Abstract
β-arrestins bind to G protein-coupled receptor kinase (GRK)-phosphorylated seven transmembrane receptors, desensitizing their activation of G proteins, while concurrently mediating receptor endocytosis, and some aspects of receptor signaling. We have used RNA interference to assess the roles of the four widely expressed isoforms of GRKs (GRK 2, 3, 5, and 6) in regulating β-arrestin-mediated signaling to the mitogen-activated protein kinase, extracellular signal-regulated kinase (ERK) 1/2 by the angiotensin II type 1A receptor. Angiotensin II-stimulated receptor phosphorylation, β-arrestin recruitment, and receptor endocytosis are all mediated primarily by GRK2/3. In contrast, inhibiting GRK 5 or 6 expression abolishes β-arrestin-mediated ERK activation, whereas lowering GRK 2 or 3 leads to an increase in this signaling. Consistent with these findings, β-arrestin-mediated ERK activation is enhanced by overexpression of GRK 5 and 6, and reciprocally diminished by GRK 2 and 3. These findings indicate distinct functional capabilities of β-arrestins bound to receptors phosphorylated by different classes of GRKs.
Keywords: angiotensin receptor, extracellular signal-regulated kinase, phosphorylation, small interfering RNA
Despite the remarkable diversity of physiological roles played by the large family of seven-transmembrane (7TM) receptors, their structural, functional, and regulatory properties are quite conserved (1). Upon binding stimulatory agonists, the receptors undergo conformational changes that promote their binding to three families of proteins, heterotrimeric G proteins, G protein-coupled receptor kinases (GRKs), and arrestins (2, 3). Interactions of the receptors with G proteins leads to second messenger-mediated signaling (1). Interaction with GRKs leads to phosphorylation of serine/threonine residues located on the cytoplasmic loops and C terminus of the receptors, a modification that promotes the high affinity binding of arrestins (visual arrestin and the ubiquitous β-arrestins) (2). This binding, in turn, physically interdicts coupling to G proteins (“desensitization”) and induces receptor internalization via clathrin-coated pits (4–6).
In addition to these classically described functions as terminators of G protein-dependent seven TM receptor signaling, recent evidence also indicates a previously undescribed role for β-arrestins as signal transducers and scaffolding molecules that can function independent of G proteins (7–10). It has been demonstrated that, upon activation of angiotensin II (AngII) type 1A (AT1A) (9), neurokinin 1 (10), and protease-activated (11) receptors, β-arrestin scaffolds the components of the extracellular signal-regulated kinase (ERK) cascade, Raf-1, MEK1, and ERK1/2, into complex with the receptors leading to activation of ERK1/2.
The AT1A receptor (AT1AR) is well known to mediate various angiotensin-dependent physiological responses such as vasoconstriction, smooth muscle cell motility and growth, and aldosterone secretion (12). Using a variety of approaches, including RNA interference, a PKC inhibitor, and a mutant AT1AR and ligand that recruit β-arrestin but fail to activate G proteins, it has been demonstrated that stimulation of AT1AR leads to ERK1/2 activation by independent Gq and β-arrestin mediated pathways (13, 14). Moreover, these two distinct mechanisms differ with respect to their kinetics (Gq rapid and transient; β-arrestin slower and persistent) as well as the distribution of the activated ERK (Gq in nucleus; β-arrestin in cytosolic vesicles) (15). Such different regulation by the G protein- and β-arrestin 2-dependent pathways strongly implies the existence of distinct physiological end points.
Based on sequence similarity, the seven GRKs have been divided into three subfamilies: GRK 1 and 7 localized to the retina; the pleckstrin homology domain containing GRK 2 and 3, which interact with Gβγ; and membrane-associated GRK 4, 5, and 6 (16). GRKs 2, 3, 5, and 6 are very widely distributed in mammalian tissues (16). Despite recent advances in understanding the mechanisms of GRK regulation as well as their patterns of expression, relatively little is known about their specificity and functional roles. We wished to examine the possibility that receptor phosphorylation by specific GRK subfamilies might play distinct roles in regulating the functional capabilities of receptor bound β-arrestin. We have used isoform-specific GRK depletion by RNA interference (17) to investigate the contribution of GRKs 2, 3, 5, and 6 to agonist-induced AT1AR phosphorylation and consequent β-arrestin-mediated internalization and signaling.
Materials and Methods
Materials. [125I]Tyr-4-AngII and [32P]Pi were from PerkinElmer. Ro-31-8425 was from Calbiochem. Polyclonal anti-GRK specific antibodies were from Santa Cruz Biotechnology. All other reagents were from Sigma unless otherwise noted.
Synthesis of Small Interfering RNAs (siRNAs). siRNAs were chemically synthesized from Dharmacon (Lafayette, CO) or Xeragon (Germantown, MD) as described (15). The siRNA sequences targeting GRKs are GRK2, 5′-AAGAAGUACGAGAAGCUGGAG-3′ (NM_001619, position 268–288); GRK3, 5′-AAGCAAGCUGUAGAACACGUA-3′ (X69117, position 376–396); GRK 5, 5′-AAGCCGUGCAAAGAACUCUUU-3′ (NM_005308, position 406–426); GRK6, 5′-AACAGUAGGUUUGUAGUGAGC-3′ (AF040751, position 724–744). Indicated position numbers are relative to the start codon. A nonsilencing RNA duplex (5′-AAUUCUCCGAACGUGUCACGU-3′), as the manufacturer indicated, was used as a control.
Cell Culture and Transfection. Human embryonic kidney (HEK) 293 cells were maintained as described (18). Thirty to 40% confluent, early passage (<15) cells in 100-mm dishes were transfected simultaneously with 20 μg of siRNA and 0.5–2 μg of plasmids encoding AT1AR by using the GeneSilencer transfection reagent (Gene Therapy Systems, San Diego) as described (14). To achieve equivalent AT1AR expression in different siRNA-transfected cells, the appropriate amounts of plasmids were used. Forty-eight hours after transfection, cells were divided for further assays that were performed in the next day. AT1AR expression was determined by radioligand binding assays as described (19) and was 200–300 fmol per mg of protein in all experiments, except receptor phosphorylation assays that required 0.8–1 pmol per mg of receptor to detect.
To rescue GRK 5 and 6 siRNA effects, 0.5 μg of plamids encoding “wobble” mutants of each kinase were transfected with corresponding siRNA into HEK293 cells. Three neutral point mutations were introduced within the siRNA target sequences for human GRK 5 and 6 by using the QuikChange site-directed mutagenesis kit (Stratagene). These mutants did not alter the amino acid sequence of the kinases, but the nucleotide substitutions rendered them insensitive to siRNA-mediated degradation. The nucleotide substitutions were as follows: GRK5 positions 411 (G → A), 417 (A → G), and 423 (C → A), and GRK6 positions 729 (T → C), 735 (T → C), and 738 (A → G) from the beginning of coding sequences, respectively.
For overexpression of GRKs, 0.5 μg of GRK-encoding plasmids and 1 μg of the plasmid encoding HA-AT1AR were transiently transfected into HEK293 cells in 100-mm dishes by using the FuGENE 6 reagent (Roche Diagnostics).
Receptor Phosphorylation Assays. To test receptor phosphorylation by GRKs, we used a mutant Flag-AT1AR, in which PKC phosphorylation sites (Ser-331, Ser-338, and Ser-348) were substituted with alanine (20). Cells were preincubated with 0.1 mCi/ml (0.2 for GRK6 siRNA-transfected cells) [32P]Pi for 1 h and then stimulated with AngII. Flag-AT1AR was immunoprecipitated, and their phosphorylation was analyzed by autoradiography as described (21).
Coimmunoprecipitation Assays. AngII-stimulated cells were subjected to covalent protein crosslinking by using the membrane-permeable, hydrolyzable crosslinker Dithiobis[succinimidyl-propionate] (DSP) (Pierce) as described (7). Coimmunoprecipitated β-arrestins w ith AT1AR were detected by immunoblotting with a 1:3,000 dilution of the A1CT rabbit polyclonal anti-β-arrestin antibody (22) and subsequently quantified by densitometry with a Fluor-S MultiImager (Bio-Rad).
Internalization Assays. Agonist-induced internalization of transiently expressed AT1AR was defined as loss of cell surface receptors after AngII treatment, measured by radioligand binding as described (19).
ERK Activation Assays. Approximately 50% confluent cells on six-well plates were serum-starved for at least 4 h. After stimulation with AngII, cellular extracts were prepared and equal amounts of protein were used to visualize phosphorylation of ERK1/2 by immunoblotting essentially as described (15). Phosphorylated ERK1/2 in immunoblots were quantified by densitometry.
Results
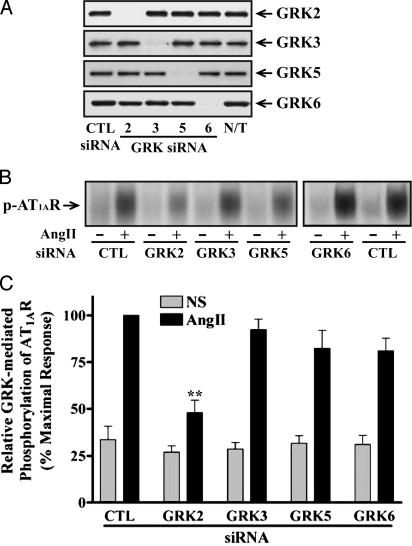
Transfection of GRK siRNAs into HEK293 cells reduced expression of the targeted GRK compared to either nonsilencing, control RNA- or non-transfected cells. All siRNAs demonstrated marked isoform specificity. These results validate our siRNAs as potent reagents capable of selectively and efficiently depleting GRK levels (>95%) in human cells (Fig. 1A).
Fig. 1.
Analysis of GRK expression in siRNA-transfected HEK293 cells and its effects on AT1AR phosphorylation. (A) Cellular extracts were prepared from the indicated siRNA-transfected cells. Equal amounts of proteins in each sample were used to determine expression of each GRK by immunoblotting with specific anti-GRK antibodies. N/T, nontransfected. (B and C) Cells were transfected with the indicated siRNAs and plasmids encoding mutant AT1AR, in which PKC phosphorylation sites were substituted. After stimulation with 100 nM AngII for 3 min, receptor phosphorylation was visualized (B) and quantified (C) as described. Values shown are expressed as percent of the phosphorylation in stimulated, control (CTL) siRNA-transfected cells and represent the mean ± SE from three independent experiments. NS, nonstimulated. Statistical significance was determined by using a one-way ANOVA (PRISM software) to correct for multiple comparisons between each GRK siRNA-transfected and control cells (**, P < 0.01).
We first tested the ability of cells transfected with siRNAs targeting the GRKs to support agonist-induced phosphorylation of AT1AR. Stimulation with AngII for 3 min led to prominent phosphorylation of the receptor in HEK293 cells, which was reduced by ≈60% in cells transfected with GRK2 siRNA (Fig. 1 B and C). The small decreases in receptor phosphorylation observed after transfection with GRK 3, 5, or 6 siRNAs were not statistically significant (Fig. 1C). It has been reported that overexpression of GRK 2, 3, and 5 significantly augments agonist-induced AT1AR phosphorylation in HEK293 cells (21).
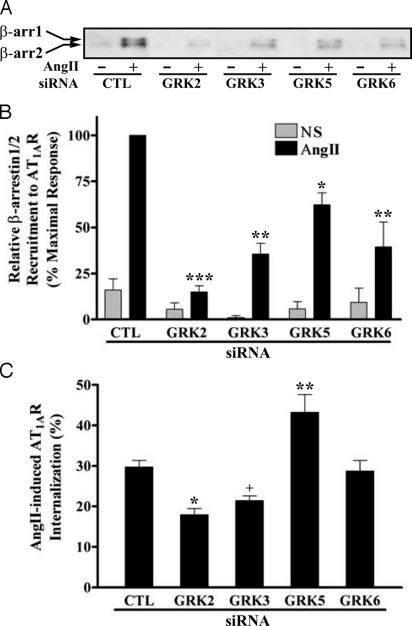
Next, we examined the effect of siRNA suppression of GRK expression on agonist-promoted β-arrestin recruitment to AT1AR and on receptor internalization. Fig. 2 A and B shows that the amount of β-arrestin coimmunoprecipitated with the receptor after agonist stimulation was significantly reduced when any of the GRK siRNAs were transfected. Reducing GRK2 expression had the strongest effect.
Fig. 2.
Effects of siRNA-mediated suppression of GRK levels on AngII-promoted β-arrestin recruitment to the receptor and AT1AR internalization. HEK293 cells were transfected with HA-AT1AR-encoding plasmids and the indicated siRNAs. (A and B) Serum-starved cells were exposed to 100 nM AngII for 2 min at 37°C, and β-arrestins (β-arrs) coimmunoprecipitated with the receptor were visualized (A) and quantified (B) as described. Values shown are expressed as percent of the level in stimulated, control (CTL) siRNA-transfected cells and represent the mean ± SE from three independent experiments. NS, nonstimulated. (C) Cells in serum-free media were cooled on ice and stimulated with 0.2 nM [125I]AngII for 5 min at 37°C. Percent of AngII-stimulated AT1AR internalization was determined as described. Data represent the mean ± SE from six independent experiments done in triplicate. Statistical significance was determined by using a one-way ANOVA or t test between each GRK siRNA-transfected and control cell (*, P < 0.05; **, P < 0.01; ***, P < 0.001 for ANOVA; +, P < 0.05 for t test).
Internalization of AT1AR was measured 5 min after AngII treatment. GRK 2 and 3 siRNA-transfected cells showed a significant reduction (40% and 28%, respectively) in AT1AR internalization; transfection with GRK6 siRNA had no effect, whereas GRK5 siRNA had a stimulatory effect on receptor internalization (Fig. 2C). Moreover, at longer times of stimulation (>30 min), the extent of receptor internalization in the GRK suppressed cells became equal to controls (data not shown), indicating that the major effect of GRK 2 and 3 depletion is to slow the rate of AT1AR internalization.
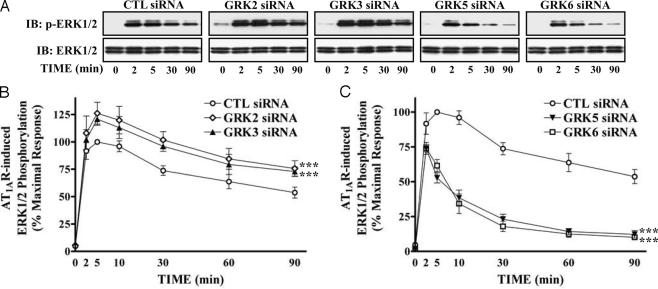
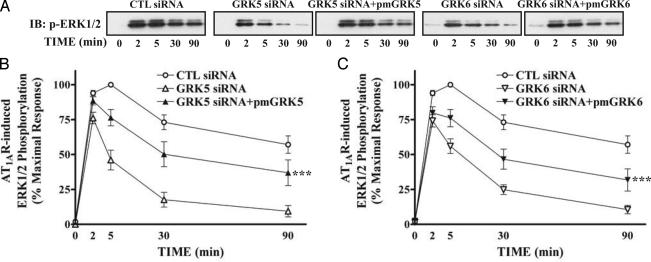
Stimulation of AT1AR activates ERK1/2 by distinct pathways dependent on either Gq or β-arrestin 2 (13, 15). We tested whether siRNA-mediated depletion of GRKs alters ERK signaling from the activated AT1AR. In AngII-stimulated, control siRNA-transfected cells, ERK1/2 activation reached maximal levels (typically, 20- to 30-fold over basal) rapidly and remained stable for up to 10 min, decreasing very slowly (Fig. 3). AngII stimulation in GRK 2 or 3 siRNA-transfected cells caused a similar kinetic pattern of ERK activation, but with elevated levels of activation of ERK1/2, compared to control siRNA-transfected cells (Fig. 3 A and B). In contrast, after depletion of GRK 5 or 6, only very rapid and transient ERK activation was observed (Fig. 3 A and C). The persistent phase of ERK activation, previously shown to be β-arresin 2-mediated, was lost.
Fig. 3.
Effects of siRNA-attenuated expression of different GRKs on the kinetic pattern of AT1AR-mediated ERK activation. siRNA-transfected cells were serum-starved and then stimulated with 100 nM AngII at 37°C for the indicated periods. Phosphorylation of ERK1/2 was visualized (A) and quantified (B and C) as described. (A Lower) Equal amounts of ERK1/2 were loaded in each sample. (B and C) Values in graphs are expressed as percent of the phosphorylation of ERK1/2 obtained at 5 min of stimulation in control (CTL) siRNA-transfected cells. Data represent the mean ± SE from at least five independent experiments. Statistical comparison of the curves was performed by using a two-way ANOVA between GRK siRNA-transfected and control cells (***, P < 0.001).
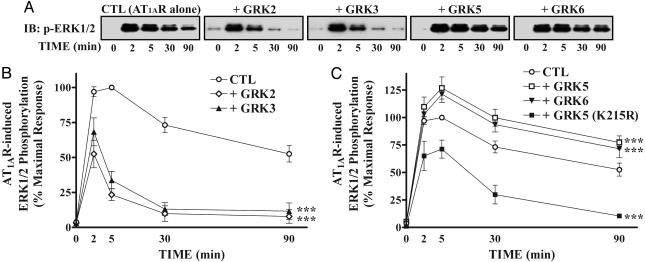
We also used overexpression approaches to test the effects of various GRKs on AT1AR-mediated ERK1/2 activation in HEK293 cells. Introduction of GRK 2 or 3 strikingly reduced primarily the late activation of ERK after AngII stimulation in a pattern similar to that observed with GRK 5 or 6 siRNA transfection (compare Figs. 3C and 4B). On the contrary, GRK 5 or 6 overexpression enhanced AngII-induced ERK activation especially at the later time points (Fig. 4 A and C), similar to the pattern observed in GRK 2 or 3 siRNA-transfected cells (Fig. 3B). Furthermore, a catalytically inactive mutant of GRK5 (K215R) not only failed to enhance, but rather inhibited ERK activity, suggesting that the catalytic activity of GRK5 is necessary for its enhancing effect on ERK activation (Fig. 4C). Taken together, these results suggest that GRK 5 and 6 empower β-arrestin 2-mediated signaling to ERK, whereas GRK 2 and 3 oppose this process.
Fig. 4.
Effects of GRK overexpression on the kinetic pattern of ERK activation after stimulation of AT1AR. (A) HEK293 cells were transiently transfected with HA-AT1AR-encoding plasmids and the indicated GRK-encoding plasmids. After serum-starvation and stimulation, phosphorylation of ERK1/2 was visualized (A) and quantified (B and C) as described in Fig. 3. (B and C) Each data point is expressed as percent of the phosphorylation of ERK1/2 obtained at 5 min of stimulation in control (CTL) cells and represents the mean ± SE from at least three independent experiments. Statistical comparison of the curves was performed by using a two-way ANOVA between GRK siRNA-transfected and control cells (***, P < 0.001).
To rescue the GRK 5 and 6 siRNA effects on AngII-induced ERK1/2 activation, “wobble” mutants of GRK 5 and 6 with three neutral point mutations within the siRNA target sequences were transfected into HEK293 cells either with control or specific GRK 5 or 6 siRNA. Fig. 5 shows that inhibition of the late ERK activation caused by deletion of GRK 5 or 6 can be substantially rescued by expression of the wobble mutants. These results further confirm that β-arrestin-2-mediated ERK activation is facilitated by GRK 5 and 6.
Fig. 5.
Rescue of GRK 5 and 6 siRNA-mediated effects on the kinetic pattern of AngII-induced ERK activation. Cells were transfected with HA-AT1AR-encoding plasmids, each indicated siRNA, and either pRK5 empty vector or plasmids expressing indicated wobble mutants of GRK (pmGRK) simultaneously. After serum starvation and stimulation, phosphorylation of ERK1/2 was visualized (A) and quantified (B and C) as described in Fig. 3. (B and C) Values in graphs are expressed as percentage of the phosphorylation of ERK1/2 obtained at 5 min of stimulation in control (CTL) siRNA-transfected cells and represent the mean ± SE from seven independent experiments. Statistical comparison of the curves was performed by using a two-way ANOVA between GRK siRNA with and without pmGRK-transfected cells in each graph (***, P < 0.001).
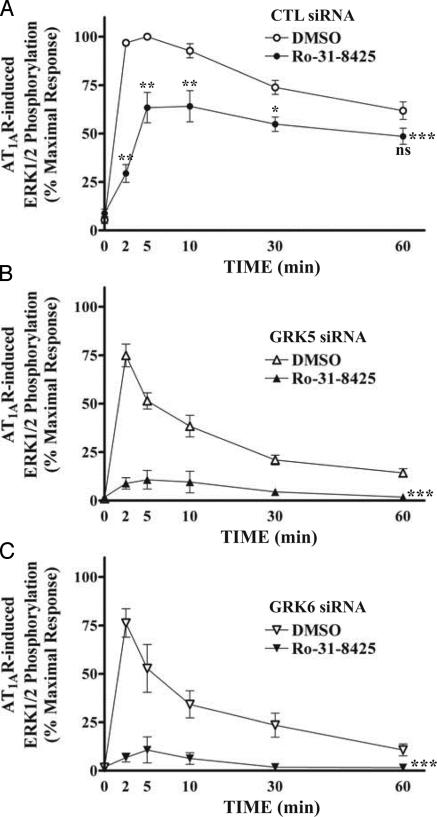
We have previously shown that AngII stimulation of the β-arrestin-2-independent (G protein-dependent) pathway to ERK activation in HEK293 cells is completely blocked by the PKC inhibitor Ro-31-8425 (13). As shown in Fig. 6A, pretreatment with the PKC inhibitor resulted in a dramatic decrease in ERK1/2 activation at early time points after AngII stimulation, but little inhibition was observed at longer time points, consistent with previous results (15). ERK activation elicited by AngII in GRK 5- or 6-depleted cells (Fig. 6 B and C) was virtually abolished by pretreatment with the PKC inhibitor. Thus, these data further support the conclusion that phosphorylation of AT1AR by GRK 5 and 6 leads to β-arrestin-2-mediated ERK activation.
Fig. 6.
Effects of the PKC inhibitor Ro-31-8425 on the kinetic pattern of AT1AR-mediated ERK activation. siRNA-transfected cells were serum-starved and then treated with vehicle only (DMSO) or 1 μM Ro-31-8425 for 15 min before stimulation. Phosphorylation of ERK1/2 was determined as described in Fig. 3. Data in each graph are expressed as percent of the phosphorylation of ERK1/2 obtained at 5 min of stimulation in control (CTL) siRNA-transfected, DMSO-treated cells and represent the mean ± SE from at least five independent experiments. Statistical comparison of the curves (***, P < 0.001) and values at each time point (A; ns, nonsignificant; *, P < 0.05; **, P < 0.01) were performed by using a two-way ANOVA between DMSO- and Ro-31-8425-treated cells.
Discussion
Transduction of receptor signals via β-arrestins is a recently appreciated signaling mechanism (23). As is the case for their other functions of receptor desensitization and endocytosis, β-arrestins must first interact with and bind to agonist-stimulated, GRK-phosphorylated 7TM receptors (24, 25). Until now, there has been little to suggest any functional specialization in these phosphorylation events. Now we have unexpectedly found that the functional consequences of β-arrestin interaction with AT1AR are determined by which of the several widely expressed GRKs phosphorylate them. As determined by siRNA-mediated depletion, GRK2 is the major kinase responsible for AT1AR phosphorylation, and accordingly for the largest fraction of β-arrestin recruitment and receptor internalization in HEK293 cells. These findings are in excellent agreement with previous results using inactivating monoclonal antibodies for GRK 2/3 or 5/6, respectively, which demonstrated that <20% of AngII-induced receptor phosphorylation in HEK293 cells could be attributed to GRK5/6 (26). Moreover, our data show that only GRK2 and the structurally very closely related GRK3 are associated with β-arrestin-mediated AT1AR endocytosis.
In striking contrast, β-arrestin-mediated ERK activation requires receptor phosphorylation by GRK 5 and 6, even though these kinases account for a relatively small fraction of the total receptor phosphorylation and β-arrestin recruitment. Interestingly, depletion of either GRK 5 or 6 alone leads to almost complete loss of β-arrestin-mediated ERK stimulation, suggesting that the concerted activity of both kinases may be necessary. Moreover, depletion of GRK2/3 or overexpression of GRK5/6 reproducibly increased β-arrestin-mediated ERK activation. This increase might be due either to a physical competition between GRK 2/3 and 5/6 for access to the receptor, or to an inhibitory effect of GRK2/3 phosphorylation on the subsequent ability of GRK5/6 to phosphorylate the receptor. The very different functional consequences of receptor phosphorylation by the different GRKs presumably are a reflection of the different sites, serine and threonine residues largely localized to the C-terminal cytoplasmic tail of the receptors, which they phosphorylate.
It can be speculated that β-arrestins bound to receptors phosphorylated on distinctive patterns of sites adopt specific conformations capable of activating different signaling effectors. The location of these phosphorylation sites may constitute a “bar code” that instructs the bound β-arrestin as to its intended function. Although details of the preferred phosphorylation consensus motifs for the different GRKs are not well defined, it is clear that there are significant differences in the preferences of the various enzymes (16). Given the large number of potential phosphorylation sites in the cytoplasmic tails of many 7TM receptors, there exists the possibility for combinatorial complexity in the patterns of phosphorylation that might lead to many different conformations of the bound β-arrestins. The detailed mapping of these sites and correlation with specific functional outcomes should be a profitable direction for future research.
Another possibility is that different patterns of receptor phosphorylation lead to differential recruitment of β-arrestins 1 and 2 with consequently different functional outcomes. It has been reported that AT1AR-mediated activation of ERK1/2 is reciprocally regulated by the two isoforms of β-arrestins, with β-arrestin 1 actually inhibiting the regulatory function of β-arrestin 2 (27). Thus, one possibility is that GRK phosphorylation of the receptor induces conformational changes that alter the preference for binding of β-arrestin 1 or 2. However, the resolution of our coimmunoprecipitation technique is such that we cannot, at present, confidently evaluate this possibility.
Finally, our data do not formally exclude the possibility that the GRKs actually differ in their phosphorylation of unidentified downstream protein involved in the β-arrestin-mediated signaling to ERK (e.g., β-arrestin 2 itself). However, neither GRK2 nor GRK5 phosphorylate β-arrestin 2 (36, 37).
Previously, GRKs have been thought to differ only with respect to their preferences for different receptors (3). However, our findings change this perspective. Strikingly, as shown in the accompanying paper, the V2 vasopressin receptor shows the very same pattern with respect to the ability of GRK 5/6 but not 2/3 to empower β-arrestin-mediated signaling to ERK (28). It will be of interest to determine the generality of these findings with respect to other 7TM receptors, as well as patterns for β-arrestin-mediated signaling pathways other than ERK. The current results also shed light on functional features that may distinguish the two nonvisual subfamilies of GRKs 2/3, and 4/5/6. However, it should be stressed that our data in no way exclude the possibility that, for other receptors, or in other cells, different functional results might be observed.
Even though GRK2 has been reported to be the major GRK responsible for the phosphorylation and desensitization of various receptors (29–32), it is also quite clear, from both in vitro and in vivo studies, that both GRK 5 and 6 also can lead to desensitization of some 7TM receptors. For example, studies with GRK knockout mice have shown that GRK5 desensitizes peripheral muscarinic cholinergic receptors (33), whereas GRK6 desensitizes central D2 dopaminergic receptors (34). Phosphorylation and decensitization of M3 muscarinic acetycholine receptors is mediated by GRK6 in a human neuroblastoma cell (35).
The present work also further validates the notion of independent G protein and β-arrestin/GRK-mediated pathways for ERK activation by AT1AR. As demonstrated in studies with β-arrestin 2 siRNA, the G protein pathway is rapid and transient, whereas the β-arrestin/GRK pathway is slower in onset and much more persistent (15). The Gq protein pathway appears to be entirely dependent on PKC, whereas the β-arrestin pathway requires both GRK 5 and 6. That these two pathways account for the major fraction of AngII-stimulated ERK activation in these cells is demonstrated by the essentially complete abolition of this activity by a combination of a PKC inhibitor together with siRNA to either GRK 5 or 6.
In summary, GRK 5 and 6 phosphorylation of AT1AR uniquely empower β-arrestin 2-mediated ERK1/2 activation, a signaling pathway antagonized by GRK2/3. This unanticipated specialization of GRK function hints at yet to be discovered combinatorial complexities in the regulation of β-arrestin-mediated transduction of 7TM receptor signaling.
Acknowledgments
We thank Donna Addison and Elizabeth Hall for excellent secretarial assistance. This work was supported by National Institutes of Health Grants RO1 HL 16037 and HL 70631 (to R.J.L.). R.J.L. is an Investigator of the Howard Hughes Medical Institute. E.R. is a Researcher from Institut National de la Recherche Agronomique.
Abbreviations: 7TM, seven-transmembrane; GRK, G protein-coupled receptor kinase; AngII, angiotensin II; AT1A, AngII type 1A; ERK, extracellular signal-regulated kinase; AT1AR, AT1A receptor; siRNA, small interfering RNA.
References
- 1.Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. (2002) Nat. Rev. Mol. Cell. Biol. 3, 639-650. [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz, R. J. (1998) J. Biol. Chem. 273, 18677-18680. [DOI] [PubMed] [Google Scholar]
- 3.Kohout, T. A. & Lefkowitz, R. J. (2003) Mol. Pharmacol. 63, 9-18. [DOI] [PubMed] [Google Scholar]
- 4.Goodman, O. B., Jr., Krupnick, J. G., Santini, F., Gurevich, V. V., Penn, R. B., Gagnon, A. W., Keen, J. H. & Benovic, J. L. (1996) Nature 383, 447-450. [DOI] [PubMed] [Google Scholar]
- 5.Laporte, S. A., Oakley, R. H., Zhang, J., Holt, J. A., Ferguson, S. S., Caron, M. G. & Barak, L. S. (1999) Proc. Natl. Acad. Sci. USA 96, 3712-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Claing, A., Laporte, S. A., Caron, M. G. & Lefkowitz, R. J. (2002) Prog. Neurobiol. 66, 61-79. [DOI] [PubMed] [Google Scholar]
- 7.Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., et al. (1999) Science 283, 655-661. [DOI] [PubMed] [Google Scholar]
- 8.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J. & Lefkowitz, R. J. (2000) Science 290, 1574-1577. [DOI] [PubMed] [Google Scholar]
- 9.Luttrell, L. M., Roudabush, F. L., Choy, E. W., Miller, W. E., Field, M. E., Pierce, K. L. & Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. USA 98, 2449-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeFea, K. A., Vaughn, Z. D., O'Bryan, E. M., Nishijima, D., Dery, O. & Bunnett, N. W. (2000) Proc. Natl. Acad. Sci. USA 97, 11086-11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeFea, K. A., Zalevsky, J., Thoma, M. S., Dery, O., Mullins, R. D. & Bunnett, N. W. (2000) J. Cell Biol. 148, 1267-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Gasparo, M., Catt, K. J., Inagami, T., Wright, J. W. & Unger, T. (2000) Pharmacol. Rev. 52, 415-472. [PubMed] [Google Scholar]
- 13.Wei, H., Ahn, S., Shenoy, S. K., Karnik, S. S., Hunyady, L., Luttrell, L. M. & Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. USA 100, 10782-10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahn, S., Nelson, C. D., Garrison, T. R., Miller, W. E. & Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. USA 100, 1740-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn, S., Shenoy, S. K., Wei, H. & Lefkowitz, R. J. (2004) J. Biol. Chem. 279, 35518-35525. [DOI] [PubMed] [Google Scholar]
- 16.Pitcher, J. A., Freedman, N. J. & Lefkowitz, R. J. (1998) Annu. Rev. Biochem. 67, 653-692. [DOI] [PubMed] [Google Scholar]
- 17.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- 18.Oakley, R. H., Laporte, S. A., Holt, J. A., Caron, M. G. & Barak, L. S. (2000) J. Biol. Chem. 275, 17201-17210. [DOI] [PubMed] [Google Scholar]
- 19.Laporte, S. A., Servant, G., Richard, D. E., Escher, E., Guillemette, G. & Leduc, R. (1996) Mol. Pharmacol. 49, 89-95. [PubMed] [Google Scholar]
- 20.Qian, H., Pipolo, L. & Thomas, W. G. (1999) Biochem. J. 343, 637-644. [PMC free article] [PubMed] [Google Scholar]
- 21.Oppermann, M., Freedman, N. J., Alexander, R. W. & Lefkowitz, R. J. (1996) J. Biol. Chem. 271, 13266-13272. [DOI] [PubMed] [Google Scholar]
- 22.Attramadal, H., Arriza, J. L., Aoki, C., Dawson, T. M., Codina, J., Kwatra, M. M., Snyder, S. H., Caron, M. G. & Lefkowitz, R. J. (1992) J. Biol. Chem. 267, 17882-17890. [PubMed] [Google Scholar]
- 23.Shenoy, S. K. & Lefkowitz, R. J. (2003) Biochem. J. 375, 503-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freedman, N. J. & Lefkowitz, R. J. (1996) Recent Prog. Horm. Res. 51, 319-351; discussion, 352-353. [PubMed] [Google Scholar]
- 25.Krupnick, J. G. & Benovic, J. L. (1998) Annu. Rev. Pharmacol. Toxicol. 38, 289-319. [DOI] [PubMed] [Google Scholar]
- 26.Oppermann, M., Diverse-Pierluissi, M., Drazner, M. H., Dyer, S. L., Freedman, N. J., Peppel, K. C. & Lefkowitz, R. J. (1996) Proc. Natl. Acad. Sci. USA 93, 7649-7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahn, S., Wei, H., Garrison, T. R. & Lefkowitz, R. J. (2004) J. Biol. Chem. 279, 7807-7811. [DOI] [PubMed] [Google Scholar]
- 28.Ren, X.-R., Reiter, E., Ahn, S., Kim, J., Chen, W. & Lefkowitz, R. J. (2005) Proc. Natl. Acad. Sci. USA 102, 1448-1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oppermann, M., Mack, M., Proudfoot, A. E. I. & Olbrich, H. (1999) J. Biol. Chem. 274, 8875-8885. [DOI] [PubMed] [Google Scholar]
- 30.Richardson, R. M., Kim, C., Benovic, J. L. & Hosey, M. M. (1993) J. Biol. Chem. 268, 13650-13656. [PubMed] [Google Scholar]
- 31.Benovic, J. L., Strasser, R. H., Caron, M. G. & Lefkowitz, R. J. (1986) Proc. Natl. Acad. Sci. USA 83, 2797-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwata, K., Luo, J., Penn, R. B. & Benovic, J. L. (2004) J. Biol. Chem. 280, 2197-2204. [DOI] [PubMed] [Google Scholar]
- 33.Gainetdinov, R. R., Bohn, L. M., Walker, J. K., Laporte, S. A., Macrae, A. D., Caron, M. G., Lefkowitz, R. J. & Premont, R. T. (1999) Neuron 24, 1029-1036. [DOI] [PubMed] [Google Scholar]
- 34.Gainetdinov, R. R., Bohn, L. M., Sotnikova, T. D., Cyr, M., Laakso, A., Macrae, A. D., Torres, G. E., Kim, K. M., Lefkowitz, R. J., Caron, M. G. & Premont, R. T. (2003) Neuron 38, 291-303. [DOI] [PubMed] [Google Scholar]
- 35.Willets, J. M., Mistry, R., Nahorski, S. R. & Challiss, R. A. (2003) Mol. Pharmacol. 64, 1059-1068. [DOI] [PubMed] [Google Scholar]
- 36.Lin, F.-T., Chen, W., Shenoy, S., Cong, M., Exum, S. T. & Lefkowitz, R. J. (2002) Biochemistry 41, 10692-10699. [DOI] [PubMed] [Google Scholar]
- 37.Kim, Y.-M., Barak, L. S., Caron, M. G. & Benovic, J. L. (2002) J. Biol. Chem. 277, 16837-16846. [DOI] [PubMed] [Google Scholar]