Abstract
The influence of the immune system on atherosclerosis involves both helper T (Th) cell and antibody responses to plaque antigens. These responses may have proatherogenic and protective effects. T-bet is a transcription factor required for Th1 differentiation and regulates the balance between Th1 and Th2 responses in inflammatory diseases. To clarify how helper T cell subset differentiation influences atherosclerosis, we compared lesion development and immune responses to plaque antigens in low-density lipoprotein receptor-deficient (Ldlr-/-) mice with or without functional T-bet genes. Atherosclerosis was significantly reduced in T-bet-deficient Ldlr-/- mice compared with Ldlr-/- controls, and the lesions that did develop in the absence of T-bet had less smooth muscle cell content. Furthermore, T-bet deficiency caused a Th2 switch in the response to the atherosclerosis-associated antigen heat shock protein-60, and a change in T-dependent isotypes of oxidized LDL-specific antibodies. Of particular significance, T-bet deficiency caused a >250% increase in the titer of E06 antibodies, which are known to be atheroprotective and whose production by B-1 B cells is enhanced by IL-5. These findings establish that T cell subset differentiation influences both T cell and antibody responses that modulate atherosclerosis, and validate the therapeutic goal of skewing T responses to atherosclerosis-associated antigens.
Keywords: cytokines, T cells, antibodies
It is now well established that the immune system is involved in atherosclerosis and exerts a profound influence on the disease progression (1). Immunohistochemical analyses show that atherosclerotic plaques contain significant numbers of T cells at all ages of lesion development, in both humans (2, 3) and rodents (4, 5). Both CD4+ and CD8+ T cells are present in plaques, but the CD4+ T cells are more abundant (6). Hypercholesterolemia and arterial disease are associated with T cell-mediated and humoral responses specific for a variety of plaque antigens, including heat shock protein-60 (hsp60), and epitopes of oxidized low-density lipoprotein (Ox-LDL) (7). A variety of studies in genetically modified mice indicate that these immune responses can influence the progression of arterial disease (8).
CD4+ cells can be categorized in functional subsets according to the types of cytokines they produce (9). T helper (Th)1 cells mainly produce IFN-γ and activate macrophages, whereas Th2 cells produce IL-4, IL-5, IL-10, and IL-13, are associated with allergic diseases, and down-regulate Th1-mediated responses (10). Both Th1 and Th2 cells may promote humoral immune responses to protein antigens, but each will stimulate production of different Ig isotypes (e.g., Th1/IFN-γ induces IgG2a whereas Th2/IL-4 induces IgG1 and IgE, in mice) (9).
Cell-mediated immune responses in atherosclerosis are believed to be Th1-dominated, based principally on the evidence that IFN-γ, but not IL-4, is usually found in lesions (11). T cells isolated from lymphoid organs of atherosclerotic animals that are specific for putative plaque antigens have a Th1 phenotype (12–14). A proatherogenic role for Th1 cells has been shown in atherosclerosis-susceptible mice that are deficient in either IFN-γ receptors (15) or the cytokine itself (13, 16). Although Th1 cells may be the primary effector T cells influencing atherosclerosis, severe hypercholesterolemia is associated with a Th1/Th2 switch of the autoimmune response in atherosclerotic ApoE-deficient mice (17), and pharmacologic enhancement of Th2 responses reduced atherogenesis in ApoE-deficient mice (18). Furthermore, recent work has established that the Th2 cytokine IL-5 promotes the production of atheroprotective E06 antibodies by B-1 B cells (19).
Helper T cell subset differentiation is regulated by lineage-restricted transcription factors that influence the expression of multiple cytokine genes (20). T-bet is a member of the T-box family of transcription factors, which induces Th1 differentiation and suppresses Th2 differentiation (21). T-bet knockout mice have a severe defect in production of IFN-γ from CD4 T, antigen-specific CD8 T, and dendritic cells, and immune responses in these mice are skewed toward a Th2 phenotype (22, 23). To determine the overall influence of helper T cell subset differentiation on atherosclerosis, we analyzed the influence of T-bet deficiency on the extent and phenotype of diet-induced atherosclerosis and plaque antigen-specific cell-mediated and humoral responses in athero-prone Ldlr-/- mice. Our findings indicate a Th1-to-Th2 shift, and the linked antibody responses accompanying this shift protect against cholesterol-diet-induced atherosclerosis.
Methods
Mice. Ldlr-/- mice (24), backcrossed 10 times onto a C57BL/6 background, purchased from The Jackson Laboratory, were crossbred with T-bet-/- mice on a C57BL/6 background (25). The doubly heterozygous progeny were intercrossed to generate double knockout T-bet-/-Ldlr-/- mice and T-bet+/+Ldlr-/- controls. Animal genotype was identified by a PCR-based assay (22, 24). There were no discernible differences in litter size or appearance of the T-bet-deficient versus control mice. There was also no significant difference in the weights of T-bet -/-Ldlr-/- and T-bet+/+Ldlr-/- mice before or after cholesterol-diet feeding. All mice were housed and bred in accordance with the institutional guidelines of Brigham and Women's Hospital and Harvard Medical School.
Study Protocol. Beginning at 5–6 weeks of age, T-bet-/-Ldlr-/- or sex-matched T-bet+/+Ldlr-/- (control) mice were fed a semipurified diet (No. D12108, Research Diets, New Brunswick, NJ) containing 40% kcal lipid, 1.25% cholesterol (26) ad libitum (n = 16, 8 males and 8 females per group). After 8 weeks, the mice were fasted overnight and killed by halothane inhalation. Blood was collected by vena cava nicking, and the arterial tree was perfused with PBS. Perfused aortas were dissected from the aortic valve to the iliac bifurcation: the aortic arches were cut and separated from the remaining aorta, then rapidly frozen in optimal cutting temperature embedding medium (OCT, Tissue-Tek, Sakura Finetek, Torrance, CA); the remaining thoracic and abdominal aorta (descending aorta) from each mouse was fixed in 10% buffered formalin.
Aortic Atherosclerotic Lesion Analysis. Two types of analyses were performed to quantify atherosclerotic lesions in the aortic arch or the descending aorta, respectively, as described (13). First, longitudinal 5-μm cryostat sections of the aortic arch, three per specimen, were stained with oil red O (ORO), and intimal area and lipid-positive areas, within a defined 2-mm stretch of aortic wall, were determined by computerized image analysis. Second, the remaining formalin-fixed descending aorta from each mouse was stained with ORO, opened longitudinally, pinned out and digitally photographed. The percentage surface area occupied by ORO-stained lesions viewed en face was determined.
Immunohistochemistry. Serial longitudinal cryostat sections of aortic arch adjacent to the oil red O-treated sections were stained with molecule-specific and isotype control antibodies as described (27). Mouse-specific antibodies included the following: anti-VCAM-1 (anti-vascular cell adhesion molecule 1) (Clone MK2, Chemicon); anti-I-A/I-E (Clone M5/114.15.2, BD Pharmingen) for class II MHC; anti-Mac3 (Clone M3/84, BD Pharmingen) for macrophages; anti-CD4 (Clone RM4-5) for CD4+ T cells; and anti-α-smooth muscle actin (Clone 1A4, Sigma) for smooth muscle cells.
Quantitative analysis of lesional content of class II MHC, vascular cell adhesion molecule 1, macrophages, and smooth muscle cells was determined by computer-assisted image analysis (13) and expressed as percentage of intimal area to normalize for overall differences between the study groups. Quantification of CD4+ staining was achieved by counting individual positively stained lesional cells in the aortic arch sections.
Serum Cholesterol Analysis. Overnight fasting serum was prepared from blood collected from individual mice at time of killing. Total serum cholesterol levels were measured by enzymatic assays (Roche Diagnostic) and expressed in milligrams per deciliter (mg/dl).
Serum Ig Analysis. Ox-LDL-specific antibodies were determined as described (28). In brief, wells were coated with malondialdehyde (MDA)-LDL and copper-oxidized (CuOx)-LDL, at 5 μg/ml in PBS/EDTA. Sera were diluted 1:100 in TBS/EDTA containing 1% BSA (BSA-TBS), and binding of IgM, IgG1, and IgG2a to wells coated with antigens was detected by chemiluminescent enzyme immunoassays using alkaline phosphatase (AP)-labeled secondary antibodies and the substrate LumiPhos (Lumigen). AP-labeled secondary antibodies used were as follows: goat anti-mouse IgM (Sigma–Aldrich); rat anti-mouse IgG1 (LO-MG1-2; Zymed); and rat anti-mouse IgG2a (R19-15; BD Biosciences–Pharmingen). For the detection of total IgG1 and IgG2a levels, a double antibody capture ELISA was used. Wells were coated with rat anti-mouse IgG1 (A85-3) or rat anti-mouse IgG2a (R11-89) at 2 μg/ml in PBS. Sera were diluted 1:200 in BSA-TBS, and binding to the wells coated with the monoclonal antibodies was detected by using 2 μg/ml biotinylated rat anti-mouse IgG1 (A85-1) or rat anti-mouse IgG2a (R19-15), respectively, followed by AP-labeled NeutrAvidin (Pierce). All anti-Ig antibodies were from BD Biosciences–Pharmingen.
For the measurement of T15 clonotypic (EO6) antibodies (29) in mouse sera, a double antibody capture assay was used as described (19). In brief, sera were diluted 1:100 in BSA-TBS, and binding of T15 clonotypic IgM antibodies (30) to wells coated with 2 μg/ml monoclonal anti-T15-idiotypic antibody AB1-2 (ATCC) was detected by using 0.1 μg/ml biotinylated AB1-2 followed by alkaline phosphatase-labeled NeutrAvidin.
In Vitro Assays of CD4+ Cytokine Secretion. Spleen and lymph nodes were removed from six T-bet-/-Ldlr-/- or T-bet+/+Ldlr-/- mice after 8 weeks of diet, and CD4+ T cells were isolated by anti-CD4 magnetic beads (Dynal, Great Neck, NY). The cells were stimulated in microwell cultures (5 × 104 per well) with plate-bound anti-CD3ε or with recombinant murine hsp60 (10 μg/ml) produced as described (31) plus syngeneic mitomycin C-treated spleen cells (5 × 105 per well). Ovalbumin (10 μg/ml) and medium alone were used as controls. Culture supernatants were removed at 48 h and analyzed by ELISA for IFN-γ, IL-4, IL-5, and IL-10 cytokines by using reagents from BD Pharmingen.
Statistical Analysis. All statistical analyses were performed by using prism software. Differences between T-bet -/-Ldlr-/- and T-bet+/+Ldlr-/- mice were analyzed by the Student t test and expressed as mean ± SEM. P ≤ 0.05 was considered significant for all analyses.
Results and Discussion
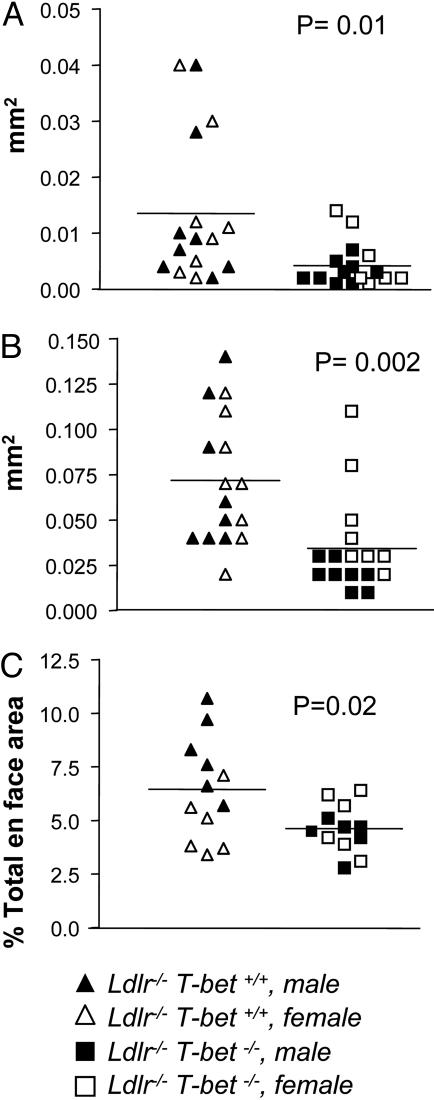
There were no statistical differences in total serum cholesterol levels between cholesterol diet-fed T-bet-/-Ldlr-/- and T-bet+/+Ldlr-/- mice (1,149 ± 35.85 mg/dl and 1,224 ± 68.23 mg/dl, respectively). There were also no significant differences in serum cholesterol levels when males and females were compared. Despite the similar cholesterol levels in the two groups of mice, atherosclerosis in both aortic arch and descending aorta were significantly reduced in T-bet-deficient mice compared with controls. The mean intimal area and lipid-positive area in the segments of aortic arch analyzed were respectively reduced by 53% and 69% in T-bet-/-Ldlr-/- mice compared with the areas in T-bet+/+Ldlr-/- mice (Fig. 1 A and B). The mean en face aortic lesional area in the descending aorta was also significantly less (28% reduction) in T-bet-deficient mice compared with controls (Fig. 1C). Interestingly, analysis by gender showed a greater effect of T-bet deficiency on extent of atherosclerosis in male mice than in female mice. T-bet-/-Ldlr-/- male mice had 81% reduction in the aortic arch intimal area of lesions and 49% reduction in descending aorta en face area compared with control males whereas there were no significant differences in the extent of atherosclerosis in T-bet-/-Ldlr-/- females vs. control females (Fig. 1). There were also significant differences in the extent of atherosclerosis between T-bet-deficient male and T-bet-deficient female mice, but no differences between control males and control females.
Fig. 1.
Reduced aortic atherosclerosis in T-bet-deficient Ldlr-/- mice. Quantification of aortic lesions was performed on digitally analyzed specimen images, as described in Methods. (A and B) Lipid and intimal positive areas, respectively, of aortic arch sections, are shown (n = 16 per group: 8 males and 8 females). (C) En face lipid positive areas of descending aorta are shown (n = 12 per group: 6 males and 6 females). Horizontal bars represent means. P values <0.05 for intergroup comparisons are shown.
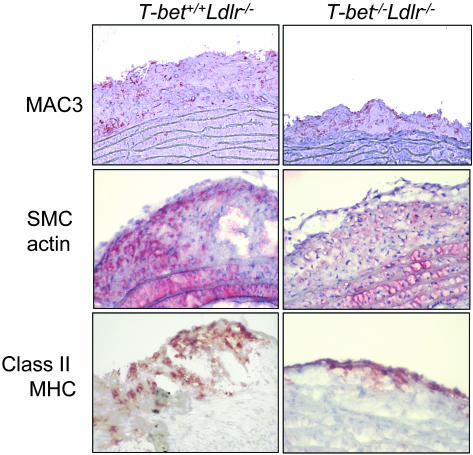
We also analyzed the phenotype of lesions after 8 weeks of proatherogenic diet (Table 1 and Fig. 2). There were no observable differences in intimal content of macrophages and CD4+ cells between T-bet-deficient and control animals. In contrast, intimal smooth muscle cell content was significantly lower in aortic arch sections of T-bet-deficient mice compared with control mice, indicating a delay in evolution of lesions toward a more complex advanced phenotype. Interestingly, we found no significant differences in the expression of the IFN-γ-regulated molecules VCAM-1 (vascular cell adhesion molecule 1) and class II MHC in aortic sections from T-bet-deficient mice compared with controls, but MHC class II was mostly present on endothelial cells overlying atherosclerotic lesions in T-bet-deficient mice, as we described in the IFN-γ-deficient Ldlr-/- mice (13). Analysis by gender of the phenotype of the lesion confirmed significant differences in smooth muscle cell content only when we compared T-bet-/-Ldlr-/- male mice vs. control males (1.3 ± 0.3 vs. 0.2 ± 0.2, P = 0.03).
Table 1. Lesion phenotype in T-bet+/+Ldlr-/- and T-bet-/-Ldlr-/- mice after 8 weeks of a high-fat/cholesterol diet.
| Mouse genotype | Macrophages, % of intimal area | Smooth muscle cells, % of intimal area | CD4-positive cells (no. of lesional cells per section) | VCAM-1, % of intimal area | Class II MHC, % of intimal area |
|---|---|---|---|---|---|
| T-bet+/+Ldlr-/- | 9.5 ± 2.2 | 1.0 ± 0.3* | 1.3 ± 0.6 | 9.7 ± 1.2 | 4.1 ± 0.8 |
| T-bet-/-Ldlr-/- | 10.3 ± 2.1 | 0.3 ± 0.2* | 1.7 ± 0.7 | 6.8 ± 1.3 | 3.3 ± 0.8 |
Values for macrophages, smooth muscle cells, CD4+ T cells, vascular cell adhesion molecule 1 (VCAM-1), and class II MHC-positive staining are expressed as mean ± SEM, determined by image analysis, as described in Methods. n = 12 (6 males and 6 females) for each group. *, P = 0.04.
Fig. 2.
Phenotype of aortic arch atherosclerotic lesions in T-bet -/-Ldlr-/- and T-bet+/+Ldlr-/- mice. Frozen sections of aortic arches taken from mice after 8 weeks of proatherogenic diet were stained with antibodies specific for macrophages (Mac3), smooth muscle cells (actin), and class II MHC, as described in Methods. Sections stained with isotype control antibodies were negative (data not shown). See Table 1 for quantification of stained areas.
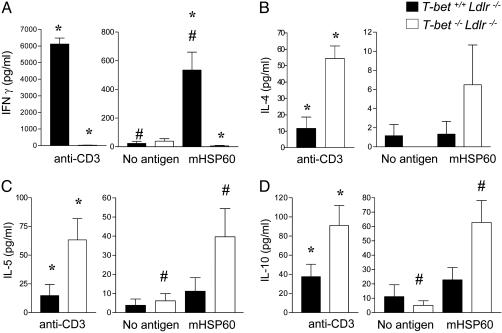
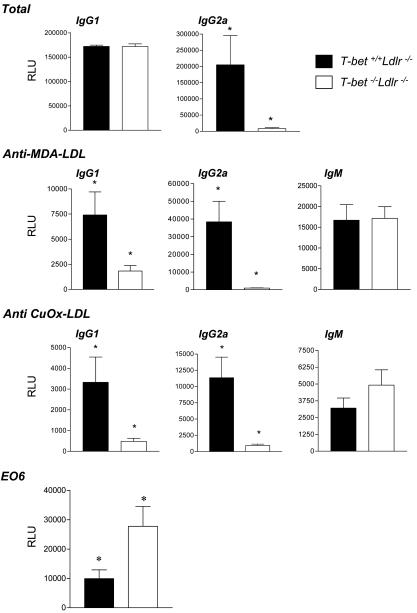
Hsp60 is a known lesional antigen implicated in the immunopathogenesis of atherosclerosis (1, 7, 13). CD4+ T cells isolated from hypercholesterolemic T-bet+/+Ldlr-/- mice produced significant amounts of IFN-γ, upon polyclonal stimulation with anti-CD3 and upon stimulation with plaque antigen hsp60, whereas T cells from T-bet-/-Ldlr-/- did not (Fig. 3). In contrast to the impaired production of IFN-γ in the absence of T-bet, significantly higher levels of IL-4, IL-5, and IL-10 were produced upon anti-CD3 stimulation of T cells from T-bet-/-Ldlr-/- mice compared with T cells from T-bet+/+Ldlr-/- controls, consistent with previous studies in T-bet-null mice (22). We also observed significant IL-5 and IL-10 secretion from hsp60-stimulated T-bet -/- CD4+ T cells but not from T-bet+/+ T cells (Fig. 3). These results indicate that a switch to a Th2-like response, notably to a specific lesional antigen, did occur in the absence of T-bet-regulated T cell differentiation. Furthermore, the serum Ig analysis showed a significant reduction of total IgG2a, indicative of suppression of proatherogenic Th-1 cellular (Fig. 4). We did not observe a significant difference in total levels of serum IgG1 between T-bet-deficient and control Ldlr-/- mice. Previous studies have also found that T-bet deficiency influences IgG2a levels more than IgG1 levels (25). To assess the impact of T-bet deficiency on the development of disease-specific anti-oxidized LDL (Ox-LDL) autoantibodies during atherogenesis in Ldlr-/- mice, we determined isotype-specific titers to two model antigens of Ox-LDL, namely MDA-modified LDL (MDA-LDL) and CuOx-LDL, at the end of the intervention period. As expected, IgG2a titers to both MDA-LDL and CuOx-LDL were significantly reduced or absent in T-bet-/-Ldlr-/- mice, compared with T-bet+/+Ldlr-/- mice. Surprisingly, IgG1 titers to both antigens were also significantly reduced in T-bet-/-Ldlr-/- mice, despite similar total levels of IgG1 in both groups (Fig. 4), suggesting specificity to the decrease of IgG1 to oxidation epitopes of atherosclerotic antigens.
Fig. 3.
Differences in cytokines production by CD4+ cells from T-bet -/-Ldlr-/- and T-bet+/+Ldlr-/- mice. CD4+ T cells were isolated from six T-bet-/-Ldlr-/- or T-bet+/+Ldlr-/- mice after 8 weeks of diet as described in Methods. The cells were stimulated in cultures with plate-bound anti-CD3ε or with recombinant murine hsp60 (mhsp60) plus syngeneic mitomycin C-treated spleen cells. Medium alone (No antigen) and ovalbumin (data not shown) were used as controls for nonspecific cytokine production. Culture supernatants were removed at 48 h and analyzed by ELISA for IFN-γ (A), IL-4 (B), IL-5 (C), and IL-10 (D). Data represent mean ± SEM of each cytokine determination. *, P < 0.05 for T-bet-/-Ldlr-/- versus T-bet+/+Ldlr-/- comparisons; #, P < 0.05 for no antigen versus murine hsp60 stimulation.
Fig. 4.
Differences in atherosclerotic antigen-specific serum immunoglobulins between T-bet-/-Ldlr-/- and T-bet+/+Ldlr-/- mice. Titers of total IgG1, total IgG2a, MDA-LDL-specific, and CuOx-LDL-specific IgM, IgG1, and IgG2a, and EO6 idiotype IgM were detected by specific ELISA from serum collected from 10 animals for each study group, after 8 weeks of diet, as described in Methods. Data represent mean ± SEM of each Ig type determination. *, P < 0.05 for T-bet-/-Ldlr-/- versus T-bet+/+Ldlr-/- comparisons. RLU, relative light units.
We further characterized IgM responses to Ox-LDL, subgroups of which may provide protection against atherosclerosis (8). Titers of IgM antibodies to MDA-LDL were similar between the two groups, but there was a small, nonsignificant increase of IgM titers to CuOx-LDL in Ldlr-/- mice lacking T-bet (Fig. 4). One immunodominant epitope of CuOx-LDL is phosphorylcholine (PC) headgroup of oxidized phospholipids, which is specifically recognized by the natural germ line-encoded IgM T15/EO6. E06 antibodies are atheroprotective (8), and their production by B-1 B cells can be enhanced by IL-5 (19). Therefore, we determined the levels of E06 antibodies in the sera of T-bet-null and control mice. At killing, T-bet-/-Ldlr-/- had a >2.5-fold increase in serum levels of EO6 compared with T-bet+/+ Ldlr-/- controls.
Immune mechanisms that impact atherogenesis are complex (7, 8). Our studies have revealed a most interesting and previously undescribed impact of T-bet deficiency, namely a Th2 shift in the T cell response to hypercholesterolemia, and a linked enhancement of serum titers of atheroprotective EO6/T15 natural IgM antibodies (19, 30). EO6/T15 IgM autoantibodies may exert atheroprotective effects by inhibiting the uptake of Ox-LDL by macrophage scavenger receptors. Although these antibodies are the result of thymus-independent activation of B-1 B cells, noncognate T cell help seems to facilitate their production (19). When mice were immunized with MDA-LDL, which does not contain oxidized phospholipids and leads to an MHC class II-restricted IgG antibody response, there was not only a strong induction of IgG1 anti-MDA-LDL antibodies, but also, unexpectedly, a strong induction of EO6/T15. Analyses indicated that MDA-LDL immunization led to a strongly biased antigen-specific expansion of Th2 cells in the spleen, with an associated secretion of Th2 cytokines, including IL-5. In turn, it was shown that the IL-5 provided noncognate help to B-1 cells and expansion of EO6-secreting cells (19). Because we have recently shown that a near monoclonal expansion of EO6/T15 antibodies in vivo, produced by immunization of mice with heat-killed Streptococcus pneumoniae, decreased the extent of atherosclerosis (30), the enhanced titer of EO6/T15 we observed in the T-bet-deficient mice may well contribute in part to the atheroprotective effect observed with T-bet deficiency. Indeed, the enhanced IgM anti-Ox-LDL antibody titer observed in the T-bet-deficient mice may contain other such atheroprotective subsets of natural antibodies as well. The expansion of such natural atheroprotective natural antibodies may be another mechanism by which a Th2 bias, as occurs with T-bet deficiency, mediates atheroprotection.
Finally, although our study was not designed to independently test the effects of T-bet deficiency in males vs. females, we found more significant differences in lesion size when we compared T-bet-/- males vs. control males than between T-bet-/- females vs. control females. We did not find significant alterations of serum cholesterol levels in male versus female animals, and therefore it is very unlikely that the gender-related effects on atherosclerosis that we observed were secondary to systemic changes in lipid metabolism. It has been shown that important sex differences are associated with the development of atherosclerosis; specifically, atherosclerotic lesions were larger and more advanced in young female than in male ApoE-null mice (32). This study also suggests that these differences may be related to sex differences in the cellular immune responses to Ox-LDLs. However, in our study, we did not find gender-related differences in the control group, but only in T-bet-deficient mice. Because female T-bet-/-Ldlr-/- mice develop more atherosclerosis than males on the same genetic background, differences in atherosclerosis extent and phenotype between T-bet-deficient mice and sex-matched controls remain significant only for the male gender.
In summary, our data demonstrate that genetically programmed biases in T cell subset differentiation and cytokine expression profiles influence atherosclerosis. Th1 cellular responses, which are suppressed in T-bet-deficient mice, are proatherogenic, and the switch of Th cell functions is able to play a protective role in atherogenesis. This study indicates that modulating adaptive T cell responses to atherosclerosis antigens can enhance the production of atheroprotective antibodies and raises the possibility that atherogenesis could be reduced by targeting the transcriptional regulation of T cell differentiation.
Acknowledgments
This work was supported by National Institutes of Health Grants P50 HL56985 (to C.B. and A.H.L.), HL56989 and HL69464 (to C.J.B. and J.L.W.), and AI48126 (to L.H.G.). C.J.B. is the recipient of a fellowship from the American Heart Association (Western Affiliates).
Abbreviations: hsp60, heat shock protein-60; LDL, low-density lipoprotein; Th, T helper; MDA, malondialdehyde; Ox, oxidized; CuOx, copper-oxidized.
References
- 1.Wick, G., Knoflach, M. & Xu, Q. (2004) Annu. Rev. Immunol. 22, 361-403. [DOI] [PubMed] [Google Scholar]
- 2.Hansson, G. K., Holm, J. & Jonasson, L. (1989) Am. J. Pathol. 135, 169-175. [PMC free article] [PubMed] [Google Scholar]
- 3.Stemme, S., Holm, J. & Hansson, G. K. (1992) Arterioscler. Thromb. 12, 206-211. [DOI] [PubMed] [Google Scholar]
- 4.Hansson, G. K., Seifert, P. S., Olsson, G. & Bondjers, G. (1991) Arterioscler. Thromb. 11, 745-750. [DOI] [PubMed] [Google Scholar]
- 5.Zhou, X., Stemme, S. & Hansson, G. K. (1996) Am. J. Pathol. 149, 359-366. [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou, X. (2003) Biomed. Pharmacother. 57, 287-291. [DOI] [PubMed] [Google Scholar]
- 7.Buono, C. & Lichtman, A. H. (2004) Trends Cardiovasc. Med. 14, 166-172. [DOI] [PubMed] [Google Scholar]
- 8.Binder, C. J., Chang, M. K., Shaw, P. X., Miller, Y. I., Hartvigsen, K., Dewan, A. & Witztum, J. L. (2002) Nat. Med. 8, 1218-1226. [DOI] [PubMed] [Google Scholar]
- 9.Abbas, A. K., Murphy, K. M. & Sher, A. (1996) Nature 383, 787-793. [DOI] [PubMed] [Google Scholar]
- 10.Romagnani, S. (2000) Ann. Allergy Asthma Immunol. 85, 9-18; quiz 18, 21. [DOI] [PubMed] [Google Scholar]
- 11.Frostegard, J., Ulfgren, A. K., Nyberg, P., Hedin, U., Swedenborg, J., Andersson, U. & Hansson, G. K. (1999) Atherosclerosis 145, 33-43. [DOI] [PubMed] [Google Scholar]
- 12.Stemme, S., Faber, B., Holm, J., Wiklund, O., Witztum, J. L. & Hansson, G. K. (1995) Proc. Natl. Acad. Sci. USA 92, 3893-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buono, C., Come, C. E., Stavrakis, G., Maguire, G. F., Connelly, P. W. & Lichtman, A. H. (2003) Arterioscler. Thromb. Vasc. Biol. 23, 454-460. [DOI] [PubMed] [Google Scholar]
- 14.Buono, C., Pang, H., Uchida, Y., Libby, P., Sharpe, A. H. & Lichtman, A. H. (2004) Circulation 109, 2009-2015. [DOI] [PubMed] [Google Scholar]
- 15.Gupta, S., Pablo, A. M., Jiang, X., Wang, N., Tall, A. R. & Schindler, C. (1997) J. Clin. Invest. 99, 2752-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitman, S. C., Ravisankar, P. & Daugherty, A. (2002) J. Interferon Cytokine Res. 22, 661-670. [DOI] [PubMed] [Google Scholar]
- 17.Zhou, X., Paulsson, G., Stemme, S. & Hansson, G. K. (1998) J. Clin. Invest. 101, 1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laurat, E., Poirier, B., Tupin, E., Caligiuri, G., Hansson, G. K., Bariety, J. & Nicoletti, A. (2001) Circulation 104, 197-202. [DOI] [PubMed] [Google Scholar]
- 19.Binder, C. J., Hartvigsen, K., Chang, M.-K., Miller, M., Broide, D., Palinski, W., Curtiss, L. K., Corr, M. & Witztum, J. L. (2004) J. Clin. Invest. 114, 427-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murphy, K. M. & Reiner, S. L. (2002) Nat. Rev. Immunol. 2, 933-944. [DOI] [PubMed] [Google Scholar]
- 21.Szabo, S. J., Kim, S. T., Costa, G. L., Zhang, X., Fathman, C. G. & Glimcher, L. H. (2000) Cell 100, 655-669. [DOI] [PubMed] [Google Scholar]
- 22.Szabo, S. J., Sullivan, B. M., Stemmann, C., Satoskar, A. R., Sleckman, B. P. & Glimcher, L. H. (2002) Science 295, 338-342. [DOI] [PubMed] [Google Scholar]
- 23.Lugo-Villarino, G., Maldonado-Lopez, R., Possemato, R., Penaranda, C. & Glimcher, L. H. (2003) Proc. Natl. Acad. Sci. USA 100, 7749-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ishibashi, S., Brown, M. S., Goldstein, J. L., Gerard, R. D., Hammer, R. E. & Herz, J. (1993) J. Clin. Invest. 92, 883-893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng, S. L., Szabo, S. J. & Glimcher, L. H. (2002) Proc. Natl. Acad. Sci. USA 99, 5545-5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lichtman, A. H., Clinton, S. K., Iiyama, K., Connelly, P. W., Libby, P. & Cybulsky, M. I. (1999) Arterioscler. Thromb. Vasc. Biol. 19, 1938-1944. [DOI] [PubMed] [Google Scholar]
- 27.Mach, F., Schonbeck, U., Sukhova, G. K., Atkinson, E. & Libby, P. (1998) Nature 394, 200-203. [DOI] [PubMed] [Google Scholar]
- 28.Horkko, S., Bird, D. A., Miller, E., Itabe, H., Leitinger, N., Subbanagounder, G., Berliner, J. A., Friedman, P., Dennis, E. A., Curtiss, L. K., et al. (1999) J. Clin. Invest. 103, 117-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw, P. X., Horkko, S., Chang, M. K., Curtiss, L. K., Palinski, W., Silverman, G. J. & Witztum, J. L. (2000) J. Clin. Invest. 105, 1731-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Binder, C. J., Horkko, S., Dewan, A., Chang, M. K., Kieu, E. P., Goodyear, C. S., Shaw, P. X., Palinski, W., Witztum, J. L. & Silverman, G. J. (2003) Nat. Med. 9, 736-743. [DOI] [PubMed] [Google Scholar]
- 31.Yi, Y., Yang, X. & Brunham, R. C. (1997) Infect. Immun. 65, 1669-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caligiuri, G., Nicoletti, A., Zhou, X., Tornberg, I. & Hansson, G. K. (1999) Atherosclerosis 145, 301-308. [DOI] [PubMed] [Google Scholar]