Abstract
The coupled evolution of land plants, CO2, and climate over the last half billion years has maintained atmospheric CO2 concentrations within finite limits, indicating the involvement of a complex network of geophysiological feedbacks. But insight into this important regulatory network is extremely limited. Here we present a systems analysis of the physiological and geochemical processes involved, identifying new positive and negative feedbacks between plants and CO2 on geological time scales. Positive feedbacks accelerated falling CO2 concentrations during the evolution and diversification of terrestrial ecosystems in the Paleozoic and enhanced rising CO2 concentrations across the Triassic–Jurassic boundary during flood basalt eruptions. The existence of positive feedbacks reveals the unexpected destabilizing influence of the biota in climate regulation that led to environmental modifications accelerating rates of terrestrial plant and animal evolution in the Paleozoic.
Keywords: carbon dioxide, climate, land plant evolution, stomatal density, weathering
Abiotic regulation of Earth's global climate on a multimillion-year time scale is achieved by the long-term inorganic carbon cycle, whereby the concentration of the greenhouse gas CO2 is controlled by its supply from volcanoes and metamorphic degassing, and removal by the chemical weathering of calcium and magnesium silicate rocks (1, 2). The advent of vascular land plants introduced a potent biotic feedback into climate regulation, with the capacity to alter the long-term atmospheric CO2 concentration through the production of organic matter for burial in sediments, and acceleration of the chemical weathering of silicate rocks (3). However, long-term changes in CO2 and climate also play an important role in driving terrestrial plant development and evolution (4–7). Plant evolution therefore not only generates global changes in environmental conditions but also feeds back on itself. This codependency creates a tightly coupled regulatory system for the long-term carbon cycle, with numerous feedback mechanisms checking runaway changes in CO2 and catastrophic planetary warming (8). Insight into these critical feedbacks is, however, extremely limited; over the last two decades, only two loops involving plants and CO2 have been postulated (9, 10).
A Systems Analysis of Plants and CO2
We characterize the network of geochemical effects of plants on atmospheric CO2 and the physiological effects of CO2 on plants using a systems analysis to reveal positive and negative geophysiological feedbacks involved with regulating the long-term carbon cycle (Fig. 1). Processes affecting CO2 on long (million year) time scales, such as evolution and weathering, are incorporated along with those occurring on much shorter time scales (see legend of Fig. 1). We include the role played by terrestrial ecosystems in regulating the land–atmosphere exchange of water vapor and recycling of precipitation, because both influence the hydrological cycle and weathering rates by altering the water–mineral contact time (11). Plain arrows indicate direct responses, and arrows with bull's-eyes indicate inverse responses. For example, an increase in CO2 leads to an increase in global mean surface temperature due to the atmospheric greenhouse effect (plain arrow i). Conversely, an increase in rock weathering leads to CO2 consumption and a decrease in atmospheric CO2 (bull's-eyed arrow g). Closed pathways linked together by an even number of arrows with bull's-eyes, or by no arrows with bull's-eyes, represent positive feedback loops (PFLs), and those with an odd number of arrows with bull's-eyes represent negative feedback loops (NFLs).
Fig. 1.
A systems analysis diagram of the geophysiological feedbacks between plants and CO2. It applies only when times of potential overheating of leaves due to high CO2 levels may have occurred. Arrows originate at causes and end at effects. Arrows with bull's-eyes represent inverse responses. Arrows without bull's-eyes represent direct responses. Letters adjacent to arrows designate paths followed by feedback loops. The time scales over which the paths operate are as follows: a, b, i, k, p, q, s, and u, 100 to 101 years; c and d, 101 to 103 years; f–h, j, m, n, r, and t, 103 to 106 years; e, >106 years.
Our systems analysis identifies five important previously unrecognized PFLs involving land plants and CO2. Those described by pathway a-b-c-d-e-f-g and its counterpart, i-k-c-d-e-f-g, involve the action of CO2 on plant evolution and the feedback of plants on chemical weathering rates (Fig. 1). Three other PFLs emerge from the effects of terrestrial ecosystem evolution on sedimentary organic carbon burial (a-b-c-d-m-n and i-k-c-d-m-n) and the intensity of the hydrological cycle (a-b-c-d-u-q-r-g) (Fig. 1). All five pathways lead to positive feedbacks, whether CO2 is rising or falling, but only if the paths at some stage involve very warm global climate states that could induce lethal overheating of leaves.
Positive feedback is initiated by a change in the global concentration of atmospheric CO2, which inversely influences the density of stomatal pores on the leaves of vascular land plants (5). Falling CO2 is accompanied by higher stomatal density, which, in turn, results in a lowering of leaf temperatures by increasing latent heat losses due to higher evapotranspiration rates (path a-b). Falling CO2 also lowers ambient temperatures, because of the atmospheric greenhouse effect, and humidity, because of the exponential effect of temperature on the saturation content of water in air (12). These environmental effects reduce the leaf-to-air water vapor deficit, allowing stomatal conductance to water vapor to increase (12) and further reductions in leaf temperature (path i-p-s).
Because of the capacity for more efficient cooling, new trees develop with larger leaves that intercept more solar radiation (path c) without the attendant risks of lethal overheating (5, 7). Larger leaves promote increases in maximum canopy size; they represent an optimal tradeoff between investment in woody supporting tissue and leaf area for photosynthetic carbon gain (13). Furthermore, higher stomatal densities reduce the diffusional limitation on photosynthesis allowing increased rates of carboxylation in the primary photosynthetic enzyme of C3 plants, ribulose-1,5-carboxylase/oxygenase (14). Increased photosynthetic capacity, coupled with greater interception of solar radiation by large leaves, facilitates the evolution of leafier, more productive plants (path d). Higher stomatal densities also permit taller plants by providing improved fine-scale control of transpiration to protect the increasing length of the xylem water pathway from cavitation (15) and distribute water and nutrients in the transpiration stream (14).
Larger plants require more nutrients and water than their smaller counterparts to support a higher biomass and transpirational stream (16). Meeting these increased demands requires more root (plus mycorrhizal) biomass and/or deeper rooting systems, as seen, for example, in contemporary vegetation where maximum rooting depth increases with progressively taller, leafier life-forms (trees > shrubs > herbs) (17). An expanding root system, in turn, increases weathering (3, 16, 18) (path f), described by the overall reaction
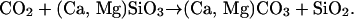 |
1 |
Eq. 1 summarizes the overall result of a wide variety of processes (3) including photosynthesis; secretion of soil organic acids and chelates by rootlets and associated symbionts; generation of CO2 by respiration of soil organic matter; reaction of organic and carbonic acids with Ca and Mg silicate minerals (here simplified in composition); the transport of dissolved Ca, Mg, and bicarbonate ions by rivers to the ocean; and the precipitation of Ca and Mg carbonates onto the seafloor. The net effect is the transfer of atmospheric CO2 to carbonate minerals that become buried in marine sediments, as succinctly represented by path g (Fig. 1), thereby completing the first proposed PFL (a-b-c-d-e-f-g). The second and complementary PFL to this one is given by i-k-c-d-e-f-g, where CO2 acts directly on leaf temperatures via the atmospheric greenhouse effect.
Two other newly recognized PFLs, a-b-c-d-m-n and i-k-c-d-m-n, stem from enhanced deposition of organic matter in sediments due to higher ecosystem productivity and biomass. These PFLs apply both to terrestrial swamplands and to the marine environment after transport of the organic matter to the sea by rivers and is especially true of woody plants because of the relative nonbiodegradability of lignin. Increased burial of organic carbon results in overall net loss of CO2 from the atmosphere (path n). Thus, both PFLs lead to a positive feedback on atmospheric CO2. The fifth PFL (pathway a-b-c-d-u-q-r-g) occurs as actively transpiring leafier ecosystems introduce more water into the atmosphere in their surroundings, and recycle it more efficiently, thereby increasing local rainfall, rock weathering rates, and the further removal of CO2 from the atmosphere (11).
Beside the four already established CO2-stabilizing pathways (i-j-g, h-e-f-g, h-m-n, and i-p-q-r-g) (Fig. 1), our analysis also identifies an NFL operating as CO2-related climate change alters the moisture content of the atmosphere and global rainfall patterns. With falling CO2, decreased moisture and rainfall leads to decreased CO2 removal via sedimentary organic carbon burial by strengthening water limitations on plant growth and decreasing the potential for wetland formation (i-p-q-t-n). The NFL i-j-g represents the well known greenhouse-weathering feedback that helps to stabilize atmospheric CO2 against changes in volcanic and metamorphic degassing or solar heating over geological time (1, 2). This feedback can operate even in the absence of life (1). NFLs h-e-f-g and h-m-n represent additional negative feedback due to the fertilization (or starvation) of plant growth by CO2 and the resulting acceleration (or deceleration) of plant-assisted weathering and organic burial in sediments, respectively (3, 9, 18, 19). The NFL i-p-q-r-g describes the negative feedback of CO2 on weathering via its effects on the hydrological cycle and weathering (11).
Paleozoic Carbon Cycle Feedbacks
Paleobotanical and sedimentary evidence indicates that PFLs a-b-c-d-e-f-g and a-b-c-d-m-n were most strongly expressed as CO2 levels began to fall during the extraordinary early evolution and diversification of land plants in the Paleozoic. Early land plants experiencing very high Early Devonian CO2 concentrations (Fig. 2) were small and typically leafless with naked simple or branched axial stems (4). As atmospheric CO2 levels subsequently declined (3) over the next 40 million years, stomatal densities rose, and maximum leaf width in several independent groups increased by a factor of up to 25 (6, 7, 20), with a diminished requirement for convective heat loss (Fig. 2). Larger leaves signaled the parallel evolution of trees, whose maximum height quickly increased, leading to the formation of stratified forests (4). By the end of the Devonian, when leaf size had reached 80% of its maximal enlargement (7), stem diameter increased logarithmically (18, 21) from 3 mm to 1.5 m, implying a rise in height from a just a few centimeters to ≈30 m (Fig. 2). Trees entrained the evolution of more complex rooting systems to provide anchorage and exploit larger volumes of soil for water and nutrients (16, 18). Along with root symbionts such as mycorrhizae, this increased nutrient removal and the surface area of the soil–root interface, both features enhancing rates of chemical weathering of silicates and increasing the rate of removal of CO2 from the atmosphere (3, 9, 16, 18). The expanding terrestrial biomass further promoted CO2 removal because of an enormous increase in organic burial of plant-derived organic matter (mostly on land but also in the sea), which tracked the rise in leaf and plant size (PFLs a-b-c-d-m-n and i-k-c-d-m-n) (Fig. 2) and was evidenced most importantly in the Mississippian and Permian by the formation of vast coal deposits (3).
Fig. 2.
Evolution of the atmosphere and land plants in the Paleozoic. (A) Modeled (○) or paleosol (•) atmospheric CO2 changes during the Late Paleozoic (3). (B) Observed (—) and modeled (––) increase in maximum width of megaphyll leaves (7). (C) Maximum plant height calculated from observed axis diameter of Devonian and Carboniferous vascular plants (18, 21, 32) (r2 = 0.66, P < 0.001). (D) Changes in terrestrial carbon burial, calculated as the difference between global and marine burial (3), the latter estimated from pyrite burial rates and a Cambrian–Silurian mean molar organic carbon/pyrite sulfur burial ratio of 1.5, assuming no changes in the proportion of burial in euxinic basins or anoxic oceans. Error bars in A indicate the range of uncertainty. The points in B represent the maximum size of megaphyll leaves calculated from fossils for 10-million-year intervals.
Given that proxy CO2 data indicate that CO2 levels stabilized at low values in the Late Paleozoic (Fig. 2 A), negative feedbacks must have operated to counterbalance these positive examples. The two most important involved reduced rates of rock weathering as CO2 levels decreased and the climate cooled (pathway i-j-g), and gradual CO2 limitation of terrestrial photosynthetic primary production eventually slowing both weathering (pathway h-e-f-g) and organic carbon burial (pathway h-m-n). The cooler climate also should have led to less rainfall and less CO2 uptake by weathering (pathway i-p-q-r-g).
End-Triassic Carbon Cycle Feedbacks
Our identified PFLs also likely enhanced rising CO2 levels during the Triassic–Jurassic boundary carbon cycle perturbation (22–25) (200 million years ago), associated with the eruption of the Central Atlantic Magmatic Province. End-Triassic to earliest Jurassic ultra-greenhouse conditions drove the global replacement of large-leaved forest taxa with those possessing narrow or highly dissected leaves (26, 27), as the former suffered probable thermal damage due to an inability to effectively shed excess latent heat to the atmosphere (24) (pathway a-b-c-d). The very hot, arid climate and physiologically challenging CO2 levels (several thousand ppm) (24, 25) conceivably then curbed primary production and limited organic carbon burial, leading to further increases in atmospheric CO2. Under these circumstances, stabilization of the rise in CO2 depends on the greenhouse-weathering negative feedback, as reflected in a marked negative osmium isotope shift in marine strata (23).
Conclusions
Our Earth-systems analysis reveals that plant responses to CO2 across a wide spectrum of time scales had far-reaching consequences for the evolution of climate and the biota. It challenges the widespread belief that destabilizing positive geophysiological feedbacks between plants, CO2, and climate on geological time scales are rare when compared with negative stabilizing feedbacks (3, 8–10). We suggest that the PFLs recognized here (Fig. 1) led to reinforcement (28), causing rates of evolution to accelerate on a grand scale. Falling CO2 concentrations are now linked to the unparalleled innovation of land plants in the Paleozoic (4, 6, 7) that, in turn, accelerated the diversification of terrestrial tetrapod and insect faunas (4, 29) and caused a marked rise in atmospheric oxygen (3), fuelling the spectacular ecological radiation of gigantism in insects (29). These consequences contrast sharply with positive feedbacks between plants and CO2 in the short-term carbon cycle that are expected to accelerate anthropogenic climate change over the 21st century (30) and that may lead to the deletion of evolutionary processes in the tropics (31).
Acknowledgments
We thank P. Falkowski, J. Ehleringer, I. Handoh, L. Hickey, C. P. Osborne, and F. I. Woodward for helpful comments and discussion on the manuscript. D.J.B. gratefully acknowledges funding of this work through the Leverhulme Trust (F/00118/G), and R.A.B. acknowledges U.S. Department of Energy Grant DE-FB02-01ER15173.
Abbreviations: NFL, negative feedback loop; PFL, positive feedback loop.
References
- 1.Walker, J. C. G., Hays, P. B. & Kasting, J. F. (1981) J. Geophys. Res. 86, 9776-9782. [Google Scholar]
- 2.Berner, R. A., Lasaga, A. C. & Garrels R. M. (1983) Am. J. Sci. 283, 641-683. [Google Scholar]
- 3.Berner, R. A. (2004) The Phanerozoic Carbon Cycle: CO2 and O2 (Oxford Univ. Press, Oxford).
- 4.Kenrick, P. & Crane, P. R. (1997) Nature 389, 33-39. [Google Scholar]
- 5.Woodward, F. I. (1987) Nature 327, 617-618. [Google Scholar]
- 6.Beerling, D. J., Osborne, C. P. & Chaloner, W. G. (2001) Nature 401, 352-354. [DOI] [PubMed] [Google Scholar]
- 7.Osborne, C. P., Beerling, D. J., Lomax, B. H. & Chaloner, W. G. (2004) Proc. Natl. Acad. Sci. USA 101, 10360-10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovelock, J. E. & Whitfield, M. (1982) Nature 296, 561-563. [Google Scholar]
- 9.Volk, T. (1987) Am. J. Sci. 287, 763-779. [Google Scholar]
- 10.Volk, T. (1989) Geology 17, 107-110. [Google Scholar]
- 11.Berner, E. K., Berner, R. A. & Moulton, K. L. (2003) Treatise Geochem. 5, 169-188. [Google Scholar]
- 12.Jones, H. G. (1992) Plants and Microclimate (Cambridge Univ. Press, Cambridge, U.K.).
- 13.Westoby, M. & Wright, I. J. (2003) Oecologia 135, 621-628. [DOI] [PubMed] [Google Scholar]
- 14.Woodward, F. I. (1998) J. Exp. Bot. 49, 471-480. [Google Scholar]
- 15.Tyree, M. T. & Zimmerman, M. H. (2002) Xylem Structure and the Ascent of Sap (Springer, Berlin).
- 16.Raven, J. A. & Edwards, D. (2001) J. Exp. Bot. 52, 381-401. [DOI] [PubMed] [Google Scholar]
- 17.Canadell, J., Jackson, R. B., Ehleringer, J. R., Mooney, H. A., Sala, O. E. & Schulze, E. D. (1996) Oecologia 108, 583-595. [DOI] [PubMed] [Google Scholar]
- 18.Algeo, T. J. & Scheckler, S. E. (1998) Philos. Trans. R. Soc. London B 353, 113-130. [Google Scholar]
- 19.Andrews, J. A. & Schlesinger, W. H. (2001) Global Biogeochem. Cycles 15, 149-162. [Google Scholar]
- 20.Boyce, C. K. & Knoll, A. H. (2002) Paleobiology 28, 70-100. [Google Scholar]
- 21.Chaloner, W. G. & Sheerin, A. (1979) in The Devonian System, eds. House, M. R., Scrutton, C. & Bassett, T. M. G. (Paleontol. Soc. Special Paper in Paleontology no. 23), pp. 145-161.
- 22.Marzoli, A., Bertrand, H., Knight, K. B., Cirilli, S., Buratti, N., Vérati, C., Nomade, S., Renne, P., Youbi, P. R., Martini, R., et al. (2004) Geology 32, 973-976. [Google Scholar]
- 23.Cohen, A. S. & Coe, A. L. (2004) Geology 30, 267-270. [Google Scholar]
- 24.McElwain, J. C., Beerling, D. J. & Woodward, F. I. (1999) Science 285, 1386-1390. [DOI] [PubMed] [Google Scholar]
- 25.Beerling, D. J. & Berner, R. A. (2002) Global Biogeochem. Cycles 16, 10.1029/2001/GB001637.
- 26.Harris, T. M. (1937) Medd. Groenl. 112, 1-114. [Google Scholar]
- 27.Wing, S. L. & Sues, H. D. (1992) in Terrestrial Ecosystems Through Time, eds. Behrensmeyer, A. K., Damuth, J. A., DiMichele, W. A., Potts, R., Sues, H. D. & Wing, S. L. (Univ. of Chicago Press, Chicago), pp. 327-416.
- 28.Odling-Smee, F. J., Laland, K. N. & Feldman, M. W. (2003) Niche Construction: The Neglected Process in Evolution (Princeton Univ. Press, Princeton).
- 29.Graham, J. B., Dudley, R., Aguilar, N. M. & Gans, C. (1995) Nature 375, 117-120. [Google Scholar]
- 30.Cox, P. M., Betts, R. A., Jones, C. D., Spall, S. A. & Totterdell, I. J. (2000) Nature 408, 184-187. [DOI] [PubMed] [Google Scholar]
- 31.Myers, N. & Knoll, A. H. (2001) Proc. Natl. Acad. Sci. USA 98, 5389-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niklas, K. J. (1993) Ann. Bot. 72, 165-172. [Google Scholar]




