Abstract
Objective
Radiofrequency ablation has been used widely for the local ablation of hepatocellular carcinoma, particularly in its early stages. The study aim was to identify significant prognostic factors and develop a predictive nomogram for patients with hepatocellular carcinoma who have undergone radiofrequency ablation. We also developed the formula to predict the probability of 3- and 5-year overall survival based on clinical variables.
Methods
We retrospectively studied 96 consecutive patients with hepatocellular carcinoma who had undergone radiofrequency ablation as a first-line treatment. Independent and significant factors affecting the overall survival were selected using a Cox proportional hazards model, and a prognostic nomogram was developed based on these factors. The predictive accuracy of the nomogram was determined by Harrell's concordance index and compared with the Cancer of the Liver Italian Program score and Japan Integrated Staging score.
Results
A multivariate analysis revealed that age, indocyanine green plasma disappearance rate, and log(des-gamma-carboxy prothrombin) level were independent and significant factors influencing the overall survival. The nomogram was based on these three factors. The mean concordance index of the nomogram was 0.74±0.08, which was significantly better than that of conventional staging systems using the Cancer of the Liver Italian Program score (0.54±0.03) and Japan Integrated Staging score (0.59±0.07).
Conclusion
This study suggested that the indocyanine green plasma disappearance rate and age at radiofrequency ablation (RFA) and des-gamma-carboxy-prothrombin (DCP) are good predictors of the prognosis in hepatocellular carcinoma patients after radiofrequency ablation. We successfully developed a nomogram using obtainable variables before treatment.
Keywords: hepatocellular carcinoma, indocyanine green plasma disappearance rate, nomogram, radiofrequency ablation, des-gamma-carboxy prothrombin
Introduction
Surgical resection should be the first-line option for patients with solitary hepatocellular carcinoma (HCC) and a well-preserved liver function (1-3); however, only 20% of patients with HCC are candidates for resection due to their tumor stage, liver function, performance status, or comorbidities (4). Radiofrequency ablation (RFA) has recently become the most frequently used treatment option for early-stage HCC and an alternative for patients with HCC who are not eligible for surgical resection (1-3,5,6). Shiina et al. reported estimated 5- and 10-year survival rates for patients undergoing RFA of 60.2% and 27.3%, respectively (7). Several studies have compared the survival prognosis between surgical resection and RFA; Livraghi et al. and Chen et al. reported that, compared with resection, RFA was less invasive and associated with fewer complications (8,9). Furthermore, Sato et al. reported that the percentages of in-hospital deaths among patients who underwent hepatectomy and RFA were 2.60% and 0.25%, respectively (10). Therefore, RFA is considered the treatment of choice for patients with single HCC.
Many staging systems have been developed to evaluate HCC severity. The Child-Pugh classification has been widely used to evaluate the liver function. Prognostic staging systems for HCC, such as the Cancer of the Liver Italian Program (CLIP) score and the Japan Integrated Staging (JIS) score, reflect the tumor, node, metastasis stage and the Child-Pugh score (11-13). Other staging systems using nomograms have recently been developed to predict the prognosis of patients with HCC (14-17). These nomograms are more sophisticated than those using conventional variables such as the CLIP or JIS score. However, prognostic nomograms for patients who have undergone local ablation therapy for HCC have not been sufficiently established.
This study's aim was to clarify the significant prognostic factors and construct a predictive nomogram for patients with HCC who have undergone RFA. Predictive outcomes using the herein-described nomogram can be obtained with widely used clinical variables, and its concordance index (c-index) can be determined (18,19). We also developed an original formula that enables easier and more rapid prediction of the 3- and 5-year overall survival (OS). This nomogram and corresponding formula may be useful for determining a treatment strategy and predicting the prognosis in clinical practice.
Materials and Methods
Patients
At Niigata University Medical and Dental Hospital, 109 patients underwent RFA as first-line treatment from January 2000 to December 2013. We excluded patients who had (i) undergone previous first-line treatments for HCC in other hospitals (n=5), (ii) undergone combined RFA and resection for multiple HCC (n=5), and (iii) been diagnosed with a simultaneous malignant tumor or recurrent tumor (n=3). Thus, the medical records for 96 consecutive patients with HCC were reviewed. All were analyzed in this study. However, the indocyanine green plasma disappearance rate (ICG-PDR) was not obtained for five patients, and the des-gamma-carboxy prothrombin (DCP), also known as protein induced by vitamin K absence of antagonist (PIVKA-II), level was not obtained for two. Therefore, the data of 89 patients were used to develop the nomogram. This retrospective study was approved by the institutional review board of Niigata University Medical and Dental Hospital (number 2041), and informed consent was waived because of the low risk associated with this study. The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki (as revised in 2008).
Diagnosis
HCC was diagnosed according to the guidelines of the Japan Society of Hepatology and the European Association for the Study of the Liver. Nodules were diagnosed as HCC requiring treatment based on typical imaging features showing areas of early arterial enhancement and delayed washout in the venous or delayed phases of dynamic computed tomography (CT) or dynamic contrast-enhanced magnetic resonance imaging (MRI) (1,2,20). Patients who were not diagnosed with typical HCC by dynamic CT or dynamic MRI underwent contrast-enhanced ultrasonography or CT arterioportography (21). Fifteen patients who could not be diagnosed by these imaging techniques underwent a tumor biopsy, and all were pathologically diagnosed with HCC (2,22).
Treatment
All nodules diagnosed as HCC were treated by RFA according to the guidelines of the Japan Society of Hepatology. The patients investigated in this study were divided into four groups according to tumor node metastasis (TNM) stages. Stage IV had only one patient, who was a 74-year-old woman with 3 stage IV HCC lesions of a maximum 26 mm diameter and a suspected 8-mm para-aortic lymph node metastasis. Her hepatic reserve was good, and RFA was performed with the objective of controlling the intrahepatic lesions. (Table 1) Thirty-one of the 96 patients in this study underwent transcatheter arterial embolization (TAE) or transcatheter arterial chemoembolization (TACE) before RFA. A 2- or 3-cm Cool-tip needle and the Cool-tip system (Covidien, Mansfield, MA, USA) were used for ablation, ensuring an ablative margin of ≥5 mm. Dynamic CT was conducted within two days of RFA to confirm the absence of an obvious remnant tumor. All blood biochemical, CT, MRI, endoscopy, and ultrasound findings were obtained within three months before RFA.
Table 1.
Demographics and Clinical Characteristics of Patients with Hepatocellular Carcinoma.
| Variable | Category | Distribution | % |
|---|---|---|---|
| Age | Years | 69.9 (8.8) | |
| Sex | Male | 57 | 59.4 |
| Female | 39 | 40.6 | |
| Child-Pugh class | A | 86 | 89.6 |
| B | 10 | 10.4 | |
| TNM stage | I | 45 | 46.9 |
| II | 35 | 36.5 | |
| III | 15 | 15.6 | |
| IV | 1 | 1.0 | |
| CLIP score | 0 | 91 | 94.8 |
| 1 | 5 | 5.2 | |
| JIS score | 0 | 38 | 39.6 |
| 1 | 38 | 39.6 | |
| 2 | 19 | 19.8 | |
| 3 | 1 | 1.0 | |
| HBs antigen | + | 16 | 16.7 |
| - | 80 | 83.3 | |
| HCV antibody | + | 65 | 67.7 |
| - | 31 | 32.3 | |
| TAE/TACE | + | 31 | 32.3 |
| - | 65 | 67.7 | |
| Esophageal varices | + | 20 | 20.8 |
| - | 76 | 79.2 | |
| Gastric varices | + | 7 | 7.3 |
| - | 89 | 92.7 | |
| Splenomegaly | + | 73 | 76.0 |
| - | 23 | 24.0 | |
| Maximum diameter | mm | 20.0 (0.78) | |
| Number of tumors | 1 | 69 | 71.9 |
| 2 | 18 | 18.8 | |
| 3 | 7 | 7.3 | |
| 4 | 2 | 2.1 | |
| Main tumor (134) | S1 | 1 | 0.7 |
| S2 | 6 | 4.5 | |
| S3 | 15 | 11.2 | |
| S4 | 8 | 6.0 | |
| S5 | 29 | 21.6 | |
| S6 | 21 | 15.7 | |
| S7 | 17 | 12.7 | |
| S8 | 37 | 27.6 | |
| Bilateral tumors | + | 12 | 12.5 |
| - | 84 | 87.5 | |
| AFP (ng/mL) | 14 (0-909) | ||
| DCP (mAU/mL) | 22 (9-2026) | ||
| AST (U/L) | 52 (20-228) | ||
| ALT (U/L) | 40 (12-270) | ||
| γ-GTP (U/L) | 47 (12-444) | ||
| ALP (U/L) | 314 (98-827) | ||
| LDH (IU/L) | 232 (126-832) | ||
| ChE (IU/L) | 182 (70.5) | ||
| Hb (g/dL) | 12.5 (1.8) | ||
| Plt (×103/µL) | 100 (35-250) | ||
| Alb (g/dL) | 3.7 (0.46) | ||
| Cre (mg/dL) | 0.7 (0.4-9.9) | ||
| T-Bil (IU/L) | 0.9 (0.1-3.7) | ||
| NH3 (µg/dL) | 64 (3-164) | ||
| PT% (%) | 79 (14) | ||
| ICG-PDR (%/min) | 10.1 (2.5-22.0) |
Data are expressed as the median (range) or the mean (standard deviation) unless otherwise indicated.
TNM: tumor node metastasis, CLIP: Cancer of the Liver Italian Program, JIS: Japan Integrated Staging, HBs: hepatitis B surface, HCV: hepatitis C virus, TAE: transcatheter arterial embolization, TACE: transcatheter arterial chemoembolization, AFP: α-fetoprotein, DCP: des-gamma-carboxy prothrombin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: γ-glutamyltranspeptidase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, ChE: cholinesterase, Hb: hemoglobin, Plt: platelet, Alb: albumin, Cre: creatinine, T-Bil: total bilirubin, NH3: ammonia, PT%: prothrombin activity percentage, ICG-PDR: indocyanine green plasma disappearance rate
Data collection
Table 1 shows the patients' demographic data and preoperative clinical factors. Information on age at the time of RFA, sex, hepatitis B surface antigen status, and hepatitis C virus antibody status were gathered in this study. The presence of esophageal and gastric varices was confirmed by endoscopy. The presence of splenomegaly and the maximum tumor diameter (mm) was confirmed by abdominal ultrasound. The number of tumors and presence or absence of bilateral tumors were also determined by dynamic CT or dynamic MRI. The levels of the tumor markers α-fetoprotein (ng/mL) and DCP (mAU/mL) were measured. The laboratory findings also included the levels of aspartate aminotransferase (U/L), alanine aminotransferase (U/L), γ-glutamyltranspeptidase (U/L), alkaline phosphatase (U/L), lactate dehydrogenase (U/L), cholinesterase (IU/L), hemoglobin (g/dL), albumin (g/dL), creatinine (mg/dL), total bilirubin (IU/L), and ammonia (μg/dL); platelet count (×103/μL); prothrombin activity percentage; and ICG-PDR (%/min). The Child-Pugh class, CLIP score, and JIS score were calculated based on the imaging and laboratory findings.
Statistical analyses
For descriptive statistics, continuous variables are presented as mean ± standard deviation or median (range), and discrete variables are presented as frequency and proportion. Normality of the distributions of continuous variables was tested by the Shapiro-Wilk test. The OS among the groups was stratified by a single factor and estimated by the Kaplan-Meier method. Significant differences were assessed by the log-rank test. In reference to previous studies, continuous variables were converted to binary variables. A Cox proportional hazards regression analysis (Cox analysis) was used to select the significant and independent prognostic factors that significantly affected OS with a forward stepwise regression method. Our nomogram was based on the variables selected by the Cox analysis using the rms package of R version 2.14.1 (19,23). Nomogram accuracy was measured by Harrell's c-index (18). Bootstraps with 1,000 resamples were used for these activities. Student's t-test was performed to compare distribution of the c-index of our nomogram with that based on the CLIP and JIS scores (11,12). All analyses were carried out using SPSS version 20.0 (IBM Corp, Armonk, NY, USA) In all analyses, a p value of <0.05 was considered significant.
Results
Baseline characteristics
Table 1 shows patients' baseline characteristics. The male and female distribution was 57 (59%) and 39 (41%), respectively, and the mean age at RFA was 69.9±8.8 years. The proportions of tumor, node, metastasis stage I, II, III, and IV tumors were 47%, 37%, 16%, and 1%, respectively, and the positivity rates for hepatitis B surface antigen and hepatitis C virus were 16.7% and 67.7%, respectively. RFA treatment was conducted for 134 tumors in 96 patients. The median maximum diameter was 18.6 (8.0-45.0) mm. With respect to tumor markers, the median α-fetoprotein and DCP levels were 14 (0-909) ng/mL and 22 (9-2026) mAU/mL, respectively. The median follow-up period for all 96 patients was 46.8 months (range 1.6-137.9 months).
Overall survival
Fifty deaths were observed during the follow-up period. The estimated 3- and 5-year OS rates were 71.1% and 56.1%, respectively (Fig. 1). Table 2 shows the estimated 1-, 3-, and 5-year OS rates and results of log-rank tests for geographic and clinical factors. The OS rate of the 54 patients ≥70 years of age was significantly lower than that of the 42 patients <70 years of age (log-rank test, p=0.004). The OS rate of 11 patients with a DCP level of ≥200 mAu/mL was significantly lower than that of 83 patients at <200 mAu/mL (log-rank test, p=0.001).
Figure 1.
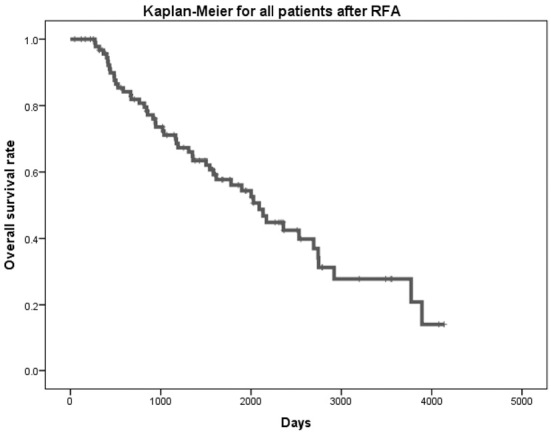
A Kaplan-Meier test for 96 patients after radiofrequency ablation. The probability of 3- and 5-year overall survival after radiofrequency ablation was 71.1% and 56.1%, respectively.
Table 2.
Estimated Survival Rate by Kaplan-Meier and Log-rank Tests.
| Factor | Category | Distribution | Estimated survival rate | Log-rank | ||
|---|---|---|---|---|---|---|
| 1-y (%) | 3-y (%) | 5-y (%) | p value | |||
| Age (years) | ≥70 | 54 | 93.9 | 61.4 | 42.7 | 0.004 |
| <70 | 42 | 100.0 | 82.4 | 71.0 | ||
| Sex | Male | 57 | 98.1 | 75.6 | 62.7 | 0.270 |
| Female | 39 | 94.9 | 65.4 | 47.2 | ||
| Child-Pugh class | A | 86 | 97.6 | 73.3 | 58.0 | 0.045 |
| B | 10 | 87.5 | 50.0 | 37.5 | ||
| TNM Stage | I, II | 80 | 96.0 | 70.2 | 59.8 | 0.255 |
| III, IV | 16 | 100.0 | 75.0 | 43.8 | ||
| CLIP score | 0 | 91 | 100.0 | 74.3 | 59.6 | <0.001 |
| 1 | 5 | 80.0 | 0.0 | 0.0 | ||
| JIS score | 0-1 | 76 | 95.8 | 70.4 | 61.1 | 0.129 |
| 2-3 | 20 | 100.0 | 73.7 | 42.1 | ||
| HBs antigen | + | 16 | 93.8 | 75.0 | 66.7 | 0.044 |
| - | 80 | 97.3 | 70.1 | 53.6 | ||
| HCV antibody | + | 65 | 98.3 | 72.8 | 55.5 | 0.341 |
| - | 31 | 93.5 | 67.7 | 56.5 | ||
| TAE/TACE | + | 31 | 96.6 | 85.4 | 59.8 | 0.577 |
| - | 65 | 96.8 | 64.6 | 54.5 | ||
| Esophageal varices | + | 20 | 94.7 | 55.7 | 31.0 | 0.030 |
| - | 76 | 97.2 | 75.2 | 62.8 | ||
| Gastric varices | + | 7 | 100.0 | 57.1 | 42.9 | 0.056 |
| - | 89 | 96.4 | 72.4 | 57.3 | ||
| Splenomegaly | + | 73 | 100.0 | 60.9 | 56.2 | 0.115 |
| - | 23 | 95.6 | 74.8 | 55.7 | ||
| Maximal diameter | ≥20 mm | 35 | 97.1 | 63.3 | 39.7 | 0.016 |
| <20 mm | 61 | 96.5 | 75.8 | 66.7 | ||
| Number of tumors | 1 | 69 | 95.4 | 72.2 | 63.8 | 0.125 |
| ≥2 | 27 | 100.0 | 68.4 | 40.2 | ||
| Bilateral tumors | + | 12 | 100.0 | 81.8 | 54.5 | 0.888 |
| - | 84 | 96.3 | 69.6 | 56.6 | ||
| AFP (ng/mL) | ≥20 | 40 | 94.9 | 60.1 | 42.1 | 0.035 |
| <20 | 56 | 98.1 | 79.5 | 67.2 | ||
| DCP (mAU/mL) | ≥200 | 11 | 90.9 | 40.4 | 15.2 | 0.002 |
| <200 | 83 | 97.5 | 74.8 | 60.6 | ||
| AST (U/L) | ≥50 | 51 | 97.9 | 66.7 | 45.1 | 0.119 |
| <50 | 45 | 95.5 | 75.9 | 67.6 | ||
| ALT (U/L) | ≥50 | 36 | 97.0 | 71.0 | 52.0 | 0.937 |
| <50 | 60 | 96.6 | 71.2 | 58.2 | ||
| γ-GTP (U/L) | ≥50 | 46 | 97.7 | 72.5 | 60.1 | 0.475 |
| <50 | 50 | 95.8 | 70.0 | 52.6 | ||
| ALP (U/L) | ≥300 | 52 | 96.0 | 65.1 | 43.7 | 0.081 |
| <300 | 44 | 97.6 | 79.2 | 72.5 | ||
| LDH (IU/L) | ≥200 | 62 | 94.8 | 66.0 | 47.1 | 0.003 |
| <200 | 34 | 100.0 | 80.4 | 72.7 | ||
| ChE (IU/L) | ≥200 | 33 | 96.9 | 83.7 | 71.4 | 0.079 |
| <200 | 63 | 96.6 | 64.4 | 47.8 | ||
| Hb (g/dL) | ≥12.0 | 60 | 96.5 | 75.8 | 60.6 | 0.021 |
| <12.0 | 36 | 97.1 | 63.2 | 48.3 | ||
| Plt (×103/µL) | ≥100 | 61 | 97.1 | 72.2 | 58.7 | 0.770 |
| <100 | 35 | 95.5 | 68.2 | 49.1 | ||
| Alb (g/dL) | ≥3.8 | 45 | 95.5 | 81.1 | 65.1 | 0.297 |
| <3.8 | 51 | 97.9 | 61.3 | 46.8 | ||
| Cre (mg/dL) | ≥0.7 | 59 | 96.4 | 66.9 | 52.5 | 0.344 |
| <0.7 | 37 | 97.2 | 77.3 | 61.3 | ||
| T-Bil (IU/L) | ≥1.0 | 39 | 100.0 | 62.2 | 50.0 | 0.065 |
| <1.0 | 57 | 94.4 | 78.4 | 61.0 | ||
| NH3 (µg/dL) | ≥60 | 51 | 97.8 | 76.0 | 61.2 | 0.383 |
| <60 | 45 | 95.5 | 64.9 | 49.5 | ||
| PT% (%) | ≥70 | 72 | 97.1 | 72.2 | 58.7 | 0.077 |
| <70 | 24 | 95.5 | 68.2 | 49.1 | ||
| ICG-PDR (%/min) | ≥10.0 | 46 | 95.5 | 80.6 | 68.6 | 0.003 |
| <10.0 | 45 | 100.0 | 66.3 | 47.5 | ||
Data are expressed as the median (range) or the mean (standard deviation) unless otherwise indicated.
TNM: tumor node metastasis, CLIP: Cancer of the Liver Italian Program, JIS: Japan Integrated Staging, HBs: hepatitis B surface, HCV: hepatitis C virus, TAE: transcatheter arterial embolization, TACE: transcatheter arterial chemoembolization, AFP: α-fetoprotein, DCP: des-gamma-carboxy prothrombin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, γ-GTP: γ-glutamyltranspeptidase, ALP: alkaline phosphatase, LDH: lactate dehydrogenase, ChE: cholinesterase, Hb: hemoglobin, Plt: platelet, Alb: albumin, Cre: creatinine, T-Bil: total bilirubin, NH3: ammonia, PT%: prothrombin activity percentage, ICG-PDR: indocyanine green plasma disappearance rate
Multivariate analysis
We performed a Cox analysis with forward stepwise regression in which all variables in the log-rank test in Table 2 were used as prognostic variable candidates. The Cox analysis indicated that age, ICG-PDR, and log(DCP) were independent and significant prognostic factors affecting the OS (Wald test: age, p=0.003; ICG-PDR, p=0.001; log(DCP), p=0.002) (Table 3).
Table 3.
Multivariate Analysis of Prognostic Factors for Overall Survival.
| Variable | Estimated regression coefficient |
SE | HR (95%CI) | p value† |
|---|---|---|---|---|
| ICG-PDR (%/min) | -0.162 | 0.047 | 0.850 (0.776-0.932) | 0.001 |
| Age (years) | 0.057 | 0.019 | 1.059 (1.019-1.100) | 0.003 |
| log (DCP) (mAu/mL) | 0.329 | 0.108 | 1.389 (1.124-1.717) | 0.002 |
SE: standard error of regression coefficient, HR: hazard ratio, CI: confidence interval, ICG-PDR: indocyanine green plasma disappearance rate, DCP: des-gamma-carboxy prothrombin
†Wald test
Nomogram development and validation
A prognostic nomogram was constructed based on the estimated regression coefficients identified in the Cox analysis and the rms package of R version 2.14.1 (Fig. 2) (17,18). The c-index of the nomogram was estimated as 0.74±0.08 using 1,000 data sets created by the bootstrap method. The estimated c-index was found to be significantly better than that of conventional staging using the CLIP score (0.54±0.03, p<0.001) and JIS score (0.59±0.07, p<0.001). Fig. 3 illustrates the calibration of this nomogram (Fig. 3). The vertical bars indicate 95% confidence intervals (CIs) on the 1,000 bootstrap analysis, and the dashed line represents the performance of an ideal nomogram. Calibration appears to be accurate for the prediction. The estimated 3- and 5-year OS rates were calculated using the following formula:
Figure 2.

Nomogram predicting the probability of 3-and 5-year overall survival after radiofrequency ablation. Each point can be determined by drawing a line straight upward from each predictor to the point axis. Total points can be calculated by summing each point. The probability of 3- and 5-year overall survival can be found by drawing a line straight down from the total points axis. ICG-PDR: indocyanine green plasma disappearance rate, DCP: des-gamma-carboxy prothrombin
Figure 3.
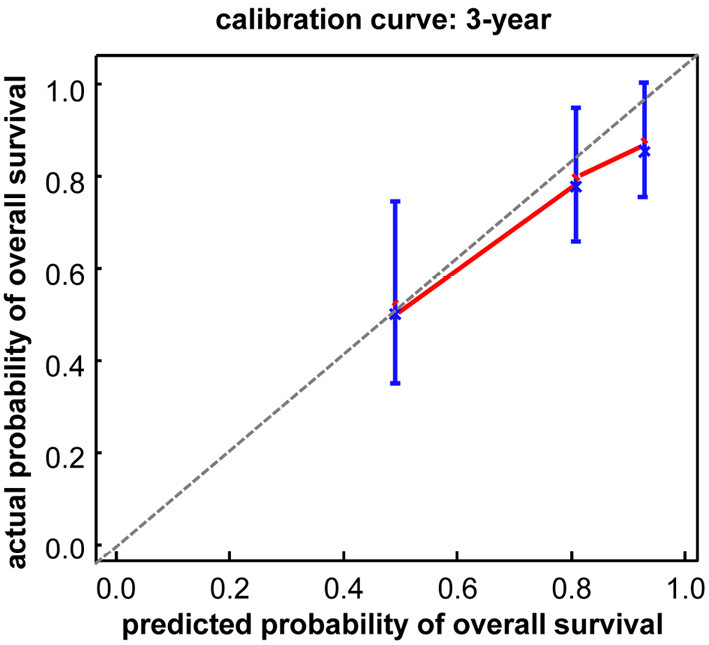
The calibration curve of the nomogram predicting survival rate. The x-axis is the prediction of the nomogram, and the y-axis is the actual survival probability by the Kaplan-Meier method.
3 years OS rate=(0.803)e(0.057×Age+-0.162×ICGPDR+0.329×log(DCP)-3.485)
5 years OS rate=(0.626)e(0.057×Age+-0.162×ICGPDR+0.329×log(DCP)-3.485)
Discussion
Nomograms are widely used to predict the cancer prognosis because of their ability to estimate the probability of an event, such as death, tailored to the profile of an individual patient. They are also often used to obtain patients' informed consent (24).
Several studies have found that age is an independent risk factor for OS after RFA (7,25-27). Kao et al. concluded that younger patients with HCC had a better OS and lower recurrence rate after RFA than older patients (27). Our results showed that the OS rate of 54 patients aged ≥70 years was significantly lower than that of 42 patients aged <70 years, and that age was an independent risk factor for OS after RFA (Table 2, 3). However, previous studies have described the efficacy and safety of RFA for elderly patients with HCC (28,29). Therefore, elderly patients with HCC should be treated according to the same strategy as that used for non-elderly patients.
In this study, log(DCP) was selected as a continuous variable for predicting the prognosis. Takahashi et al. concluded that DCP was the best prognostic predictor post-RFA (30). Hagiwara et al. suggested that HCC frequently infiltrated the portal vein in patients with a DCP level of ≥100 mAU/mL (31). Asaoka et al. reported that DCP level is the most useful predisposing parameter for development of vascular invasion (32). They found that patients with microvascular invasion had a poor prognosis and other treatment strategies should therefore be explored for such patients. In the present study, the OS rate of 11 patients with a DCP level of ≥200 mAu/mL was significantly lower than that of 83 patients at <200 mAu/mL (Table 2). This suggests that patients with a DCP level of ≥200 mAu/mL have a poor prognosis. We should consider the possibility of microvascular invasion undetectable by pre-RFA diagnostic imaging in patients with a DCP level of ≥200 mAu/mL. The presence of microvascular invasion makes it difficult to completely cure HCC with a single RFA treatment. In such cases, resection should also be considered for patients with a good liver function.
The ICG-PDR is correlated with the liver function and is used to determine the need for liver transplantation against acute liver failure (33,34). Kaneko et al. concluded that selection criteria for hepatectomy based on the ICG-PDR are useful (35). Hemming et al. and Scheingraber et al. reported similar findings (36,37). In the present study, the ICG-PDR was calculated based on three separate blood samples (5, 10, and 15 minutes after intravenous injection). Thus, the calculation of the ICG-PDR requires collection of multiple samples, necessitating time and labor. However, a noninvasive technique that monitors the ICG-PDR via a finger clip using transcutaneous pulse spectrophotometry was recently developed. Several studies have reported this transcutaneous measurement of the ICG-PDR to be sufficiently accurate (38,39). Furthermore, the ICG-PDR is affected by the hepatic blood flow and has several associated disadvantages. For example, it tends to be low in patients with factors that compromise hepatic hemodynamics, such as extrahepatic shunting, because the patient's liver function is not accurately reflected (40,41). No researchers have reported the usefulness of the ICG-PDR in predicting the survival prognosis of patients with HCC. Our study selected the ICG-PDR as a continuous variable by a Cox analysis (p=0.001). In our nomogram, the ICG-PDR had the longest line, with 100 points, showing it is the most useful variable for predicting the prognosis of patients with HCC post-RFA. Therefore, the ICG-PDR appears to be an extremely important factor for determining the treatment strategy, including recommendations for hepatectomy, and we strongly recommend checking the ICG-PDR before treatment. Kaneko et al. concluded that an ICG-PDR of ≥ 6.0 in patients undergoing portal resection is valid because of the acceptable morbidity and mortality associated with this criterion (35). We propose the same criterion (ICG-PDR ≥ 6.0) for RFA.
To our knowledge, this is the first prognostic nomogram to use ICG-PDR for patients with HCC. Compared with a regression formula for the precise estimation of survival, nomograms are more useful in clinical settings because they provide an easily accessible visual representation of the approximate estimated rate and the effect of different factors on survival.
Certain limitations associated with the present study warrant mention. This was a single-center retrospective study, and the method of selecting treatment strategies may have introduced bias. We evaluated 89 patients, which is fewer than in previous studies of nomograms for HCC. Because a limited number of cases were used to develop this nomogram and calculate the c-index, external validation is needed.
The authors state that they have no Conflict of Interest (COI).
References
- 1.El-Serag HB. Hepatocellular carcinoma. N Engl J Med 365: 1118-1127, 2011. [DOI] [PubMed] [Google Scholar]
- 2.European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer . EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56: 908-943, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, Matsui O, Izumi N, Liver Cancer Study Group of Japan, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer 3: 458-468, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borie F, Bouvier AM, Herrero A, et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol 98: 505-509, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer 120: 2824-2838, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa K, Kokudo N, Makuuchi M, et al. Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 58: 724-729, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Shiina S, Tateishi R, Arano T, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol 107: 569-577, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livraghi T, Meloni F, Di Stasi M, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology 47: 82-89, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg 243: 321-328, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, Tateishi R, Yasunaga H. Mortality and morbidity of hepatectomy, radiofrequency ablation, and embolization for hepatocellular carcinoma: a national survey of 54,145 patients. J Gastroenterol 47: 1125-1133, 2012. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka N, Okamoto E, Oriyama T, et al. A prediction scoring system to select the surgical treatment of liver cancer. Further refinement based on 10 years of use. Ann Surg 219: 342-346, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 38: 207-215, 2003. [DOI] [PubMed] [Google Scholar]
- 13.The Cancer of the Liver Italian Program (CLIP) investigators A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients. Hepatology 28: 751-755, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Cho CS, Gonen M, Shia J, et al. A novel prognostic nomogram is more accurate than conventional staging systems for predicting survival after resection of hepatocellular carcinoma. J Am Coll Surg 206: 281-291, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Shim JH, Jun MJ, Han S, et al. Prognostic nomograms for prediction of recurrence and survival after curative liver resection for hepatocellular carcinoma. Ann Surg 261: 939-946, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Han S, Shim JH. A patient-based nomogram for predicting overall survival after radiofrequency ablation for hepatocellular carcinoma. J Vasc Interv Radiol 26: 1787-1794, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Peng ZW, Chen MS. Prognostic nomogram for patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. J Hepatol 63: 122-130, 2015. [DOI] [PubMed] [Google Scholar]
- 18.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15: 361-387, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE Jr. Regression Modeling Strategies. R Package version 3.4-0 [Internet]. [cited 2015 March 11] Available from: http://CRAN.R-project.org/package=rms. [Google Scholar]
- 20.Kokudo N, Hasegawa K, Akahane M, et al. Evidence-based clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2013 update (3rd JSH-HCC Guidelines). Hepatol Res 2015. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 21.Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am J Gastroenterol 105: 610-612, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Sangiovanni A, Manini MA, Iavarone M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut 59: 638-644, 2010. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 31: 1188-1195, 2013. [DOI] [PubMed] [Google Scholar]
- 24.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol 26: 1364-1370, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Waki K, Aikata H, Katamura Y, et al. Percutaneous radiofrequency ablation as first-line treatment for small hepatocellular carcinoma: results and prognostic factors on long-term follow up. J Gastroenterol Hepatol 25: 597-604, 2010. [DOI] [PubMed] [Google Scholar]
- 26.Toshikuni N, Takuma Y, Goto T, Yamamoto H. Prognostic factors in hepatitis C patients with a single small hepatocellular carcinoma after radiofrequency ablation. Hepatogastroenterology 59: 2361-2366, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Kao WY, Chiou YY, Hung HH, et al. Younger hepatocellular carcinoma patients have better prognosis after percutaneous radiofrequency ablation therapy. J Clin Gastroenterol 46: 62-70, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Hiraoka A, Michitaka K, Horiike N, et al. Radiofrequency ablation therapy for hepatocellular carcinoma in elderly patients. J Gastroenterol Hepatol 25: 403-407, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Takahashi H, Mizuta T, Kawazoe S, et al. Efficacy and safety of radiofrequency ablation for elderly hepatocellular carcinoma patients. Hepatol Res 40: 997-1005, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi S, Kudo M, Chung H, et al. PIVKA-II is the best prognostic predictor in patients with hepatocellular carcinoma after radiofrequency ablation therapy. Oncology 75 (Suppl 1): 91-98, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Hagiwara S, Kudo M, Kawasaki T, et al. Prognostic factors for portal venous invasion in patients with hepatocellular carcinoma. J Gastroenterol 41: 1214-1219, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Asaoka Y, Tateishi R, Nakagomi R, et al. Frequency of and predictive factors for vascular invasion after radiofrequency ablation for hepatocellular carcinoma. PLoS One 9: e111662, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merle U, Sieg O, Stremmel W, Encke J, Eisenbach C. Sensitivity and specificity of plasma disappearance rate of indocyanine green as a prognostic indicator in acute liver failure. BMC Gastroenterol 9: 91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quintero J, Miserachs M, Ortega J, et al. Indocyanine green plasma disappearance rate: a new tool for the classification of paediatric patients with acute liver failure. Liver Int 34: 689-694, 2014. [DOI] [PubMed] [Google Scholar]
- 35.Kaneko K, Shirai Y, Wakai T, Yokoyama N, Akazawa K, Hatakeyama K. Low preoperative platelet counts predict a high mortality after partial hepatectomy in patients with hepatocellular carcinoma. World J Gastroenterol 11: 5888-5892, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hemming AW, Scudamore CH, Shackleton CR, Pudek M, Erb SR. Indocyanine green clearance as a predictor of successful hepatic resection in cirrhotic patients. Am J Surg 163: 515-518, 1992. [DOI] [PubMed] [Google Scholar]
- 37.Scheingraber S, Richter S, Igna D, Flesch S, Kopp B, Schilling MK. Indocyanine green disappearance rate is the most useful marker for liver resection. Hepatogastroenterology 55: 1394-1399, 2008. [PubMed] [Google Scholar]
- 38.Sakka SG, Koeck H, Meier-Hellmann A. Measurement of indocyanine green plasma disappearance rate by two different dosages. Intensive Care Med 30: 506-509, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Halle BM, Poulsen TD, Pedersen HP. Indocyanine green plasma disappearance rate as dynamic liver function test in critically ill patients. Acta Anaesthesiol Scand 58: 1214-1219, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Purcell R, Kruger P, Jones M. Indocyanine green elimination: a comparison of the LiMON1 and serial blood sampling methods. ANZ J Surg 76: 75-77, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Levesque E, Martin E, Dudau D, Lim C, Dhonneur G, Azoulay D. Current use and perspective of indocyanine green clearance in liver diseases. Anaesth Crit Care Pain Med 35: 49-57, 2015. [DOI] [PubMed] [Google Scholar]


