Abstract
The evolutionary success of reef-building corals in nutrient-poor tropical waters is attributed to endosymbiotic dinoflagellates. The algae release photosynthetic products to the coral animal cells, augment nutrient flux, and enhance the rate of coral calcification. Natural abundance of stable isotopes (δ13C and δ18O) provides answers to modern and paleobiological questions about the effect of photosymbiosis on sources of carbon and oxygen in coral skeletal calcium carbonate. Here we compare 17 species of symbiotic and nonsymbiotic corals to determine whether evidence for photosymbiosis appears in stable isotopes (δ13C and δ15N) of an organic skeletal compartment, the coral skeletal organic matrix (OM). Mean OM δ13C in symbiotic and nonsymbiotic corals was similar (-26.08‰ vs. -24.31‰), but mean OM δ15N was significantly depleted in 15N in the former (4.09‰) relative to the latter (12.28‰), indicating an effect of the algae on OM synthesis and revealing OM δ15N as a proxy for photosymbiosis. To answer an important paleobiological question about the origin of photosymbiosis in reef-building corals, we applied this proxy test to a fossil coral (Pachythecalis major) from the Triassic (240 million years ago) in which OM is preserved. Mean OM δ15N was 4.66‰, suggesting that P. major was photosymbiotic. The results show that symbiotic algae augment coral calcification by contributing to the synthesis of skeletal OM and that they may have done so as early as the Triassic.
Keywords: symbiosis, calcification, paleobiology
Productivity and growth of scleractinian corals in shallow, nutrient-poor tropical marine waters is significantly enhanced by endosymbiotic dinoflagellates. The algae release products of photosynthesis to animal cells, take up, retain, and recycle inorganic nutrients, and augment the rate of skeletal calcification because it can be two to three times faster in the light than in the dark (1, 2). Natural abundance of stable isotopes (δ13C and δ18O) has answered paleobiological and modern questions about the effect of photosymbiosis on sources of carbon and oxygen in coral skeletal calcium carbonate (3, 4). Similarly, δ13C and δ15N have provided information on how coral algae and animal cells use carbon and nitrogen (5–11). Here we investigate natural abundance of stable isotopes (δ13C and δ15N) in another coral skeletal compartment, the skeletal organic matrix (OM).
The scleractinian coral skeleton is a two-phase composite structure consisting of fiber-like crystals of aragonitic calcium carbonate intimately associated with an intrinsic OM (12). The OM is generally <0.1% of the total skeleton by weight (13) but is believed to initiate nucleation of calcium carbonate and provide a framework for crystallographic orientation and species-specific architecture (14–16). Inhibition of matrix synthesis by emetin or cycloheximide brings coral calcification to a halt without affecting photosynthesis or respiration (17).
Decalcification of coral skeleton yields water-soluble and -insoluble fractions, which include glycoproteins and glycosaminoglycans (13, 18–25). Matrix glycoprotein contains sulfated polysaccharide and protein rich in acidic amino acids, especially aspartate and glutamate (13). Matrix monosaccharides and amino acids vary qualitatively and quantitatively depending on species. As such they are useful in taxonomy (21, 22, 26) and discrimination between modern symbiotic and nonsymbiotic corals (22, 27). The first sequence of a coral OM protein has been described (28).
The morphological relationship between organic and mineral phases in coral unit crystals is poorly understood. OM is synthesized by cells of the calicoblastic epithelium and then secreted into the adjacent subepithelial space in which it initiates and controls calcification (17, 24, 29–31). Electron microscopy of thin sections of the coral tissue–skeleton interface reveals a 3-μm-thick reticulum of acid polysaccharide-rich fibrils in this space (24, 29). When corals are exposed to a matrix precursor such as [14C]aspartate, the amino acid is incorporated rapidly into animal tissue protein and appears in matrix proteins within 20 min (17).
There is a metabolic link between symbiont photosynthesis and OM synthesis as coral algae fix 14CO2 and release 14C-labeled products to coral animal cells. After decalcification of the skeleton, 14C can be detected in OM (32–34).
Recognizing the fundamental role of skeletal OM in coral calcification, we analyzed δ13C and δ15N in algae, animal tissue, and OM of 17 species of modern scleractinian corals. We compared symbiotic and nonsymbiotic forms to determine whether activities of the algae influence the natural abundance of OM stable isotopes. The data reveal a highly significant difference between symbiotic and nonsymbiotic corals with respect to OM δ15N but not with respect to OM δ13C, establishing OM δ15N as a potential proxy for photosymbiosis. We wondered whether such a proxy could be used to obtain retrospective information in fossil corals. In a seminal paper, Stanley and Swart (35) used skeletal mineral data (carbonate δ13C and δ18O) as a proxy for photosymbiosis in Late Triassic corals. The remarkable discovery of aragonite crystals and skeletal OM in a Triassic coral, Pachythecalis major (36, 37), enabled us to pursue this question by using OM δ15N. Accordingly, we analyzed the extant OM (δ13C, δ15N) and skeletal carbonate (δ13C and δ18O) in P. major. The resulting data support the contention that P. major was photosymbiotic.
Methods
We analyzed eight symbiotic and nine nonsymbiotic modern species of scleractinian corals including Oculina spp. The latter are facultative symbioses containing algae in shallow water and lacking them (aposymbiotic) in shaded habitats and deep water (Table 1). The corals were collected fresh from the sea or aquaria or obtained as dry “museum” skeletons. Replicate colonies of a few species were included. Algae and animal tissue from freshly collected corals were analyzed also.
Table 1. Corals used in this study.
| Collection site | Species | Collection depth, m |
|---|---|---|
| Modern symbiotic | ||
| New Caledonia | Acropora sp. | 4; f |
| Fungia danai (Edwards and Haime, 1851) | 4; f | |
| Merulina sp. | 4; f | |
| Pavona decussata (Dana, 1846) | 4; f | |
| Pocillopora damicornis (Linnaeus, 1758) | 4; f | |
| Red Sea | Stylophora pistillata (Esper, 1797) | 1, 5, 10, 15, 20, 30; f |
| Hawaii | Fungia scutaria (Verril, 1864) | 1; f |
| Pocillopora damicornis (Linnaeus, 1758) | 1, 10; f | |
| North Carolina | Oculina arbuscula (symbiotic) | 1; ds |
| Oculina arbuscula (aposymbiotic) | 6; ds | |
| Florida | Oculina varicosa (aposymbiotic) | 100; ds |
| Monaco | Stylophora sp. | aq; ds |
| Acropora sp. | aq; ds | |
| Modern nonsymbiotic | ||
| Celtic Sea | Caryophyllia ambrosia (Alcock, 1898) | unk; ds |
| New Caledonia | Dendrophyllia alcocki (Wells, 1954) | 412; ds |
| Enallopsammia sp. | 435; ds | |
| Paracyathus sp. | 425; ds | |
| Solenosmilia variabilis (Duncan, 1873) | unk; ds | |
| Stephanocyathus spiniger (Marenzeller, 1888) | 250; ds | |
| Great Barrier Reef | Tubastrea coccinea (Lesson, 1829) | 12–15; f |
| Tubastrea micrantha (Ehrenberg, 1834) | 12–15; f | |
| Hawaii | Tubastrea coccinea (Lesson, 1829) | 4; f |
| New Caledonia | Tubastrea micrantha (Ehrenberg, 1834) | 2; f |
| Indian Ocean | Madrepora oculata (Linnaeus, 1758) | unk; ds |
| Mediterranean Sea | Madrepora oculata (Linnaeus, 1758) | unk; ds |
| Fossil | ||
| Turkey | Pachythecalis major Cuif 1975 | n/a |
aq, aquarium; ds, dried skeleton; f, freshly collected; n/a, not applicable; unk, unknown.
Freshly collected corals were processed according to skeletal architecture (perforate or imperforate). Animal tissue was removed from imperforate skeletons with a jet of sea water (Waterpik, Teledyne, Ft. Collins, CO) and from perforate skeletons by crushing to a coarse sand, shaking the slurry in 100 ml of filtered seawater, and decanting the supernatant containing the algae and animal tissue (32). In both cases, the resulting tissue homogenates were centrifuged to separate algae from animal tissue (38). Tissue fractions were acidified with 0.1 M HCl to dissolve any residual skeletal calcium carbonate and remove seawater bicarbonate, lyophilized, and stored at -20°C. Tissue-free skeletons, including dry museum specimens, were soaked in sodium hypochlorite (commercial bleach) overnight, rinsed with distilled water, and dried in an oven at 50°C. They then were crushed to a coarse sand in a ceramic mortar and pestle, treated with NaOH (2 M, 80°C, 15 min) to remove any residual extrinsic organic material such as animal tissue and endolithic organisms (see below), rinsed in distilled water, oven-dried (50°C), and then reduced to a fine powder in a motor-driven agate mill (Retsch Type RMO, Bioblock Scientific, Haan, Germany). Approximately 15–50 g of skeletal powder was decalcified by dropwise addition of 2 M HCl over several hours until the powder dissolved and the evolution of bubbles ceased. The resulting slightly turbid solution, containing aggregate matrix (i.e., soluble and insoluble fractions) was neutralized with 0.1 M NaOH and then dialyzed (Spectra/Por, Spectrum Laboratories, Houston; molecular-mass cutoff, 3.5 kDa) for 24 h at 5°C against distilled water (four changes of 6 liters with stirring). The dialyzed material was lyophilized and weighed. Recoveries ranged from 0.01% to 1.5% by weight (data not included here). Our OM weights were generally higher than those reported previously (13). Because different extraction methods and different operational definitions of OM (i.e., soluble vs. aggregate) make comparison with previous studies difficult, we initiated a separate investigation to determine OM weights by thermogravimetry. Preliminary results indicate that OM weights averaged 1.6% for the coral Stylophora pistillata, suggesting that recovery may depend on extraction protocol and that percent OM weights may now be expected to exceed previous estimates (Puverel, S., Houlbreque, F., Tambutte, E., Zoccola, D., Payan, P., Tambutte, S., and D.A., unpublished data).
Depending on age, location within a colony, and species, scleractinian skeletons may harbor endolithic filamentous algae and fungi (39). Following the convention used in all previous studies of OM, skeletons containing endolithic filamentous algae, identified by their green color, were discarded.
Three specimens of the Triassic coral P. major (Upper Triassic/Lower Norian) were collected from the Alakir Valley in Turkey. The largest (specimen 1), a conical white calyx (50 mm high; distal diameter, 40 mm; proximal diameter, 25 mm) encased in gray micrite, was processed at the University of California, Los Angeles. The calyx walls were 5–10 mm thick. The specimen was cut transversely with a diamond saw into 1-cm-thick slabs. Calyx wall material was removed from each slab with a diamond drill (Dremel, Racine, WI) and yielded 49.17 g of finely powdered skeleton. A few milligrams were saved for carbonate δ13C and δ18O analyses. To avoid destruction of OM in the finely powdered skeleton, we omitted treatment with hot NaOH. The powder was dissolved in 2 M HCl as described above. The solution was adjusted to pH 7.0 with 0.1 M NaOH, dialyzed, and lyophilized, yielding 0.59 g of tan powder (1.19% of skeleton weight). Approximately 23 g of the micrite was also powdered. It behaved like a carbonate-rich sediment, dissolving in 2 M HCl with concomitant evolution of bubbles. The solution was neutralized, dialyzed, and lyophilized, yielding 0.4 g of residue (1.7% of total weight). Specimens 2 and 3 were processed in Orsay and treated as described above except that after decalcification, the solution was centrifuged (3000 × g, 10 min) to remove particulate material. The supernatant was desalted on a Sephadex G-25 column (Amersham Pharmacia) and purified further by ultrafiltration (Pall-Filtron cells) using molecular-mass cutoffs of 3.0 kDa for sample 2 and 0.5 kDa for sample 3 and then lyophilized.
Stable isotopes were measured in the departments of Geology (University of Texas) and Earth Sciences (University of California, Santa Cruz). At the University of Texas, dried samples of algae, tissue, and OM were pulverized and treated with 10% HCl to remove any residual CaCO3. Samples were analyzed by combustion at 850°C in quartz tubes (40) so that both δ13C and δ15N values were obtained from the same sample. Carbon is reported relative to the Pee Dee Belemnite standard, and nitrogen isotopic compositions were normalized by using the N-1 standard (+1.36‰) and are reported relative to atmospheric nitrogen. Both isotopic compositions were reproducible to better than 0.3‰. At the University of California, Santa Cruz, isotopes of algae, tissue, and OM were analyzed with a Carlo Erba 2500 elemental analyzer attached to a VG Optima gas-source mass spectrometer. Sample powder (4–40 mg) was flash-combusted by O2 in a He stream, and sample gases were converted to CO2 and N2 on heated catalyst columns. CO2 and N2 then were separated on a chromatographic column before injection into the mass spectrometer. The SD of replicate analyses of an internal laboratory gelatin standard was <0.2‰ for both carbon and nitrogen values. Isotopes of P. major skeletal carbonate (δ13C and δ18O) were analyzed with a VG Micromass Autocarb coupled to the mass spectrometer. Each sample was reacted in a bath of 100% phosphoric acid at 90°C. Average precision, as determined for replicate analyses of calcite (NBS-19) and CM (a lab standard), was <0.1‰ for both δ13C and δ18O. Laboratory bias was not a concern, because both laboratories consistently discerned, with a high level of significance, the difference between OM δ15N in symbiotic vs. nonsymbiotic corals. Stable isotope data are expressed relative to standards using the conventional per mil (‰) notation.
Stable isotope data were evaluated statistically by using minitab 13 (Minitab, State College, PA). Means were compared by using one-way ANOVA. When more than two means were compared, we used Tukey's procedure for pairwise comparison. The family error rate was 0.05. Individual error rates are reported with the results.
Results
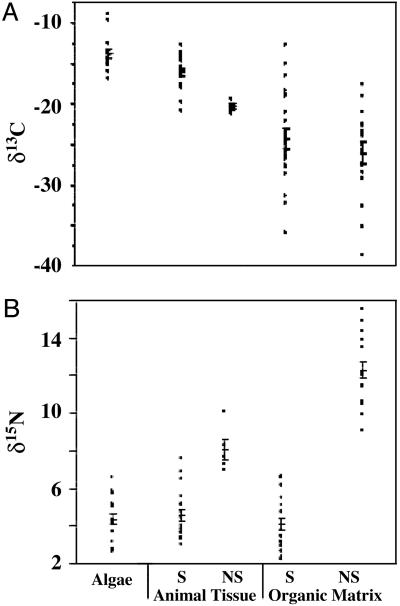
Fig. 1 shows the range of δ13C and δ15N values in modern symbiotic and nonsymbiotic species. In the symbiotic group there is a modest 8‰ range in δ13C in algae (-9‰ to -16.8‰) and animal tissue (-12.8‰ to -20.8‰) and a substantial 23‰ range (-12.7‰ to -35.8‰) in the skeletal OM. The mean δ13C values for algae, animal, and OM compartments (-13.8‰, -16.5‰, and -24.3‰, respectively; Table 2) indicate progressive depletion of 13C from producer (algae) to consumer (animal tissue and OM) compartments. OM δ13C is depleted by almost 8‰ relative to animal tissue, a feature that supports the argument that OM is not contaminated by animal tissue. Similarly, the range of δ13C values is modest in nonsymbiotic animal tissue (-19.4‰ to -21.2‰) and substantial in OM (-17.6‰ to -38.5‰) (Fig. 1 A). Animal tissue and OM are both depleted in 13C, the latter by ≈6‰ relative to animal tissue. Mean OM δ13C in symbiotic and nonsymbiotic corals (Table 2) are not significantly different [ANOVA: F(1, 38) = 0.98; P > 0.33].
Fig. 1.
Range of δ13C(A) and δ15N(B) values in algae, animal tissue, and OM in symbiotic (S) and nonsymbiotic (NS) corals. Shown are mean ‰ ± SEM (horizontal bars).
Table 2. Mean stable isotope values (‰ ± SD of the mean) (n) for coral organic fractions.
| Isotope | Organic fraction | Symbiotic | Nonsymbiotic | P. major |
|---|---|---|---|---|
| δ13C | Algae | -13.84 ± 2.02 (17) | n/a | |
| Animal | -16.53 ± 2.22 (17) | -20.30 ± 0.83 (5) | ||
| Organic matrix | -24.31 ± 5.60 (23) | -26.08 ± 5.52 (17) | -23.23 ± 2.60 (3) | |
| δ15N | Algae | 4.35 ± 1.23 (18) | n/a | |
| Animal | 4.56 ± 1.40 (17) | 8.08 ± 1.23 (5) | ||
| Organic matrix | 4.09 ± 1.51 (24) | 12.28 ± 1.81 (17) | 4.66 ± 2.72 (3) |
Data for Oculina spp. are included with nonsymbiotic groups (see Results and Discussion). n/a, not applicable.
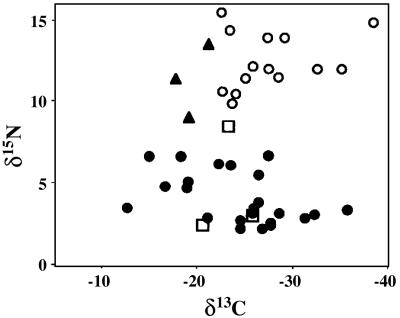
δ15N in modern symbiotic corals ranges in algae from 2.6‰ to 6.6‰, in animal tissue from 3.0‰ to 7.6‰, and in OM from 2.2‰ to 6.7‰ (Fig. 1B). The mean OM δ15N is 4.09‰ ± 1.51‰ (Table 2). δ15N in nonsymbiotic species ranges from 7.0‰ to 10.1‰ in animal tissue and 9.9‰ to 15.5‰ in OM (Fig. 1B), revealing a progressive enrichment of 15N in animal tissue and OM (Table 2). Nonsymbiotic δ15N OM is enriched by 4.4‰ over nonsymbiotic animal tissue to 12.28‰ ± 1.81‰. In contrast, mean δ15N values in algae, animal tissue, and OM in symbiotic corals are similar. There is no trend in δ15N that would indicate trophic enrichment. In this respect, OM δ15N in symbiotic corals seems uncoupled from conditions that affect δ13C. The most striking observation is the significant difference in mean OM δ15N between symbiotic and nonsymbiotic corals [ANOVA: F(1, 39) = 247.7; P << 0.001; Figs. 1B and 2 and Table 2]. The latter are more enriched than the former by an average of 8.73‰. These data indicate that the algae have a significant effect on the magnitude of coral OM δ15N, rendering it potentially useful as a proxy for photosymbiosis in coral paleobiological research.
Fig. 2.
Paired δ13C/δ15N values (‰) for OM. •, symbiotic; ○, nonsymbiotic; ▴, Oculina spp.; □ P. major.
Some species exhibit unusual δ15N values. The mean Fungia scutaria OM δ15N (6.4‰ ± 0.3‰) is significantly higher than that of other symbiotic corals [3.5‰ ± 1‰; ANOVA: F(1, 22) = 43.02; P < 0.001]. The reason is obscure but may be related to the fact that F. scutaria is a large, solitary polyp; the other symbiotic species are all colonial.
The mean OM δ15N for both symbiotic and aposymbiotic Oculina spp. is significantly higher still (11.4‰ ± 2.2‰) and is not significantly different from the mean OM δ15N for nonsymbiotic corals (12.28‰ ± 1.81‰; ANOVA: F(1, 15) = 0.927; P > 0.35; Fig. 2). OM δ15N in Oculina spp. is clearly aligned with the nonsymbiotic group.
To determine whether OM δ15N is a useful proxy for photosymbiosis, we analyzed stable isotopes of the Triassic coral P. major. OM δ13C in the three specimens of P. major were -23.3‰, -25.8‰, and -20.6‰. The average (-23.23‰) is not significantly different from OM δ13C for modern symbiotic or nonsymbiotic scleractinians (individual error rate = 0.0196; Table 2), confirming that this isotope is not a useful organic marker for photosymbiosis. δ13C and δ18O in skeletal carbonate (3.5‰ and -3.5‰, respectively) falls within the range of data obtained by Stanley and Swart (35) for this species (see Discussion). The δ13C of the micrite organic fraction was -28.9‰.
OM δ15N in P. major specimens 1–3 are 8.5‰, 3.0‰, and 2.47‰, respectively. The mean value (4.65‰ ± 1.92‰) is not significantly different from that of modern symbiotic corals and is significantly different from that of nonsymbiotic corals (individual error rate = 0.0105; Fig. 2 and Table 2). Because δ15N of the organic residue recovered after dissolution of micrite was -0.2‰, contamination of OM by micrite constituents is not a concern. We conclude from these data that P. major was very likely photosymbiotic.
Discussion
Gautret et al. (22) first observed significant biochemical differences in OM isolated from modern symbiotic and nonsymbiotic coral skeletons. Here we show that these coral ecotypes also differ significantly with respect to OM δ15N and that OM δ15N is a useful proxy for photosymbiosis in paleobiological research on corals.
Differences in absolute value of δ15N depends on coral nutritional lifestyle and δ15N of source nitrogen. Nonsymbiotic corals exhibit a strict heterotrophic nutritional lifestyle. They feed mainly on particulate organic nitrogen sources already enriched in 15N. After assimilation of these sources, the coral animal tissue δ15N is enriched further. In this study it averages 8.08‰ (Table 2). In contrast, symbiotic corals exhibit both phototrophic and heterotrophic nutritional lifestyles. δ15N of the algae, animal, and matrix compartments is depleted in 15N and averages 4.33‰ (Table 2). This value is near the low end of the general range of δ15N values (-3‰ to 20‰) previously reported for marine organisms (41) and might be explained by the phototrophic uptake and assimilation of 15N-depleted dissolved inorganic ammonium by the symbiotic algae. Phototrophy may well predominate over heterotrophy in symbiotic corals, because even at the low ambient concentrations associated with coral reefs, ammonium uptake is sufficient to supply the daily demand of a symbiotic coral for new nitrogen (42). The similarity of δ15N in symbiotic coral compartments might be the result of nutrient recycling, which could bring ammonium assimilation products to some equilibrium δ15N value in algae and animal tissue, including OM precursors (1, 10, 11, 43). It is interesting to note that in several symbiotic corals from the Great Barrier Reef, OM δ15N is less enriched in 15N than algae or animal tissue and averages ≈1.3‰ (11). Such a trend might result from synthesis of OM proteins unique to those species. Finally, in some Caribbean corals, particularly Montastrea cavernosa living below 10 m, δ15N of algae and animal tissue is <1.00‰ (8). To this observation we add the recent discovery of cyanobacteria living in apparent stable endosymbiosis in animal cells of M. cavernosa (44). These cyanobacteria are capable of fixing dissolved atmospheric nitrogen and releasing products of nitrogen fixation to algae and animal cells. Because δ15N of atmospheric nitrogen is ≈0.00‰, we would expect δ15N of ammonium nitrogen, the first stable product of nitrogen fixation, to be relatively depleted in 15N but with room for enrichment during assimilation by the algae. This scenario provides an alternative explanation as to why δ15N in symbiotic corals is depleted in 15N but awaits confirmation that symbiotic corals generally contain cyanobacteria and nonsymbiotic corals do not.
In contrast to OM δ15N, OM δ13C is not a useful proxy for photosymbiosis, but the values of δ13C in algae, animal tissue, and OM do evoke an interesting hypothesis. Symbiotic algae (δ13C = -13.84‰; Table 2) are customarily enriched in 13C (7, 45, 46). Ninety percent or more of this carbon is translocated to the host animal cells (1). The animal cells also acquire carbon by feeding on demersal zooplankton, the δ13C of which averages approximately -19‰ (47). The combination of translocation, holozoic feeding, and concomitant isotopic fractionation results in animal tissue depleted in 13C by 1–3‰, consistent with producer–consumer trophic dynamics (48). Here, the mean for animal tissue is -16.5‰ (Table 2), but there is a further depletion of 13C in OM in both symbiotic and nonsymbiotic corals of ≈7–8‰. This depletion is 3-fold larger than that usually attributed to trophic dynamics. We hypothesize that fractionation of carbon isotopes is substantial during synthesis of OM in animal calicoblast cells. The resulting large isotopic difference between animal cells and OM is maintained because newly synthesized OM is not retained by the calicoblast cells but secreted immediately and mineralized rapidly. That is, animal tissue δ13C is not “diluted” by retention of a pool of isotopically lighter OM precursors. This hypothesis is supported by the data of Allemand et al. (17), who used [14C]aspartate to show that synthesis of OM and concomitant CaCO3 precipitation is rapid, occurring within 20 min of introduction of the labeled precursor. There also may be intrinsic biochemical differences between animal tissue and OM, which include preferential incorporation of 13C-depleted (or -enriched) amino acids into OM (16).
The interpretation that P. major was very likely photosymbiotic is consistent with the findings of Stanley and Swart (35), who used a method based on skeletal carbonate δ13C and δ18O to determine whether fossil corals, including P. major, may have contained photosynthetic endosymbionts (3). They compared modern symbiotic and nonsymbiotic scleractinians with Triassic and Jurassic fossils. Data from 13 Triassic species were similar to modern symbiotic corals with respect to carbonate δ18O and enriched with respect to carbonate δ13C. The authors concluded that Late Triassic corals were very likely symbiotic. Jurassic corals were clearly aligned with nonsymbiotic corals.
The alignment of Oculina spp. with nonsymbiotic corals with respect to OM δ15N is not surprising. These species exemplify temperature- and light-dependent facultative symbioses in temperate, often turbid, warm-water habitats along the coast of North Carolina and Florida (49, 50). Algae are present in Oculina arbuscula in shallow water (1 m) but in densities (cells per cm2) often an order of magnitude less than that normally found in tropical symbiotic corals. Given the lower annual rates of insolation, frequent episodes of increased turbidity at the latitude and depth of collection, and greater dependence on heterotrophic nutrition (50), the flux of organic nitrogen between algae, animal tissue, and OM in shallow-water O. arbuscula would seem to be insufficient to generate a symbiosis-specific OM δ15N signature. O. arbuscula from 6 m, collected from dark habitats (e.g., under ledges), and Oculina varicosa from 100 m are both aposymbiotic and depend largely on heterotrophic nutrition. As such, they also behave as nonsymbiotic corals.
As an extraction product, skeletal OM is vulnerable to contamination by fragments of animal tissue and by endoliths. This potential problem has not yet been addressed critically in scleractinian corals. We treated pieces of intact skeleton with sodium hypochlorite, and coarsely ground skeleton with NaOH, to remove any residual animal tissue fragments. This step is especially crucial to the decontamination of perforate skeletons. The finding that the skeletal OM in both symbiotic and nonsymbiotic corals has δ13C values very different (Table 2) from those of the algae and animal tissue argues strongly against the interpretation that OM represents “trapped” remnants of host tissue (51). Corals also differ in the extent of visible contamination by endolithic algae. Although green skeletons can be discarded easily, white skeletons may still contain chlorophyll-free algal filaments and colorless fungal hyphae. We carried out one trial experiment in which a large piece of green skeleton (F. scutaria) was processed by our method to determine whether the OM stable-isotope signature differed from similarly processed F. scutaria white skeleton. OM δ13C from the single large sample of green skeleton (-22.2‰) fell within the range observed for white skeleton (-23.0‰ ± 6.38‰; n = 3). OM δ15N from the green skeleton (6.2‰) was similar to that for OM δ15N from white skeleton (6.46‰ ± 0.32‰; n = 3), suggesting in a preliminary way that endoliths (including fungi) were removed by the extraction protocol. Because sample sizes were small, this interpretation is still subject to confirmation. A less likely alternative interpretation is that endoliths have survived the extraction protocol, have isotope signatures intrinsically similar to those of OM, and also differ significantly in abundance in symbiotic and nonsymbiotic corals.
The effects of diagenesis on P. major OM might be expected to include, at a minimum, selective loss of amino acids (52) and thereby raise questions about the meaning of the OM δ15N signal. However, Cuif (36) described the remarkable status of preservation of P. major aragonite crystals and the extant OM associated with these crystals. The proportion of acidic, alkaline, basic, aromatic, and alcoholic amino acids in the extant OM and the pattern of sulfated polysaccharides in P. major wall structures is comparable to those of modern coral OM (19, 53). The difference in OM δ15N for the three specimens of P. major (8.5‰, 3.01‰, and 2.47‰) could have resulted from different extraction protocols. Sample 1 consisted of aggregate (insoluble and soluble) OM, because it was obtained without centrifugation and dialyzed by using 3.5-kDa cutoff membranes. Samples 2 and 3 consisted only of soluble OM having been subjected to centrifugation and passage through ultrafiltration cells with molecular mass cutoffs of 3.0 and 0.5 kDa, respectively. Classically, centrifugation is used to separate insoluble OM from the soluble OM (15). Insoluble OM represents ≈66% of the total OM in corals (D.A., unpublished data) and is viewed as a structural framework only (14). By omitting centrifugation, sample 1 very likely includes more insoluble OM than samples 2 and 3. On the other hand, by using ultrafiltration pore sizes with molecular mass cutoffs of 3 and 0.5 kDa, more of the low molecular mass constituents are retained in the soluble OM, including any small peptides that may occur in fossil specimens. These observations suggest that more attention should be given to low molecular mass soluble fractions in studies of coral OM. The function of soluble OM in corals has never been demonstrated experimentally. By inference from data on molluscan shell soluble OM (54), it is hypothesized to be one of mineral nucleation and growth modulation.
With respect to scleractinian evolution, P. major skeletal microstructure is comparable to that of Permian plerophyllids (37), the major difference between the two families being the mineralogy. Whereas in all Paleozoic corals, plerophyllid skeletons were calcitic, the evolution of biomineralization in P. major has led to production of modern aragonitic units. This divergence suggests that photosymbiosis may have played a role in coral skeletogenesis after the complete decay of Rugosa during the Upper Permian.
Coral skeletons have been proven to be good proxy physiological and environmental recorders. However, all the data come from analysis of the mineral fraction (55). Here we demonstrate that OM δ15N is a good proxy recorder of photosymbiosis, providing paleobiological information reaching back to the Paleozoic. The difference between symbiotic and nonsymbiotic OM δ15N demonstrates unambiguously an effect of photosymbiosis on OM synthesis (22, 27). It now seems that symbiotic algae may control calcification by both modification of physicochemical parameters within the coral polyps (56, 57) and augmenting the synthesis of OM (17).
Acknowledgments
We thank Paul Koch (University of California, Santa Cruz) and Rachel Eustice (University of Texas, Austin) for stable isotope analyses; Sandra Brooke and Jay Stachowicz for Oculina spp.; Lior Vaki for Stylophora pistillata; Ove Hoegh-Guldberg for Tubastrea micrantha; Helmut Zibrowius for deep-water nonsymbiotic corals; Kim Selkoe for laboratory assistance at the University of California, Los Angeles; Stéphanie Reynaud-Vaganay and Christine Ferrier-Pagès for valuable suggestions on our manuscript; Steve Obrebski for statistical analyses; and the Centre Scientifique de Monaco for research support.
Abbreviation: OM, organic matrix.
References
- 1.Muscatine, L. (1990) in Coral Reefs, Ecosystems of the World, ed. Dubinsky, Z. (Elsevier, Amsterdam), pp. 75-84.
- 2.Gattuso, J.-P., Allemand, D. & Frankignoulle, M. (1999) Am. Zool. 39, 160-183. [Google Scholar]
- 3.Swart, P. K. (1983) Earth Sci. Rev. 19, 51-80. [Google Scholar]
- 4.Grottoli, A. G. & Wellington, G. M. (1999) Coral Reefs 18, 29-41. [Google Scholar]
- 5.Land, L. S., Lang, J. C. & Smith, B. N. (1975) Limnol. Oceanogr. 20, 283-287. [Google Scholar]
- 6.Goreau, T. J. (1977) Proc. R. Soc. London B 196, 291-315. [Google Scholar]
- 7.Muscatine, L., Porter, J. W. & Kaplan, I. R. (1989) Mar. Biol. (Berlin) 100, 185-193. [Google Scholar]
- 8.Muscatine, L. & Kaplan, I. R. (1994) Pac. Sci. 48, 304-312. [Google Scholar]
- 9.Heikoop, J. M., Dunn, J., Risk, M. J., Sandeman, I. M., Schwarcz, H. P. & Waltho, N. (1998) Limnol. Oceanogr. 43, 909-920. [Google Scholar]
- 10.Sammarco, P. W., Risk, M. J., Schwarcz, H. P. & Heikoop, J. M. (1999) Mar. Ecol. Prog. Ser. 180, 131-138. [Google Scholar]
- 11.Hoegh-Guldberg, O., Muscatine, L., Goiran. C., Siggaard, D. & Marion, G. (2004) Mar. Ecol. Prog. Ser. 280, 105-114. [Google Scholar]
- 12.Cuif, J.-P., Dauphin, Y. & Gautret, P. (1999a) Int. J. Earth Sci. 88, 582-592. [Google Scholar]
- 13.Constantz, B. R. & Weiner, S. (1988) J. Exp. Zool. 248, 253-258. [Google Scholar]
- 14.Towe, K. M. (1972) Biomineralization 4, 1-7. [Google Scholar]
- 15.Lowenstam, H. A. & Weiner, S. (1989) On Biomineralization (Oxford Univ. Press, New York).
- 16.Addadi, L. & Weiner. (1985) Proc. Natl. Acad. Sci. USA 82, 4110-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allemand, D., Tambutté, E., Girard, J.-P. & Jaubert, J. (1998) J. Exp. Biol. 201, 2001-2009. [DOI] [PubMed] [Google Scholar]
- 18.Isa, Y. & Okazaki, M. (1987) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 87, 507-512. [Google Scholar]
- 19.Cuif, J.-P., Denis, A., Gautret, P., Marin, F., Mastandrea, A. & Russo, F. (1992) C. R. Acad. Sci. 314, 1097-1102. [Google Scholar]
- 20.Gautret, P. & Marin, F. (1993) C. R. Acad. Sci. 316, 1319-1325. [Google Scholar]
- 21.Cuif, J.-P. & Gautret, P. (1995) C. R. Acad. Sci. 320, 273-278. [Google Scholar]
- 22.Gautret, P., Cuif, J.-P. & Freiwald, A. (1997) Facies 36, 189-194. [Google Scholar]
- 23.Dauphin, Y. & Cuif, J.-P. (1997) Electrophoresis 18, 1180-1183. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg, W. M. (2001) Tissue Cell 33, 376-387. [DOI] [PubMed] [Google Scholar]
- 25.Fricain, J. C., Alouf, J., Bareille, R., Rouais, F. & Rouvillain, J. L. (2002) Biomaterials 23, 273-679. [DOI] [PubMed] [Google Scholar]
- 26.Gautret, P. & Aubert, F. (1993) C. R. Acad. Sci. 316, 1445-1491. [Google Scholar]
- 27.Cuif, J.-P., Dauphin, Y., Freiwald, A., Gautret, P. & Zibrowius, H. (1999) Comp. Biochem. Physiol. A Physiol. 123, 269-278. [Google Scholar]
- 28.Fukuda, I., Ooki, S., Fujita, T., Murayama, E., Nagasawa, H., Isa, Y. & Watanabe, T. (2003) Biochem. Biophys. Res. Commun. 304, 11-17. [DOI] [PubMed] [Google Scholar]
- 29.Johnston, I. S. (1980) Int. Rev. Cytol. 67, 171-214. [Google Scholar]
- 30.Puverel, S., Tambutté, E., Bouchot, A., Zoccola, D., Payan, P., Cuif, J.-P. & Allemand, D., in 8th International Symposium of Bioorganic Mineralization, eds. Kobayashi, I. & Sasagawa, I. (Kurokawa, Niigata, Japan), pp. 2-26, in press.
- 31.Clode, P. L. & Marshall, A. T. (2003) Protoplasma 220, 153-161. [DOI] [PubMed] [Google Scholar]
- 32.Muscatine, L. & Cernichiari, E. (1969) Biol. Bull. (Woods Hole, Mass.) 137, 506-523. [DOI] [PubMed] [Google Scholar]
- 33.Pearse, V. B. (1971) in Experimental Coelenterate Biology, eds. Lenhoff, H. M., Muscatine, L. & Davis, L.V. (Univ. of Hawaii Press, Honolulu), pp. 239-245.
- 34.Rinkevich, B. (1991) Symbiosis 10, 175-193. [Google Scholar]
- 35.Stanley, G. D. & Swart, P. K. (1995) Paleobiology 21, 179-199. [Google Scholar]
- 36.Cuif, J.-P. (1972) C. R. Acad. Sci. 274, 1272-1275. [Google Scholar]
- 37.Cuif, J.-P. (1975) Geobios Mem. Spec. 8, 157-180. [Google Scholar]
- 38.Steen, R. G. & Muscatine, L. (1987) Biol. Bull. (Woods Hole, Mass.) 172, 246-263. [Google Scholar]
- 39.Bentis, C. J., Kaufman, L. & Golubic, S. (2000) Biol. Bull. (Woods Hole, Mass.) 198, 254-260. [DOI] [PubMed] [Google Scholar]
- 40.Lajtha, K. & Michener, R. H. (1994) Stable Isotopes in Ecology and Environmental Science (Blackwell Scientific, Oxford).
- 41.Owens, N. P. J. (1987) Adv. Mar. Biol. 24, 389-451. [Google Scholar]
- 42.Hoegh-Guldberg, O. & Williamson, J. (1999) Mar. Biol. 133, 561-570. [Google Scholar]
- 43.Uhle, M. E., Macko, S., Spero, H. J., Lea, D. W., Ruddiman, W. F. & Engel, M. H. (1999) Limnol. Oceanogr. 44, 1968-1977. [Google Scholar]
- 44.Lesser, M. P., Mazel, C. H., Gorbunov, M. Y. & Falkowski, P. (2004) Science 305, 997-1000. [DOI] [PubMed] [Google Scholar]
- 45.Risk, M. J., Sammarco, P. W. & Schwarcz, H. P. (1994) Mar. Ecol. Prog. Ser. 106, 121-130. [Google Scholar]
- 46.Reynaud-Vaganay, S., Juillet-Leclerc, A., Jaubert, J. & Gattuso, J.-P. (2001) Paleogeogr. Paleoclimatol. Paleoecol. 175, 393-404. [Google Scholar]
- 47.Land, L. S., Lang, J. C. & Barnes, D. J. (1977) Geochim. Cosmochim. Acta 41, 169-172. [Google Scholar]
- 48.Rau, G. H., Mearns, A. J., Young, D. R., Olson, R. J., Schafer, H. A. & Kaplan, I. R. (1983) Ecology 64, 1314-1318. [Google Scholar]
- 49.Reed, J. (1981) in Proc. 4th Int. Coral Reef Symp., eds. Gomez, E. D., Birkeland, C. E. & Buddemeier, R. W. (Univ. of the Philippines, Quezon City), Vol. 2, 201-206. [Google Scholar]
- 50.Miller, M. W. (1995) Mar. Ecol. Prog. Ser. 122, 217-225. [Google Scholar]
- 51.Barnes, D. J. (1970) Science 170, 1305-1308. [DOI] [PubMed] [Google Scholar]
- 52.Whitelaw, M. J., Batts, B. D., Murray-Wallace, C. V. & McRae, C. R. (2001) Proc. Linn. Soc. N. S. W. 123, 225-234. [Google Scholar]
- 53.Cuif, J.-P., Dauphin, Y., Doucet, J., Salaome, M. & Susini, J. (2003) Geochim. Cosmochim. Acta 67, 75-83. [Google Scholar]
- 54.Gotliv, B.-A., Addadi, L. & Weiner, S. (2003) Chembiochem 4, 522-529. [DOI] [PubMed] [Google Scholar]
- 55.Barnes, D. J. & Lough, J. M. (1996) Glob. Change Biol. 2, 569-582. [Google Scholar]
- 56.Furla, P., Bénazet-Tambutté, S., Jaubert, J. & Allemand, D. (1998) Am. J. Physiol. 274, R303-R310. [DOI] [PubMed] [Google Scholar]
- 57.Furla, P., Galgani, I., Durand, I. & Allemand, D. (2000) J. Exp. Biol. 203, 3445-3457. [DOI] [PubMed] [Google Scholar]