Abstract
The past quarter century has seen an unprecedented increase in the number of new and emerging infectious diseases throughout the world, with serious implications for human and wildlife populations. We examined host persistence in the face of introduced vector-borne diseases in Hawaii, where introduced avian malaria and introduced vectors have had a negative impact on most populations of Hawaiian forest birds for nearly a century. We studied birds, parasites, and vectors in nine study areas from 0 to 1,800 m on Mauna Loa Volcano, Hawaii from January to October, 2002. Contrary to predictions of prior work, we found that Hawaii amakihi (Hemignathus virens), a native species susceptible to malaria, comprised from 24.5% to 51.9% of the avian community at three low-elevation forests (55–270 m). Amakihi were more abundant at low elevations than at disease-free high elevations, and were resident and breeding there. Infection rates were 24–40% by microscopy and 55–83% by serology, with most infected individuals experiencing low-intensity, chronic infections. Mosquito trapping and diagnostics provided strong evidence for year-round local transmission. Moreover, we present evidence that Hawaii amakihi have increased in low elevation habitats on southeastern Hawaii Island over the past decade. The recent emergent phenomenon of recovering amakihi populations at low elevations, despite extremely high prevalence of avian malaria, suggests that ecological or evolutionary processes acting on hosts or parasites have allowed this species to recolonize low-elevation habitats. A better understanding of the mechanisms allowing coexistence of hosts and parasites may ultimately lead to tools for mitigating disease impacts on wildlife and human populations.
Keywords: Hemignathus virens, host–parasite coevolution, Plasmodium relictum, Culex quinquefasciatus
The past quarter century has seen an unprecedented increase in the number of new and emerging infectious diseases throughout the world, with serious implications for human and wildlife populations (1). This rise in the emergence of new infectious diseases is attributed to many factors, among them human alteration of habitats, transportation of vectors and pathogens, and climate and weather patterns, including anthropogenic climate change (2, 3). Vector-borne diseases in particular may undergo geographic range shifts and large changes in abundance with climate change because rising temperatures will affect vector distribution, parasite development, and transmission rates (4).
Identifying the factors that allow for coexistence of hosts and parasites has been a topic of intensive study in the ecological literature for decades (5, 6). Modeling and empirical studies have identified host and vector abundance, vector competence and behavior, host community, spatial and metapopulation dynamics, host demography, seasonality, parasite virulence, and host resistance, among others, as being of importance (7, 8). A better understanding of the mechanisms of host–parasite coexistence may ultimately lead to tools for manipulating species, habitats, and landscapes to mitigate the impacts of infectious disease on wildlife and human populations (9).
We examined host persistence in the face of introduced vector-borne disease in the context of introduced avian malaria, introduced vectors, and native forest bird hosts in Hawaii. The introduction of mosquito-borne avian malaria (Plasmodium relictum) and its primary vector, the southern house mosquito (Culex quinquefasciatus), to Hawaii decimated populations of native Hawaiian honeycreepers throughout the lowlands (<1,300 m) of Hawaii (10, 11). The decline of native birds provides a classic example of the profound effect of invasive diseases on naïve natural populations (12). Anthropogenic climate change and its interactions with the disease system are predicted to threaten the persistence of several remaining endemic species in Hawaii (13).
The avian malaria–forest bird host system in Hawaii provides an ideal system for investigating the interaction of vector-borne disease, climate, and biotic factors on host populations (see also ref. 14). Because of its isolation, Hawaii has a simplified (and therefore potentially tractable) host, vector, and pathogen system, interacting over a gradient of climate and habitat conditions from coastal scrub and wet montane forest to alpine desert at 4,170 m (15). Because avian malaria arrived in Hawaii early in the last century, the host–parasite relationship is <100 years old (11).
Endemic Hawaiian forest birds are extremely susceptible to infection with P. relictum, suffering mortality rates of 65–90% after being bitten by a single infective mosquito (16–18). The physiological effects of avian malaria include severe anemia, destruction of mature erythrocytes, declines in food consumption and activity levels, and loss of up to 30% of body weight (17, 19). Individuals that survive acute infection develop concomitant immunity to homologous strains of the parasite, but remain infective to mosquitoes, probably for life (20).
Warner (10) and van Riper et al. (11) provided compelling evidence that the near absence of native forest birds below elevations of 900 m in Hawaii was due to the presence of the Culex mosquito vector and associated disease transmission, demonstrating that disease could limit the distribution, range, and abundance of natural populations. van Riper et al. (11) developed a simple but powerful model to predict the intensity of malaria transmission in Hawaiian forests by overlaying mosquito and host distributions over a 0- to 1,800-m altitudinal gradient. Malaria transmission was greatest in mid-elevation (700–1,300 m) forests where highly susceptible native forest birds overlapped in distribution with mosquitoes. Native birds were found to be almost entirely absent from low-elevation study sites because of high mosquito densities, and malaria prevalence in the predominantly alien, disease resistant lowland avifauna was low. This model has guided all subsequent research and management, and the observation that native birds are absent from low elevations is widely cited (e.g., refs. 4 and 13). Unfortunately, as a result, low elevation forests have been ignored as suitable native bird habitat and the ecological processes occurring between hosts, vectors, and parasites in these areas have not been studied.
Here, we document the recent emergent phenomenon of large, resident populations of a native Hawaiian forest bird, the Hawaii amakihi (Hemignathus virens), in low-elevation habitats on Hawaii, despite extremely high prevalence of avian malaria and evidence of local transmission, and provide evidence that amakihi populations have increased at low elevations over the past decade. These observations are particularly important in light of predictions of global climate change and its predicted impact on remaining Hawaiian forest bird populations (13). That amakihi are recovering in the presence of avian malaria suggests hope for other coevolving host–parasite systems.
Methods
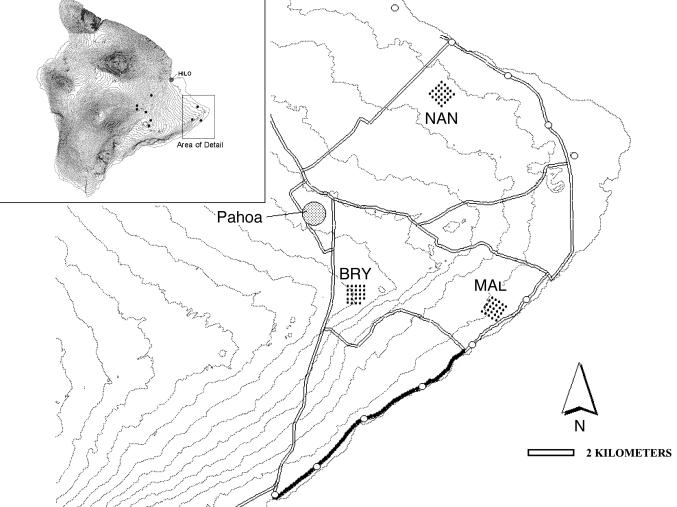
Study Area. This study was conducted as part of a larger collaborative research effort on the Biocomplexity of Introduced Avian Diseases in Hawaii. The study area comprises ≈1,100 km2 on the eastern (windward) flanks of Mauna Loa and Kilauea volcanoes on the southeast corner of the island of Hawaii (Fig. 1). We established nine 1-km2 study plots, each containing five 1-km-long transects spaced 200 m apart. Study plots were distributed along the elevational gradient from 25 to 1,800 m, and were stratified into three elevational classes: two high elevation [>1,650 m above sea level (ASL)], four mid-elevation (1,000–1,300 m ASL), and three low elevation (<300 m ASL). Mean monthly temperatures ranged from 23°C at low elevation sites to ≈13°C at high-elevation sites (15). All study plots were in wet forest (2,000–5,000 mm annual rainfall) dominated by ohia (Metrosideros polymorpha), the primary canopy tree in Hawaiian forests. Mid-canopy tree species found at most sites included Myrsine lessertiana, Ilex anomala, and Cheirodendron trigynum. We focus on data from three low-elevation study sites in this paper. Bryson's Cinder Cone (BRY, 270 m ASL) is a tall-statured, closed-canopy forest of ohia and tree ferns (Cibotium sp.) on an ≈550-year-old a'a lava and ash substrate (21). In contrast, Malama Ki Forest Reserve (MAL, 25 m ASL) and Nanawale Forest Reserve (NAN, 85 m ASL) are short-statured, open-canopy ohia forests on relatively young (160–210 years old), smooth (pahoehoe), and rough (a'a) lava, respectively.
Fig. 1.
Map of eastern Hawaii Island showing locations of three low-elevation study grids [Nanawale (NAN), Bryson's Cinder Cone (BRY), and Malama Ki (MAL)] and bird survey routes in low elevation Puna district. Stations sampled by Reynolds et al. (24) in 1994 are indicated by open circles. The route sampled by David (25) in 1995 is shown as a solid line. Dotted lines are 50-m elevational contours. (Inset) Entire Hawaii Island with locations of all nine Biocomplexity study grids.
Bird Sampling. Mist-netting was conducted from January through October, 2002, within a 50-hectare subplot within each of nine study areas, using 18–24 mistnets (12 m × 2.6 m, 32-mm mesh) placed at a height of 6 m on galvanized metal poles. Nets were operated for ≈6 h each day between 0630 and 1400 hours Hawaii standard time, for 2–4 days/month (mean = 296, SD = 117 mistnet h/month), for a total of 23,974 mistnet h and 3,329 captures of 2,350 individual birds. All captured birds were banded with U.S. Fish and Wildlife Service numbered aluminum leg bands and processed according to standard protocol (22); native birds were also banded with three colored leg bands for individual identification. From each bird, we drew 100 μl of whole blood by jugular venipuncture into a heparinized syringe with a 28-gauge needle for preparation of blood smears and collection of plasma and packed cells for serological and DNA analysis (see below). Birds were aged and sexed by using a combination of plumage, morphometric, skull pneumatization, and breeding characters. The proportion of birds in breeding condition was determined by the swelling of the cloacal protuberance in males, and development of a brood patch in females (22). Productivity was measured as the ratio of hatch-year birds (HY; birds fledged in calendar year 2002) to after-hatch-year birds (birds fledged in 2001 or earlier) captured in May (the first month in which HY birds were observed) through August 2002 (when HY birds began their first prebasic molt and could no longer be reliably distinguished from adults). Only the first capture of each individual bird in a given year was used in analyses unless otherwise noted.
We conducted variable-circular plot (VCP) counts (23) on each study area in March, May, and September 2002, at 25 stations located 200 m apart throughout each 1-km2 grid, for a total of 675 counts at 225 stations. Four observers were trained and calibrated in distance estimation before conducting point counts. Observers recorded the horizontal distance to all birds seen or heard during 8-min counts conducted between sunrise and 1130 hours during favorable weather.
To examine distribution and abundance of Hawaii amakihi in low elevation habitats outside of our study areas, we conducted roadside surveys along two transects in coastal Puna district (0–55 m ASL) that were first surveyed in the early to mid 1990s. We matched surveys by month to control for variation in amakihi singing behavior over the course of the breeding season. In February 2004, we sampled 11 stations spaced 3.2 km apart along a 35-km section of the coastal road (hereafter, the “Reynolds transect,” Fig. 1) by using VCP methodology as described above. These 11 stations were originally surveyed in January and February 1994 by using 8-min, 30-m fixed and unlimited-radius point count methodology by Reynolds et al. (24). Between April 8 and 17, 2004, we surveyed 79 stations spaced 150 m apart along an 11.5-km subsection of the Reynolds transect (hereafter, the “David transect,” Fig. 1); these stations were originally sampled in April 1995 by using standard 8-min VCP counts by David (25). Although VCP methodology allows estimation of densities from count data, we converted all data to frequency (percentage of stations with at least one amakihi detection) and birds per station (mean amakihi detected per 8-min, unlimited-radius point count) to allow comparisons with Reynolds et al. (24).
Diagnostics. Upon capture, a 100-μl blood sample was taken by jugular venipuncture with a heparinized 28-gauge insulin syringe for malarial diagnostics. A thin blood smear was made immediately, air dried, and fixed with methanol. The remaining blood was spun with a portable centrifuge to separate plasma from red blood cells. Plasma from each bird was tested for antibodies to P. relictum by using an ELISA (26), and red cells were frozen for genetic studies. Absorbance values were expressed as a percent ELISA value (%EV) of positive and negative Pekin duckling plasma controls that were run on each plate. The %EV was calculated as (mean absorbance of triplicate samples - the mean absorbance of triplicate negative controls)/(mean absorbance of triplicate positive controls - mean absorbance of triplicate negative controls) × 100. We used a cutoff %EV of 25 to classify birds as antibody positive or negative. Birds testing within a range of five points above or below a %EV of 25 were retested by immunoblotting (16, 20) to verify ELISA results.
Blood smears were stained with 6% phosphate-buffered Giemsa, pH 7.0, for 1 h, rinsed with tap water, air dried, and examined by microscopy to diagnose acute infections (<30 days old), because at this stage of the infection antibody titers are too low to be detected by ELISA or immunoblotting. One hundred ×500 fields were examined on each slide, which was equivalent to a search effort of ≈30,000 erythrocytes per sample. Birds were scored as infected if they had detectable parasitemias by microscopy or antibodies to P. relictum. Intensity of infection in birds with detectable parasitemias was estimated by counting total numbers of erythrocytes in a subset of 10 microscope fields by digital image analysis (27) and then extrapolating to 100 fields to obtain an estimate of total erythrocytes in 100 fields. Total number of infected red blood cells in the 100 fields was then expressed as parasitemia per 1,000 erythrocytes. We were unable to detect infections that were earlier than 4–8 days, when parasites were undergoing initial rounds of multiplication in fixed tissues of the bird and when numbers of parasites in the peripheral circulation was extremely low. In addition, because acute malaria infection decreases activity levels in Hawaiian honeycreepers (17, 19), we expect mistnet sampling to underestimate the prevalence of acute infection in a population.
Vector Sampling and Diagnostics. We operated modified miniature Centers for Disease Control (CDC) light traps baited with 300 g of dry ice (CO2) to sample host-seeking mosquito populations. We also operated alfalfa infusion-baited oviposition (“gravid”) traps to sample the population of gravid females. CDC traps were hung in the forest canopy at 4–12 m in height, located at least 100 m apart, at 25 systematic-random stations throughout each study site. Gravid traps were placed on the forest floor in paired location with the CDC traps. Sampling was conducted at 4- to 6-week intervals for 4 or 9 days, respectively. Traps were baited 1 h before sunset, and mosquitoes were collected the following morning. Mosquitoes were transported alive from the field and maintained on sucrose solution at constant room temperature (21°C) until dissection.
Malarial infection was determined by microscopic examination (×400) of midguts and salivary glands. Infected mosquitoes included those with early stage oocysts and/or later stage sporozoites. We present data for C. quinquefasciatus only, because laboratory susceptibility trials have shown this species to be extremely effective vectors of avian malaria, whereas other common mosquitoes in the Hawaiian lowlands (Aedes albopictus and Wyeomyia mitchelli) are largely refractory to infection (28).
Results
Native Hawaii amakihi comprised between 24.5% and 51.9% of the avian community at the three low-elevation study sites (BRY: 228 individual amakihi of 439 total birds captured, n = 2,105 mistnet h; NAN: 145 amakihi in 513 captures, n = 1,747 mistnet h; MAL: 66 amakihi in 269 captures, n = 2,188 mistnet h). The overall capture rate of individual amakihi at low-elevation study areas was 7.3 per 100 mistnet h (n = 6,040 mistnet h). Native Elepaio (Chasiempis sandwichensis) and Apapane (Himatione sanguinea) were also detected, but in much lower abundances: only one Elepaio (at BRY) and three Apapane (one at BRY and two at MAL) were captured. The balance of the avian community was composed of nonnative species, principally Japanese White-eye (Zosterops japonicus; 36.9–54.6%), Northern Cardinal (Cardinalis cardinalis; 4.1–9.3%), House Finch (Carpodacus mexicanus; 0–9.9%), Zebra Dove (Geopelia striata; 0–7.8%), and a few (<3% each) Nutmeg Mannikin (Lonchura punctulata), Spotted Dove (Streptopelia chinensis), and Melodious Laughing-Thrush (Garrulax canorus).
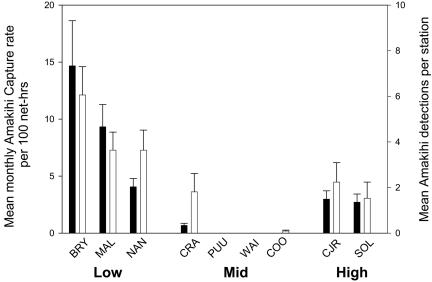
Amakihi were more abundant at low-elevation study sites than at high elevation, and relatively rare at middle elevations, based on both monthly mistnetting and quarterly VCP survey results (Fig. 2). Nearly 100% of low-elevation males and females were in breeding condition at the peak of the breeding season, and on average, 41 ± 3.9% SE (n = 157) of the population during the May–August breeding season was made up of HY birds (BRY, 36 ± 6% SE; MAL, 44 ± 6%; NAN, 44 ± 8%).
Fig. 2.
Relative abundance of Hawaii amakihi on nine study areas along an altitudinal gradient on Mauna Loa Volcano, Hawaii, February to October 2002, as measured by mist-net sampling (filled bars) and VCP censuses (open bars). Mistnet capture rates include all captures except same-day recaptures.
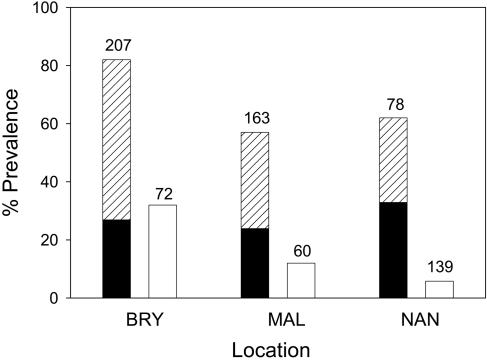
Infection rates of avian malaria in amakihi in low-elevation forests were 24–40% by microscopy and 55–83% by serology (Fig. 3). High seroprevalence and associated low-intensity parasitemias (mean = 0.95 infected erythrocyte per 1,000 erythrocytes, range = 0.00029–37.293, n = 112) indicate that most infected individuals had chronic infections and were survivors of prior acute infections.
Fig. 3.
Proportion of Hawaii amakihi (left bars) and southern house mosquitoes (right bars) that were diagnosed to be infected with P. relictum at three low-elevation study areas on Mauna Loa volcano, February to October 2002. Infections in amakihi were diagnosed by serological methods (hatched bars) and blood smears (filled bars). Infections in mosquitoes were diagnosed by microscopic examination of midguts and salivary glands, and represent those with early stage oocysts and/or later stage sporozoites. Numbers above bars represent sample size.
Southern house mosquitoes were present at all low-elevation study sites throughout the sampling period (mean = 0.34 mosquitoes per trap night, SE = 0.02, n = 2,633 trap nights). Relative abundance was greatest at Nanawale (NAN: mean = 0.622 mosquitoes per trap night, SE = 0.051, n = 863; BRY: mean = 0.25, SE = 0.027, n = 916; MAL: mean = 0.143, SE = 0.017, n = 854). An average of 15% of southern house mosquitoes (n = 379) were positive for malaria, harboring early stage oocysts and/or later stage sporozoites (Fig. 3).
VCP surveys along the 35-km Reynolds transect in February 2004 detected 13 amakihi at 4 of 11 stations (36.4%, mean amakihi per station = 1.18, SD = 1.9) compared with 0 amakihi at the same 11 stations in February 1994 (ref. 24; Fisher's exact test, P = 0.0902). Along the David transect in April 2004, we detected a total of 62 amakihi at 33 of 79 stations (41.8%, mean amakihi per station = 0.78, SD = 1.09), compared with 0 amakihi at the same 79 stations in April 1995 (ref. 25; χ2 = 39.2, df = 1, P < 0.0001).
Discussion
Although scattered observations of native Hawaiian forest birds at low elevations have been recorded previously, the occurrence and prevalence of infection in these populations was unknown. van Riper et al. (11) and Scott et al. (29) noted the presence of amakihi in the leeward dry forest in Puna district, Hawaii Island. In windward wet forests in Puna, researchers noted amakihi of unknown infection status at 400 m in 1984 (ref. 30 and P. Banko, personal communication) and at 120 m in 1993 (24). On Oahu, vanderWerf (31, 32) reported low-elevation elepaio and amakihi of unknown infection status, and Sheehata et al. (33) reported low-elevation Hawaii amakihi that were negative for P. relictum by PCR, suggesting that birds had either not been exposed to malaria or were refractory to infection.
The present study documents high abundance of resident, breeding Hawaii amakihi in low-elevation forests, with high prevalence of avian malaria and evidence for local transmission. Furthermore, significant increases in amakihi distribution and abundance on low elevation southeastern Hawaii Island have taken place over the past decade. These findings present a paradigm for understanding the spatial and temporal dynamics of avian malaria in Hawaii, and suggest that ecological or evolutionary processes acting on hosts or parasites in these populations have led to coexistence of avian malaria and Hawaii amakihi.
Mechanisms of Coexistence of Hawaii Amakihi and Avian Malaria. Prevalence of avian malaria in low elevation Hawaii amakihi populations during this study was the highest documented in Hawaii (C.T.A., unpublished data), and was extremely high relative to the Nearctic and Neotropics, where the overall prevalence of Plasmodium ranges from 1.9% to 3.8% by microscopy (34, 35). What factors lead to these high prevalence rates, and how are amakihi able to persist in the face of high transmission rates of a highly pathogenic emergent disease?
Earlier accounts hypothesized that Amakihi could persist in low-elevation dry forests where mosquito oviposition sites were limiting, and mosquito populations (and therefore malaria transmission) were at a minimum (11, 29). Although mosquito capture rates in our low-elevation forested sites were low relative to those in urban or continental situations (36, 37), they are typical of forested habitat on Hawaii (28). Moreover, infection rates of mosquitoes were quite high, ≈15%; in comparison, infection rates of mosquitoes in areas of endemic human malaria are typically only ≈1% (38). The presence of mosquito populations year-round in low-elevation study sites, coupled with high rates of malarial infection in these vectors, provides strong evidence for year-round local transmission and contributes to the unusually high prevalence of malaria in lowland amakihi.
In addition to vector ecology, host community structure and composition can have profound effects on disease dynamics in ecological systems (39, 40). Japanese White-eye, the introduced species that is most abundant in low-elevation forests, are for the most part refractory to infection (C.T.A., unpublished data), and these exotics may act as “sinks” for infected mosquito bites, reducing efficiency of transmission; thus, van Riper's observations of an alien-dominated lowland bird community where malaria was rare (11). In contrast, native birds such as amakihi and apapane (which were absent from van Riper's study areas) are important reservoirs for avian malaria because those individuals that survive acute infection remain infective to mosquitoes for life (20). Amakihi made up a large proportion of the community (25–52%) at our low elevation study sites, and we speculate that their abundance, coupled with year-round presence of mosquitoes, allows malaria prevalence to reach extremely high levels. Consistent with this, prevalence of malaria in both mosquitoes and amakihi was highest at Bryson's Cinder Cone, the low-elevation site where native (reservoir) birds made up the largest proportion of the community and not, as one might have expected, where relative abundance of vectors was highest.
Unquestionably, the most exciting explanation for amakihi recovery is that lowland amakihi have evolved genetic resistant to Plasmodium relictum, allowing them to repopulate the resource-rich lowlands. In Hawaii, environmental conditions vary across the elevational gradient, creating a variety of selection regimes on hosts, parasites, and vectors. Because malaria is endemic in the lowlands, with year-round vector populations and high rates of disease transmission, selection on hosts, vectors, and pathogens is expected to be particularly intense there, with resulting changes in parasite life-history traits and virulence and/or genetically based, heritable host resistance. In contrast, at middle elevations, malaria is more episodic/epidemic in nature and therefore less likely to drive rapid selection (28, 41). Definitive tests of these hypotheses await experimental infections of serologically negative birds, in progress. [van Riper et al. (11) conducted experimental infections of amakihi from high vs. mid elevations, but serological methods to determine the exposure history and acquired immunity of the birds were not available at the time, making the results difficult to interpret.] If direct evidence ultimately supports this interpretation, then the Hawaii amakihi and Plasmodium relictum story will take its place in the literature alongside only a very few other examples of evolution and coevolution of vertebrate hosts and pathogens occurring within a few hundred generations (see ref. 42).
It remains to be determined whether the populations of amakihi at low elevations are self-sustaining (have stable or positive population growth rates), or whether they are maintained by immigration from other populations in the landscape, presumably from drier or higher habitats with lower incidences of disease. If environmental conditions in low-elevation forests are favorable, amakihi could potentially produce enough young to compensate for disease-related mortality, or good nutrition might mitigate disease effects on individual mortality (43, 44). These ideas are particularly interesting because they suggest that management that affects host demographic rates (e.g., habitat restoration affecting nesting habitat quality or food resources) could “tip the scales” toward host persistence. Alternatively, low-elevation forests may be “ecological disease traps” (after the nest predation “ecological trap” of Gates and Gysel, ref. 45), habitat that is attractive to hosts (and thus harbors high abundance of hosts) but where population growth rate is negative because of disease-related mortality. We recognize that a variety of other factors are likely to be at work in this system, and that they are not likely to be mutually exclusive.
Implications for Conservation of Hawaiian Forest Birds. The present study opens up a myriad of new questions about how this emergent disease has shaped the distribution, abundance, behavior, and evolution of native Hawaiian forest birds. Understanding the biocomplexity of genetic, immunological, epidemiological, demographic, ecological, and landscape factors in the persistence of low-elevation amakihi populations may hold the key to preservation of the remaining endemic Hawaiian avifauna.
Warner (10) and van Riper et al.'s (11) groundbreaking work on avian malaria in Hawaii has guided considerable subsequent research and vitally important conservation actions. However, it has also inadvertently fostered a widespread belief that Hawaiian forest birds are doomed to extinction by avian disease wherever Culex mosquitoes are present. Conservation strategies for native forest birds have, understandably, focused primarily on high-elevation habitats near the current core of endangered species' ranges, where Culex mosquitoes and disease transmission are rare, and have excluded conservation of low elevation forests. Isolated and relictual populations of native species below 900-m elevation were assumed to be doomed to extinction, and were therefore a low priority for conservation. As a result, potentially important populations of several native species have been lost or reduced through habitat loss and degradation (24, 30), and any potential for evolutionary change in those populations has disappeared along with them. This research supports the idea that the lowlands are not a wasteland for Hawaiian birds, but rather are a theater where a complicated and dynamic interplay of coevolutionary forces is occurring.
Conservation biology practitioners have debated the value of conserving isolated or peripheral populations (46), and some argue that conservation efforts aimed at such populations are relatively ineffective (47). Recent reviews have challenged the assumption that peripheral populations are less viable than core populations, and demonstrated the importance of peripheral populations in population persistence across a variety of taxa (48). The present study highlights the importance of ecological and evolutionary processes taking place in the periphery of species' ranges, and is the best example to date of the true conservation significance of metapopulations and remnant populations in the face of environmental change.
Acknowledgments
We thank Kamehameha Schools, Hawaii Volcanoes National Park, and Hawaii Division of Forestry and Wildlife for permission to work on their properties. We thank our colleagues on the Biocomplexity of Introduced Avian Diseases in Hawaii research group (J. Ahumada, A. Dobson, L. Eggert, S. Jarvi, R. Fleischer, D. Fonseca, N. Keygobadi, and M. Samuel) for discussion, critical insight, and collaboration; W. Steiner and D. Helweg for support; P. Banko and R. David for access to unpublished data and field notes; and P. Banko, M. Reynolds, M. Samuel, J. M. Scott, and C. van Riper III for their insight and critical comments on earlier versions of the manuscript. Many volunteer field assistants provided critical assistance with data collection. This work was supported by National Science Foundation Grant DEB 0083944 and by the U.S. Geological Survey Invasive Species and Wildlife and Terrestrial Resources programs.
Abbreviations: ASL, above sea level; BRY, Bryson's Cinder Cone; MAL, Malama Ki Forest Reserve; NAN, Nanawale Forest Reserve; HY, hatch year; VCP, variable-circular plot.
References
- 1.Daszak, P., Cunningham, A. A. & Hyatt, A. D. (2000) Science 287, 443-449. [DOI] [PubMed] [Google Scholar]
- 2.Schrag, S. J. & Wiener, P. (1995) Trends Ecol. Evol. 10, 319-324. [DOI] [PubMed] [Google Scholar]
- 3.Dobson, A. & Foufopoulos, J. (2001) Philos. Trans. Royal Soc. London B 356, 1001-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harvell, C. D., Mitchell, C. E., Ward, J. R., Altizer, S., Dobson, A. P., Ostfeld, R. S. & Samuel, M. D. (2002) Science 296, 2158-2162. [DOI] [PubMed] [Google Scholar]
- 5.Kermack, W. O. & McKendrick, A. G. (1927) Proc. R. Soc. London Ser. A 115, 700-721. [Google Scholar]
- 6.Anderson, R. M. & May, R. M. (1991) Infectious Diseases of Humans: Dynamics and Control (Oxford Univ. Press, Oxford).
- 7.Grenfell, B. T. & Dobson, A. P., eds. (1995) Ecology of Infectious Diseases in Natural Populations (Cambridge Univ. Press, Cambridge, U.K.).
- 8.Poulin, R., Morand, S. & Skorping, A., eds. (2000) Evolutionary Biology of Host-Parasite Relationships: Theory Meets Reality (Elsevier, Amsterdam).
- 9.Cleaveland, S., Hess, G. R., Dobson, A. P., Laurenson, M. K., McCallum, H. I., Roberts, M. G. & Woodroffe, R. (2002) in The Ecology of Wildlife Diseases, eds. Hudson, P. J., Rizzoli, A., Grenfell, B. T., Heesterbeek, H. & Dobson, A. P. (Oxford Univ. Press, New York), pp. 139-150.
- 10.Warner, R. E. (1968) Condor 70, 101-120. [Google Scholar]
- 11.van Riper, C., III, van Riper, S. G., Goff, M. L. & Laird, M. (1986) Ecol. Monogr. 56, 327-344. [Google Scholar]
- 12.Gulland, F. M. D. (1995) in Ecology of Infectious Diseases in Natural Populations, eds. Grenfell, B. T. & Dobson, A. P. (Cambridge Univ. Press, Cambridge, U.K.), pp. 20-51.
- 13.Benning, T. L., LaPointe, D., Atkinson, C. T. & Vitousek, P. M. (2002) Proc. Natl. Acad. Sci. USA 99, 14246-14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitousek, P. M. (1993) Pacific Sci. 49, 2-16. [Google Scholar]
- 15.Juvik, S. P. & Juvik, J. O. (1998) Atlas of Hawaii (Univ. of Hawaii Press, Honolulu), 3rd Ed.
- 16.Atkinson, C. T., Woods, K. L., Dusek, R. J., Sileo, L. S. & Iko, W. M. (1995) Parasitology 111, S59-S69. [DOI] [PubMed] [Google Scholar]
- 17.Atkinson, C. T., Dusek, R. J., Woods, K. L. & Iko, W. M. (2000) J. Wildl. Dis. 36, 197-204. [DOI] [PubMed] [Google Scholar]
- 18.Atkinson, C. T., Lease, J. K., Drake, B. M. & Shema, N. P. (2001) Condor 103, 209-218. [Google Scholar]
- 19.Yorinks, N. & Atkinson, C. T. (2000) Auk 117, 731-738. [Google Scholar]
- 20.Atkinson, C. T., Dusek, R. J. & Lease, J. K. (2001) J. Wildl. Dis. 37, 20-27. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe, E. W. & Morris, J. (1996) Geologic Map of the Island of Hawaii (U.S. Geological Survey, U.S. Dept. of Interior, Honolulu).
- 22.Pyle, P. (1997) Identification Guide to North American Birds (Slate Creek, Bolinas, CA), Part 1.
- 23.Reynolds, R. T., Scott, J. M. & Nussbaum, R. A. (1980) Condor 82, 309-313. [Google Scholar]
- 24.Reynolds, M. H., Camp, R. J., Nielson, B. M. B. & Jacobi, J. D. (2003) Bird Cons. Int. 13, 175-187. [Google Scholar]
- 25.David, R. E. (1995) Faunal Survey of Helco SSPP Unit 71-Rauenhorst, Kehena-Keekee Homestead, Puna, Hawaii (R. M. Towill Corporation and Hawaii Electric Light Co., Honolulu).
- 26.Graczyk, T. K., Cranfield, M. R. & Shiff, C. J. (1993) J. Parasitol. 79, 879-885. [PubMed] [Google Scholar]
- 27.Gering, E. & Atkinson, C. T. (2004) J. Parasitol. 90, 879-881. [DOI] [PubMed] [Google Scholar]
- 28.LaPointe, D. A. (2000) Ph.D. dissertation (Univ. of Hawaii, Manoa).
- 29.Scott, J. M., Mountainspring, S., Ramsey, F. L. & Kepler, C. B. (1986) Stud. Avian Biol. 9, 1-431. [Google Scholar]
- 30.Warshauer, R. (1984) Elepaio 45, 48-51. [Google Scholar]
- 31.VanderWerf, E. A. (1997) Elepaio 57, 125-126. [Google Scholar]
- 32.VanderWerf, E. A. (1998) in The Birds of North America, eds. Poole, A. & Gill, F. (Birds of North America, Philadelphia), no. 344.
- 33.Sheehata, C., Freed, L. & Cann, R. (2001) Stud. Avian Biol. 22, 264-273. [Google Scholar]
- 34.Greiner, E. C., Bennett, G. F., White, E. M. & Coombs, R. F. (1975) Can. J. Zool. 53, 1762-1787. [DOI] [PubMed] [Google Scholar]
- 35.White, E. M., Greiner, E. C., Bennett, G. F. & Herman, C. M. (1978) Rev. Biol. Trop. 26, 43-102. [PubMed] [Google Scholar]
- 36.Meyer, R. P. (1991) J. Am. Mosq. Control Assoc. 7, 467-475. [PubMed] [Google Scholar]
- 37.Reisen, W. K. & Pfuntner, A. R. (1987) J. Am. Mosq. Control Assoc. 3, 601-606. [PubMed] [Google Scholar]
- 38.Warrell, D. A. & Gilles, H. M. (2002) Essential Malariology (Oxford Univ. Press, New York), 4th Ed.
- 39.Lyles, A. M. & Dobson, A. P. (1993) J. Zoo Wildl. Med. 24, 315-326. [Google Scholar]
- 40.Schmidt, K. A. & Ostfeld, R. S. (2001) Ecology 82, 609-619. [Google Scholar]
- 41.Jarvi, S. I., Atkinson, C. T. & Fleischer, R. C. (2001) Stud. Avian Biol. 22, 254-263. [Google Scholar]
- 42.Altizer, S., Harvell, D. & Friedle, E. (2003) Trends Ecol. Evol. 18, 589-596. [Google Scholar]
- 43.Klassing, K. C. (1998) Poultry Sci. 77, 1119-1125. [DOI] [PubMed] [Google Scholar]
- 44.Shankar, A. H. (2000) J. Infect. Dis. 182, S37-S53. [DOI] [PubMed] [Google Scholar]
- 45.Gates, J. E. & Gysel, L. W. (1978) Ecology 59, 871-883. [Google Scholar]
- 46.Nielsen, J. L., Scott, J. M. & Aycrigg, J. L. (2001) Endangered Species Update 18, 194-197. [Google Scholar]
- 47.Peterson, A. T. (2001) Endangered Species Update 18, 29. [Google Scholar]
- 48.Channell, R. & Lomolino, M. V. (2000) Nature 403, 84-86. [DOI] [PubMed] [Google Scholar]