molecular sieves and cooled to -50°C. Trimethylsilylmethyl trifluoromethanesulfonate (TMSOTf) (3.33 mg, 0.0198 mmol) was added, and the mixture was stirred at -50°C for 1 h. The mixture was allowed to warm to -20°C, and another 3.33 mg of TMSOTf was added. The mixture was then slowly allowed to warm to room temperature and stirred for 3 h. The solution was then diluted with EtOAc and filtered over Celite. The organic layer was washed with saturated aqueous NaHCO3 and brine, dried (MgSO4), and concentrated. The residue was purified by column chromatography on silica gel (toluene:EtOAc 12:1) to give 128 mg (67.5%, 0.134 mmol) of 23. A general procedure for transformation of 23 to 24 can be found in the supporting information.
Fig. 4.
Synthesis of glycolipids. Reagents and conditions were as follows. a, 22, TMSOTf, 67%. b, (i) TFA, DCM; (ii) HBTU, myristic acid or (S)-2-acetoxymyristic acid, n-morpholine, ≈92%, two steps. c, H2, 20% Pd(OH)2, (ii) LiOH, H2O:THF:MeOH, 38%, two steps. d, 26, TMSOTf, 60%. e, (i) TFA, DCM, (ii) HBTU, myristic acid or (S)-2-acetoxymyristic acid, n-morpholine, ≈92%, two steps. f, (i) NaOMe, MeOH, (ii)Pd/C, H2, EtOH, 90%, two steps. g, 30, TMSOTf, 62%. h, (i) TFA, DCM, (ii) HBTU, nervonic acid, n-morpholine, 60%, two steps. i, (i) NaOMe, MeOH, quant; (ii) Bu2SnO, MeOH; (iii) Me3N·SO3, THF, 95%. j and k, (i) LDA, TMSOOTMS, (ii) H+, MeOH, 30%, two steps; then LiOH, H2O:MeOH, 81%; l, novozyme 435, CH2=CHOAc, 54% based on S isomer.
Compound 24 (36 mg, 0.03 mmol), dissolved in 6 ml of EtOAc, was added to 36 mg of 20% (wt) palladium hydroxide in 1 ml of EtOAc and saturated with hydrogen. The reaction vessel was purged with hydrogen, and the mixture was stirred at room temperature overnight. The reaction mixture was filtered, and the solvent was evaporated. The above hydrogenated compound was dissolved in 2 ml of THF, 1 ml of water, and 1 ml of methanol. LiOH (9 mg, 0.14 mmol) was added to the solution, and the reaction was stirred at room temperature for 4 h. We added 100 mg of Na2CO3, and the mixture was stirred for 30 min. The solvent was evaporated, and the remaining residue was purified on silica gel by column chromatography (CH2Cl2:MeOH 4:1) to give 7.8 mg of 1 (38%, 0.0114 mmol, two steps).
Sulfation of 32. After deprotection of compound 32 (14 mg, 0.017 mmol), Bu2SnO (6.5 mg, 0.0259 mmol) dissolved in 1 ml of MeOH was added. The mixture was refluxed under argon for 2 h. After evaporation of the solvent, Me3N·SO3 (5 mg, 0.035 mmol) dissolved in 1 ml of THF was added, and the reaction was allowed to proceed at room temperature for 6 h. The solvent was then removed under reduced pressure, and the residue was dissolved in CHCl3/MeOH 1:1 (1 ml) and loaded onto an ion exchange column (Dowex, 50 × 8Na+ form). After elution with CHCl3/MeOH 1:1, the mixture was concentrated and purified by column chromatography (CH2Cl2:MeOH 5:1) to give 7 (14.4 mg, 95%).
1.2 Hybridoma Assay. CD1d-reactive T cell hybridomas with an invariant Vα14i T cell antigen receptor α chain have been described (27). T cell hybridomas were stimulated with the indicated glycolipids that were added either to plates coated with soluble CD1d, or with CD1d-transfected A20 B lymphoma cells, according to published protocols (28). As a measure of T cell activation, IL-2 release into the tissue culture medium was measured after a 16-h culture by an ELISA assay.
Generation of Vα24i Human NKT Cell Line. Human NKT cell lines, expressing the Vα24i T cell receptor as well as CD161, were generated as follows. Anti-CD161 mAbs, and anti-CD14 mAbs, each coupled to magnetic beads (Miltenyi Biotec, Auburn, CA), were used sequentially to isolate CD161+ cells and CD14+ cells from leukopaks. Immature dendritic cells were generated from the CD14+ cells after a 2-day incubation in the presence of 300 units/ml GM-CSF (R & D Systems) and 100 units/ml IL-4 (R & D Systems). After irradiation with 2,000 rad, the immature dendritic cells were cocultured with syngeneic CD161+ cells in the presence of 100 ng/ml α-galactosylceramide and 10 units/ml IL-2 (Invitrogen) for 10–14 days. After stimulating the CD161+ cells a second time with α-galactosylceramide-pulsed, irradiated immature dendritic cells, NKT cell lines were shown flow-cytometrically to express both CD161+ and Vα24i T cell antigen receptor (99% purity).
In Vitro Cytokine Secretion Assay Using Human NKT Cell Lines. IFN-γ and IL-4 secretion by the Vα24i human NKT cell line was determined by ELISA (BD Pharmingen) after culture for 16 h. For these assays, 1 × 105 Vα24i human NKT cells were cocultured with 4 × 105 irradiated, immature CD14+ dendritic cells, in the presence of the glycolipid compounds at 10 μg/ml in a 96-well flat-bottom plate.
In Vitro CD1d-Dimer Assay Using a Human NKT Cell Line. We incubated 1 μg of soluble divalent human CD1d-IgG1 fusion protein (human CD1d-IgG1 dimers, BD Pharmingen) overnight with 10 M each glycolipid at 32°C and at neutral pH according to the manufacturer's protocol. The glycolipid-loaded CD1d-IgG1 dimers were incubated with human NKT cells at 4°C for 60 min, followed by incubation with PE-coupled anti-mouse IgG1 mAb (A85-1). The cells were also surface-stained with a PerCP-coupled anti-CD3 mAb (BD Pharmingen).
Results and Discussion
Synthesis. We first synthesized the glycolipids of bacterial origin (1, 2, 5, and 6) of which the structures are similar to α-GalCer either at the sugar or lipid moiety and tested their CD1d-mediated NKT cell activation activities. Analogs 3 and 4 were prepared to probe the effect of the carboxyl group on the sugar and the α-hydroxyl group on the lipid. Compounds 7–9 contain a3″-sulfate group with an α-or β-glycosidic linkage. Compounds 10–13 were prepared to probe the effect of the 2″ modification of α-GalCer. Analogs of α-GalCer (16–18) with modification of the lipid moiety were also prepared to probe their interaction with CD1d and the subsequent effect on NKT cell activation.
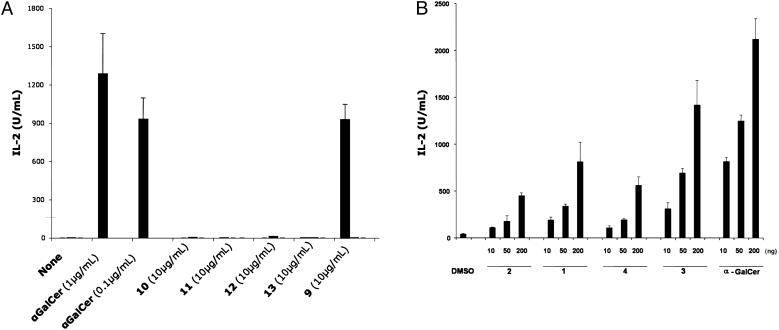
Murine 1.2 Hybridoma Assay. Mouse Vα14i NKT cells immortalized by cell fusion provide a convenient method for assaying the ability of the synthetic glycolipids to activate T cells. As shown in Fig. 5A, 3″-O-sulfo-α-GalCer, 9, stimulated significant IL-2 release from the hybridomas when used at 10 μg/ml. Dose–response curves indicated, however, that this compound was somewhat less active than αGalCer (data not shown) in this model. By contrast, every modification of the 2″-OH position of the galactose (compounds 10–13) that was tested abolished all biological activity. These data indicate that the Vα14i NKT cell response to glycolipids apparently is more sensitive to modifications of the 2 position than the 3 position. The B. burgdorferi glycolipids (5 and 6) and compounds 14 and 15 were moderately active in the 1.2 hybridoma assay. However, IL-2 secretion could only be detected when large quantities of the glycolipids were used to stimulate the hybridoma cells (unpublished data).
Fig. 5.
IL-2 secretion by murine 1.2 hybridomas when stimulated by the indicated glycolipids loaded on CD1d-coated plates. (A) IL-2 secretion profile of 3″-O-sulfo-α-GalCer compared with α-GalCer and analogs. (B) Dose-dependent secretion of IL-2 by Sphingomonas GSLs and analogs. CD1d molecules (10 μg/ml in PBS) were coated in a 96-well plate by incubation for 1 h at 37°C. IL-2 release was measured after 16 h of culture in a sandwich ELISA.
CD1d-coated plates were used to assay response of the hybridomas to the Sphingomonas glycolipids (Fig. 5B). A substantial level of IL-2 secretion can be observed for all compounds. The structure of the sugar head group significantly affected the activation of the hybridomas. α-GalCer and the galactose analog, 3, consistently solicited greater IL-2 secretion when compared with the galacturonic acid derivatives. Also affecting activity was the (S)-2-hydroxy of the fatty amide tail. A fully saturated tail was more greatly favored, suggesting that the α-hydroxyl group is not optimal. This finding is in agreement with previous structure–activity studies (8) of the marine sponge glycolipid, α-GalCer, which showed the same result. In fact, the (S)-2-hydroxy appeared to have a greater affect on activity as compound 4, a galactose analog, that was less able to activate IL-2 secretion when compared with 1, the galacturonic acid compound without the α-hydroxyl fatty amide. Although 3 and 4 have not been reported as natural products, both could be precursors to compounds 1 and 2.
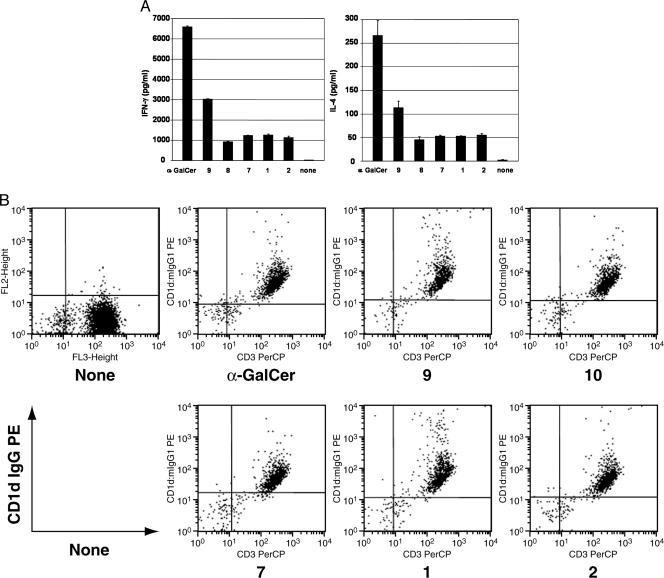
Recognition of Glycolipids by the Human NKT Cell Line Results in Cytokine Secretion. We assessed IFN-γ and IL-4 secretion from a Vα24i NKT cell line, after stimulation with irradiated, syngeneic CD14+ immature dendritic cells in the presence of 10 μg/ml glycolipids and 10 units/ml IL-2 (Fig. 6A). Stimulation of the NKT cell line by each glycolipid compound resulted in significant IFN-γ and IL-4 secretion, when compared with the negative control. Whereas greater IFN-γ and IL-4 secretion was observed after stimulation by the potent NKT cell agonist, α-GalCer, secretion of IFN-γ and IL-4 by NKT cells stimulated by 3″-O-sulfo-α-galactosylceramide was approximately half that of α-GalCer but twice that induced by the other glycolipids. Surprisingly, β-linked sulfatides 7 and 8 were observed to elicit both IFN-γ and IL-4 production. In fact, the level of cytokine secretion was comparable to the GSLs. Other α-GalCer analogs with an acetyl side chain or a shortened backbone were also tested, and some activity was also observed (data not shown).
Fig. 6.
Human Vα24i NKT cells release IL-4 and IFN-γ in response to the indicated glycolipids. Flow cytometric analyses also show that Vα24i NKT cells bind to the various glycolipids in response to the indicated glycolipids. (A) Human Vα 24i NKT cells respond to synthetic Sphingomonas and sulfatide glycolipids. IFN-γ and IL-4 release after 16 h of culture by 1 × 105 Vα24i human NKT cell line in response to culture with 4 × 105 autologous immature CD14+ dendritic cells pulsed with the indicated glycolipid antigens at 10 μg/ml. The negative control wells contained similar numbers of NKT cells and dendritic cells, cultured without added glycolipid. Data are mean ± SD of duplicate wells. (B) Human Vα 24i NKT cells bind to glycolipids in the context of CD1d. Flow cytometric analysis of a human Vα24i human NKT cell line with human CD1d dimers that were unloaded or loaded with 10 M indicated glycolipid antigen. The cells were also stained with anti-human CD3-PerCP.
Human NKT Cell Lines Bind to Glycolipids in the Context of CD1d. Although glycolipids stimulate the NKT cell line, we cannot invariably conclude that the glycolipids are presented by CD1d molecules and are capable of triggering the Vα24i T cell receptor expressed by the NKT cells. Therefore, to confirm the glycolipid antigen reactivity to the Vα24i T cell receptor at the single-cell level, we stained a human NKT cell line with human CD1d dimers loaded with different glycolipids, using unloaded CD1d dimers as a negative control. Each glycolipid-loaded dimer nearly universally stained the human NKT cells, whereas the unloaded dimer did not stain these cells (Fig. 6B).
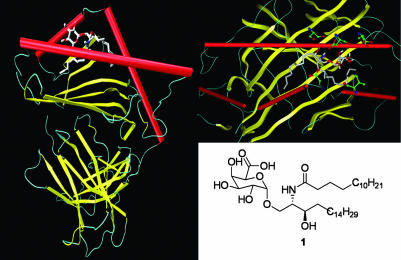
Computer Modeling of GSL Complexed to mCD1d. To further understand the interaction of bacterial glycolipid 1 with CD1d, a model generated by autodock 3.0 (29) of GSL 1 complexed with the crystal structure of mCD1d was shown in Fig. 7 (4). In this model, the fatty acyl chain extends into the F′ pocket, and the sphingosine chain extends toward the A′ pocket. This allowed the polar head group to be oriented such that it was exposed for recognition by a T cell antigen receptor. Numerous favorable contacts could be observed between mCD1d and the GSL. Among them, possible hydrogen bonding included interactions between the carboxylate of the sugar and the backbone carbonyl of Asp-80, and the amide nitrogen of the fatty-acid tail with the Asp-80 side chain.
Fig. 7.
Computer modeling of GSL-1 docked to the crystal structure of mCD1d. The two acyl tails fit nicely into the hydrophobic pockets of the protein, allowing for the sugar head group to be presented for NKT cell recognition.
Studies (30) have shown mCD1d to be somewhat accommodating in terms of lipid tail length on NKT cell reactivity. However, changes in the lipid length, composition, or addition of an α-hydroxyl group on the fatty acid could cause a slight shift in orientation of the sugar, thereby affecting how the CD1d/glycolipid complex is recognized by the T cell receptor. This could also be true of the substitution of galacturonic acid for galactose. The perturbation caused by having the 6″-OH oxidized to a carboxylic acid caused only moderate changes in NKT cell reactivity.
Conclusion
In summary, a number of glycolipids were synthesized and tested for their ability to activate NKT cells. Of particular interest were sulfatides 7–9 and the Sphingomonas GSLs, which showed considerable activity as measured by cytokine production. Although none of the compounds were as potent as α-GalCer, specific responses were obtained to an α-GalCer analog, 3″-O-sulfo-α-GalCer, 9, but not to any of the four compounds having a modification of the equatorial 2″-OH of the galactose. Furthermore, glycolipid/CD1d dimer staining of human Vα24i NKT cells showed reaction toward the Sphingomonas GSLs (GSL-1 and GSL-2) and sulfatides 7–9 when compared with α-GalCer. We were also able to demonstrate responses to the Sphingomonas compounds in vivo, and mice deficient for NKT cells have a reduced ability to clear bacteria from the liver (Y.K., D.W., G. Kim, G.-W.X., M.A.P., M.T., K. Kawahara, C.-H.W., and M.K., unpublished work).
Bacterial α-galacturonosyl ceramides have been shown to elicit activity from both mouse and human NKT cell lines. As measured by cytokine release and CD1d dimer/glycolipid staining of a human NKT cell line, 3″-O-sulfo-GalCer, 9, had comparable potency to the exceptional agonist α-GalCer, showing that some flexibility can be accommodated at the 3″-OH position of galactose. Any modification at the 2″-OH position almost completely abolished activity. The Sphingomonas GSLs are reactive to the same population of human Vα24i NKT cells as α-GalCer, showing that bacterium-derived antigens are also able to activate NKT cells.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health, the Skaggs Institute, and the Irlene Diamond Foundation.
Abbreviations: α-GalCer, α-galactosyl-ceramide; GSL, glycosphingolipid; mCD1d, murine CD1d; NKT cell, natural killer T cell; THF, tetrahydrofuran.
References
- 1.Porcelli, S. A. & Modlin, R. L. (1999) Annu. Rev. Immunol. 17, 297-329. [DOI] [PubMed] [Google Scholar]
- 2.Joyce, S. & Kaer, L. V. (2003) Curr. Opin. Immunol. 15, 95-104. [DOI] [PubMed] [Google Scholar]
- 3.Calabi, F., Jarvis, J. M., Martin, L. & Milstein, C. (1989) Eur. J. Immunol. 19, 285-292. [DOI] [PubMed] [Google Scholar]
- 4.Zeng, Z., Castano, A. R., Segelke, B. W., Stura, E. A., Peterson, P. A. & Wilson, I. A. (1997) Science 277, 339-345. [DOI] [PubMed] [Google Scholar]
- 5.Zajonc, D. M., Elsliger, M. A., Teyton, L. & Wilson, I. A. (2003) Nat. Immunol. 4, 808-815. [DOI] [PubMed] [Google Scholar]
- 6.Gadola, S. D., Zaccai, N. R., Harlos, K., Shepherd, D., Castro-Palomino, J. C., Ritter, G., Schmidt, R. R., Jones, E. Y. & Cerundolo, V. (2002) Nat. Immunol. 3, 721-726. [DOI] [PubMed] [Google Scholar]
- 7.Batuwangala, T., Shepherd, D., Gadola, S. D., Gibson, K. J. C., Zaccai, N. R., Fersht, A. R., Besra, G. S., Cerundolo, V. & Jones, E. Y. (2004) J. Immunol. 172, 2382-2388. [DOI] [PubMed] [Google Scholar]
- 8.Morita, M., Motoki, K., Akimoto, K., Natori, T., Sakai, T., Sawa, E., Yamaji, K., Koezuka, Y., Kobayashi, E. & Fukushima, H. (1995) J. Med. Chem. 38, 2176-2187. [DOI] [PubMed] [Google Scholar]
- 9.Singh, N., Hong, S., Scherer, D. C., Serizawa, I., Burdin, N., Kronenberg, M., Koezuka, Y. & Van Kaer, L. (1999) J. Immunol. 163, 2373-2377. [PubMed] [Google Scholar]
- 10.Hong, S., Wilson, M. T., Serizawa, I., Wu, L., Singh, N., Naidenko, O. V., Miura, T., Haba, T., Scherer, D. C., Wei, J., et al. (2001) Nat. Med. 7, 1052-1056. [DOI] [PubMed] [Google Scholar]
- 11.Burdin, N., Brossay, L. & Kronenberg, M. (1999) Eur. J. Immunol. 29, 2014-2025. [DOI] [PubMed] [Google Scholar]
- 12.Gapin, M. K. L. (2002) Nat. Rev. Immunol. 8, 557-568. [DOI] [PubMed] [Google Scholar]
- 13.Brossay, L., Chioda, M., Burdin, N., Koezuka, Y., Casorati, G., Dellabona, P. & Kronenberg, M. (1998) J. Exp. Med. 188, 1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, K., Scotet, E., Niemeyer, M., Koebernick, H., Zerrahn, J., Maillet, S., Hurwitz, R., Kusar, M., Bonnevile, M., Kaufmann, S., et al. (2004) Proc. Natl. Acad. Sci. USA 101, 10685-10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu, D. Y., Segal, N. H., Sidobre, S., Kronenberg, M. & Chapman, P. B. (2003) J. Exp. Med. 198, 173-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch, J., Gumperz, J., Robinson, C., Skold, M., Roy, C., Young, D. C., Lafleur, M., Moody, D. B., Brenner, M. B., Costello, C. E., et al. (2003) J. Biol. Chem. 278, 47508-47515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang, G., Schmieg J., Tsuji, M. & Franck, R. W. (2004) Angew. Chem. Int. Ed. 43, 3818-3822. [DOI] [PubMed] [Google Scholar]
- 18.Goff, R. D., Gao, Y., Mattner, J., Zhou, D., Yin, N., Cantu, C., Teyton, L., Bendelac, A. & Savage, P. B. (2004) J. Am. Chem. Soc. 126, 13602-13603. [DOI] [PubMed] [Google Scholar]
- 19.Shamshiev, A., Gober, H.-J., Donda, A., Mazorra, Z., Mori, L. & De Libero, G. (2002) J. Exp. Med. 195, 1013-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawahara, K., Kubota, M., Sato, N., Tsuge, K. & Seto, Y. (2002) FEMS Microbiol. Lett. 214, 289-294. [DOI] [PubMed] [Google Scholar]
- 21.Kumar, H., Belperron, A., Barthold, S. W. & Bockenstedt, L. K. (2000) J. Immunol. 165, 4797-4801. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Menachem, G., Kubler-Kielb, J., Coxon, B., Yergey, A. & Schneerson, R. (2003) Proc. Natl. Acad. Sci. USA 100, 7913-7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawahara, K., Moll, H., Knirel, Y. A., Seydel, U. & Zahringer, U. (2000) Eur. J. Biochem. 267, 1837-1846. [DOI] [PubMed] [Google Scholar]
- 24.Plettenburg, O., Bodmer-Narkevitch, V. & Wong, C. H. (2002) J. Org. Chem. 67, 4559-4564. [DOI] [PubMed] [Google Scholar]
- 25.Williams, L., Zhang, Z., Shao, F., Carroll, P. J. & Joullie, M. M. (1996) Tetrahedron 52, 11673-11694. [Google Scholar]
- 26.Deng, S. Y. B., Xie, J. & Hui, Y. (1999) J. Org. Chem. 64, 7265-7266. [DOI] [PubMed] [Google Scholar]
- 27.Sidobre, S., Hammond, K. J. L., Benazet-Sidobre, L., Maltsev, S. D., Richardson, S. K., Ndonye, R. M., Howell, A. R., Sakai, T., Besra, G. S., Porcelli, S. A. & Kronenberg, M. (2004) Proc. Natl. Acad. Sci. USA 101, 12254-12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elewaut, D., Lawton, A. P., Nagarajan, N. A., Maverakis, E., Khurana, A., Honing, S., Benedict, C. A., Sercarz, E., Bakke, O., Kronenberg, M. & Prigozy, T. I. (2003) J. Exp. Med. 198, 1133-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morris, G. M., Goodsell, D. S., Halliday, R. S., Huey, R., Hart, W. E., Belew, R. K. & Olson, A. J. (1998) J. Comput. Chem. 19, 1639-1662. [Google Scholar]
- 30.Brossay, L., Naidenko, O., Burdin, N., Matsuda, J., Sakai, T. & Kronenberg, M. (1998) J. Immunol. 161, 5124-5128. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.