Abstract
Background
Chronic obstructive pulmonary disease (COPD) is a commonly diagnosed condition in people older than 50 years of age. In advanced stage of this disease, integrated care (IC) is recommended as an optimal approach. IC allows for holistic and patient-focused care carried out at the patient’s home. The aim of this study was to analyze the impact of IC on costs of care and on demand for medical services among patients included in IC.
Material/Methods
The study included 154 patients diagnosed with advanced COPD. Costs of care (general, COPD, and exacerbations-related) were evaluated for 1 year, including 6-months before and after implementing IC. The analysis included assessment of the number of medical procedures of various types before and after entering IC and changes in medical services providers.
Results
Direct medical costs of standard care in advanced COPD were 886.78 EUR per 6 months. Costs of care of all types decreased after introducing IC. Changes in COPD and exacerbation-related costs were statistically significant (p=0.012492 and p=0.017023, respectively). Patients less frequently used medical services for respiratory system and cardiovascular diseases. Similarly, the number of hospitalizations and visits to emergency medicine departments decreased (by 40.24% and 8.5%, respectively). The number of GP visits increased after introducing IC (by 7.14%).
Conclusions
The high costs of care in advanced COPD indicate the need for new forms of effective care. IC caused a decrease in costs and in the number of hospitalization, with a simultaneous increase in the number of GP visits.
MeSH Keywords: Delivery of Health Care, Integrated; Economics; Pulmonary Disease, Chronic Obstructive
Background
COPD from pharmacoeconomic perspective
Chronic obstructive pulmonary disease (COPD) is a common chronic condition in people over the age of 50 years. Rapid population aging in developed countries is considered to be the main reason for higher prevalence of chronic diseases, including COPD. These changes will require some specific actions undertaken by health system decision-makers [1].
According to WHO estimates, there are more than 65 million people worldwide suffering from COPD. This is also an important health problem in Poland. It is estimated that 10.1–10.2% of Poles have COPD [2,3]. This is equivalent to approximately 2 million people, out of whom, 20% have an advanced stage of the disease (stage III and IV according to GOLD classification of obturation) [4].
COPD imposes a heavy economic burden on health systems. Expenditures are approximately EUR 38.6 billion a year, of which 40–75% is spent on treatment of exacerbations requiring hospitalization [5–9]. Therefore, preventing exacerbations is the biggest medical and economic challenge. Similarly, in Poland, unstable disease with frequent exacerbations is related to expenditures higher by PLN 1600/year (≈EUR 366.60) than in patients with stable disease [10]. In-hospital treatment of an exacerbation is up to 10 times more expensive than outpatient treatment [11].
Thus, to achieve more effective management of the disease, particularly in advanced COPD, an innovative type of care was proposed. The Integrated Care Model (ICM) is an intervention developed in Gdansk (Pomeranian Province, Poland). The main goal of ICM was to decrease the number of exacerbations and stabilize patient condition, thereby limiting National Health Fund (NHF) expenditures.
The intervention – Integrated Care Model (ICM)
ICM includes general and specialist care combined with home support for patients, and intensive education of patients and their relatives.
All actions are synchronized by the program coordinator, who is also responsible for supervising the proper use of drugs by the patients and coordinating their visits to GPs and pulmonologists (Figure 1).
Figure 1.
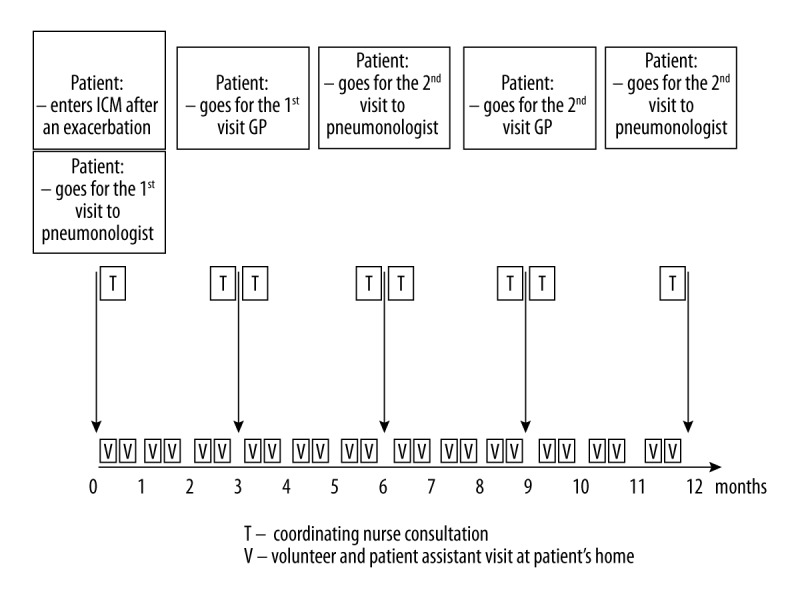
Scheme of ICM provided for Gdansk (Poland), made by the authors, based on Damps-Konstańska et al. [30].
As shown in Figure 1, the coordinator is responsible for providing ongoing support to the patients. This is a non-medical staff member who has completed a specialist course in a coordination of treatment of chronically ill patients, organized by a hospice foundation, who has participated in educational training on COPD, according to the educational program developed by one of the present co-authors – Iwona Damps-Konstanska, MD, PhD.
The coordinator provides patient support by:
– preparing the schedule of medical and non-medical activities for ICM patients;
– communicating with all ICM members;
– contacting patients by phone once every 2 weeks to assess their general health condition, if they are medication-compliant, and if they require attention of medical or non-medical staff;
– coordinating education of patients and their families by organizing trainings.
Within ICM there are 2 multidisciplinary teams working in close collaboration to assure optimal patient-oriented care.
-
The ICM medical team consists of:
– pulmonologists;
– medical doctors in other specializations, such as endocrinologists, cardiologists, or gastroenterologists (but only within standard care cooperation);
– GPs;
– nurses.
-
The ICM non- medical team consists of:
– patient assistants;
– dietitians;
– psychologists;
– physiotherapists;
– social workers;
– priests.
All team members are cooperating not only with each other but also with patients and their relatives. The innovative element is to integrate the activities of medical specialists and non-medical staff and to provide home support for patients with poor self-management and problems with compliance with medical recommendations.
Aim
The aim of this study was to estimate COPD costs and to analyze the impact of introducing integrated care (IC) on the public budget.
Secondary endpoints included the evaluation of:
– direct medical costs for patients included and not included in ICM;
– changes in costs before and after including patients in ICM;
– changes in the frequency of using specific types of medical services.
Material and Methods
Study population
The study included 175 patients diagnosed with advanced COPD. The mean age of the study group was 71 years old (46–88 years) and 65% were men. The baseline characteristics are presented in Table 1.
Table 1.
Baseline characteristics of the study participants divided in two subgroups ICM-yes and ICM-no.
| ICM-yes | ICM-no | |
|---|---|---|
| Age (average) | 72 | 71 |
| Age (median) | 71 | 70 |
| Sex | Male (n= 31; 70.45%) | Male (n=82; 62.60%) |
| Female (n=13; 29.54%) | Female (n=49; 37.40%) |
Cost analysis
This was a “before-after” study. The first period, called “intro-6m” (meaning 6 months before introducing ICM), included 6 months of standard treatment received by all the patients. After 6 months, the study group was divided into 2 subgroups:
– subgroup 1 called “ICM-no” continued standard type of care (n=131);
– subgroup 2 called “ICM-yes” received integrated care (n=44).
The observation lasted for another 6 months after dividing patients into subgroups (the period called “intro+6m”). Based on prior studies on IC, the study included patients who fulfilled the specified criteria (Figure 2).
Figure 2.
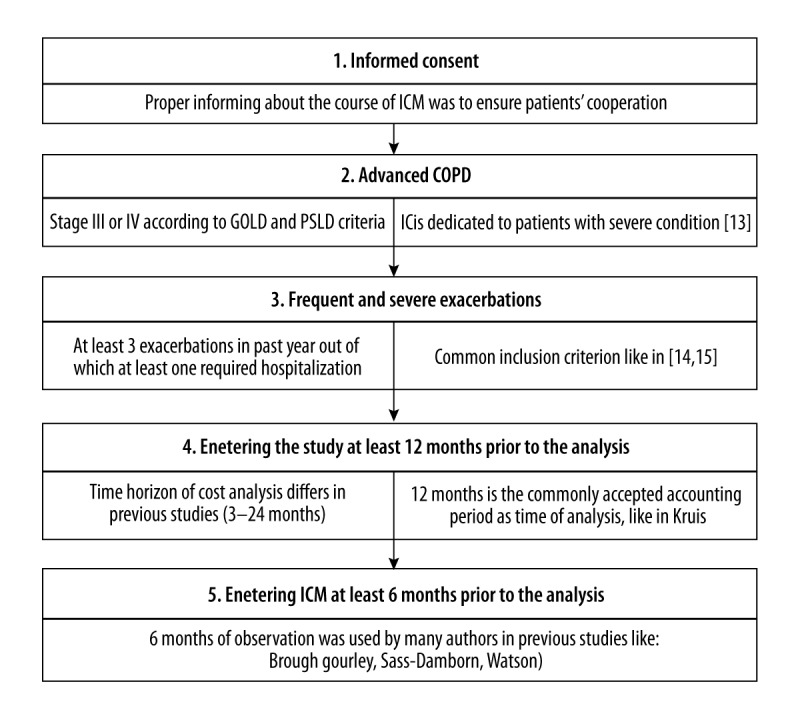
Criteria for including patients in the particular study groups [31–38].
Finally, the cost analysis included 154 patients. The dropouts from the study were a result of: death during the observation period (n=16), lack of COPD-related costs (n=2), and exacerbation costs (n=87). The cost analysis was divided into 3 stages. The types of costs analyzed in the study are presented in Table 2.
Table 2.
Stages of cost analysis with types of costs included.
| Stage of analysis | Type of costs | Type of procedures included |
|---|---|---|
| I | General | All medical procedures realized for patients |
| II | COPD related | Medical procedures realized due to COPD and other diseases of the respiratory system (the list of DRG codes of diseases and conditions requiring medical procedures included in stage II is available in Supplementary Table 1) |
| III | Exacerbation related (ER) | Medical procedures realized due to exacerbations of COPD (the list of DRG codes of diseases and conditions requiring medical procedures included in stage III is available in Supplementary Table 2). |
Selection of DRG (Diagnosis-Related Groups) codes to describe the list of services included in the particular stages of the analysis was done on the basis of the clinical expertise of the 2 authors independently. The cost data were provided by the NHF and covered the time period from September 2012 to the end of June 2014.
The analysis included the following steps:
Calculating costs of standard care for all the patients included in the study;
Calculating costs of IC for patients fulfilling the specified criteria (Figure 2);
Estimating changes in values of costs of all types (Table 2);
Assessing changes in types of medical procedures after replacing SC with IC;
Assessing changes in types of medical services providers engaged into taking care of patients after replacing SC with IC.
Statistical analysis
All calculations were carried out using Microsoft Excel spreadsheets and the STATISTICA, StatSoft Inc. version 8.0. statistical package. The normality of the variables distribution and variance equality of a studied feature in groups were tested using both an appropriate Shapiro-Wilk’s test and a variance equality test. When assessing changes in time, the Wilcoxon’s matched-pairs signed-rank test was used. In all the calculations, the statistical significance level was set to p<0.05.
Results
First, the standard care costs for 6 months were evaluated for all the patients included in the cost analysis (n=154). The average half-year cost was PLN 3870.26 (≈EUR 886.78), whereas the maximal cost exceeded PLN 4500 (≈ EUR 10310.69) (Table 3).
Table 3.
Direct medical costs of standard care in patients with COPD divided into: general costs, system related costs of COPD and other diseases of the respiratory system and related costs of exacerbations of COPD (in PLN).
| Type of cost | N | Mean | Median | Dominant | Dominant representation | Minimal value | Maximal value |
|---|---|---|---|---|---|---|---|
| General costs in intro−6m | 154 | 3870.26 (≈886.78 EUR) | 1087.18 (≈249.10 EUR) | 0 | 18 | 0 | 45456.91 (≈10415.38 EUR) |
| COPD costs in intro−6m | 152 | 1599.03 (≈366.38 EUR) | 91.35 (≈20.93 EUR) | 0 | 46 | 0 | 20071.54 (≈4598.92 EUR) |
| ER costs in intro−6m | 65 | 1571.28 (≈360.02 EUR) | 0 | 0 | 34 | 0 | 20010.64 (≈4584.97 EUR) |
The next step was to assess the changes in values of 3 costs types after implementation of ICM to the group of patients who fulfilled the criteria and were included in ICM. The summary data on cost changes is presented in Table 4.
Table 4.
Direct medical costs of three types of before and after, including patients in ICM (group ICM-yes) (in PLN and EUR) with results of Wilcoxon’s Matched-Pairs Signed-Rank test.
| Period | N | Mean | Median | Dominant | Dominant representation | Min. value | Max. value | p value |
|---|---|---|---|---|---|---|---|---|
| General costs | ||||||||
| Intro−6m | 41 | 5627.29 (≈1289.36EUR) | 3708.72 (»849.77 EUR) | 0 | 1 | 30.45 (≈6.98 EUR) | 29671.15 (≈6798.45 EUR) | 0.079114 |
| Intro+6m | 41 | 3576.79 (≈819.54 EUR) | 2388.4 (»547.25 EUR) | 0 | 2 | 0 | 16765.1 (≈3841.33 EUR) | |
| COPD and other respiratory system disease costs | ||||||||
| Intro−6m | 41 | 3191.43 (≈731.24EUR) | 2168.1 (»496.77 EUR) | 0 | 3 | 0 | 20071.54 (≈4598.92 EUR) | 0.012492 |
| Intro+6m | 41 | 1741.31 (≈398.98 EUR) | 157.5 (»36.09 EUR) | 0 | 6 | 0 | 9314.65 (≈2134.23 EUR) | |
| Exacerbation costs | ||||||||
| Intro−6m | 26 | 2443.61 (≈559.9EUR) | 1872 (»428.92EUR) | 0 | 7 | 0 | 20010.64 (≈4584.97EUR) | 0.017023 |
| Intro+6m | 26 | 735.17 (≈168.45EUR) | 0 | 0 | 18 | 0 | 5539.5 (≈1269.25EUR) | |
General costs
General costs were analyzed in 2 consecutive periods: before and after including patients into ICM. We found that general direct medical costs decreased. Both average and median values were reduced to PLN 2050.50 (≈EUR 469.82) and PLN 1319.93 (≈EUR 302.43), respectively, after 6 months of ICM. However, the Wilcoxon’s signed-rank test showed no statistical significance of the differences in the distribution of parameters (p=0.079114).
COPD costs
The costs related to COPD and other respiratory system diseases decreased significantly (Wilcoxon’s Matched-Pairs Signed-Rank p=0.012492). Both average and median values were reduced to PLN 1450.12 (≈EUR 332.26) and PLN 2010.60 (≈EUR 460.68), respectively, after 6 months of ICM.
Exacerbation costs
The last stage of cost analysis was to assess exacerbation-related costs. These costs also decreased significantly (Wilcoxon’s matched-pairs signed-rank p=0.017023). Both average and median costs were lowered to PLN 1708.44 (≈EUR 391.45) and PLN 1872.00 (≈EUR 428.92), respectively, after 6 months of ICM. Median costs after introducing ICM were PLN 0 (EUR 0). Eighteen (approximately 70%) of the 26 patients included in this stage of the cost analysis had no exacerbation-related costs during 6 months of ICM.
Changes in the number of medical procedures provided for patients
After including patients in ICM, the number of medical procedures used in treating their health condition changed. There was a decrease in the number of medical services provided in most of the cases, except from gastrointestinal diseases (Figure 3).
Figure 3.
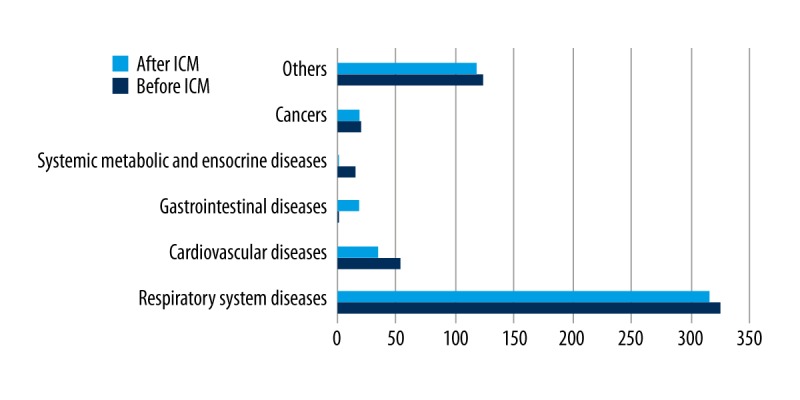
The number of medical services provided before and after ICM in treatment of health conditions.
A similar comparison was done for the type of medical services provider. The number of services provided by GPs increased along with a decrease in the number of hospitalizations and services provided by emergency departments and paramedic teams (Figure 4).
Figure 4.
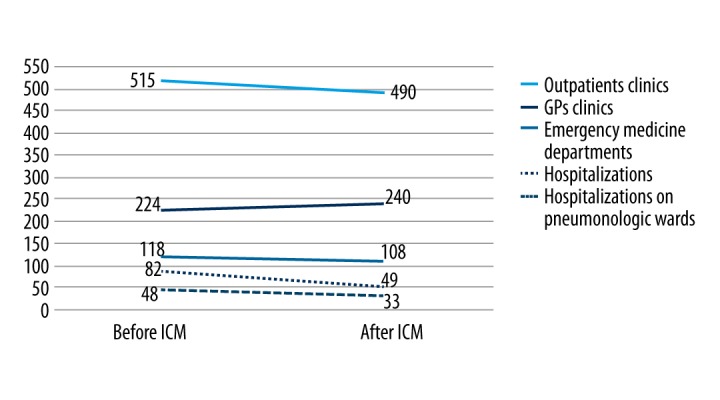
The number of medical services provided by different types of providers before and after ICM.
Discussion
According to numerous studies, IC is a relevant and essential medical technology in the management of advanced COPD [12–14]. At the present time, it seems reasonable to determine the economic effectiveness of this technology with the use of pharmacoeconomic tools. In Poland, the role of pharmacoeconomic analyses is systematically growing and they are currently used for assessing all new and expensive procedures. They are particularly important in selecting optimal medical technologies for managing chronic diseases, including COPD, as these conditions are related to particularly high costs [11–17].
Back in 2007, in Poland, it was found that the average direct medical costs of COPD were PLN 4027 per 1 year (≈EUR 922.69) [10]. In the present study, after 8 years, the average costs were PLN 3191.43 per 6 months (≈EUR 731.24) when standard care was used. Nevertheless, in our study, we decided to analyze not only COPD-related costs, but also costs of all procedures provided for patients, as it has already been proven that assessing only COPD costs is not sufficient for the meticulous calculation of actual economic burden, mainly due to the presence of multiple co-morbidities [10,18,19].
Furthermore, earlier studies showed that severity of COPD is related to cost of care [8], especially costs due to frequent hospitalizations [20]. This is one reason why ICM should be indicated for patients with advanced COPD with severe and very severe obstruction of the airways.
The cost analysis proved that patients fulfilling the criteria for participation in ICM were in a worse health status, which was confirmed by higher costs of care in the primary assessment. By comparing costs calculated for all patients (n=154) and for the ICM-yes group in the period before introducing ICM, we noticed that the costs were lower: 31% lower for general costs, 49% lower for COPD costs, and 36% lower for exacerbation costs. This confirms that the correct inclusion criteria were planned for the study, as the intention of the authors was to make ICM available for the patients in more severe condition.
In numerous “before-after” studies, authors found that implementing IC results in savings. In the Steuten et al. study, the decrease in direct medical costs was EUR 42 [21]. In our study, the difference between average costs was even greater (PLN 2050.48 ≈EUR 489). Even after taking into account that ICM requires additional financing (PLN 915.18/6 months/1patient ≈EUR 209.69), in 1 year it is still possible to save EUR 543 per patient. This shows that the construction of the model proposed in Gdansk is well adjusted to the needs of COPD patients and might be an important tool for managing COPD and limiting health system expenditures. ICM is a complex intervention that includes medical and non-medical staff support provided at patients’ homes, cooperation of specialist care with GPs, and supervision of adherence to medical recommendations (for a comparison of elements available for patients, see Table 5). A similar observation concerning the positive impact of integrated care on costs was found in 2014 by van Boven et al. [22], who reported savings of EUR 227 per patient.
Table 5.
Comparison of studies dealing with integrated care.
| Author (year) | IC programme country | Types of intervention used | Main assumptions of ICM | ||
|---|---|---|---|---|---|
| Base for comparison – main assumption of ICM Poland | |||||
| Bandurska et al. (2016) | Poland |
|
|
||
| Comparison with IC studies | |||||
| Author (year) | IC programme country | Types of intervention used | Similarities (ICM)In study design.In IC model construction.In results obtained | Differences (ICM)In study design.In IC model construction.In results obtained | Positive impact of IC |
| Boven et al. (2014) | Belgium |
|
|
|
|
| Steuten et al. | Netherlands |
|
|
|
|
| Hermiz et al. (2002) | Australia |
|
|
|
|
| Casas et al. (2006) | Belgium, Spain |
|
|
|
|
| Titova et al. (2015) | Norway |
|
|
|
|
| Boland et al. (2015) | Netherlands |
|
|
|
|
It is worth noting, though, that no statistically significant changes were found in general costs. However, after entering ICM, patients visited specialists such as dermatologists or dentists more often, which influenced the general costs. We perceive this phenomenon as positive, and it might indicate that health status improved and patients were able to use medical services that were out of reach for them before entering ICM because of poor health.
The statistically significant decrease in the COPD costs indicates that fewer procedures were provided after including patients in ICM. We believe that many health needs were satisfied by ICM and no additional procedures were necessary. Therefore, ICM seems to be appropriate for patients with advanced COPD.
Numerous have studies concentrated on the significance of preventing exacerbations that require hospitalizations [23]. This is particularly important because an exacerbation requiring hospitalization usually results in significant deterioration in health status [6].
The decrease noticed in the exacerbation-related costs may also be considered as a decreasing number of exacerbations. Before ICM was implemented, approximately 47.7% of patients required medical services typical for exacerbations (Table 3). In the group of patients meeting the criteria of inclusion to ICM, the prevalence was even higher; exacerbation-related procedures were provided for 73% of patients. After 6 month of ICM in the group “ICM-yes” only 31% of patients required procedures provided for exacerbations. A similar observation was recently made by Titova et al., who noticed a difference in the number of hospitalizations between patients receiving IC and those receiving regular care (12.6% reduction in the first year and 46.5% reduction during the second year of follow-up, in comparison to 8.3% increase in the first year and no change during the second year of follow-up) [24].
These results seem to be encouraging. Furthermore, it is worth noting that ICM seems to be more effective than many IC models described by other authors [22,25]. For example, in a recent study by Hernandez et al., there was no reduction in the number of hospital admission found in IC in comparison with SC for stable patients [26]. Some authors found no economic efficacy of integrated care [27]. In a recently published cluster study on cost-effectiveness of IC in COPD, no economic benefit was found [28]. Table 5 shows differences and similarities between studies dealing with IC in COPD.
As contemporary knowledge of the economic effectiveness of integrated care is still not clear, the present study, conducted in Gdansk, proves ICM is an effective tool. We hope our results will be important in the worldwide discussion regarding designing effective models of care for patients and the healthcare system finances.
Conclusions
We found that ICM is a beneficial intervention for patients and the public payer. After including patients in ICM, their demand for medical services changed. The patients were rarely hospitalized and used services provided by outpatient clinics more often. Fewer procedures were continued in treating respiratory system disorders, which might indicate an improvement in the health of ICM patients.
The costs of care provided for advanced COPD patients are high, which indicates a need to evaluate traditionally used medical services and to looking for more effective ones, both from the medical and economic point of view. It seems that ICM meets these criteria, as medical costs of all analyzed types decreased when standard care was replaced by IC. Statistically significant changes were observed in costs related to COPD and exacerbations.
The obtained results show that ICM can be an effective tool to manage advanced COPD, because it reduces the number of exacerbations and therefore limits public expenditures.
Implications
This article is particularly relevant in countries where integrated care is still being implemented [29]. For specialists already using integrated care, this study confirms that IC can benefit COPD patients and the healthcare system. In countries that are still adjusting their health system to current and future challenges (e.g., demographic changes), the results obtained in Gdansk can be a guide to choosing a beneficial model of care. After an analysis of the literature on IC, it is clear that scientific discussion on the effectiveness of this type of care is still open.
Limitations
This study has some limitations. The number of patients included in ICM was small because ICM still has no stable source of financing and is not financed from the budget of the NHF. Another limitation was using the public payer perspective in the cost analysis instead of the societal one, which is considered to be the most extensive and complete. In Poland, conducting cost analysis from the societal perspective is very difficult due to a limited access to data that is highly dispersed and collected on a fragmentary basis by various institutions. Due to using the public payer perspective, it was impossible to assess indirect costs.
Supplementary Tables
Supplementary Table 1.
List of diseases and related health problems requiring procedures qualified to costs of COPD and other diseases of respiratory system
| ICD-10 code | Name | Reason for inclusion |
|---|---|---|
| I26 | Pulmonary embolism | Frequent cause of death |
| I26.9 | Pulmonary embolism without mention of acute cor pulmonale | Frequent cause of death |
| I27 | Other pulmonary heart diseases | Frequent cause of death |
| I27.8 | Other specified pulmonary heart diseases | Frequent cause of death |
| I27.9 | Pulmonary heart disease, unspecified | Frequent cause of death |
| J00 | Acute nasopharyngitis | Frequent occurrence in patients with COPD |
| J02 | Acute pharyngitis | Frequent occurrence in patients with COPD |
| J03 | Acute tonsillitis | Frequent occurrence in patients with COPD |
| J04 | Acute laryngitis and tracheitis | Frequent occurrence in patients with COPD |
| J06 | Acute upper respiratory infections of multiple and unspecified sites | Frequent occurrence in patients with COPD |
| J13 | Pneumonia due to Streptococcus pneumoniae | Frequent occurrence in patients with COPD |
| J15 | Bacterial pneumonia, not elsewhere classified | Frequent occurrence in patients with COPD |
| J15.1 | Pneumonia due to Pseudomonas | Frequent occurrence in patients with COPD |
| J15.4 | Pneumonia due to other streptococci | Frequent occurrence in patients with COPD |
| J15.5 | Pneumonia due to Escherichia coli | Frequent occurrence in patients with COPD |
| J15.6 | Pneumonia due to other aerobic Gram-negative bacteria | Frequent occurrence in patients with COPD |
| J15.8 | Other bacterial pneumonia | Frequent occurrence in patients with COPD |
| J15.9 | Bacterial pneumonia, unspecified | Frequent occurrence in patients with COPD |
| J16 | Pneumonia due to other infectious organisms, not elsewhere classified | Frequent occurrence in patients with COPD |
| J16.8 | Pneumonia due to other specified infectious organisms | Frequent occurrence in patients with COPD |
| J18 | Pneumonia, organism unspecified | Frequent occurrence in patients with COPD |
| J18.9 | Pneumonia, unspecified | Frequent occurrence in patients with COPD |
| J20 | Acute bronchitis | Frequent occurrence in patients with COPD |
| J20.9 | Acute bronchitis, unspecified | Frequent occurrence in patients with COPD |
| J21 | Acute bronchiolitis | Frequent occurrence in patients with COPD |
| J22 | Unspecified acute lower respiratory infection | Frequent occurrence in patients with COPD |
| J31 | Chronic rhinitis, nasopharyngitis and pharyngitis | In connection with the use of inhalers |
| J32.4 | Chronic pansinusitis | Frequent occurrence in patients with COPD |
| J37 | Chronic laryngitis and laryngotracheitis | Frequent occurrence in patients with COPD |
| J39 | Other diseases of upper respiratory tract | Frequent occurrence in patients with COPD |
| J39.2 | Other diseases of pharynx | Frequent occurrence in patients with COPD |
| J40 | Bronchitis, not specified as acute or chronic | Frequent occurrence in patients with COPD |
| J41 | Simple and mucopurulent chronic bronchitis | Frequent occurrence in patients with COPD |
| J41.0 | Simple chronic bronchitis | Frequent occurrence in patients with COPD |
| J41.8 | Mixed simple and mucopurulent chronic bronchitis | Frequent occurrence in patients with COPD |
| J42 | Unspecified chronic bronchitis | Frequent occurrence in patients with COPD |
| J43 | Emphysema | Frequent occurrence in patients with COPD |
| J43.8 | Other emphysem | Frequent occurrence in patients with COPD |
| J43.9 | Emphysema, unspecified | Frequent occurrence in patients with COPD |
| J44 | Other chronic obstructive pulmonary disease | Code assigned for COPD |
| J44.0 | Chronic obstructive pulmonary disease with acute lower respiratory infection | Frequent occurrence in patients with COPD |
| J44.1 | Chronic obstructive pulmonary disease with acute exacerbation, unspecified | Frequent occurrence in patients with COPD |
| J44.8 | Other specified chronic obstructive pulmonary disease | Frequent occurrence in patients with COPD |
| J44.9 | Chronic obstructive pulmonary disease, unspecified | Frequent occurrence in patients with COPD |
| J45 | Asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.0 | Predominantly allergic asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.1 | Nonallergic asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.8 | Mixed asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.9 | Asthma, unspecified | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J46 | Status asthmaticus | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J47 | Bronchiectasis | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J84 | Other interstitial pulmonary diseases | Frequent occurrence in patients with COPD |
| J84.1 | Other interstitial pulmonary diseases with fibrosis | Frequent occurrence in patients with COPD |
| J84.8 | Other specified interstitial pulmonary diseases | Frequent occurrence in patients with COPD |
| J84.9 | Interstitial pulmonary disease, unspecified | Frequent occurrence in patients with COPD |
| J85.1 | Abscess of lung with pneumonia | Frequent occurrence in patients with COPD |
| J93 | Pneumothorax | Frequent occurrence in patients with COPD |
| J93.1 | Spontaneous tension pneumothorax | Frequent occurrence in patients with COPD |
| J93.8 | Other pneumothorax | Frequent occurrence in patients with COPD |
| J94 | Other pleural conditions | Frequent occurrence in patients with COPD |
| J96 | Respiratory failure, not elsewhere classified | Frequent occurrence in patients with COPD |
| J96.0 | Acute respiratory failure | Frequent occurrence in patients with COPD |
| J96.1 | Chronic respiratory failure | Frequent occurrence in patients with COPD |
| J96.9 | Respiratory failure, unspecified | Frequent occurrence in patients with COPD |
| J98 | Other respiratory disorder | Frequent occurrence in patients with COPD |
| J98.4 | Other disorders of lung | Frequent occurrence in patients with COPD |
| J98.8 | Other specified respiratory disorders | Frequent occurrence in patients with COPD |
| J98.9 | Respiratory disorder, unspecified | Frequent occurrence in patients with COPD |
| J99 | Respiratory disorders in diseases classified elsewhere | Frequent occurrence in patients with COPD |
| K13 | Other diseases of lip and oral mucosa | In connection with the use of inhalers |
| L25 | Unspecified contact dermatitis | In connection with the use of inhalers |
| R04 | Haemorrhage from respiratory passages | Frequent occurrence in patients with COPD |
| R04.2 | Haemoptysis | Frequent occurrence in patients with COPD |
| R05 | Cough | Frequent occurrence in patients with COPD |
| R06 | Abnormalities of breathing | Frequent occurrence in patients with COPD |
| R06.0 | Dyspnoea | Frequent occurrence in patients with COPD |
| R07 | Pain in throat and chest | Frequent occurrence in patients with COPD |
| R07.1 | Chest pain on breathing | Frequent occurrence in patients with COPD |
| R07.3 | Other chest pain | Frequent occurrence in patients with COPD |
| R07.4 | Chest pain, unspecified | Frequent occurrence in patients with COPD |
| R09.8 | Other specified symptoms and signs involving the circulatory and respiratory systems | Frequent occurrence in patients with COPD |
| R91 | Abnormal findings on diagnostic imaging of lung | Frequent occurrence in patients with COPD |
Supplementary Table 2.
The list of diseases and related health problems requiring procedures qualified to costs of exacerbations of COPD
| ICD-10 code | Name | Reason for inclusion |
|---|---|---|
| J44.1 | Chronic obstructive pulmonary disease with acute exacerbation, unspecified | Assigned for exacerbation of COPD |
| J44.0 | Chronic obstructive pulmonary disease with acute lower respiratory infection | Frequently used for exacerbation of COPD |
| J22 | Unspecified acute lower respiratory infection | Frequently used for exacerbation of COPD |
| J96 | Respiratory failure, not elsewhere classified | Frequently used for exacerbation of COPD |
| J96.0 | Acute respiratory failure | Frequently used for exacerbation of COPD |
| J96.9 | Respiratory failure, unspecified | Frequently used for exacerbation of COPD |
| J46 | Status asthmaticus | Frequently used for exacerbation of COPD |
Acknowledgments
We wish to thank all the people engaged in ICM.
Footnotes
Conflict of interest
Authors declare no conflict of interest.
Source of support: Novartis Grant, ST-553 grant, Town Council of Gdańsk
References
- 1.World Health Organization. Global Status Report on noncommunicable diseases 2014. [cited 2016 Oct 3]; Available from: http://www.who.int/nmh/publications/ncd-status-report-2014/en/ [DOI] [PubMed]
- 2.Niepsuj G, Kozielski J, Niepsuj K. Chronic obstructive pulmonary disease in citizens of Zabrze. Wiad Lek. 2002;55:354–59. [PubMed] [Google Scholar]
- 3.Pływaczewski R, Bednarek M, Jonczak L, Zieliński J. Prevalence of COPD in citizens of right-shore Warsaw. Pneumonol Alergol Pol. 2003;71:329–35. [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease. Global Strategy For The Diagnosis, Management, And Prevention Of Chronic Obstructive Pulmonary Disease Updated 2015. [cited 2016 Sept 15]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2015.pdf.
- 5.Hoogendoorn M. Dissertation. Erasmus University Rotterdam; the Netherlands: 2011. Economic impact of COPD. Empirical and model-based studies on the cost-effectiveness of treatment option. [Google Scholar]
- 6.Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67:957–63. doi: 10.1136/thoraxjnl-2011-201518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solter-Caluna J, Martinez-Garcia M, Sanchez P, et al. Severe acute exacerbations and mortality in patients with chronic obstrutive pulmonary disease. Thorax. 2005;60:957–65. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilleman D, Dewan N, Malesker M, Friedman M. Pharmacoeconomic evaluation of COPD. Chest. 2000;118:1276–85. doi: 10.1378/chest.118.5.1278. [DOI] [PubMed] [Google Scholar]
- 9.Celli B, MacNee W ATS/ERS Task Force. Standards for the diagnosis and treatment of patients with COPD: A summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 10.Jahnz-Różyk K, Targowski T, From S, et al. Costs of chronic obstructive pulmonary disease in patients treated in ambulatory care in Poland. Pneum Alergol Pol. 2011;79:337–42. [PubMed] [Google Scholar]
- 11.Jahnz-Różyk K, Targowski T, From S. Costs of exacerbations of chronic obstructive pulmonary disease in primary and secondary care in 2007 – results of multicenter Polish study. Pol Merk Lek. 2009;XXVI:208–14. [PubMed] [Google Scholar]
- 12.Elkington H, White P, Addington-Hall J, et al. The healthcare needs of chronic obstructive pulmonary disease patients in the last year of life. Palliat Med. 2005;19(6):485–91. doi: 10.1191/0269216305pm1056oa. [DOI] [PubMed] [Google Scholar]
- 13.MacPherson A, Walshe C, O’Donnell V, Vyas A. The views of patients with severe chronic obstructive pulmonary disease on advance care planning: A qualitative study. Palliat Med. 2013;27(3):265–72. doi: 10.1177/0269216312440606. [DOI] [PubMed] [Google Scholar]
- 14.Cilione C, Lorenzi C, Dell Orso D, et al. Predictors of change in exercise capacity after comprehensive copd inpatient rehabilitation. Med Sci Monit. 2002;8:CR740–45. [PubMed] [Google Scholar]
- 15.Koligat D, Szulc I, Kostusiak M, et al. Pharmacoeconomics among physicians – in theory and practice. Nowiny Lekarskie. 2012;81:129–35. [Google Scholar]
- 16.Czech M. The role and meaning of pharmacoeconomics. Czasopismo Aptekarskie. 2008;12:46–49. [Google Scholar]
- 17.Chang K, Nash D. The role of pharmacoeconomic evaluations in disease management. Pharmacoeconomics. 1998;14:11–17. doi: 10.2165/00019053-199814010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Ford ES, Murphy LB, Khavjou O, et al. Total and state-specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >18 years in the United States for 2010 and projections through 2020. Chest. 2015;147:31–45. doi: 10.1378/chest.14-0972. [DOI] [PubMed] [Google Scholar]
- 19.Kruis A, Boland M, Assendelft W, et al. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: Results of cluster randomised trial. BMJ. 2014:349. doi: 10.1136/bmj.g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lusuardi C, Lucioni F, De Benedetto, et al. GOLD severity stratification and risk of hospitalization for COPD exacerbations. Monaldi Arch Chest Dis. 2008;69(4):164–69. doi: 10.4081/monaldi.2008.378. [DOI] [PubMed] [Google Scholar]
- 21.Steuten L, Vrijhoef B, Van Merode F, et al. Evaluation of a regional disease management programme for patients with asthma or chronic obstructive pulmonary disease. Int J Qual Health Care. 2006;18(6):429–36. doi: 10.1093/intqhc/mzl052. [DOI] [PubMed] [Google Scholar]
- 22.van Boven JF, Tommelein E, Boussery K, et al. Improving inhaler adherence in patients with Chronic Obstructive Pulmonary Disease: A cost-effectiveness analysis. Respir Res. 2014;15:66. doi: 10.1186/1465-9921-15-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Apps M, Mukherjee D, Abbas S, et al. A Chronic Obstructive Pulmonary Disease (COPD) service integrating community and hospital services can improve patient care and reduce hospital stays. Am J Respir Crit Care Med. 2016;193:A1523. [Google Scholar]
- 24.Titova E, Steinshamn S, Indredavik B, Henriksen AH. Long term effects of an integrated care intervention on hospital utilization in patients with severe COPD: A single centre controlled study. Respir Res. 2015;16:8. doi: 10.1186/s12931-015-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Steuten L, Vrijhoef B, Van Merode F, et al. Evaluation of regional disease management programme for patients with asthma or chronic obstructive pulmonary disease. Int J Quality Health Care. 2006;18:429–36. doi: 10.1093/intqhc/mzl052. [DOI] [PubMed] [Google Scholar]
- 26.Hernández C, Alonso A, Garcia-Aymerich J, et al. Effectiveness of community-based integrated care in frail COPD patients: A randomised controlled trial. NPJ Prim Care Respir Med. 2015;25:15022. doi: 10.1038/npjpcrm.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoogendoorn M, van Wetering CR, Schols AM, Rutten-van Molken MP. Is INTERdisciplinary COMmunity-based COPD management (INTERCOM) cost-effective? Eur Respir J. 2010;35:9–87. doi: 10.1183/09031936.00043309. [DOI] [PubMed] [Google Scholar]
- 28.Boland M, Kruis A, Tsiachristas A, et al. Cost-effectiveness of integrated COPD care: the RECODE cluster randomised trial. BMJ Open. 2015;5(10):e007284. doi: 10.1136/bmjopen-2014-007284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gabrani J, Gabrani A, Hamer S, et al. Primary healthcare reforms through the lens of innovation – Comparative Case Studies from Western Balkans and Eastern Europe. Management in health. 2015;XIX/1:3–10. [Google Scholar]
- 30.Damps-Konstańska I, Cynowska B, Kuziemski K, et al. [Integrated care for patients with chronic obstructive pulmonary disease (COPD) in family doctor’s practice]. Forum Medycyny Rodzinnej. 2012;6:14–23. [in Polish] [Google Scholar]
- 31.Koff PB, Jones RH, Cashman JM, et al. Proactive integrated care improves quality of life in patients with COPD. Eur Respir J. 2009;33:1031–38. doi: 10.1183/09031936.00063108. [DOI] [PubMed] [Google Scholar]
- 32.Casas A, Troosters T, Garcia-Aymerich J, et al. Integrated care prevents hospitalizations for exacerbations in COPD patients. Eur Respir J. 2006;28:123–30. doi: 10.1183/09031936.06.00063205. [DOI] [PubMed] [Google Scholar]
- 33.Hermiz O, Comino E, Marks G, et al. Randomized controlled trial of home based care of patients with chronic obstrucctive pulmonary disease. BMJ. 2002;325:938. doi: 10.1136/bmj.325.7370.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruis LA, Boland MR, Schoonveldel CH, et al. RECODE: Design and baseline results of a cluster randomized trial on cost-effectiveness of integrated COPD management in primary care. BMC Pulm Med. 2013;13:17. doi: 10.1186/1471-2466-13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brough FK, Schmidt CD, Rasmussen T, Boyer M. Comparison of two teaching methods for self-care training for patients with chronic obstructive pulmonary disease. Patient Couns Health Educ. 1982;4:111–16. doi: 10.1016/s0190-2040(82)80008-6. [DOI] [PubMed] [Google Scholar]
- 36.Gourley DR, Gourley GA, Solomon DK. Development, implementation, and evaluation of a multicenter pharmaceutical care outcomes study. J Am Pharm Assoc (Wash) 1998;38:567–73. doi: 10.1016/s1086-5802(16)30370-9. [DOI] [PubMed] [Google Scholar]
- 37.Sassi-Dambron DE, Eakin EG, Ries AL, Kaplan RM. Treatment of dyspnea in COPD: a controlled clinical trial of dyspnea management strategies. Chest. 1995;107:724–29. doi: 10.1378/chest.107.3.724. [DOI] [PubMed] [Google Scholar]
- 38.Watson PB, Town GI, Holbrook N, et al. Evaluation of a self-management plan for chronic obstructive pulmonary disease. Eur Respir J. 1997;10:1267–71. doi: 10.1183/09031936.97.10061267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
List of diseases and related health problems requiring procedures qualified to costs of COPD and other diseases of respiratory system
| ICD-10 code | Name | Reason for inclusion |
|---|---|---|
| I26 | Pulmonary embolism | Frequent cause of death |
| I26.9 | Pulmonary embolism without mention of acute cor pulmonale | Frequent cause of death |
| I27 | Other pulmonary heart diseases | Frequent cause of death |
| I27.8 | Other specified pulmonary heart diseases | Frequent cause of death |
| I27.9 | Pulmonary heart disease, unspecified | Frequent cause of death |
| J00 | Acute nasopharyngitis | Frequent occurrence in patients with COPD |
| J02 | Acute pharyngitis | Frequent occurrence in patients with COPD |
| J03 | Acute tonsillitis | Frequent occurrence in patients with COPD |
| J04 | Acute laryngitis and tracheitis | Frequent occurrence in patients with COPD |
| J06 | Acute upper respiratory infections of multiple and unspecified sites | Frequent occurrence in patients with COPD |
| J13 | Pneumonia due to Streptococcus pneumoniae | Frequent occurrence in patients with COPD |
| J15 | Bacterial pneumonia, not elsewhere classified | Frequent occurrence in patients with COPD |
| J15.1 | Pneumonia due to Pseudomonas | Frequent occurrence in patients with COPD |
| J15.4 | Pneumonia due to other streptococci | Frequent occurrence in patients with COPD |
| J15.5 | Pneumonia due to Escherichia coli | Frequent occurrence in patients with COPD |
| J15.6 | Pneumonia due to other aerobic Gram-negative bacteria | Frequent occurrence in patients with COPD |
| J15.8 | Other bacterial pneumonia | Frequent occurrence in patients with COPD |
| J15.9 | Bacterial pneumonia, unspecified | Frequent occurrence in patients with COPD |
| J16 | Pneumonia due to other infectious organisms, not elsewhere classified | Frequent occurrence in patients with COPD |
| J16.8 | Pneumonia due to other specified infectious organisms | Frequent occurrence in patients with COPD |
| J18 | Pneumonia, organism unspecified | Frequent occurrence in patients with COPD |
| J18.9 | Pneumonia, unspecified | Frequent occurrence in patients with COPD |
| J20 | Acute bronchitis | Frequent occurrence in patients with COPD |
| J20.9 | Acute bronchitis, unspecified | Frequent occurrence in patients with COPD |
| J21 | Acute bronchiolitis | Frequent occurrence in patients with COPD |
| J22 | Unspecified acute lower respiratory infection | Frequent occurrence in patients with COPD |
| J31 | Chronic rhinitis, nasopharyngitis and pharyngitis | In connection with the use of inhalers |
| J32.4 | Chronic pansinusitis | Frequent occurrence in patients with COPD |
| J37 | Chronic laryngitis and laryngotracheitis | Frequent occurrence in patients with COPD |
| J39 | Other diseases of upper respiratory tract | Frequent occurrence in patients with COPD |
| J39.2 | Other diseases of pharynx | Frequent occurrence in patients with COPD |
| J40 | Bronchitis, not specified as acute or chronic | Frequent occurrence in patients with COPD |
| J41 | Simple and mucopurulent chronic bronchitis | Frequent occurrence in patients with COPD |
| J41.0 | Simple chronic bronchitis | Frequent occurrence in patients with COPD |
| J41.8 | Mixed simple and mucopurulent chronic bronchitis | Frequent occurrence in patients with COPD |
| J42 | Unspecified chronic bronchitis | Frequent occurrence in patients with COPD |
| J43 | Emphysema | Frequent occurrence in patients with COPD |
| J43.8 | Other emphysem | Frequent occurrence in patients with COPD |
| J43.9 | Emphysema, unspecified | Frequent occurrence in patients with COPD |
| J44 | Other chronic obstructive pulmonary disease | Code assigned for COPD |
| J44.0 | Chronic obstructive pulmonary disease with acute lower respiratory infection | Frequent occurrence in patients with COPD |
| J44.1 | Chronic obstructive pulmonary disease with acute exacerbation, unspecified | Frequent occurrence in patients with COPD |
| J44.8 | Other specified chronic obstructive pulmonary disease | Frequent occurrence in patients with COPD |
| J44.9 | Chronic obstructive pulmonary disease, unspecified | Frequent occurrence in patients with COPD |
| J45 | Asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.0 | Predominantly allergic asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.1 | Nonallergic asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.8 | Mixed asthma | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J45.9 | Asthma, unspecified | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J46 | Status asthmaticus | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J47 | Bronchiectasis | Difficult in differentiation with COPD (especially at the level of care primary care) |
| J84 | Other interstitial pulmonary diseases | Frequent occurrence in patients with COPD |
| J84.1 | Other interstitial pulmonary diseases with fibrosis | Frequent occurrence in patients with COPD |
| J84.8 | Other specified interstitial pulmonary diseases | Frequent occurrence in patients with COPD |
| J84.9 | Interstitial pulmonary disease, unspecified | Frequent occurrence in patients with COPD |
| J85.1 | Abscess of lung with pneumonia | Frequent occurrence in patients with COPD |
| J93 | Pneumothorax | Frequent occurrence in patients with COPD |
| J93.1 | Spontaneous tension pneumothorax | Frequent occurrence in patients with COPD |
| J93.8 | Other pneumothorax | Frequent occurrence in patients with COPD |
| J94 | Other pleural conditions | Frequent occurrence in patients with COPD |
| J96 | Respiratory failure, not elsewhere classified | Frequent occurrence in patients with COPD |
| J96.0 | Acute respiratory failure | Frequent occurrence in patients with COPD |
| J96.1 | Chronic respiratory failure | Frequent occurrence in patients with COPD |
| J96.9 | Respiratory failure, unspecified | Frequent occurrence in patients with COPD |
| J98 | Other respiratory disorder | Frequent occurrence in patients with COPD |
| J98.4 | Other disorders of lung | Frequent occurrence in patients with COPD |
| J98.8 | Other specified respiratory disorders | Frequent occurrence in patients with COPD |
| J98.9 | Respiratory disorder, unspecified | Frequent occurrence in patients with COPD |
| J99 | Respiratory disorders in diseases classified elsewhere | Frequent occurrence in patients with COPD |
| K13 | Other diseases of lip and oral mucosa | In connection with the use of inhalers |
| L25 | Unspecified contact dermatitis | In connection with the use of inhalers |
| R04 | Haemorrhage from respiratory passages | Frequent occurrence in patients with COPD |
| R04.2 | Haemoptysis | Frequent occurrence in patients with COPD |
| R05 | Cough | Frequent occurrence in patients with COPD |
| R06 | Abnormalities of breathing | Frequent occurrence in patients with COPD |
| R06.0 | Dyspnoea | Frequent occurrence in patients with COPD |
| R07 | Pain in throat and chest | Frequent occurrence in patients with COPD |
| R07.1 | Chest pain on breathing | Frequent occurrence in patients with COPD |
| R07.3 | Other chest pain | Frequent occurrence in patients with COPD |
| R07.4 | Chest pain, unspecified | Frequent occurrence in patients with COPD |
| R09.8 | Other specified symptoms and signs involving the circulatory and respiratory systems | Frequent occurrence in patients with COPD |
| R91 | Abnormal findings on diagnostic imaging of lung | Frequent occurrence in patients with COPD |
Supplementary Table 2.
The list of diseases and related health problems requiring procedures qualified to costs of exacerbations of COPD
| ICD-10 code | Name | Reason for inclusion |
|---|---|---|
| J44.1 | Chronic obstructive pulmonary disease with acute exacerbation, unspecified | Assigned for exacerbation of COPD |
| J44.0 | Chronic obstructive pulmonary disease with acute lower respiratory infection | Frequently used for exacerbation of COPD |
| J22 | Unspecified acute lower respiratory infection | Frequently used for exacerbation of COPD |
| J96 | Respiratory failure, not elsewhere classified | Frequently used for exacerbation of COPD |
| J96.0 | Acute respiratory failure | Frequently used for exacerbation of COPD |
| J96.9 | Respiratory failure, unspecified | Frequently used for exacerbation of COPD |
| J46 | Status asthmaticus | Frequently used for exacerbation of COPD |


