Abstract
We report the case of a mother and two children who shared a mitochondrial DNA A3243G mutation. The mother had diabetes mellitus, neurogenic bladder, bradykinesia, dystonia, and slowly progressive cerebellar ataxia. Her two daughters were diagnosed with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes at adolescence. They all presented with gastrointestinal symptoms at an advanced clinical stage. They were diagnosed with chronic intestinal pseudo-obstruction, and they were resistant to therapy. The mother and her youngest daughter died from aspiration pneumonia because of vomiting. The determination of chronic intestinal pseudo-obstruction is an important prognostic factor in patients with the mitochondrial DNA A3243G variant.
Keywords: chronic intestinal pseudo-obstruction, mitochondrial DNA A3243G mutation, MELAS, familial occurrence
Introduction
Mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) is a devastating multisystem syndrome that is characterized by progressive encephalopathy and stroke-like episodes leading to disability and early death. MELAS is most commonly associated with the mitochondrial DNA A3243G mutation. In addition to neurological symptoms, carriers of the mitochondrial DNA A3243G mutation experience a wide spectrum of symptoms involving multiple organs (1). This mutation can give rise to gastrointestinal symptoms, including bloating, dysphagia, recurrent vomiting and anorexia, chronic diarrhea, and gastrointestinal pseudo-obstruction. Gastrointestinal symptoms are reported in 15% of patients with mitochondriopathy (2). Chronic intestinal pseudo-obstruction (CIPO), which is one of the more severe gastrointestinal disorders, is characterized by signs of intestinal obstruction and radiographic evidence of a dilated bowel in the absence of any mechanical obstruction (3). We herein present the case of a mother and her two children with a mitochondrial DNA A3243G mutation who presented with chronic intestinal pseudo-obstruction at an advanced clinical stage.
Case Report
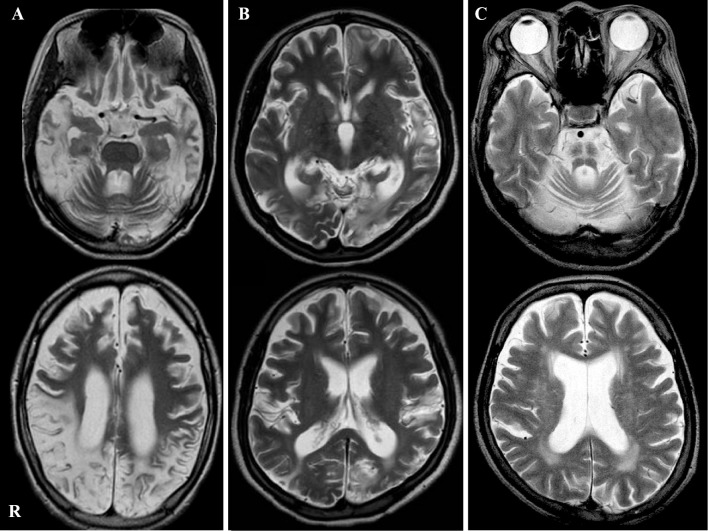
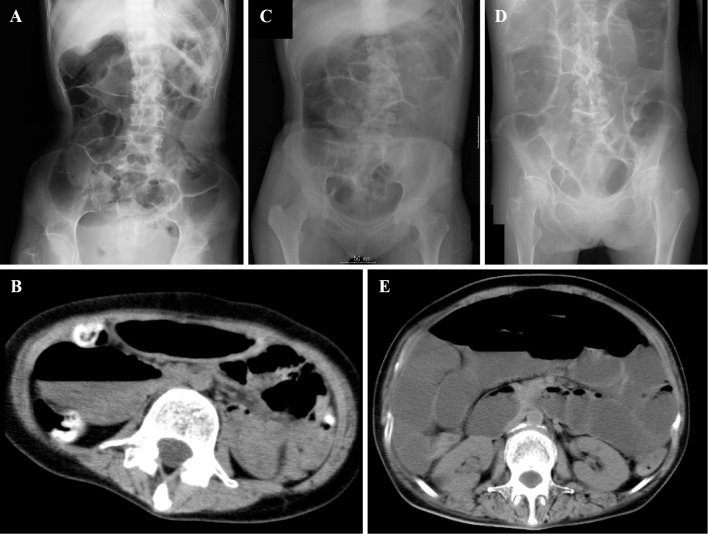
Patient 1 was a woman who was born at full term with normal early development. She presented with her first stroke-like episode, including headache, vomiting, and blurred vision, at 10 years of age. A genetic analysis of the mitochondrial DNA from a blood sample demonstrated the presence of the A3243G mutation, which confirmed the diagnosis of MELAS. Although she was treated with L-carnitine, coenzyme Q10, and arginine, she was repeatedly admitted to hospital because of stroke-like episodes such as headache, vomiting, and convulsions. At 23 years of age, she was admitted due to abdominal pains and vomiting. On a physical examination at admission, short stature (140 cm) and low body weight (25.1 kg) were noted. Her abdomen was distended, and decreased bowel sounds were observed. During a neurological examination, she could not speak or decipher our instructions. A cranial nerve examination was unremarkable with the exception of hearing impairment. She had muscle weakness and atrophy in her body trunk and four limbs. Her deep tendon reflexes were generally increased and the patient was bilaterally positive for Babinski's sign. A laboratory analysis revealed the following findings: white blood cell count, 16,100 /μL; C-reactive protein, 2.0 mg/dL; creatine kinase (CK), 105 U/L; lactate, 14.7 mg/dL (normal range: 3.3-14.9 mg/dL); and pyruvate, 0.6 mg/dL (normal range: 0.3-0.9 mg/dL). Brain T2-weighted magnetic resonance imaging (MRI) showed multiple infarcts and diffuse brain atrophy (Fig. 1A). A simple abdominal radiograph showed remarkably dilated intestinal loops (Fig. 2A). Abdominal computed tomography showed no signs of either a definite obstructive level or bowel wall thickening (Fig. 2B). After admission, she abstained from eating, and lactate-free fluid and antibiotics were administered intravenously. Her abdominal distention gradually recovered over the next several days. However, she was subsequently unable to take in any food or liquid by mouth, and she was therefore tube-fed. She then began vomiting again, and several methods of therapy, including mosapride citrate, erythromycin, bifidobacterium, and distigmine bromide had no effect. Pressure was decreased with the use of a nasogastric tube, and total parenteral nutrition was administered through a central venous line. At six months after admission, she died from aspiration pneumonia due to vomiting; she was 23 years of age.
Figure 1.
Brain T2-weighted magnetic resonance imaging of Patients 1 (A) and 2 (B) showing multiple infarcts and diffuse brain atrophy. Brain T2-weighted magnetic resonance imaging of Patient 3 (C) showing the hot cross bun sign at the pons and brain stem and cerebellum atrophy.
Figure 2.
Simple abdominal radiographs of Patients 1 (A), 2 (C), and 3 (D) and abdominal computed tomographic scans of Patients 1 (B) and 3 (E) showing remarkably dilated intestinal loops.
Patient 2 was the older sister of Patient 1. She was born at full term and her early development was normal. She presented with her first stroke-like episode, including headache and vomiting, at 12 years of age. Brain T2-weighted MRI showed multiple infarcts and diffuse brain atrophy (Fig. 1B). A genetic analysis of mitochondrial DNA of a blood sample demonstrated the presence of the A3243G mutation, which confirmed the diagnosis of MELAS. Like her sister, she was also repeatedly admitted to hospital for stroke-like episodes such as headache, vomiting, and convulsions. At 22 years of age, she was admitted to our hospital because of vomiting. A simple abdominal radiograph showed remarkably dilated intestinal loops (Fig. 2C). A small bowel series did not show a definite obstructive level. Total parenteral nutrition was administered through a central venous catheter because of recurrent vomiting, and she ultimately died due to renal failure because of focal sclerosing glomerulonephritis at 22 years of age.
Patient 3 was the mother of Patients 1 and 2. She was diagnosed with diabetes mellitus at 39 years of age and with urinary retention at 46 years of age. She developed a staggering gait and presented bradykinesia at 47 years of age. She had rigidity of the upper limbs and dystonia of the lower limbs at 49 years of age, and showed forced crying and forced laughing at 50 years of age. At 51 years of age, she was admitted to our hospital because of fever, abdominal pains, and vomiting. The physical examination at admission revealed that she was 147.5 cm tall and weighed 47.8 kg. Her abdomen was distended, and her bowel sounds were decreased. A neurological examination revealed clear sensorium and a euphoric mood. A cranial nerve examination was unremarkable except for a slow singsong voice. Muscle weakness (grade 4-5 on a manual muscle test) was observed in her body trunk and four limbs. Her vibratory sensation was slightly decreased on the bilateral medial malleolus, while her pain and touch sensations were intact. She did not have orthostatic hypotension. There was severe gait and limb ataxia in the finger-nose-finger test and heel-knee test. Her deep tendon reflexes were generally increased and she was bilaterally positive for Babinski's sign. She used a wheelchair due to difficulty in walking because of ataxia. A laboratory analysis revealed the following: white blood cell count, 16,000 /μL; C-reactive protein, 35.4 mg/dL; CK, 16 U/L; hemoglobin A1c, 7.1%; lactate, 19.4 mg/dL (normal range: 3.3-14.9 mg/dL); and pyruvate, 1.38 mg/dL (normal range: 0.3-0.9 mg/dL). Simple abdominal radiography revealed remarkably dilated intestinal loops (Fig. 2D). Abdominal computed tomography did not demonstrate signs indicating either a definite obstructive level or bowel wall thickening (Fig. 2E). Although her abdominal distension initially recovered following the administration of erythromycin and bifidobacterium, she subsequently had multiple relapses. An ileus tube was inserted, but was ineffective. Total parenteral nutrition was administered through a central venous line. Brain T2-weighted MRI showed atrophy of the brain stem and cerebellum, and a hot cross bun sign at the pons (Fig. 1C). A genetic analysis of mitochondrial DNA from a blood sample demonstrated the presence of the A3243G mutation. She died from aspiration pneumonia due to vomiting at 54 years of age. Autopsy was not permitted in these three cases. A summary of the cases is presented in Table.
Table.
Characteristics of Chronic Intestinal Pseudo-obstruction in Patients with the Mitochondrial DNA A3243G Mutation.
| Patient 1 (youngest daughter) | Patient 2 (oldest daughter) | Patient 3 (mother) | |
|---|---|---|---|
| Age at the onset of mitochondrial disease (years) | 10 | 12 | 39 |
| Sex | Female | Female | Female |
| Clinical manifestation | Headache, episodic vomiting, stroke-like episodes, seizure, blurred vision |
Headache, episodic vomiting, stroke-like episodes, seizure, blurred vision | Diabetes mellitus, dystonia, ataxia, urinary retention, forced laughing, forced crying |
| Brain MRI | Multiple infarcts, brain atrophy | Multiple infarcts, brain atrophy | A hot-cross-bun sign of the pons; brain stem and cerebellum atrophy |
| Age at the onset of CIPO (years) | 23 | 22 | 51 |
| Age of death (years) | 23 | 22 | 54 |
| Cause of death | Aspiration pneumonia | Chronic renal failure | Aspiration pneumonia |
CIPO: chronic intestinal pseudo-obstruction, MRI: magnetic resonance image
Discussion
A genetic analysis of the mitochondrial DNA from blood samples demonstrated the presence of the A3243G mutation in a mother and her two children. The most important finding was that despite the differences in the patients' neurological symptoms, they all presented with CIPO at an advanced clinical stage, which was diagnosed according to the consensus criteria (3). The mitochondrial DNA A3243G mutation is the most frequent heteroplasmic mitochondrial DNA mutation. Individuals with the mitochondrial DNA A3243G mutation may manifest either syndromic or non-syndromic mitochondrial disorders such as kidney disease, MELAS, inherited progressive external opthalmoplegia, isolated myopathy, cardiomyopathy, and maternally inherited diabetes and deafness syndrome. In this familial case, the two daughters (Patients 1 and 2) manifested the classical signs of MELAS, including headache, vomiting, and convulsive seizures. However, their mother (Patient 3) manifested different clinical signs, which included diabetes, urinary retention, dystonia, cerebellar ataxia, forced crying, and forced laughing. Her brain T2-weighted MRI showed characteristic changes with brain stem and cerebellum atrophy and a hot cross bun sign at the pons; these findings are commonly observed in patients with the cerebellar variant of multiple system atrophy. However, the hot cross bun sign has also been observed in patients with spinocerebellar ataxia type 2 and spinocerebellar ataxia type 3 (4). The mitochondrial DNA A3243G mutation is associated with various central nervous system manifestations, such as dystonia (5), parkinsonism (6), and ataxia (7-9). Pathologically, patients with the mitochondrial DNA A3243G mutation show diffuse atrophy of the cerebellar cortex, gliosis of the cerebellar white matter, and cactus formation in Purkinje cells with the accumulation of mitochondria (10-12). Although we cannot exclude the possibility that the mother had both mitochondrial disease and spinocerebellar degeneration, we attributed the atrophy of the brain stem and cerebellum and the hot cross bun sign at the pons to mitochondrial disease. The precise mechanism that results in different clinical manifestations, even within a single family, from the mitochondrial DNA A3243G mutation remains unknown.
Despite these differences, all three of our patients presented with CIPO at an advanced clinical stage. CIPO syndrome is a clinical entity that is characterized by repeated episodes of intestinal obstruction without evidence of a mechanical obstructive lesion (13). CIPO develops in either idiopathic or secondary form; the latter can be categorized as disease-induced (such as connective tissue disorders, muscular dystrophies, infiltrative diseases, generalized nerve disease, endocrine disease, metabolic disease, and others) or drug-induced (antidepressants and anti-anxiety drugs, phenothiazines, and others) (13). CIPO has been reported in several patients with the mitochondrial DNA A3243G mutation (14-21). In a previous study, CIPO was found to develop in 40% of MELAS patients and the frequency was higher when the onset of mitochondrial disease occurred in young age (14). Among patients with mitochondrial disease, mutated mitochondrial DNA and normal mitochondrial DNA exist in various ratios at each level of organization, and the ratio of mutated mitochondrial DNA differs among individuals as well as at different levels of organization and among individual cells (17). The rate of heteroplasmy of the mitochondrial A3243G mutation also varies considerably among individuals, even within a single family (17). The percentage of mutated mitochondrial DNA molecules in the blood is strongly correlated with the age of onset in patients with MELAS (1). Unfortunately, the percentages of mutant mitochondrial DNA in the blood were not estimated in our cases. To our knowledge, there have only been three previous reports of familial cases of CIPO associated with the mitochondrial A3243G mutation (17-19). However, in these previous reports, CIPO was not related to the direct cause of death, unlike our cases. The most frequent causes of death in patients with mitochondrial disease are cardiopulmonary failure, status epilepticus, and pulmonary embolism (22). Given that there are few reports of CIPO as the direct cause of death in mitochondrial disease (23), this case report highlights the importance of careful clinical observation, including gastrointestinal symptoms, in the management of patients with mitochondrial disease.
Surgical treatment for CIPO is not considered to be an option, as this intervention can increase lactic acidosis. The use of mosapride citrate, erythromycin, bifidobacterium, distigmine bromide, octreotide, neostigmine, coenzyme Q10, and a cofactor of the respiratory chain of the mitochondria has been proposed. However, as in our patients, the effect of these treatments is generally transient or limited, and the prognosis is often poor, with many cases ending in death (22). Thus, CIPO may reflect a severe end-stage symptom of mitochondrial cytopathy associated with the A3243G mutation.
The pathophysiology of CIPO due to the mitochondrial DNA A3243G mutation remains uncertain; however, several hypotheses have been put forward. First, the accumulation of numerous enlarged, abnormal mitochondria was observed in the intestinal smooth muscle cells and mucosal layer (20), and cytochrome c oxidase deficiency was specifically confined to the smooth muscle cells of the muscularis mucosa, the outer muscularis externa, the inner muscularis externa, and the smooth muscle lining of large arterioles. In addition, the mitochondrial DNA A3243G mutation was nearly homoplastic at these sites (24). These findings suggest that the pathogenesis of CIPO is related to myogenicity. Alternatively, in one reported case of CIPO, no mutated mitochondrial DNA was found in any digestive tract samples, suggesting that dysmotility is related to the autonomic nervous system (21). In addition, MELAS patients have been reported to show endothelial dysfunction, which results in a decreased vasodilatory capacity in the small arteries (25), and to have ischemic colitis associated with the A3243G mutation (26), which suggests vascular involvement in the pathogenesis of CIPO.
In conclusion, we herein reported a familial case of CIPO associated with the mitochondrial DNA A3243G mutation. This case highlights that CIPO is an important prognostic factor in patients with the mitochondrial DNA A3243G variant. Thus, careful clinical observation, including the observation of gastrointestinal symptoms, is required in the management of patients with the mitochondrial DNA A3243G mutation.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Ma Y, Fang F, Cao Y, et al. Clinical features of mitochondrial DNA m.3243A>G mutation in 47 Chinese families. J Neurol Sci 291: 17-21, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Hom XB, Lavine JE. Gastrointestinal complications of mitochondrial disease. Mitochondrion 4: 601-607, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Ohkubo H, Iida H, Takahashi H, et al. An epidemiologic survey of chronic intestinal pseudo-obstruction and evaluation of the newly proposed diagnostic criteria. Digestion 86: 12-19, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Liu CS, Wu HM, Wang PS, Chang MH, Soong BW. The ‘hot cross bun’ sign in the patients with spinocerebellar ataxia. Eur J Neurol 16: 513-516, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Sudarsky L, Plotkin GM, Logigian EL, Johns DR. Dystonia as a presenting feature of the 3243 mitochondrial DNA mutation. Mov Disord 14: 488-491, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Hara K, Yamamoto M, Anegawa T, Sakuta R, Nakamura M. Mitochondrial encephalomyopathy associated with parkinsonism and a point mutation in the mitochondrial tRNA(Leu)(UUR)) gene. Rinsho Shinkeigaku 34: 361-365, 1994. (in Japanese, Abstract in English). [PubMed] [Google Scholar]
- 7.Petruzzella V, Zoccolella S, Amati A, et al. Cerebellar ataxia as atypical manifestation of the 3243A>G MELAS mutation. Clin Genet 65: 64-65, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Tanahashi C, Nakayama A, Yoshida M, Ito M, Mori N, Hashizume Y. MELAS with the mitochondrial DNA 3243 point mutation: a neuropathological study. Acta Neuropathol 99: 31-38, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Kageyama Y, Ichikawa K, Fujioka A, Tsutsumi A, Yorifuji S, Miyoshi K. An autopsy case of mitochondrial encephalomyopathy with prominent degeneration in olivo-ponto-cerebellar system. Acta Neuropathol 83: 99-103, 1991. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi Z, Tsunemi T, Miake H, Tanaka S, Watabiki S, Morokuma Y. A mother and a child with maternally inherited diabetes and deafness (MIDD) showing atrophy of the cerebrum, cerebellum and brainstem on magnetic resonance imaging (MRI). Intern Med 44: 328-331, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Mori O, Yamazaki M, Ohaki Y, et al. Mitochondrial encephalomyopathy with lactic acidosis and stroke like episodes (MELAS) with prominent degeneration of the intestinal wall and cactus-like cerebellar pathology. Acta Neuropathol 100: 712-717, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Truong DD, Harding AE, Scaravilli F, Smith SJ, Morgan-Hughes JA, Marsden CD. Movement disorders in mitochondrial myopathies. A study of nine cases with two autopsy studies. Mov Disord 5: 109-117, 1990. [DOI] [PubMed] [Google Scholar]
- 13.Hirano I, Pandolfino J. Chronic intestinal pseudo-obstruction. Dig Dis 18: 83-92, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Sekino Y, Inamori M, Yamada E, et al. Characteristics of intestinal pseudo-obstruction in patients with mitochondrial diseases. World J Gastroenterol 18: 4557-4562, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiyonobu T, Noda R, Yoshida M, et al. Intestinal pseudo-obstruction in a patient with mitochondrial myopathy, encephalopathy, lactic acidosis, and stroke-like episodes (MELAS) associated with phenytoin therapy. Brain Dev 30: 430-433, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Kuroiwa T, Kuwata T, Nakayama T, et al. Mitochondrial encephalomyopathy showing prominent microvacuolation and necrosis of intestinal smooth muscle cells: a case diagnosed by rectal biopsy. Acta Neuropathol 96: 86-90, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Verny C, Amati-Bonneau P, Letournel F, et al. Mitochondrial DNA A3243G mutation involved in familial diabetes, chronic intestinal pseudo-obstruction and recurrent pancreatitis. Diabetes Metab 34: 620-626, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Li JY, Hsieh RH, Peng NJ, et al. A follow-up study in a Taiwanese family with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes syndrome. J Formos Med Assoc 106: 528-536, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Shimotake T, Furukawa T, Inoue K, Iwai N, Takeuchi Y. Familial occurrence of intestinal obstruction in children with the syndrome of mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS). J Pediatr Surg 33: 1837-1839, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Hiel JA, Verrips A, Keyser A, et al. Ileus in mitochondrial encephalomyopathy, lactic acidosis and stroke-like episodes. Neth J Med 53: 27-31, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Chinnery PF, Jones S, Sviland L, et al. Mitochondrial enteropathy: the primary pathology may not be within the gastrointestinal tract. Gut 48: 121-124, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klopstock T, Jaksch M, Gasser T. Age and cause of death in mitochondrial diseases. Neurology 53: 855-857, 1999. [DOI] [PubMed] [Google Scholar]
- 23.Amiot A, Tchikviladze M, Joly F, et al. Frequency of mitochondrial defects in patients with chronic intestinal pseudo-obstruction. Gastroenterology 137: 101-109, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Betts J, Barron MJ, Needham SJ, Schaefer AM, Taylor RW, Turnbull DM. Gastrointestinal tract involvement associated with the 3243A>G mitochondrial DNA mutation. Neurology 70: 1290-1292, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Koga Y, Akita Y, Junko N, et al. Endothelial dysfunction in MELAS improved by l-arginine supplementation. Neurology 66: 1766-1769, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Hess J, Burkhard P, Morris M, Lalioti M, Myers P, Hadengue A. Ischaemic colitis due to mitochondrial cytopathy. Lancet 346: 189-190, 1995. [PubMed] [Google Scholar]