Abstract
A duodenal polyp was found during a health check of a 71-year-old asymptomatic man. Duodenoscopy demonstrated a pedunculated, smooth-surfaced tumor of 18 mm in size, protruding from the minor papilla. Endoscopic ultrasonography demonstrated a homogeneously low-echoic submucosal tumor. Enhanced computed tomography and magnetic resonance imaging demonstrated a well-enhanced duodenal tumor without obvious metastasis. A tumor biopsy revealed a well-differentiated neuroendocrine tumor, and laparotomic transduodenal polypectomy with regional lymph node dissection was performed. The histology of the surgical specimen revealed gangliocytic paraganglioma consisting of three cell types: endocrine, ganglion, and spindle cells. There has been no recurrence in >5 years after surgery.
Keywords: gangliocytic paraganglioma, duodenum, minor papilla, diagnosis, treatment, prognosis
Introduction
Gangliocytic paraganglioma (GP) is a rare nonepithelial tumor mostly located in the 2nd and 3rd portion of the duodenum. There is a wide distribution in the age of the patients (15-84 years), with a slight predominance of males (114:76) (1). The biological behavior of GP is less aggressive when limited to the submucosal layer; however, GP sometimes extends beyond the submucosa and metastasizes to the lymph nodes and the liver (1). Death is reported to occur after the progression of the disease (2).
The GP tumor is histologically composed of three tissue types: endocrine cells, ganglion cells, and spindle cells. Many theories have been proposed with respect to the histogenesis of GP, but there is currently no satisfactory explanation. To date, GP of the duodenal major papilla has been reported in a large number of cases, whereas that of the minor papilla is quite rare. We herein describe the case of a patient with a GP protruding from the minor papilla who was successfully treated by laparotomic window surgery (3).
Case Report
A 71-year-old man was referred to Shizuoka Cancer Center Hospital for the investigation of a duodenal polyp that was detected during upper gastrointestinal endoscopy, which was performed for a health check. The patient had no specific symptoms. The histology of a forceps biopsy specimen taken at the previous hospital showed only a normal duodenal mucosa with Brunner glands. He had a smoking habit (20 cigarettes per day) and a history of hypertension, but no history of obvious melena or abdominal pain. With regard to his family history, his father had duodenal cancer and his mother had lung cancer. The laboratory data on admission demonstrated mild renal dysfunction [BUN, 21.0 mg/dL (normal range, 6-20 mg/dL); creatinine, 1.09 mg/dL (normal range, 0.61-1.04 mg/dL)] and an increased level of serum urinary acid [9.8 mg/dL (normal range, 3.7-7.0 mg/dL)]. The serum levels of various hormones (gastrin, insulin, and vasoactive intestinal peptide) and tumor markers [carcinoembryonic antigen (CEA): 1.6 ng/mL (normal: ≤5.0 ng/mL), cancer antigen (CA) 19-9: ≤2.0 U/mL (normal: ≤37 U/mL), neuron-specific enolase (NSE): 7.1 ng/mL (normal: ≤10 ng/mL), and pro-gastrin-releasing peptide (proGRP): 46.1 pg/mL (normal: <70 pg/mL)] were within the normal ranges.
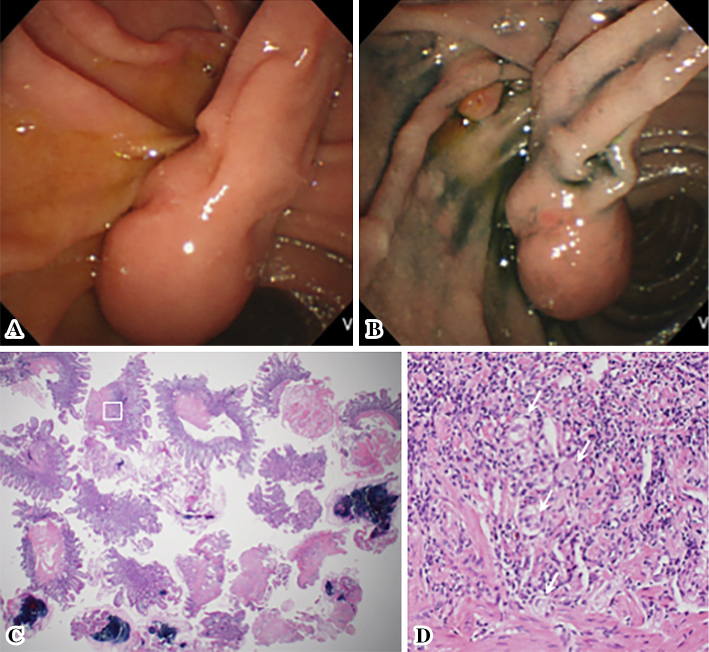
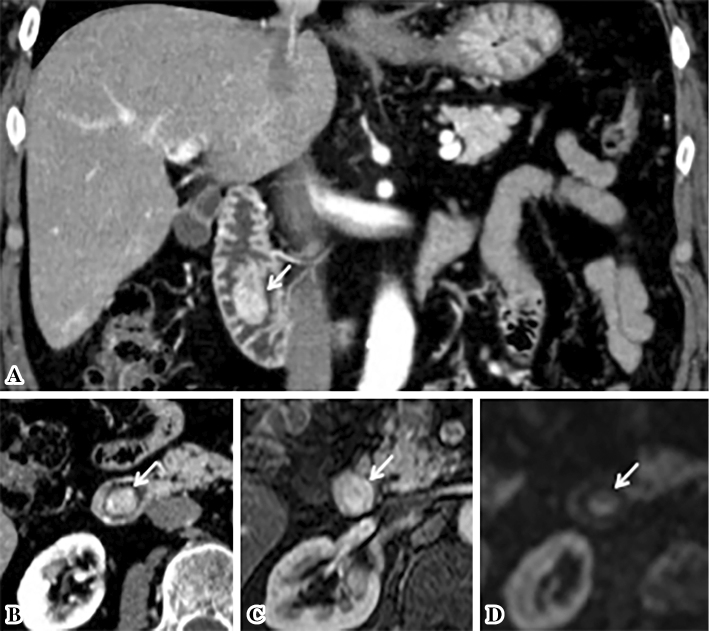
Duodenoscopy demonstrated a pedunculated tumor with a smooth surface, at the duodenal minor papilla (Fig. 1A and B). The histological examination of multiple boring biopsies (Fig. 1C and D) of the tumor showed the infiltration of ganglion-like cells, which were positive for chromogranin-A and synaptophysin. Endoscopic ultrasonography demonstrated that a tumor of 17 mm in size located within the submucosa with a homogeneously low echoic interior (Fig. 2). Enhanced computed tomography (CT) and magnetic resonance image (MRI) (Fig. 3A-C) demonstrated the marginal enhancement of the tumor, but no metastasis. Diffusion-weighted MRI showed a high-intensity signal within the tumor (Fig. 3D). Magnetic resonance cholangiopancreatography was normal, but the Santorini's duct was not visible. 18F-fluolodeoxyglucose positron emission tomography (FDG-PET) indicated no abnormal uptake in the duodenum, but the tumor was positive at the pulmonary apex area (SUV max: 3.70) (Fig. 4), which suggested malignancy. Transbronchial needle aspiration cytology and brushing cytology were performed from the left B1 branch, and revealed clusters of adenocarcinoma cells. The duodenal biopsy findings indicated that the duodenal tumor was a well-differentiated neuroendocrine tumor (a so-called carcinoid) or a GP; hence, the lung cancer was treated first as it was a more life-threatening disease. Upper segmentectomy of the left lung was performed prior to the resection of the duodenal tumor. A surgical specimen showed a well-differentiated adenocarcinoma with a mixed bronchial-alveolar carcinoma subtype of 20 mm in size, without lymph node metastasis (0/10).
Figure 1.
An endoscopic view of the duodenal tumor. An upward directed view showing a submucosal tumor with a smooth surface and a long stalk (A). An overhead view after spraying with indigo carmine shows the major papilla at the anal side of the stalk root (B). Forceps boring biopsies of the tumor, which were performed 22 times (C) [Hematoxylin and Eosin (H&E) staining, ×12.5], demonstrated a very small number of ganglion-like cells infiltration (D) [H&E staining, ×200; corresponding to the white square in (C)].
Figure 2.
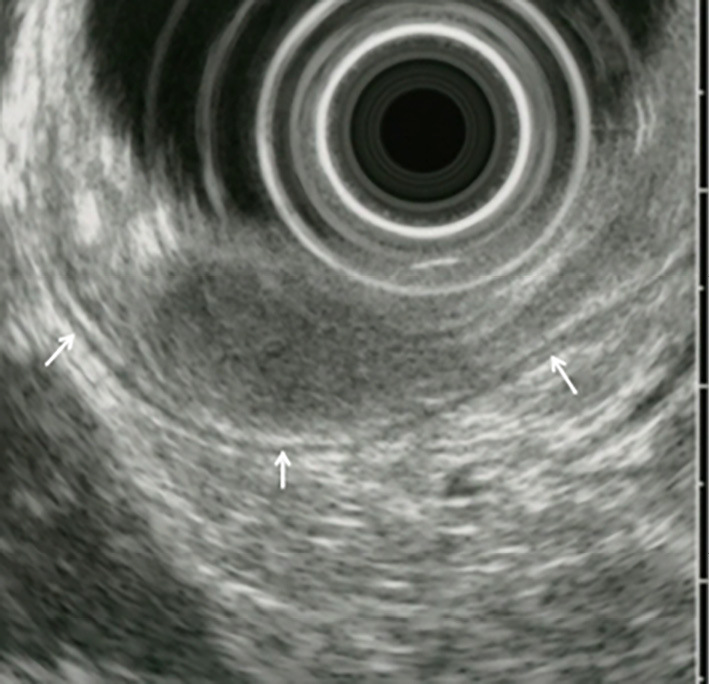
Endoscopic ultrasonography (EUS) showing a homogeneously low-echoic duodenal polyp of 17 mm in size, and the intact muscular propria of the duodenum (arrows).
Figure 3.
Cross-section images. A high vascular tumor protruding into the duodenal lumen, as depicted by enhanced computed tomography (CT) (A: coronal view, B: horizontal view) and by enhanced magnetic resonance imaging (MRI) (C). Diffusion-weighted MRI showing a high-intensity signal at the tumor (D). White arrows indicate the tumor.
Figure 4.
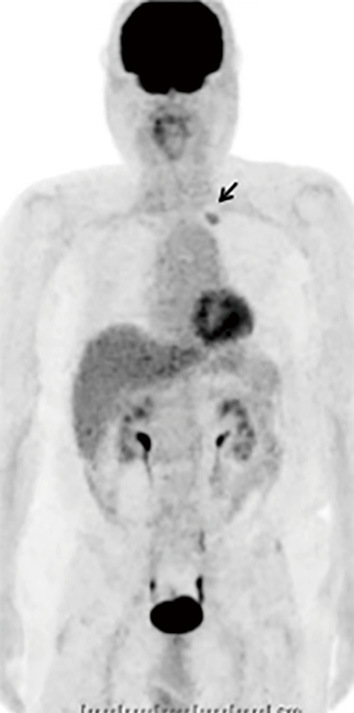
18F-fluolodeoxyglucose positron emission tomography (FDG-PET) showing the abnormal uptake of FDG at the apex area of the left lung.
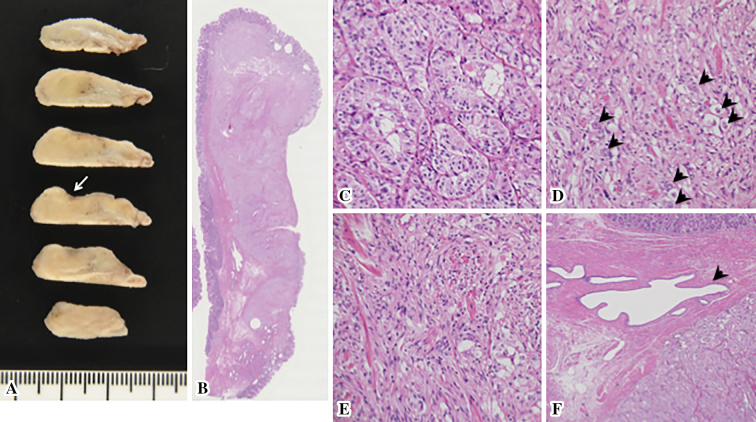
Three months after the diagnosis of the minor papilla tumor, the patient underwent laparotomic duodenal polypectomy. The tumor at the duodenal papilla was either carcinoid tumor or GP, both of which have shown the potential to metastasize to the lymph nodes, even in cases involving small tumors (1,4,5). Thus, regional lymph node dissection was also added. The macroscopic examination of the surgical specimen showed a whitish-yellow, well-demarcated tumor with erosion, probably corresponding to the biopsy site (Fig. 5A). The histological examination of the duodenal tumor showed a GP of 18 mm in size, which consisted of three neoplastic components: endocrine cells, spindle cells and ganglion-like cells (Fig. 5B-F). This tumor was limited to within the submucosa, the surgical margin was negative and no lymph node metastasis was detected (0/4). Immunohistochemistry revealed that all of the tissue types in the tumor were positive for synaptophysin and NSE (Table 1). The Ki-67 labeling index of the tumor was 1.1% (number of counted cells: >1,000). The patient underwent follow-up examinations at six-month intervals. He is currently in good health today, with no evidence of recurrence at 67 months.
Figure 5.
The histology of the resected duodenal tumor. A macroscopic view of the thin-sliced polyp demonstrating a whitish-yellow, well-demarcated tumor with an erosive area probably corresponding to the biopsy site (white arrow) (A). A loupe view of the polyp (B) [Hematoxylin and Eosin (H&E) staining]. A magnified view of the tumor histology showing three components: endocrine cells (C), ganglion cells (arrowheads) (D) and spindle cells (E) (H&E staining, ×200). Santorini’s duct running alongside the endocrine tumor component (arrowhead) (F) (H&E staining, ×40).
Table 1.
Immunohistochemical Findings for Each of Three Types of Tumor Component.
| Endocrine cell | Spindle cell | Ganglion-like cell | |
|---|---|---|---|
| Neuron specific enolase | (++) | (++) | (++) |
| Synaptophysin | (++) | (+-++) | (+-++) |
| S-100 | (+) | (++) | (+) |
| Chromogranin A | (+) | (-) | (-) |
| Somatostatin | (++) | (-) | (+) |
| Pancreatic polypeptide | (++) | (-) | (+) |
(++): diffusely positive, (+-++): heterogeneously positive, (+): weakly and/or focally positive, (-): negative
Discussion
GP is a rare tumor, which is mostly recognized in the duodenum. It rarely develops in other organs, including the esophagus, mediastinum (6), thymus (7), lung (8), and ovary (9). According to a search of the PubMed database, 45 cases of the duodenal GP have been reported during the last decade (2007-2016) (Table 2) (2,3,10-48). This tumor frequently develops in middle-aged and elderly individuals (mean, 51.2 years; range, 16-92 years) and predominantly affects men (27 men vs. 18 women). The major symptoms at the onset of disease were abdominal pain and melena. GP often develops in the 2nd and 3rd portions of the duodenum, especially at the major papilla of Vater (1), and usually appears as a pedunculated or polypoid, submucosal tumor, with a mean size of 26.5 mm (range, 10-50 mm) (Table 2).
Table 2.
Reported Cases of Ganglyocytic Paraganglioma of the Duodenum (2007-2016, n=45).
| No. | ref. | Age | Sex | Symptom at onset | Location | Size (mm) | Macroscopic-type | Depth | LN | Treatment | Status, Follow-up period (mth) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 10 | 51 | M | fatigue, dyspnea | MPV | 14 | polypoid mass | N.A | (-) | ER | NED, 14 |
| 2 | 11 | 66 | M | epigastralgia, vomiting | minor papilla | 20 | SMT | SM | (-) | ER | NED, 24 |
| 3 | 12 | 53 | M | no symptom | 2nd portion | 13 | pedunculated SMT | SM | N.A | ER | N.A |
| 4 | 13 | 75 | F | melena, anemia | 2nd portion | 40 | pedunculated tumor | N.A | (-) | LapTDTR | NED, 24 |
| 5 | 14 | 38 | F | abdominal pain | near MPV | 15 | polypoid | MP | (+) | ER → PPPD | N.A |
| 6 | 15 | 48 | M | abdominal pain | 2nd portion | 35 | subepithelial tumor | N.A | N.A | local excision | N.A |
| 7 | 16 | 67 | M | no symptom | MPV | 15 | ball-like lesion | N.A | N.A | LTDR | N.A |
| 8 | 17 | 17 | F | abdominal pain, weight loss | 2nd~3rd portion | N.A | mass | SS | (+) | PPPD | N.A |
| 9 | 18 | 60s | M | no symptom | anal of MPV | 25 | polypoid SMT | SM | N.A | PD | NED, 12 |
| 10 | 19 | 70 | F | abdominal pain | near MPV | 19 | polypoid mass | N.A | N.A | ER → LTDR | NED, 48 |
| 11 | 20 | 56 | F | melena | MPV | 25 | SMT | MP | (-) | PPPD | NED, 16 |
| 12 | 21 | 61 | M | epigastralgia, melena | MPV | 30 | lobulated SMT | MP | (+) | PPPD | NED, 6 |
| 13 | 22 | 52 | M | vomitting | 3rd portion | 35 | SMT | MP | (-) | LTDR | N.A |
| 14 | 23 | 62 | F | abdominal pain | MPV | 20 | polypoid mass | SM | (-) | laparotomy | NED, 12 |
| 15 | 24 | 61 | M | abdominal pain, weight loss | 2nd portion | 15 | submucosal nodule | N.A | (+) | PPPD | NED, 12 |
| 16 | 25 | 33 | F | abdominal pain, vomitting | MPV | 39 | polypoid mass | N.A | N.A | local excision | NED, 12 |
| 17 | 26 | 16 | M | melena, dyspnea | MPV | 35 | polypoid | MP | (+) | PPPD | NED, 36 |
| 18 | 27 | 20 | F | melena, anemia | 2nd portion | 50 | polypoid mass | MP | (-) | PPPD | NED, 11 |
| 19 | 28 | 52 | F | N.A | near minor papilla | 10 | nodular mass | MP | (+) | PD | alive*, 27 |
| 20 | 29 | 53 | M | abdominal pain, vomitting | MPV | 25 | SMT | N.A | (-) | LTDR | N.A$ |
| 21 | 30 | 51 | F | melena, anemia | MPV | 25 | polypoid mass | SS | (+) | PD | NED, 96 |
| 22 | 31 | 54 | F | no symptom | near MPV | 15 | SMT | N.A | N.A | LTDR | NED, 30 |
| 23 | 32 | 16 | M | abdominal pain, weight loss | 3rd portion | 25 | pedunculated SMT | SM | (-) | LTDR | NED, 15 |
| 24 | 33 | 67 | M | melena, anemia | 2nd portion | 30 | submucosal nodule | SM | (-) | local excision | NED, 15 |
| 25 | 34 | 57 | M | abdominal pain, vomitting | MPV | 30 | mass | N.A | (+) | laparotomy | alive*, 8 |
| 26 | 35 | 56 | F | abdominal pain, weight loss | near MPV | 18 | mass | N.A | (+) | PPPD | N.A |
| 27 | 36 | 32 | M | melena | 2nd portion | 23 | SMT | SM | N.A | LTDR | N.A |
| 28 | 37 | 92 | F | jaundice | near MPV | 20 | SMT | SM | (-) | ER | N.A |
| 29 | 38 | 72 | F | weight loss | near MPV | 37 | polypoid mass | SM | (-) | local excision | NED, 17 |
| 30 | 2 | 47 | M | abdominal pain | near MPV | 30 | mass | pancreas | (+) | PD | dead, 13# |
| 31 | 39 | 56 | F | dyspepsia | near MPV | 29 | subepithelial tumor | N.A | N.A | ER | NED, 6 |
| 32 | " | 56 | F | anemia | 2nd portion | 50 | lobulated mass | N.A | N.A | ER → LTDR | NED, 30 |
| 33 | " | 46 | F | no symptom | MPV | N.A | enlarged papilla | N.A | N.A | ER | NED, 23 |
| 34 | " | 70 | M | no symptom | 2nd portion | 20 | subepithelial tumor | N.A | (-) | ER | NED, 12 |
| 35 | 3 | 50 | M | abdominal pain | MPV | 30 | mass | SM | N.A | LTDR | NED, 36 |
| 36 | 40 | 47 | M | abdominal pain | MPV | 40 | polypoid | pancreas | (+) | PD | NED, 24 |
| 37 | 41 | 48 | M | melena, weight loss | 4th portion | 40 | polypoid | SM | N.A | SD | NED, 24 |
| 38 | 42 | 48 | M | no symptom | 2nd portion | 15 | subepithelial tumor | SM | N.A | ER | N.A |
| 39 | 43 | 41 | F | abdominal pain | MPV | 20 | polypoid | SM | (-) | ER | NED, 6 |
| 40 | 44 | 38 | M | hematochezia, anemia | 2nd portion | 15 | polypoid | SM | (-) | local excision | NED, 12 |
| 41 | 45 | 70 | M | no symptom | MPV | 23 | SMT | SM | (-) | LTDR | N.A |
| 42 | 46 | 50 | M | melena | 3rd portion | 25 | SMT | MP | (-) | LTDR | N.A |
| 43 | 47 | 42 | M | melena, dizziness | 3rd portion | 30 | polypoid | SM | N.A | local excision | not followed |
| 44 | " | 49 | M | abdominal pain | MPV | 40 | mass | pancreas | (+) | PD | NED, 36 |
| 45 | 48 | 45 | M | abdominal pain, melena | MPV | 15 | mass (pedunculated) | SM | (+) | LTDR | alive, 3** |
F: female, M: male, LN: lymph node metastasis, MPV: major papilla Vater, ER: endoscopic resection, PD: pancreaticoduodenectomy, PPPD: pylorus-preserving pancreaticoduodenectomy, NED: no evidence of disease, SMT: submucosal tumor, SM: submucosa, MP: muscular propria, SS: subserosa, LapTDR: laparoscopic transduodenal resection, LTDR: laparotomic
transduodenal resection, SD: segmental duodenectomy, *alive with liver metastasis, **alive with regional lymph node recurrence, $accompanied with esophageal adenocarcinoma, #died of disease with metastasis to the pelvis and liver
GP has generally been regarded as a neuroendocrine tumor that produces a variety of hormones (1). It has been hypothesized that a hamartoma-like mechanism is involved in the development of GP from misplaced embryonic pancreatic tissue (1,49,50). Although there are only two reported cases of a GP of the duodenal minor papilla (11,51), this theory can be compatible with the viewpoint of its favored location, as the endocrine cell micronest and (ectopic) pancreatic tissue are often recognized within the minor papilla (52). However, the high incidence in the duodenum and not the pancreas suggests that some other factors remain to be elucidated (e.g., digestive or chemical stimuli such as pancreatic juice and/or bile exposure).
A preoperative diagnosis of GP is generally difficult due to its rarity and because various other submucosal tumors, including neuroendocrine tumors, gastrointestinal stromal tumors (GISTs), smooth muscle tumors, lipomas, aberrant pancreas, Brunner gland's hyperplasia, cysts, and lymphoma, also develop in the duodenum. In addition, the reported diagnostic rate of forceps biopsy specimens is only 11% (1). In our case, the biopsy performed in the previous hospital was not diagnostic, and our biopsy demonstrated only a small number of infiltrating ganglion-like cells, despite the fact that we performed repeated sampling 22 times (Fig. 1C and D). Hence, when only a spindle cell component is obtained, it may be diagnosed as a GIST, while it might be diagnosed as a neuroendocrine tumor when only an endocrine cell component is found. The EUS findings are important for the differential diagnosis as well as for determining the extent of the tumor. The difference in tumor size can be subtle between cases with (29.8 mm) and without (23.5 mm) lymph node metastasis. Nevertheless, the ratio of lymph node metastasis differs significantly in tumors that are located within the submucosa (2.4%, 1/42) and those that extend beyond the submucosa (16.7%, 11/66)(p<0.05) (1). A preoperative EUS examination is therefore critical for deciding suitable treatment.
Our treatment of an 18-mm neuroendocrine tumor or a tumor that is located at the minor papilla is to perform an open transduodenal resection with regional lymph node dissection, as this operation results in a good prognosis. As mentioned above, a small size is not a definitive factor for predicting lymph node metastasis. According to a summary by Park et al. (5), up to 36% (8/22) of tumors of ≤2 cm in size had lymph node metastasis. Even in cases of carcinoid tumors, a small size does not guarantee that there will be no metastasis (4). Although the overall survival after tumor resection is favorable (1,5), progressive disease with recurrence and tumor-associated death have been reported for this tumor (2,14,53). Hence, in our case, despite the tumor's small size, the EUS finding of the tumor extent (within the submucosa) and the absence of regional lymph node swelling (as determined by preoperative CT), we selected a laparotomic transduodenal operation, which enabled us to perform a local tumor resection as well as regional lymph node dissection. If the tumor had appeared to extend beyond the submucosa or if lymph node metastasis was suspected, we would have selected pancreatoduodenectomy.
In conclusion, GP should be listed as a differential diagnosis of duodenal submucosal tumors, especially tumors with a pedunculated shape. GP often develops at the major Vater and rarely at the minor papilla. Surgical resection must be carefully performed, while keeping the risk of lymph node metastasis in mind.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Okubo Y, Wakayama M, Nemoto T, et al. Literature survey on epidemiology and pathology of gangliocytic paraganglioma. BMC Cancer 11: 187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li B, Li Y, Tian XY, Luo BN, Li Z. Malignant gangliocytic paraganglioma of the duodenum with distant metastases and a lethal course. World J Gastroenterol 20: 15454-15461, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rafiullah , Tanimu S, Onitilo AA. Gangliocytic paraganglioma, a rare ampullary tumour treated with open transduodenal resection and sphincteroplasty. BMJ Case Rep 2014: bcr-2013-202941, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matsubayashi H, Matsunaga K, Sasaki K, Yamaguchi Y, Hasuike N, Ono H. Small carcinoid tumor of papilla of the Vater with lymph node metastases. J Gastrointest Cancer 39: 61-65, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Park HK, Han HS. Duodenal gangliocytic paraganglioma with lymph node metastasis. Arch Pathol Lab Med 140: 94-98, 2016. [DOI] [PubMed] [Google Scholar]
- 6.Weinrach DM, Wang KL, Blum MG, Yeldandi AV, Laskin WB. Multifocal presentation of gangliocytic paraganglioma in the mediastinum and esophagus. Hum Pathol 35: 1288-1291, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Yang JW, Han J, Lee HW, Cho SY, Kim HK. A rare case of thymic gangliocytic paraganglioma. J Pathol Transl Med 50: 165-167, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin D, Hu Y, Xing X, et al. Pulmonary gangliocytic paraganglioma: a case report and review of the literature. Int J Clin Exp Pathol 7: 432-437, 2014. [PMC free article] [PubMed] [Google Scholar]
- 9.Mahdavi A, Silberberg B, Malviya VK, Braunstein AH, Shapiro J. Gangliocytic paraganglioma arising from mature cystic teratoma of the ovary. Gynecol Oncol 90: 482-485, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Chahal P, Prasad GA, Sanderson SO, Gostout CJ, Levy MJ, Baron TH. Endoscopic resection of nonadenomatous ampullary neoplasms. J Clin Gastroenterol 41: 661-666, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Loew BJ, Lukens FJ, Navarro F, Roy M, Mattia A, Howell DA. Successful endoscopic resection of a gangliocytic paraganglioma of the minor papilla in a patient with pancreas divisum and pancreatitis (with video). Gastrointest Endosc 65: 547-550, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Morita T, Tamura S, Yokoyama Y, et al. Endoscopic resection of a duodenal gangliocytic paraganglioma. Dig Dis Sci 52: 1400-1404, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Parini U, Nardi M Jr, Loffredo A, Fabozzi M, Roveroni M. Laparoscopic resection of duodenal gangliocytic paraganglioma. A case report. Chir Ital 59: 551-558, 2007. [PubMed] [Google Scholar]
- 14.Witkiewicz A, Galler A, Yeo CJ, Gross SD. Gangliocytic paraganglioma: case report and review of the literature. J Gastrointest Surg 11: 1351-1354, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Suk FM, Lin YH, Hsieh MC. Clinical challenges and images in GI. Duodenal gangliocytic paraganglioma. Gastroenterology 135: 361, 714, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Chen J, Sharon E, Morgenstern S, Geller A, Greif F. Local surgical resection of gangliocytic paraganglioma of the duodenal papilla. Isr Med Assoc J 11: 311-312, 2009. [PubMed] [Google Scholar]
- 17.Mann CM, Bramhall SR, Buckels JA, Taniere P. An unusual case of duodenal obstruction-gangliocytic paraganglioma. J Hepatobiliary Pancreat Surg 16: 562-565, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Ohtsuki Y, Watanabe R, Kimura M, et al. Immunohistochemical and electron microscopic studies of a case of duodenal gangliocytic paraganglioma. Med Mol Morphol 42: 245-249, 2009. [DOI] [PubMed] [Google Scholar]
- 19.Sánchez-Pérez MA, Luque-de León E, Muñoz-Juárez M, Moreno-Paquentin E, Genovés-Gómez H, Torreblanca-Marín MA. Duodenal gangliocytic paraganglioma. Can J Surg 52: E27-E28, 2009. [PMC free article] [PubMed] [Google Scholar]
- 20.Kwon J, Lee SE, Kang MJ, Jang JY, Kim SW. A case of gangliocytic paraganglioma in the ampulla of Vater. World J Surg Oncol 8: 42, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okubo Y, Yokose T, Tuchiya M, et al. Duodenal gangliocytic paraganglioma showing lymph node metastasis: a rare case report. Diagn Pathol 5: 27, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sucandy I, Ayers G, Bertsch DJ. Surgical management of endoscopically unresectable duodenal gangliocytic paraganglioma in a patient with partial upper gastrointestinal obstruction. N Am J Med Sci 2: 547-551, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Von Loga K, Konig A, Zustin J. Periampullary gangliocytic paraganglioma: a case report. In Vivo 24: 321-324, 2010. [PubMed] [Google Scholar]
- 24.Fiscaletti M, Fornelli A, Zanini N, et al. Segmental groove pancreatitis and duodenal gangliocytic paraganglioma with lymph node metastasis: a newly described association. Pancreas 40: 1145-1147, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Lytras D, Kalaitzakis E, Hatfield AR, et al. Recurrent acute pancreatitis as primary manifestation of gangliocytic paraganglioma of the ampulla. Pancreas 40: 985-986, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Ogata S, Horio T, Sugiura Y, Aiko S, Aida S. Duodenal gangliocytic paraganglioma with regional lymph node metastasis and a glandular component. Pathol Int 61: 104-107, 2011. [DOI] [PubMed] [Google Scholar]
- 27.Parray FQ, Lone IM, Chowdri NA, et al. Emergency pancreaticoduodenectomy in duodenal paraganglioma: case report. ISRN Surg 2011: 268674, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowsell C, Coburn N, Chetty R. Gangliocytic paraganglioma: a rare case with metastases of all 3 elements to liver and lymph nodes. Ann Diagn Pathol 15: 467-471, 2011. [DOI] [PubMed] [Google Scholar]
- 29.Assef MS, Carbonari AP, Araki O, et al. Gangliocytic paraganglioma of the duodenal papilla associated with esophagogastric adenocarcinoma. Endoscopy 44 Suppl 2 UCTN: E165-E166, 2012. [DOI] [PubMed] [Google Scholar]
- 30.Barret M, Rahmi G, Duong van Huyen JP, Landi B, Cellier C, Berger A. Duodenal gangliocytic paraganglioma with lymph node metastasis and an 8-year follow-up: a case report. Eur J Gastroenterol Hepatol 24: 90-94, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Čečka F, Jon B, Repák R, Kohout A, Šubrt Z, Ferko A. Gangliocytic paraganglioma of the duodenum. Can J Gastroenterol 26: 778-779, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nuño-Guzmán CM, Arróniz-Jáuregui J, Alvarez-López F, et al. Obstructing gangliocytic paraganglioma in the third portion of the duodenum. Case Rep Gastroenterol 6: 489-495, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu GC, Wang KL, Zhang ZT. Gangliocytic paraganglioma of the duodenum: a case report. Chin Med J (Engl) 125: 388-389, 2012. [PubMed] [Google Scholar]
- 34.Amin SM, Albrechtsen NW, Forster J, Damjanov I. Gangliocytic paraganglioma of duodenum metastatic to lymph nodes and liver and extending into the retropancreatic space. Pathologica 105: 90-93, 2013. [PubMed] [Google Scholar]
- 35.Dustin SM, Atkins KA, Shami VM, Adams RB, Stelow EB. The cytologic diagnosis of gangliocytic paraganglioma: a case report. Diagn Cytopathol 41: 650-653, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Narang V, Behl N, Sood N, Puri H. Gangliocytic paraganglioma of duodenum. Case Rep Pathol 2013: 378582, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sathyamurthy A, Choudhary A, Ng D, et al. Obstructive jaundice due to a rare periampullary tumor. World J Gastrointest Oncol 5: 195-197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asimakopoulou NI, Papakonstantinou PE, Lasithiotakis KG, et al. Recurrent episodes of acute pancreatitis due to duodenal gangliocytic paraganglioma: report of a case. JOP 15: 201-205, 2014. [DOI] [PubMed] [Google Scholar]
- 39.Park SJ, Kim DH, Lim H, et al. Endoscopic resection as a possible radical treatment for duodenal gangliocytic paraganglioma: a report of four cases. Korean J Gastroenterol 63: 114-119, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Shi H, Han J, Liu N, et al. A gangliocytic patially glandular paraganglioma with lymph node metastasis. Diagn Pathol 9: 63, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tatangelo F, Cantile M, Pelella A, et al. Duodenal gangliocytic paraganglioma, a rare entity among GEP-NET: a case report with immunohistochemical and molecular study. Diagn Pathol 9: 54, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trawick E, Salaria S, Yachimski P. Endoscopic resection of an ampullary gangliocytic paranganglioma. Gastrointest Endosc 79: 716-717, 2014. [DOI] [PubMed] [Google Scholar]
- 43.Yang JI, Choi JS, Lee GH, et al. A case of ampullary gangliocytic paraganglioma. Korean J Intern Med 29: 375-378, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boeriu A, Dobru D, Georgescu R, Mocan S, Boeriu C. Gangliocytic paraganglioma: a rare cause of gastrointestinal bleeding. J Gastrointestin Liver Dis 24: 109-112, 2015. [DOI] [PubMed] [Google Scholar]
- 45.Juarez-Parra MA, Guzman-Huerta EA, Ochoa-Rodriguez G, et al. Gastric gist with syncronous ampullary gangliocytic paraganglyoma: a novel presentation of an incomplete Carney's triad. J Gastrointest Cancer 46: 317-321, 2015. [DOI] [PubMed] [Google Scholar]
- 46.Loftus TJ, Kresak JL, Gonzalo DH, Sarosi GA Jr, Behrns KE. Duodenal gangliocytic paraganglioma: a case report and literature review. Int J Surg Case Rep 8C: 5-8, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang B, Zou Y, Zhang H, Xu L, Jiang X, Sun K. Duodenal gangliocytic paraganglioma: report of two cases and review of literature. Int J Clin Exp Pathol 8: 9752-9759, 2015. [PMC free article] [PubMed] [Google Scholar]
- 48.Lei L, Cobb C, Perez MN. Functioning gangliocytic paraganglioma of the ampulla: clinicopathological correlations and cytologic features. J Gastrointest Oncol 7: S107-S113, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perrone T, Sibley RK, Rosai J. Duodenal gangliocytic paraganglioma. An immunohistochemical and ultrastructural study and a hypothesis concerning its origin. Am J Surg Pathol 9: 31-41, 1985. [PubMed] [Google Scholar]
- 50.Burke AP, Helwig EB. Gangliocytic paraganglioma. Am J Clin Pathol 92: 1-9, 1989. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura T, Ozawa T, Kitagawa M, et al. Endoscopic resection of gangliocytic paraganglioma of the minor duodenal papilla: case report and review. Gastrointest Endosc 55: 270-273, 2002. [DOI] [PubMed] [Google Scholar]
- 52.Suda K. Histopathology of the minor duodenal papilla. Dig Surg 27: 137-139, 2010. [DOI] [PubMed] [Google Scholar]
- 53.Dookhan DB, Miettinen M, Finkel G, Gibas Z. Recurrent duodenal gangliocytic paraganglioma with lymph node metastases. Histopathology 22: 399-401, 1993. [DOI] [PubMed] [Google Scholar]