Abstract
The primary objective of this study was to assess the association between short-term changes in ambient fine particulate matter (PM2.5) and first hospital admissions for ischemic stroke. We identified 63,956 first hospital admissions for ischemic stroke from the Beijing Medical Claim Data for Employees from January 1, 2010, through June 30, 2012. A generalized additive Poisson model was applied to explore the association between PM2.5 and admissions for ischemic stroke. We also explore the effect modification of risk by age and gender. The exposure–response relationship between PM2.5 and admissions for ischemic stroke was approximately linear, with a relatively stable response at lower concentrations (<100 μg/m3) and a steeper response at higher concentrations. A 10 μg/m3 increase in the same-day PM2.5 concentration was associated with 0.31% (95% CI, 0.17–0.45%, P < 1.57 × 10−5) increase in the daily admissions for ischemic stroke. The association was also statistically significant at lag 1, 2, 3, 0–2 and 0–4 days. Subgroup analyses showed that the association was not different between patients ≥65 years and <65 years old or between males and females. In conclusion, short-term exposure to PM2.5 was positively associated with first hospital admissions for ischemic stroke in Beijing, China.
Introduction
Stroke is the second most common cause of death and the third leading cause of disability-adjusted life years globally1, 2. In 2010, approximately 16.9 million new cases of stroke were diagnosed and 5.9 million stroke-related deaths occurred worldwide3. In China, stroke is the leading cause of death and adult disability, with an estimated 2.5 million new stroke cases and 1.6 million deaths from stroke occurring each year. In line with other countries, ischemic stroke is the predominant subtype of stroke in China, accounting for 43–79% of all stroke events4. Despite major improvements in primary and secondary prevention for stroke over the last decades, the global incidence of stroke continues to increase, particularly in economically transitioning countries including China due to the rapid growth and aging of population as well as changes in environmental and lifestyle factors3. Additionally, stroke is responsible for considerable patient suffering and imposes a substantial economic burden to society4, 5. Therefore, it is of great significance to identify modifiable risk factors for stroke from public health perspective.
A mounting body of evidence suggests that air pollution is an important risk factor for cardiovascular morbidity and mortality6–8, accounting for over three million deaths worldwide annually9. With the rapid industrialization and economic growth in the past several decades, air pollution has become an increasingly serious concern in China. Haze-fog pollution, an atmospheric phenomenon caused by the presence of dust, smoke and other dry particles at high concentration, has frequently occurred in China in recent years10. From 2013 to 2014, the annual mean concentrations of ambient fine particulate matter (PM2.5, particles with an aerodynamic diameter ≤2.5 μm) for all of the 31 provincial capital cities in China failed to reach the Chinese Ambient Air Quality Standards (CAAQS) Grade I standard (15 μg/m3), and only three cities met the CAAQS Grade II standards (35 μg/m3). Beijing, the capital of China, was experiencing more serious haze-fog pollutions because of coal burning and adverse weather conditions as well as the automobile exhaust emitted by the rapidly increasing transportation vehicles11, 12. The severe haze-fog pollution has attracted extensive public concern due to the adverse health effects.
A meta-analysis that combined the results from prior studies using mortality and/or admission data indicated that various gaseous pollutants including carbon monoxide, sulphur dioxide, nitrogen dioxide, and PM10 (particles with an aerodynamic diameter ≤10 μm) were significantly associated with increased risk of ischemic stroke13. However, only a mere handful of studies have been undertaken which specifically evaluated the health effects of PM2.5 on ischemic stroke, due primarily to the lack of PM2.5 monitoring data. Researchers have suggested that PM2.5 was more harmful to human health than PM10, because PM2.5 can penetrate more deeply into the lung and carry larger concentrations of adsorbed or condensed toxic air pollutants per unit mass with its greater surface area14, 15. In addition, previous studies were primarily conducted in Western developed countries; however, because of the considerable differences in the level of pollution, weather patterns, and population susceptibility, the association between PM2.5 and ischemic stroke is still unclear in developing countries.
The primary interest outcome in prior studies that evaluated the short-term effects of PM2.5 on ischemic stroke hospitalizations was the daily total number of admissions regardless of first or repeated admissions, and all admissions were treated as independent events. A meta-analysis reported that 1-year and 5-year stroke recurrence rates were 11.1% and 26.4%, respectively16. Thus, admissions for recurrent stroke may account for a considerable proportion of daily hospital admissions for stroke. While first-ever and recurrent stroke share similar risk factors and clinical manifestations, their clinical characteristics and pathogenetic features are not exactly the same17, 18. Furthermore, several studies indicated that a history of the disease had considerable influence on the association between air pollution and the specific health outcome7, 19. On the other hand, repeated admissions could cause a temporal dependence among the hospitalization counts, leading to an underestimation of the variance of air pollution risk estimates20. Based on these findings, it seems plausible that air pollution may have a differential effect on first-ever vs. recurrent stroke events. Therefore, studies that specifically examine the association between PM2.5 and first-ever stroke are needed to better understand the real impact of ambient fine particulate matter on public health.
The primary objective of this study was to examine the short-term effect of PM2.5 on first admissions for ischemic stroke in Beijing, China.
Methods
Data collection
Data on daily admissions for ischemic stroke from January 1, 2010, through June 30, 2012, was obtained from Beijing Medical Claim Data for Employees (BMCDE). Medical claim data for all working or retired employees who are covered by basic medical insurance in Beijing were recorded in BMCDE. The medical information recorded in BMCDE includes basic demographics (age and sex), admission date, medication use, clinical diagnosis in Chinese and corresponding International Classification of Diseases, 10th Revision (ICD-10) codes, and reimbursement information. The information on brain computed tomography or magnetic resonance imaging use was also recorded in the database. Hospital admissions for ischemic stroke were identified according to the principal diagnosis, using the ICD-10 code of I63. Specifically, subjects who had been hospitalized for stroke (I60–I64) during the five years preceding the index event were excluded, and we focused on the first admissions for ischemic stroke in this study. We also used the corresponding Chinese diagnoses to check the identified cases, ensuring that only the first admissions could be included in this study.
The air pollution data, hourly PM2.5 concentration from January 1, 2010, through June 30, 2012, was obtained from a web platform (http://www.stateair.net/web/historical/1/1.html) run by the US embassy, which established an ambient air quality monitoring station on the rooftop of embassy building located in Chaoyang district, Beijing. A prior study has indicated that the PM2.5 concentrations obtained from the monitoring station exhibited approximately the same trend as citywide PM2.5 observations21. Furthermore, 79.2% of Beijing’s total population resided within a 40-km radius of the U.S. embassy ambient monitoring station. All areas of high population density (>5000 people/km2), 97.8% (44/45) of the tertiary hospitals and 79.3% (69/87) of the secondary hospitals in Beijing that admit ischemic stroke cases located within a 40-km radius of the monitoring station. It has been suggested that the monitoring data could be used as a good proxy for personal exposure among individuals residing <40 km from the monitoring station8, 14. In addition, only urban residents were included in this study to further reduce exposure misclassification. To allow for the effects of weather conditions, meteorological data on daily 24-hour average temperature (°C) and relative humidity (%) was obtained from the Chinese Meteorological Bureau over the same period.
Statistical Analysis
Daily average PM2.5 concentration, daily admissions for ischemic stroke, and weather conditions were linked by date and, thus could be analyzed with a time-series design. Because daily hospital admission for ischemic stroke was rare, a generalized additive Poisson model was applied to explore the association between PM2.5 and ischemic stroke, after adjusting for day of week (DOW), calendar time, public holiday, and daily average temperature and relative humidity. The penalized spline (ps) function of calendar time with 10 degrees of freedom (df) was used to adjust for seasonality and long-term trends8. We also used the ps functions of daily average temperature (df = 3) and relative humidity (df = 3) to allow for the potential nonlinear confounding effects of weather conditions22, 23. The DOW and public holiday were also incorporated in the model to control for the difference in the baseline hospital admission rates for each day. After the basic model was established, the variable of PM2.5 concentration was introduced. The final model was described below:
where, E(Yt) is the expected number of admissions for ischemic stroke on day t; α is the model intercept; PM2.5t−i is the mean PM2.5 concentration on day t, and i is the day lag; β represents regression coefficient; DOW is the day of the week; public holiday is a binary variable indicating a public holiday on day t (coded as 0 indicates no holiday, and 1 indicates a holiday); ps() indicates penalized spline function; Temp0 and RH0 indicate the daily mean temperature and relative humidity on the current day, respectively. The results are presented as the percentage change and 95% confidence interval (CI) in the daily ischemic stroke admissions per 10 μg/m3 increase in PM2.5 concentration. Smoothing function was used to graphically analyze the exposure-response relationship between the log-relative risk of ischemic stroke and PM2.5 concentration.
To examine the temporal association of PM2.5 concentration with ischemic stroke admission, we fitted the models with different lag structures from the current day (lag0) up to 4 lag days (lag4). Considering that single-day lag models may underestimate the effect of pollutant24, we also evaluated the cumulative effects using 3-day (lag0–2) and 5-day (lag0–4) moving averages of PM2.5 concentrations.
Stratified analyses were conducted to examine whether the association differed by age (≥65 years and <65 years) and gender. The Z-test was applied to test the statistical significance of differences by age or gender25. We also conducted sensitivity analyses to examine the robustness of the results in terms of the df in the smooth function of time trend (8–12), daily mean temperature (2–6) and daily relative humidity (2–6).
All statistical analyses were carried out using R Programming Language (V.3.2.2, R Development Core Team) with the “mgcv” and “nlme” packages. All statistical tests were two-sided, and tests of statistical significance were set at P < 0.05.
Results
Table 1 summarizes the basic characteristics for our study. A total of 63,956 first hospital admissions for ischemic stroke were identified from BMCDE database between January 1, 2010 and June 30, 2012. There were 66.5% male patients, and 56.7% patients were ≥65 years old. The mean (standard deviation, SD) age of the ischemic stroke patients was 66.4 (12.1) years.
Table 1.
Demographic characteristics of Ischemic Stroke Admissions in Beijing, China, January 1, 2010 to June 30, 2012.
| Variable | No. |
|---|---|
| Total | 63,956 |
| Gender | |
| Male (%) | 42,529 (66.5) |
| Female (%) | 21,427 (33.5) |
| Age (year) (mean ± SD) | 66.4 ± 12.1 |
| <65 (%) | 27,706 (43.3) |
| ≥65 (%) | 36,250 (56.7) |
The summary statistics for daily ischemic stroke admissions, air pollution and weather conditions from January 1, 2010 to June 30, 2012 are shown in Table 2. The mean daily count for ischemic stroke admission was 70 during the study period. The overall mean daily PM2.5 concentration was 99.5 μg/m3, with a range from 7.2 to 492.8 μg/m3. According to the CAAQS Grade II standards for daily mean concentrations of PM2.5 (75 μg/m3), 45.4% (414 days) of the daily PM2.5 concentrations were up to the standard. However, in terms of the WHO Air Quality Guidelines for 24-hour average PM2.5 concentration (25 μg/m3), only 13.6% (124 days) days met the standard. The means (SD) of temperature and relative humidity were 12.6 °C (11.6 °C) and 48.6% (20.3%), respectively.
Table 2.
Summary statistics for daily number of hospital admissions for ischemic stroke, daily fine particulate matter (PM2.5) concentrations and weather conditions in Beijing, China, January 1, 2010 to June 30, 2012.
| Variable | Mean ± SD | Minimum | Percentile | Maximum | ||
|---|---|---|---|---|---|---|
| 25th | 50th | 75th | ||||
| Daily admission | 70.1 ± 43.8 | 1 | 12 | 86 | 102 | 167 |
| PM2.5 (μg/m3) | 99.5 ± 75.3 | 7.2 | 42.5 | 82.8 | 133.3 | 492.8 |
| Temperature(°C) | 12.6 ± 11.6 | −12.5 | 1.5 | 14.1 | 23.8 | 34.5 |
| Relative humidity (%) | 48.6 ± 20.3 | 9 | 30 | 48 | 66 | 92 |
There was a clear dose–response relationship between PM2.5 concentration and the count of daily admissions for ischemic stroke (Fig. 1). The relationship was approximately linear, with a tiny fluctuation at lower concentrations (<100 μg/m3) and a sharper response at higher concentrations.
Figure 1.
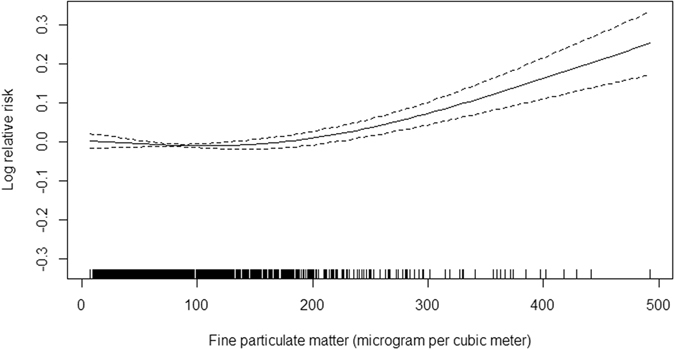
The smoothed exposure-response curves of daily average PM2.5 concentrations at current-day against ischemic stroke hospital admission. Note: The X-axis is the current-day (lag 0 day) fine particulate matter (PM2.5) concentrations (mg/m3). Y-axis is the predicted log (relative risk (RR), after adjusting for calendar time, day of the week, public holiday, current-day temperature, and relative humidity, is shown by the solid line, and the dotted lines represent the 95% CI.
Table 3 shows the association between PM2.5 concentration and ischemic stroke admissions for different lag structures. We observed significant associations between ischemic stroke admissions and PM2.5 concentrations on the current day (lag0) and lag 1, 2 and 3 days after adjustment for calendar time, day of the week, public holiday, and weather conditions. A 10 μg/m3 increase in PM2.5 concentration at lag 0, 1, 2 and 3 days corresponded to a 0.31% (95% CI, 0.17–0.45%), 0.48% (95% CI, 0.35–0.60%), 0.48% (95% CI, 0.37–0.58%), and 0.32% (95% CI, 0.22–0.43%) increase in ischemic stroke admissions, respectively. An increment of 10 μg/m3 in 3-day (percentage change, 0.80%; 95% CI, 0.64–0.96%) and 5-day (percentage change, 0.75%; 95% CI, 0.58–0.93%) average PM2.5 concentrations were also significantly associated with increased ischemic stroke admissions.
Table 3.
Percentage change with 95% CI in ischemic stroke admissions associated with a 10 μg/m3 increase in fine particulate matter (PM2.5) concentration for different lag structures.
| Lag days | Percentage change | 95% CI | P |
|---|---|---|---|
| Lag 0 days | 0.31 | 0.17–0.45 | 1.57e-05 |
| Lag 1 days | 0.48 | 0.35–0.60 | 1.35e-14 |
| Lag 2 days | 0.48 | 0.37–0.58 | <2e-16 |
| Lag 3 days | 0.32 | 0.22–0.43 | 2.62e-09 |
| Lag 4 days | 0.06 | −0.05–0.16 | 0.308 |
| Lag 0–2 days | 0.80 | 0.64–0.96 | <2e-16 |
| Lag 0–4 days | 0.75 | 0.58–0.93 | <2e-16 |
Stratified analyses showed that the association between PM2.5 and ischemic stroke was not different between patients ≥65 years and <65 years old or between males and females (P > 0.05) (Table 4).
Table 4.
Percentage change with 95% CI in ischemic stroke admissions associated with a 10 μg/m3 increase in fine particulate matter (PM2.5) concentration by gender and age.
| Gender | Age (year) | |||||
|---|---|---|---|---|---|---|
| Male | Female | P value | <65 | ≥65 | P value | |
| Lag 0 days | 0.23(0.05–0.40) | 0.39(0.18–0.61) | 0.257 | 0.38(0.16–0.59) | 0.25(0.06–0.43) | 0.381 |
| Lag 1 days | 0.51(0.36–0.65) | 0.38(0.19–0.57) | 0.293 | 0.52(0.34–0.70) | 0.44(0.28–0.60) | 0.515 |
| Lag 2 days | 0.47(0.34–0.60) | 0.47(0.30–0.65) | 0.999 | 0.48(0.32–0.64) | 0.46(0.32–0.60) | 0.854 |
| Lag 3 days | 0.35(0.22–0.48) | 0.29(0.11–0.47) | 0.596 | 0.32(0.16–0.48) | 0.33(0.19–0.47) | 0.927 |
| Lag 4 days | 0.07(−0.06–0.20) | 0.03(−0.15–0.22) | 0.724 | 0.13(−0.03–0.30) | 0.002(−0.14–0.15) | 0.341 |
| Lag0–2 days | 0.78(0.58–0.98) | 0.72(0.46–0.97) | 0.720 | 0.86(0.61–1.11) | 0.74(0.52–0.95) | 0.480 |
| Lag0–4 days | 0.76(0.54–0.97) | 0.72(0.43–1.01) | 0.758 | 0.83(0.57–1.09) | 0.68(0.45–0.91) | 0.397 |
Table 5 shows the percentage changes in ischemic stroke admissions in relation to a 10 μg/m3 increase in PM2.5, under different df for calendar time, temperature and relative humidity. The risk estimates were stable when df for calendar time was 10, while different df for temperature and relative humidity had little effect on the estimates, suggesting that the results on the association of PM2.5 concentration with ischemic stroke admissions was robust in this study.
Table 5.
Percentage change with 95% CI in ischemic stroke admissions associated with a 10 μg/m3 increase in fine particulate matter (PM2.5) concentration on the same day, by different degree of freedom (df) for calendar time, temperature, and relative humidity.
| Variable | df | Percentage change | 95% CI | P value |
|---|---|---|---|---|
| Calendar time | 8 | 0.20 | 0.06–0.33 | 0.00474 |
| 9 | 0.20 | 0.06–0.33 | 0.00445 | |
| 10* | 0.31 | 0.17–0.45 | 1.57e-05 | |
| 11 | 0.31 | 0.17–0.45 | 1.78e-05 | |
| 12 | 0.30 | 0.16–0.44 | 2.7e-05 | |
| Temperature | 2 | 0.31 | 0.17–0.45 | 1.57e-05 |
| 3* | 0.31 | 0.17–0.45 | 1.57e-05 | |
| 4 | 0.38 | 0.23–0.52 | 2.1e-07 | |
| 5 | 0.37 | 0.23–0.51 | 3.96e-07 | |
| 6 | 0.37 | 0.22–0.51 | 4.55e-07 | |
| Relative humidity | 2 | 0.31 | 0.17–0.45 | 1.57e-05 |
| 3* | 0.31 | 0.17–0.45 | 1.57e-05 | |
| 4 | 0.31 | 0.16–0.45 | 2.38e-05 | |
| 5 | 0.31 | 0.17–0.45 | 2.11e-05 | |
| 6 | 0.32 | 0.17–0.46 | 1.3e-05 |
*The df value used in this study model.
Discussion
The present study provides strong evidence of the association between PM2.5 and ischemic stroke in Beijing. The short-term exposure to PM2.5 was significantly associated with the first admission for ischemic stroke accounting for temperature, relative humidity, day of week, public holiday, long-term trends and seasonality of stroke events. To the best of our knowledge, this is the first city-level investigation of the relationship between PM2.5 and first admission for ischemic stroke in a real and severe air pollution environment. Although the magnitude of increased risk of ischemic stroke due to PM2.5 exposure was relatively small, the number of ischemic stroke events attributable to PM2.5 may be high due to the high incidence of ischemic stroke and the fact that the overwhelming majority of the public is exposed to ambient fine particulate matter, suggesting potentially large public health implications.
There has been numerous epidemiological and experiments research suggesting a possible link between air pollution and higher risk of ischemic stroke26. Some pathophysiological hypotheses have been proposed to explain the possible association between short-term effects of air pollution and ischemic stroke. Researchers have suggested that exposure to air pollution had adverse effects on vascular endothelial function, increased activity of the sympathetic nervous system and systemic inflammation, resulting in vasoconstriction, increased plasma viscosity, and increased risk of blood clotting and thrombosis27–29. These pathophysiological responses could potentially place individuals at higher risk of ischemic stroke. However, direct evidence on the association between PM2.5 and ischemic stroke was limited and the findings remain equivocal. A comprehensive review identified only six studies published before January 2014 that specifically examined the association between short-term exposure to PM2.5 and the risk of ischemic stroke hospitalization, and the pooled results suggested a non-significant increased risk of hospital admission for ischemic stroke per 10 μg/m3 increase in PM2.5 30. Wellenius et al. investigated association between short-term ambient air pollution and risk of ischemic stroke in 1,705 patients admitted from Boston, USA. They found that PM2.5 was positively associated with ischemic stroke for the <24 hours lag period31. However, a study conducted in Canada, including 9,202 admissions for acute ischemic stroke, found no association between short-term increases in PM2.5 and ischemic stroke risk32. The heterogeneity of results across studies may due to differences in demographic characteristics of study population, meteorological patterns, and PM2.5 levels.
In this study, the estimate scaled to a 10 μg/m3 increase in PM2.5 was relatively lower when compared to previous reports. A meta-analysis that combined the risk estimates derived from previous studies reported a 1.0% increase in the daily ischemic stroke admissions per 10 μg/m3 increase in the level of PM2.5 30. The differences in the definition of health outcomes across studies may be responsible for the weaker effect of PM2.5 observed in this study. Considerable attention has been focused on overall hospitalizations including admissions for both first-ever and recurrent stroke in previous studies32–34, possibly combining effects with different sensitivities to PM2.5. In contrast, only the first hospital admissions for ischemic stroke were included in this study. Epidemiological evidence indicated that individuals with a history of cardiovascular disease were more susceptible to air pollution. Patients with myocardial infarction35, congestive heart disease36, 37, or chronic obstructive pulmonary disease38 were demonstrated to have higher risk of death on days with heavy air pollution. Stroke survivors were at higher risk of recurrent ischemic stroke in relation to increased level of PM10 39. Therefore, it is reasonable to suppose that PM2.5 may exert greater adverse effect on recurrent stroke than first-ever stroke, which may partly explain the relatively weaker effect observed in this study. Differences in characteristics of study population and pollutant, meteorological pattern, and exposure assessment strategy are also likely to contribute to the heterogeneity across studies. Given that this is the first study to evaluate the association between PM2.5 and first admissions for ischemic stroke in a badly polluted environment, the findings should be interpreted with caution and future studies are warranted to confirm the current results for PM2.5.
Identifying the dose-response relationship for ischemic stroke in relation to PM2.5 concentration is of public health and regulatory interest. Nevertheless, as prior studies were primarily conducted in Western countries with slight PM2.5 pollution, the exposure-response relationship in a real and severe air pollution environment remains unclear. In this study, we conducted a dose-response analysis to explore the pattern and scope of the adverse response. We observed an approximately linear exposure-response relationship, which is consistent with a time-stratified case-crossover study conducted in USA that indicated a linear relationship between PM2.5 and risk of acute ischemic stroke31. It is worth noting that, in that study, even in concentration ranges well below the present US National Ambient Air Quality Standards (20 μg/m3), PM2.5 was also significantly associated with increased risk of acute ischemic stroke, which is contrast to a relatively shallower response at low concentrations in our study. A recent time–series study conducted in China evaluated the exposure-response relationship for respiratory emergency visits in relation to fine particulate air pollution40. The exposure–response curve was virtually flat at low levels of PM2.5 (<200 μg/m3) and became sharp at high levels, which is consistent with our results. Our findings were also supported by a study involving 369,469 ischemic heart disease cases suggesting that PM2.5 at levels below 75 μg/m3 would not significantly increase the risk of ischemic heart disease8. Based on these findings, we hypothesized that there might be a threshold concentration at which PM2.5 become harmful enough to impose an adverse impact on the development and progression of ischemic stroke. This hypothesis may be able to partly explain the non-significant association between short-term exposure to PM2.5 and ischemic stroke observed in other areas where the PM2.5 concentrations were too low to have significant effects on ischemic stroke32, 34. Future studies are needed to clarify this quite important issue.
Possibly because of the higher PM2.5 concentration in Beijing, a stronger temporal association was noted in this study when compared to prior reports of a 1–2 day lag31, 33. Nevertheless, it is important to note that the pollution exposure assessment was based on the date of hospital admission rather than the time of stroke onset in this study, resulting in a substantial exposure misclassification and underestimates of pollutants effects41. A study conducted in USA, including 1,101 hospital admissions for acute ischemic stroke, found that hospital admission occurred at a median of one calendar day after onset of symptoms, and this delay could cause an underestimation of the association between ambient particulate matter exposure and stroke42. Therefore, the lag effects of PM2.5 exposure and ischemic stroke should be interpreted cautiously.
Examining the modification effects of individual characteristics will help to identify susceptible population and develop specific interventions for subgroups. We did not observe significant effect modification of risk by age, which is in consistent with a study indicating that air pollution risk estimates did not differ substantially between patients ≤65 years and >65 years for both ischemic and hemorrhagic stroke43. However, the American Heart Association made an updated scientific statement on the association between particulate matter air pollution and cardiovascular disease, suggesting that the elderly are more sensitive to the elevated PM2.5 level; however, the evidence is limited9. Elderly people are very likely to spend a greater proportion of time indoors or wear a face mask outdoors when PM2.5 pollution is severe, thus decreasing personal exposure44, which may cover up the real age-specific effects of PM2.5. The gender subgroup analysis showed that the associations with PM2.5 were not different between men and women, which are in line with previous reports43, 45.
Compared with prior studies that focused on the association in developed countries where PM2.5 pollution was mild, our study was conducted in a heavily polluted city. Given the high level of pollution, we were able to evaluate the exposure-response relationship in a wide range of PM2.5 concentration and have an opportunity to reveal a more complete picture of the association. Additionally, we specifically used the first admissions as the health outcome, providing a novel insight into the underlying mechanism of the association.
This study was subject to certain limitations. First, the use of air pollution data deriving entirely from one single monitoring station is expected to cause exposure measurement error, leading to underestimate the pollutant effects46. Second, we had no access to monitoring data on other air pollutants, such as sulfur dioxide, nitrogen dioxide, carbon monoxide and ozone, thus limiting our ability to explore the independent effect of PM2.5. Therefore, our findings should be interpreted with caution, and additional studies are warranted to examine the independent effect of PM2.5 on first hospital admission for ischemic stroke. Another limitation is our inability to differentiate the ischemic stroke subtypes, because that information was not available in our database. Future studies are needed to examine whether the acute effects of PM2.5 differed across strata defined by ischemic stroke etiology. Finally, the retrospective data collection may bring about bias from diagnostic and coding inaccuracy. However, both ICD-10 codes and corresponding Chinese diagnoses were used to identify eligible ischemic stroke hospitalizations, which significantly reduced bias from coding inaccuracy47, 48. Given the robustness of the association in all lag models, stratified analyses and sensitivity analyses, and large sample size in the present study, these potential limitations are unlikely to have compromised our results.
In conclusions, our results indicated that short term elevation of PM2.5 was associated with increased risk of ischemic stroke among populations in Beijing. The present study contributed to the limited scientific literature about the short-term effects of particulate matter air pollution on ischemic stroke in developing countries and additional research on this topic is warranted.
Acknowledgements
This work was supported by the Key Project of Natural Science Funds of China [No. 81230066] and the National Natural Science Fund Projects of China [No. 81473043].
Author Contributions
Y.H. contributed to the study concept. Y.H. had full access to all the data in the study and take responsibility for the integrity of the data. Y.T., X.X. and H.L. contributed to the statistical analysis and tables’ development of this article. Y.T., Y.W., Y.C., J.S. and K.S. interpreted the findings and drafted the article. All the authors contributed to the critical revision of the article for important intellectual content.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 3.Feigin VL, et al. Global and regional burden of stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2014;383:245–254. doi: 10.1016/S0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Wang D, Wong KS, Wang Y. Stroke and stroke care in China: huge burden, significant workload, and a national priority. Stroke. 2011;42:3651–3654. doi: 10.1161/STROKEAHA.111.635755. [DOI] [PubMed] [Google Scholar]
- 5.Tong X, George MG, Gillespie C, Merritt RK. Trends in Hospitalizations and Cost Associated with Acute Ischemic Stroke by Age, United States 2003–2012. Stroke. 2016;47:A183–A183. doi: 10.1161/STROKEAHA.116.013124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 7.Pope CA, 3rd, et al. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- 8.Xie W, et al. Relationship between fine particulate air pollution and ischaemic heart disease morbidity and mortality. Heart. 2015;101:257–263. doi: 10.1136/heartjnl-2014-306165. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, et al. Atmospheric aerosol compositions in China: spatial/temporal variability, chemical signature, regional haze distribution and comparisons with global aerosols. Atmospheric Chemistry and Physics. 2012;12:779–799. doi: 10.5194/acp-12-779-2012. [DOI] [Google Scholar]
- 11.Li L, Liu DJ. Study on an air quality evaluation model for Beijing City under haze-fog pollution based on new ambient air quality standards. Int J Environ Res Public Health. 2014;11:8909–8923. doi: 10.3390/ijerph110908909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(Barely) living in smog: China and air pollution. (Lancet, 383(9920), 845, doi:10.1016/S0140-6736(14)60427-X, 2014 Mar 8). [DOI] [PubMed]
- 13.Shah, A. S. et al. Short term exposure to air pollution and stroke: systematic review and meta-analysis. Bmj24 (2015). [DOI] [PMC free article] [PubMed]
- 14.Wilson WE, Suh HH. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. J Air Waste Manag Assoc. 1997;47:1238–1249. doi: 10.1080/10473289.1997.10464074. [DOI] [PubMed] [Google Scholar]
- 15.Pope CA, 3rd, Dockery DW. Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc. 2006;56:709–742. doi: 10.1080/10473289.2006.10464485. [DOI] [PubMed] [Google Scholar]
- 16.Mohan KM, et al. Risk and cumulative risk of stroke recurrence: a systematic review and meta-analysis. Stroke. 2011;42:1489–1494. doi: 10.1161/STROKEAHA.110.602615. [DOI] [PubMed] [Google Scholar]
- 17.Howard G, et al. Differences in the role of black race and stroke risk factors for first vs. recurrent stroke. Neurology. 2016;86:637–642. doi: 10.1212/WNL.0000000000002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jorgensen HS, Nakayama H, Reith J, Raaschou HO, Olsen TS. Stroke recurrence: predictors, severity, and prognosis. The Copenhagen Stroke Study. Neurology. 1997;48:891–895. doi: 10.1212/WNL.48.4.891. [DOI] [PubMed] [Google Scholar]
- 19.Henrotin JB, et al. Evidence of the role of short-term exposure to ozone on ischaemic cerebral and cardiac events: the Dijon Vascular Project (DIVA) Heart. 2010;96:1990–1996. doi: 10.1136/hrt.2010.200337. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y, et al. The effect of coarse ambient particulate matter on first, second, and overall hospital admissions for respiratory disease among the elderly. Inhal Toxicol. 2005;17:649–655. doi: 10.1080/08958370500189420. [DOI] [PubMed] [Google Scholar]
- 21.Wang JF, Hu MG, Xu CD, Christakos G, Zhao Y. Estimation of citywide air pollution in Beijing. PLoS ONE. 2013;8:8. doi: 10.1371/annotation/5fa9cfb4-9964-4586-845d-d8205f318d68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominici F, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. Jama. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kan H, et al. Differentiating the effects of fine and coarse particles on daily mortality in Shanghai, China. Environ Int. 2007;33:376–384. doi: 10.1016/j.envint.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bell ML, Samet JM, Dominici F. Time-series studies of particulate matter. Annu Rev Public Health. 2004;25:247–280. doi: 10.1146/annurev.publhealth.25.102802.124329. [DOI] [PubMed] [Google Scholar]
- 25.Altman DG, Bland JM. Interaction revisited: the difference between two estimates. Bmj. 2003;326:219. doi: 10.1136/bmj.326.7382.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateen FJ, Brook RD. Air pollution as an emerging global risk factor for stroke. JAMA. 2011;305:1240–1241. doi: 10.1001/jama.2011.352. [DOI] [PubMed] [Google Scholar]
- 27.Lucking AJ, et al. Diesel exhaust inhalation increases thrombus formation in man. Eur Heart J. 2008;29:3043–3051. doi: 10.1093/eurheartj/ehn464. [DOI] [PubMed] [Google Scholar]
- 28.Lucking AJ, et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123:1721–1728. doi: 10.1161/CIRCULATIONAHA.110.987263. [DOI] [PubMed] [Google Scholar]
- 29.Gurgueira SA, Lawrence J, Coull B, Murthy GG, Gonzalez-Flecha B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environ Health Perspect. 2002;110:749–755. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Eliot MN, Wellenius GA. Short-term changes in ambient particulate matter and risk of stroke: a systematic review and meta-analysis. J Am Heart Assoc. 2014;3:000983. doi: 10.1161/JAHA.114.000983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wellenius GA, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172:229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Donnell MJ, Fang J, Mittleman MA, Kapral MK, Wellenius GA. Fine particulate air pollution (PM2.5) and the risk of acute ischemic stroke. Epidemiology. 2011;22:422–431. doi: 10.1097/EDE.0b013e3182126580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lisabeth LD, et al. Ambient air pollution and risk for ischemic stroke and transient ischemic attack. Ann Neurol. 2008;64:53–59. doi: 10.1002/ana.21403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan CC, Chuang KJ, Chien LC, Chen WJ, Chang WT. Urban air pollution and emergency admissions for cerebrovascular diseases in Taipei, Taiwan. Eur Heart J. 2006;27:1238–1244. doi: 10.1093/eurheartj/ehi835. [DOI] [PubMed] [Google Scholar]
- 35.Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15:143–149. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- 36.Goldberg MS, et al. Identification of persons with cardiorespiratory conditions who are at risk of dying from the acute effects of ambient air particles. Environ Health Perspect. 2001;4:487–494. doi: 10.1289/ehp.01109s4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon HJ, Cho SH, Nyberg F, Pershagen G. Effects of ambient air pollution on daily mortality in a cohort of patients with congestive heart failure. Epidemiology. 2001;12:413–419. doi: 10.1097/00001648-200107000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Sunyer J, et al. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- 39.Oudin, A., Forsberg, B. & Jakobsson, K. Air pollution and stroke. Epidemiology. 23(3), 505–6, doi:10.1097/EDE.0b013e31824ea667) (2012 May). [DOI] [PubMed]
- 40.Xu, Q. et al. Fine Particulate Air Pollution and Hospital Emergency Room Visits for Respiratory Disease in Urban Areas in Beijing, China, in 2013. PLoS ONE11 (2016). [DOI] [PMC free article] [PubMed]
- 41.Zeger SL, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environ Health Perspect. 2000;108:419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lokken RP, et al. Air pollution and risk of stroke: underestimation of effect due to misclassification of time of event onset. Epidemiology. 2009;20:137–142. doi: 10.1097/EDE.0b013e31818ef34a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang F, et al. Particulate Matter and Hospital Admissions for Stroke in Beijing, China: Modification Effects by Ambient Temperature. J Am Heart Assoc. 2016;5:003437. doi: 10.1161/JAHA.116.003437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts J. China: the air pollution capital of the world. The Lancet. 2005;366:1761–1762. doi: 10.1016/S0140-6736(05)67711-2. [DOI] [PubMed] [Google Scholar]
- 45.Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21:689–700. doi: 10.1007/s10654-006-9050-9. [DOI] [PubMed] [Google Scholar]
- 46.Goldman GT, et al. Impact of exposure measurement error in air pollution epidemiology: effect of error type in time-series studies. Environ Health. 2011;10:10–61. doi: 10.1186/1476-069X-10-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benesch C, et al. Inaccuracy of the International Classification of Diseases (ICD-9-CM) in identifying the diagnosis of ischemic cerebrovascular disease. Neurology. 1997;49:660–664. doi: 10.1212/WNL.49.3.660. [DOI] [PubMed] [Google Scholar]
- 48.Miller ML, Wang MC. Accuracy of ICD-9-CM coding of cervical spine fractures: implications for research using administrative databases. Ann Adv Automot Med. 2008;52:101–105. [PMC free article] [PubMed] [Google Scholar]


