Abstract
Exposure to estrogen in the neonatal period affects prostatic growth and leads to an increased incidence of prostatic intraepithelial neoplasia in later life. The effects of neonatal estrogen are clearly dependent on estrogen receptor (ER) α because they do not occur in ERα-knockout mice. Because ERα is expressed in the stroma, but not in the epithelium, of the adult ventral prostate, the concept of indirect estrogen effects through stromal signaling has been proposed. Here, we show that during the first 4 weeks of life, there are profound and rapid changes in the ER profile in the mouse ventral prostate. ERα is abundant in the stroma during week 1, but by week 2 it is exclusively epithelial, and then by week 4, ERα is lost and ERβ is dominant in the prostatic epithelium. The presence of ERα is associated with a high proliferation index, and ERβ is associated with quiescence. Branching morphogenesis was altered in ERα-/-, but not in ERβ-/-, mice. We conclude that imprinting and branching morphogenesis of the ventral prostate are mediated by estrogen acting directly on epithelial and stromal ERα during the first 2 weeks of life.
Keywords: branching morphology; immunohistochemistry; androgen receptor; proliferation; 5α-androstane-3β,17β-diol
Exposure of neonatal mice to the nonsteroidal estrogen diethylstilbestrol disturbs prostatic development, alters epithelial cell differentiation, and predisposes mice as they age to prostatic hyperplasia and dysplasia analogous to human prostatic intraepithelial neoplasia (1–3). This delayed effect of a brief neonatal exposure to estrogens is called imprinting. One characteristic of imprinting is that there is a specific time window during development when it occurs. For imprinting of the prostate by estrogen, the window corresponds to the first week of postnatal life. From published studies, imprinting of the prostate occurs before day 15 of life, coincident with differentiation and ductal morphogenesis (4). Estrogen receptor (ER) α knockout (ERα-/-) mice are resistant to imprinting by neonatal diethylstilbestrol (5), whereas ERβ knockout (ERβ-/-) mice respond like wild-type (WT) mice. These data suggest that ERα mediates the effects of neonatal estrogens on the prostate. In the adult rodent ventral prostate (VP), ERβ is the only ER expressed in the epithelium, whereas ERα is expressed in the stroma (6). To explain the role of ERα in imprinting of the prostate, it was hypothesized that estrogen acts on stromal ERα to stimulate growth factor release, and these growth factors cause epithelial proliferation (1, 5).
The issue of which ER mediates the action of estrogens at different developmental stages of prostatic growth and differentiation has direct implications for understanding the development of prostatic disease and for the design of pharmaceuticals for the treatment of prostate cancer and benign prostatic hyperplasia. Prostate cancer is the most common malignancy in men in Western society (7–9). Both the etiology and the mechanisms involved in the progression of prostate cancer have been, and continue to be, extensively investigated, but to date the causes of this disease remain unknown. Phytoestrogen-containing diets are reported to protect against prostate cancer (10). Because many phytoestrogens have a higher affinity for ERβ than ERα (11) and because ERβ has antiproliferative actions, the beneficial effects of phytoestrogens may be mediated by ERβ. Conversely, some phytoestrogens and xenoestrogens do not discriminate between the two ERs, and exposure to such agents in the neonatal period may lead to the activation of ERα and prostatic disease in later life (12).
If estrogenic action in the prostate epithelium is indirect by means of growth factors from the stroma, growth factor release and/or growth factor receptor activation could be natural targets for pharmacological modulation of growth of the prostate epithelium. If estrogen elicits epithelial proliferation by means of epithelial ERα, then this receptor could be the appropriate target for reducing epithelial proliferation.
In this study, we used ERα-/-, ERβ-/-, and CYP7B1 knockout (CYP7B1-/-) mice to show that in the neonatal period, ERα, not ERβ, is the predominant ER in mouse VP and that ERα mediates both stromal and epithelial growth.
Materials and Methods
Animals. ERβ-/- mice from our colony were used (13). ERα-/- mice were purchased from Taconic, and CYP7B1-/- mice were provided by Richard Lathe (Centre for Genome Research and Centre for Neuroscience, University of Edinburgh, Edinburgh, U.K.) (14) and were bred in our laboratory. These animals were housed in Huddinge University Hospital Animal Facility in a controlled environment on a 12-h light/12-h dark illumination schedule and fed a standard pellet diet with water provided ad libitum. Animals were asphyxiated by CO2, and tissues were fixed in 4% paraformaldehyde for immunohistochemical study and frozen in liquid nitrogen for mRNA and protein studies. We followed the guidelines for care and use of experimental animals that were issued by Stockholm's Södra Djurförsöksetiska Nämnd.
RNA Extraction and RT-PCR. RNA extraction was performed by using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed by using random hexamers and Moloney murine leukemia virus reverse transcriptase (Invitrogen) in a total volume of 20 μl. In total, 1 μl of synthesized cDNA was subjected to PCR amplification by using Taq polymerase (Promega). PCR primers and conditions for ERα were as follows: forward, 5′-GCC GAG GAG GGA GAA TGT TG-3′; reverse, 5′-CGC CAG ACG AGA CCA ATC AT-3′ (57.0°C, 35 cycles). The housekeeping gene, β-actin, was used as a control with the following primers and conditions: forward, 5′-TGG CAC CAC ACC TTC TAC AA-3′; reverse, 5′-TCA CGC ACG ATT TCC CTC TC-3′ (58.1°C, 30 cycles). The PCR products were separated on a 1% agarose gel and visualized by ethidium bromide staining under UV illumination.
Chemicals and Antibodies. We purchased 5α-androstane-3β,17β-diol (3βAdiol) from Sigma, and BrdUrd was purchased from Roche (Mannheim, Germany). Rabbit polyclonal anti-ERα (MC-20) and anti-androgen receptor (anti-AR; N-20) antibodies were from Santa Cruz Biotechnology. Chicken polyclonal anti-ERβ 503 and rabbit polyclonal anti-ERβ ligand-binding domain antibodies were produced in our laboratory (15). Mouse monoclonal anti-BrdUrd antibody was from Pharmingen. Biotinylated anti-rabbit and anti-mouse goat antibodies were purchased from Vector Laboratories.
Adsorption of Antibodies. Fresh uterus was obtained from 2-month-old WT mice. Samples were homogenized in 500 mM NaCl in PBS with proteinase inhibitor mixture tablet (Boehringer Mannheim) and centrifuged at 100,000 × g for 1 h at 4°C. The supernatant was diluted 4-fold in 0.1 M sodium bicarbonate (pH 8.8) and incubated with activated CH Sepharose at 4°C overnight. The pellet was recovered by centrifugation, washed with PBS, and stored in PBS containing 0.02% sodium azide at 4°C. The anti-ERα antibody (MC-20) was incubated with coupled Sepharose gel at 4°C overnight to remove antibodies interacting with uterine proteins.
Immunohistochemical Staining. The representative blocks of paraffin-embedded tissues were cut at 4-μm thickness, dewaxed, and rehydrated. Antigens were retrieved by boiling in 10 mM citrate buffer (pH 7.0) for 15 min. The sections were incubated in 0.5% H2O2 in PBS for 30 min at room temperature to quench endogenous peroxidase, then incubated in 0.5% Triton X-100 in PBS for 15 min. For BrdUrd staining, sections were incubated additionally in 2 M HCl for 10 min and in solution mixed equally with 0.05 M sodium tetraborate (pH 8.5) and 0.05 M NaCl in 0.2 M boric acid for 15 min at room temperature after blocking endogenous peroxidase activity, then incubated in 0.5% Triton X-100 in PBS for 5 min at room temperature. To block the nonspecific binding, sections were incubated in 10% normal serum prepared from the host of secondary antibodies for 1 h at 4°C. Sections were incubated with the following antibodies and dilutions: anti-ERα (1:120), anti-ERβ (1:100), anti-AR (1:300), and anti-BrdUrd (1:100) in 3% BSA in PBS overnight at 4°C. After washing, sections were incubated with the corresponding secondary antibodies (all in 1:200 dilutions) for 1 h at room temperature. The Vectastain ABC kit (Vector) was used for the avidin–biotin complex (ABC) method according to the manufacturers' instructions. Peroxidase activity was visualized with 3,3′-diaminobenzidine (DAKO). The sections were lightly counterstained with hematoxylin. Negative controls were incubated without primary antibody.
Preparation of Cytosol for Sucrose Density Gradient Centrifugation. Tissue was frozen in liquid nitrogen and pulverized in a Dismembrator (Braun, Melsungen, Germany) for 45 s. Pulverized tissue was added to buffer composed of 10 mM Tris·HCl (pH 7.5), 1.5 mM EDTA, and 5 mM sodium molybdate, using 1 ml per 100 mg of tissue. Cytosol was obtained by centrifugation of the homogenate at 204,000 × g for 1 h in a 70Ti rotor at 4°C. Cytosols from adult WT mouse uterus and ERα-/- VP were used for positive and negative controls, respectively, of ERα.
Sucrose Density Gradient and Western Blotting. Sedimentation studies were carried out as described in ref. 16. Cytosols were incubated for 3 h at 0°C with 10 nM [3H]estradiol-17β (Amersham Pharmacia) in the presence or absence of 50-fold excess of cold estradiol, and the unbound steroid was removed with dextran-coated charcoal. Sucrose density gradients [10–30% (wt/vol)] were prepared in a buffer containing 10 mM Tris·HCl (pH 7.5), 1.5 mM EDTA, 1 mM α-monothioglycerol (Sigma), and 10 mM KCl. Samples of 200 μl were layered on 3.5-ml gradients and centrifuged at 4°C for 18 h at 300,000 × g in a Beckman L-70K ultracentrifuge with an SW-60Ti rotor. Successive 100-μl fractions were collected from the bottom by paraffin oil displacement, using a collector of our own design, and assayed for radioactivity by scintillation counting. The fractions were divided into two, half used for scintillation counting and half for Western blotting. Proteins were precipitated with trichloroacetic acid, and the precipitates were washed in methanol on a bed of dry ice for 30 min. Proteins were recovered by centrifugation. Protein pellets were dissolved in SDS sample buffer and resolved on SDS/polyacrylamide gels in Tris·glycine buffer, by using gradient gels with 4–20% acrylamide (NOVEX, San Diego). Transfer to poly(vinylidene difluoride) membranes was performed by electroblotting in Tris·glycine buffer. Molecular weight markers were Precision Plus protein standards (Bio-Rad). Primary antibody dilution was 1:1,000 for ERα MC-20. After washing, membranes were incubated with the corresponding biotinylated secondary antibodies, and then the Vectastain ABC kit (Vector) was used for the ABC method according to the manufacturers' instructions. The enhanced chemiluminescence system ECL Plus (Amersham Pharmacia) was used for visualization.
Counting of Proliferating Cells. For proliferation studies, BrdUrd was administered to mice by s.c. injection (100 mg/kg of body weight) 2 h before they were killed. Three individual areas were selected from the glands of each mouse. The total number of cells in the field and BrdUrd-positive cells were counted. The data were expressed as a percentage of BrdUrd-positive epithelial cells.
Exposure of Neonatal Mice to 3βAdiol. To observe the effect of 3βAdiol on neonatal VP, 2-week-old mice were used. Two mice were treated with 3βAdiol (10 μg per animal, 1.4 mg/kg of body weight) three times every 24 h by s.c. injection, and two control mice were treated with vehicles instead. They were injected with 100 mg/kg BrdUrd 2 h before they were killed.
Ductal Branching Morphogenesis of VP. The basic method of Ko and Ko (17) was followed. VPs were dissected into chilled Hanks' balanced salt solution (Ca- and Mg-free) under a microdissection microscope. After pictures were taken, dissected tissues were separated into single lobes and incubated in 0.5% collagenase in HBSS for 1 h at 35°C to remove stroma. The ducts of stroma-depleted VP were carefully unfolded on slide glass and pictures taken. The specimens then were dried completely and mounted after staining with Giemsa solution for 10 min. Thereafter, the number of primary and secondary branches from main ducts were counted.
Results
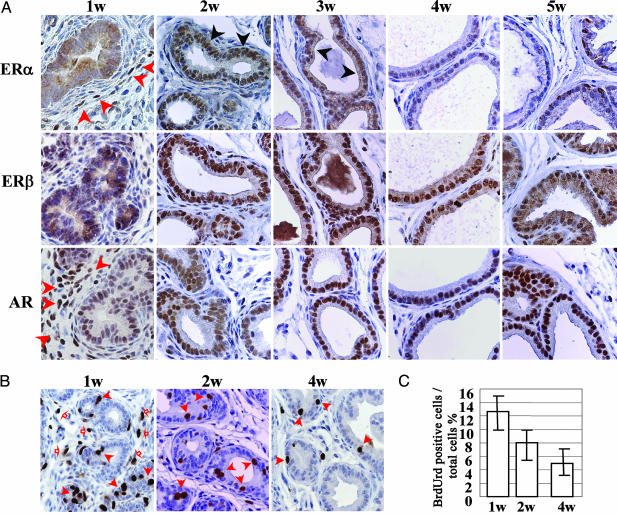
Expression of ERα, ERβ, and AR in VP by Immunohistochemistry. VPs of 1-, 2-, 3-, 4-, and 5-week-old WT mice were examined immunohistochemically for ERα,ERβ, and AR (Fig. 1A). In the epithelium, neither ERα nor ERβ was detectable in the first week of life, but both receptors were expressed in epithelium of 2- and 3-week-old-mice. After 3 weeks of age, ERα levels declined and no ERα-positive epithelial cells were detectable by week 4. ERβ, conversely, continued to increase toward adult levels, which were achieved by 6 weeks. Weak AR staining was observed in the nuclei of ductal epithelial cells throughout the neonatal period and increased during puberty. Profound changes also occurred in the expression of nuclear receptors in the stroma. At 1 week of age there were very high levels of ERα and AR in almost all stromal cells. Expression of both receptors declined after week 1, and by week 2, very few stromal cells were ERα positive, and AR was undetectable.
Fig. 1.
Expression of nuclear receptors and cell proliferation in neonatal VP. (A) Immunohistochemical staining of ERα,ERβ, and AR in neonatal VP. VPs of WT mice aged 1, 2, 3, 4, and 5 weeks were collected. ERα staining was observed in the nuclei of epithelial cells from 2- and 3-week-old mice (black arrow) but not in 1-week-old mice or in mice older than 4 weeks. ERα staining also could be seen in a certain number of stromal cells in all samples, particularly in those from 1-week-old mice (red arrow). ERβ staining was seen in epithelial cells of mice older than 2 weeks. AR staining was observed in nuclei of ductal epithelial cells of mice of all ages studied; only 1-week-old mice showed positive staining in the stroma (red arrow). Antibodies and dilutions used are as follows: anti-ERα MC-20 (1:120), anti-ERβ (1:100), and anti-AR N-20 (1:300). (B and C) Examination of cell proliferation in neonatal VP. At the age of 1, 2, and 4 weeks, WT mice were killed 2 h after s.c. injection of BrdUrd (100 mg/kg). (B) Dissected VP was fixed and sectioned for immunostaining with an anti-BrdUrd antibody. Red filled arrows show positive staining in epithelial cells. Red open arrows show positive staining in stroma. Many proliferating cells in stroma can be seen in 1-week-old mice, whereas almost no staining can be observed after 2 weeks of age. (C) The BrdUrd staining ratio in epithelial cells was 12.4 ± 2.5% in 1-week-old mice, 8.1 ± 1.5% in 2-week-old mice, and 5.0 ± 2.0% in 4-week-old mice.
Cell Proliferation During the Neonatal Period. After administration of BrdUrd 2 h before mice were killed, the percentages of BrdUrd-positive cells in the epithelium of VPs of WT mice were 12.4 ± 2.5%, 8.1 ± 1.5%, and 5.0 ± 2.0% at 1, 2, and 4 weeks of age, respectively (Fig. 1 B and C). In 1-week-old mice, most of the stromal cells, which expressed both AR and ERα, were BrdUrd-positive. There were very few BrdUrd-positive cells in the stroma of mice older than 2 weeks of age.
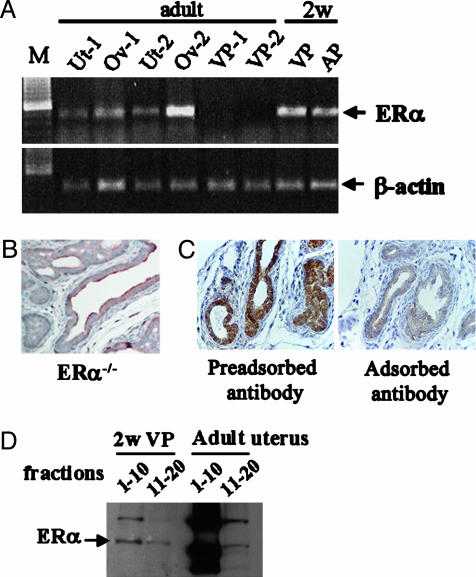
ERα Expression in 2-Week-Old Mouse VP. Several supportive techniques were used to confirm the presence of ERα in neonatal VP epithelium. They were RT-PCR, estradiol binding analyzed by sucrose density gradient centrifugation, and Western blotting. The specificity of the ERα antibody was checked by preadsorption with adult uterine cytosol and use of ERα-/- mouse prostates.
RT-PCR was used to evaluate expression of ERα mRNA in VPs and anterior prostates (APs) from 2-week-old males. Adult mouse uteri and ovaries were used as positive controls and adult VPs as negative controls. VPs from three neonatal mice were pooled for RNA extraction and cDNA preparation. ERα amplification was observed in cDNA from adult uterus and ovary, but not from adult VP. ERα fragments were amplified from both neonatal VP and AP (Fig. 2A). Stroma cells in adult VP express ERα, but stroma is very sparse and the level of ERα in the adult prostate is too low for detection under the conditions we used.
Fig. 2.
Expression of ERα in VP of 2-week-old mice. (A)ERα mRNA detection by RT-PCR. RNA was prepared from uterus, ovary, and VP of 2-month-old female (n = 2) and male (n = 2) mice. RNA was extracted from pooled AP and VP from three 2-week-old mice. RT-PCR conditions are described in Materials and Methods. M, DNA marker; Ut, uterus; Ov, ovary. (B) Immunohistochemical staining of ERα in an ERα-/- mouse. No positive signal was detectable in 2-week-old ERα-/- VP. (C) Specificity of the ERα antibody. The positive staining obtained with the ERα antibody (Left) was completely extinguished after adsorption of ERα antibody with high salt extracts from adult uterus (Right). (D)ERα detection by Western blotting. The fractions corresponding to the 4S and 8S peaks obtained by sucrose gradients were used for Western blotting. Both 2-week-old VP and 8-month-old uterus showed a specific ERα band. The antibody used was anti-ERα MC-20 (1:1,000 dilution).
When VPs of 2-week-old ERα-/- and WT mice were compared by immunohistochemistry, ERα was detected in both stroma and epithelium of WT mice, but no ERα was detected in either stroma or epithelium of ERα-/- mice (Fig. 2B). Preadsorption of the ERα antibody with high salt extracts of adult uteri resulted in loss of positive signals in VP (Fig. 2C).
Sucrose gradient sedimentation with [3H]estradiol as ligand confirmed the presence of ERα in the 2-week-old mouse VP. In this system, ERβ sediments in the 4 S region (fractions 11–20), whereas ERα sediments as an 8 S peak (fractions 1–10) (16). A specific 8S [3H]estradiol binding peak was present in extracts from 2-week-old VP as well as from adult uterus (Fig. 2D).
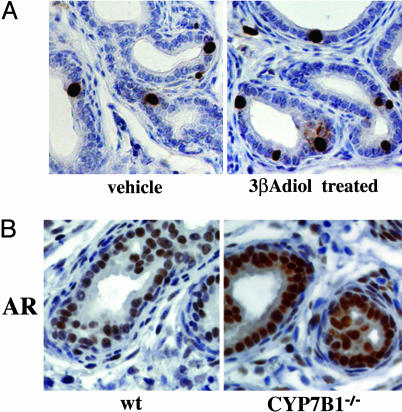
Effect of 3βAdiol on Neonatal Prostatic Proliferation. We previously reported that 3βAdiol is the most likely ligand of ERs in VP (18). Brief exposure of 2-week-old mice to 3βAdiol resulted in a 1.9-fold increase in BrdUrd incorporation into DNA in the VP (Fig. 3A). The dosage of 3βAdiol was calculated on the basis of two considerations: (i) the relative affinity of 3βAdiol for ERα and ERβ, and (ii) the fact that 3βAdiol is 25- to 30-fold less potent than estradiol (19). The increased BrdUrd incorporation indicates that, unlike the case with the adult prostate where 3βAdiol interacts with ERβ to inhibit proliferation, in the neonatal period 3βAdiol interacts with ERα and induces proliferation.
Fig. 3.
Effect of 3βAdiol on cell proliferation and AR in VP. (A) The effect of 3βAdiol on proliferation in the neonatal VP. Two-week-old WT mice were treated with 3βAdiol and killed 2 h after s.c. injection of BrdUrd (100 mg/kg). Dissected VP was fixed and sectioned for immunostaining with anti-BrdUrd antibody. The percentage of BrdUrd-positive cells in the epithelium was 2.32 ± 0.65% in WT mice and was increased 2-fold to 4.43 ± 1.34% after treatment with 3βAdiol. (B) Immunohistochemical staining for AR in 2-week-old ERα-/-, CYP7B1-/-, and WT mice. Intensity of AR staining in the epithelial cells of CYP7B1-/- mice was higher than it was in WT mice. Antibodies used were anti-AR N-20 (1:300 dilution).
AR Expression in 2-Week-Old CYP7B1-/- and WT VPs. Because ERα is coexpressed in cells with AR (Fig. 1 A), the question arose as to whether ERα induces AR. Because of the difficulty in interpreting experiments where estradiol is administered to male mice, we used another mouse model for hyperestrogenicity, the CYP7B1-/- mouse. CYP7B1 is a member of the P450 superfamily. It is responsible for inactivation of 3βAdiol, and in mice in which the CYP7B1 gene is inactivated, tissue levels of 3βAdiol are elevated. The level of AR in epithelial cells of VP of CYP7B1-/- mice is higher than that in WT mice (Fig. 3B). These data suggest that in the VP, ERα increases AR.
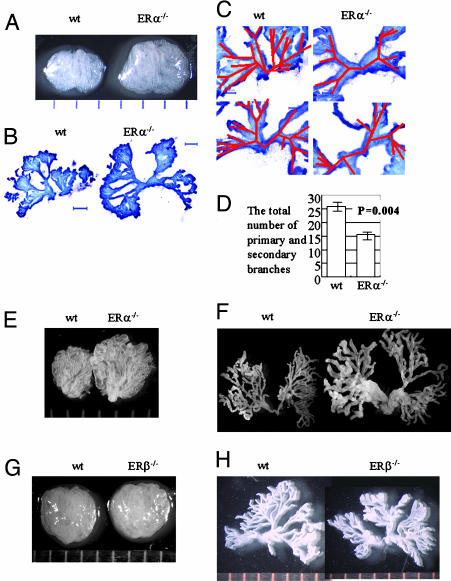
Morphological Examination of VP in ERα-/-, ERβ-/-, and WT Mice by Whole-Mount Sectioning. If ERα in the neonatal prostate is involved in branching morphogenesis, one could expect substantial differences in prostate morphology between WT and ERα-/- mice. Whole mounts of 4-week-old ERα-/- and WT mice and 9-week-old ERα-/-, ERβ-/-, and WT mice were examined. Both at 4 and 9 weeks of age, ERα-/- mice had larger VPs with looser stroma compared with their WT littermates (Fig. 4 A and E). ERβ-/- VP could not be distinguished from that of WT littermates on the basis of size or stromal density (Fig. 4G).
Fig. 4.
Role of ER in morphology of the VP. The VPs of 4-week-old ERα-/- and WT mice and 9-week-old ERα-/-,ERβ-/-, and WT mice were examined under a light microscope. (A–D) VP from 4-week-old ERα-/- and WT littermates. (A) The lobes of VP were dissected and photographed under a dissecting microscope. (B) A view of the whole ductal tree from WT and ERα-/- mice. VPs were separated into two parts, digested with collagenase, and examined for branching morphology. (Scale bars: 500 μm.) (C) Higher magnification to show primary branches from WT and ERα-/- mice with tracing of the primary and secondary branches shown in red. (D) The total number of primary and secondary branches in WT and ERα-/- mice were counted and compared. In ERα-/- mice there was significantly less branching than WT mice. (E and F)VP from 9-week-old ERα-/- mice and WT littermates. (E) VPs were dissected, separated into two lobes, and photographed under a dissecting microscope. (F) Branching morphology of WT and ERα-/- mice. (G and H) VP from 9-week-old ERβ-/- and WT littermate mice. (G) VPs were dissected, and pictures were taken under microscope. (H) Branching morphology of WT and ERβ-/- mice.
There were clear differences in ductal morphology between ERα-/- and WT mice (Fig. 4 B and C). Normally, the VP has one to three primary ducts that divide into secondary and tertiary branches. The secondary branches are close to the base of the main ducts, which was the structure in the VP of 4-week old WT mice. However, in ERα-/- mouse VP, the number of secondary branches was reduced, and secondary branching began significantly further away from the base of the major trunk (Fig. 4C). The total number of primary and secondary branches was 26.0 ± 1.8 in WT and 15.5 ± 1.3 in ERα-/- mice (P = 0.004) (Fig. 4D). In 9-week-old ERα-/- mice, ducts were wider and more flattened with thinner walls, suggesting that the stroma is not capable of providing support for normal ductal growth (Fig. 4F). The ductal tree in ERβ-/- mice was not different from that of WT mice (Fig. 4H). These data suggest that ductal morphogenesis takes place within the first 4 weeks of postnatal life and that ERα plays the major role in determining the structure of the adult VP.
Discussion
A brief exposure to estrogen in the neonatal period permanently programs the prostate in terms of its growth, morphology, responsiveness to androgens, and susceptibility to diseases. In mice, neonatal estrogenization leads to squamous metaplasia in the adult prostate (3). Tissue recombination experiments have shown that induction of squamous metaplasia by estrogen in the AP requires participation of ERα in the epithelium and stroma (20). The AP is different from the VP because it normally expresses ERα in both epithelium and stroma. In the adult mouse VP, ERα is not detectable in the epithelium. Because adult VP epithelium is devoid of ERα, the current view is that imprinting is mediated by stromal ERα (1, 5).
In the present study, we show that ERα is abundant in VP epithelium of 2- to 3-week-old mice and is associated with rapid ductal proliferation and branching morphogenesis. To confirm that our immunohistochemical staining did represent ERα in neonatal VP, we analyzed the ERα mRNA and protein expression in VP by several methods. With RT-PCR, ERα sequences were amplified from cDNA prepared from 2-week-old, but not from adult, mouse VP (Fig. 2 A). The specificity of the ERα antibody was confirmed by the lack of signals in ERα-/- mice and the quenching of signals after preadsorption with uterine cytosol.
Expression of ERα in different lobes of the prostate has been intensely examined in neonatal rats. Unlike the data presented in this paper, immunohistochemical studies with the rat VP showed that ERα is located in the periductal smooth muscle cells but not in epithelial cells (21). It is not clear whether these results reflect true differences between rats and mice or whether they are artifacts due to technical differences between labs or antibody idiosyncrasies. Yamashita (22) has suggested that ERα is present in mouse but not in rat prostate, and Asano et al. (23), using RT-PCR, detected ERα in 2-week-old mouse prostates.
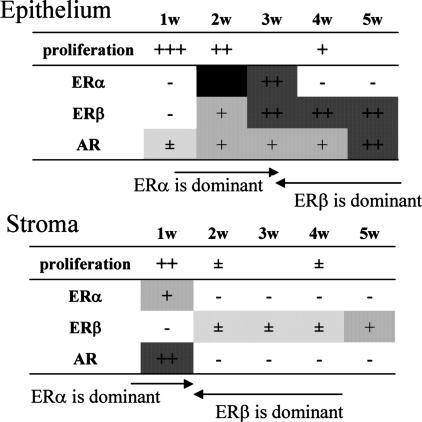
The pattern of expression of ERα, ERβ, and AR in the first 5 weeks of life is summarized in Fig. 5. Between weeks 2 and 4, the VP epithelium switches from ERα-dominant highly proliferative tissue to an ERβ-dominant quiescent tissue. Earlier on, between weeks 1 and 2, the stroma also changes from a highly proliferating, ERα-expressing state to a quiescent state with very low levels of ERα. This remarkable switching of ERs marking the end of proliferation/growth and the beginning of differentiation/functional activation of the prostate is accompanied by changes in expression of AR. We speculate that stromal proliferation/growth is mediated by synergistic action of AR and ERα.
Fig. 5.
Summary of nuclear receptor expression and proliferation index in the epithelium and stroma of VP from 1- to 5-week-old mice. In epithelial cells, ERα is dominant at 2–3 weeks of age and disappears after 4 weeks. ERβ and AR are detectable from 2 weeks of age and dominate by 4 weeks. In the stroma, ERα and AR, but not ERβ, are already present in 1-week-old mice. After 2 weeks of age, AR is lost and expression of ERα is reduced. ERβ is more abundant in stroma after 2 weeks of age.
Most of the ductal branching morphogenesis of VP occurs during the first 15 days of postnatal life and is complete by week 4 (4, 24), suggesting that epithelial that epithelial proliferation is highest before androgen levels increase at puberty. In the present study, the proliferation index, measured by BrdUrd incorporation into DNA, showed that in both stromal and epithelial cells, proliferation is highest in 1-week-old mice and decreases over the following 4 weeks. At the time of puberty, the proliferation index is very low (Fig. 1 B and C).
The limited branching in VPs of ERα-/- mice observed in 4-week-old mice (Fig. 4 B–D) clearly shows that ERα in the early postnatal VP is important for initiation of ductal branching morphogenesis, affecting the structure of the prostate in later life.
Because the branching pattern in ERβ-/- mice was not different from that of WT mice (Fig. 4H), we conclude that there is an exclusive and absolute role for ERα in the process of ductal formation in the VP. Ductal branching morphogenesis involves coordinated growth of both supportive stroma and epithelium forming ducts. Stromal proliferation, which also appears to be ERα-dependent, occurs earlier than epithelial proliferation. Loss of ERα and cessation of proliferation in the stroma occur in the first week of life. In ERα-/- mice early stromal proliferation must be affected, which may account for the very loose stromal structure in the adult VP. The loose stroma cannot support the ductal tree, which might explain why the VP ducts in ERα-/- mice become wider as mice age. A similar function for ERα in the growth of mammary gland stroma is suggested in ref. 25.
In the mammary gland, estradiol is well established as the ligand for ERα. There is less certainty about the ERα ligand in the neonatal prostate. We have reported previously that the 5α-dihydrotestosterone metabolite 3βAdiol is an alternative endogenous ligand for ERβ in VP (18). 3βAdiol inhibits prostatic epithelial proliferation through ERβ and down-regulates AR (26). During the first 5 days of postnatal life, the neonatal testis expresses 5α-reductase, 3α-hydroxysteroid dehydrogenase, and 3β-hydroxysteroid dehydrogenase type 1, which can convert 5α-dihydrotestosterone into 3βAdiol (27). The most abundant hydroxysteroid dehydrogenase in the prostate is 17β-hydroxysteroid dehydrogenase type 7, which is an oxoreductase converting 5α-dihydrotestosterone into 3βAdiol (28).
3βAdiol can bind to both ERα and ERβ but not AR (11). Brief exposure of 2-week-old mice to 3βAdiol resulted in epithelial cell proliferation in VP (Fig. 3A). Because ERα is the only ER in VP at this age, we conclude that ERα is activated by 3βAdiol and mediates the proliferative signal.
A role for ERα in induction of AR is supported by the weak expression of AR in 2-week-old ERα-/- mice and the expression of AR in CYP7B1-/- mice (Fig. 3B). In CYP7B1-/- mice, 3βAdiol accumulates in tissues, ERα is activated, and AR is induced. This opposing effect of ERα and ERβ on AR expression could provide new insights into hormonal control of prostatic growth and function and perhaps suggest new therapeutic targets for treatment of prostatic diseases. Moreover, AR and ERα have been shown to interact directly and regulate transcription of each other (29). Both AR and ERα normally enhance proliferation, whereas ERβ is antiproliferative.
Our study shows that ERα is responsible for the basic construction of prostate glands before puberty. Thereafter ERβ takes over the main steroid receptor role in prostate development and together with AR regulates differentiation and functional activity. Although we still cannot explain the mechanism of imprinting, we can say that ERα signaling in both the stroma and epithelial compartments can be disrupted by neonatal treatment with estrogen. Because ERα mediates cell proliferation, inappropriate exposure of either epithelium or stroma to estrogen can permanently alter the ratio of stromal to epithelial cells. In addition, extended exposure of the stromal cells to estrogen could prolong the period of proliferation and postpone differentiation, which could result in loss of production of stromal factors that would normally be signaling the epithelial cells.
Acknowledgments
We thank Jóse Inzunza for managing ERα-/- mice and Christina Thulin-Andersson, AnnMarie Witte, and Patricia Humire for excellent technical assistance. This study was supported by grants from the Wenner-Gren Foundation, the Swedish Cancer Fund, KaroBio AB, and Konung Gustav V:s och Drottning Victorias Stiftelse.
Abbreviations: ER, estrogen receptor; ERα-/-, ERα knockout; ERβ-/-, ERβ knockout; VP, ventral prostate; AP, anterior prostate; AR, androgen receptor; CYP7B1-/-, CYP7B1 knockout; 3βAdiol, 5α-androstane-3β,17β-diol; ABC, avidin–biotin complex.
See Commentary on page 1269.
References
- 1.Prins, G. S., Birch, L., Habermann, H., Chang, W. Y., Tebeau, C., Putz, O. & Bieberich, C. (2001) Reprod. Fertil. Dev. 13, 241-252. [DOI] [PubMed] [Google Scholar]
- 2.vom Saal, F. S., Timms, B. G., Montano, M. M., Palanza, P., Thayer, K. A., Nagel, S. C., Dhar, M. D., Ganjam, V. K., Parmigiani, S. & Welshons, W. V. (1997) Proc. Natl. Acad. Sci. USA 94, 2056-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strauss, L., Makela, S., Joshi, S., Huhtaniemi, I. & Santti, R. (1998) Mol. Cell. Endocrinol. 144, 83-93. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi, N., Sugimura, Y., Kawamura, J., Donjacour, A. A. & Cunha, G. R. (1991) Biol. Reprod. 45, 308-321. [DOI] [PubMed] [Google Scholar]
- 5.Prins, G. S., Birch, L., Couse, J. F., Choi, I., Katzenellenbogen, B. & Korach, K. S. (2001) Cancer Res. 61, 6089-6097. [PubMed] [Google Scholar]
- 6.Adams, J. Y., Leav, I., Lau, K. M., Ho, S. M. & Pflueger, S. M. (2002) Prostate 52, 69-81. [DOI] [PubMed] [Google Scholar]
- 7.Hsing, A. W., Tsao, L. & Devesa, S. S. (2000) Int. J. Cancer 85, 60-67. [DOI] [PubMed] [Google Scholar]
- 8.Crawford, E. D. (2003) Urology 62, 3-12. [DOI] [PubMed] [Google Scholar]
- 9.Quinn, M. & Babb, P. (2002) BJU Int. 90, 162-173. [DOI] [PubMed] [Google Scholar]
- 10.Shirai, T., Asamoto, M., Takahashi, S. & Imaida, K. (2002) Toxicology 181–182, 89-94. [DOI] [PubMed] [Google Scholar]
- 11.Kuiper, G. G., Lemmen, J. G., Carlsson, B., Corton, J. C., Safe, S. H., van der Saag, P. T., van der Burg, B. & Gustafsson, J.-Å. (1998) Endocrinology 139, 4252-4263. [DOI] [PubMed] [Google Scholar]
- 12.Rajfer, J. & Coffey, D. S. (1978) Invest. Urol. 16, 186-190. [PubMed] [Google Scholar]
- 13.Krege, J. H., Hodgin, J. B., Couse, J. F., Enmark, E., Warner, M., Mahler, J. F., Sar, M., Korach, K. S., Gustafsson, J.-Å. & Smithies, O. (1998) Proc. Natl. Acad. Sci. USA 95, 15677-15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose, K., Allan, A., Gauldie, S., Stapleton, G., Dobbie, L., Dott, K., Martin, C., Wang, L., Hedlund, E., Seckl, J. R., et al. (2001) J. Biol. Chem. 276, 23937-23944. [DOI] [PubMed] [Google Scholar]
- 15.Saji, S., Jensen, E. V., Nilsson, S., Rylander, T., Warner, M. & Gustafsson, J.-Å. (2000) Proc. Natl. Acad. Sci. USA 97, 337-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greene, G. L., Closs, L. E., Fleming, H., DeSombre, E. R. & Jensen, E. V. (1977) Proc. Natl. Acad. Sci. USA 74, 3681-3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ko, S. W. & Ko, K. S. (2002) Am. J. Roentgenol. 179, 1646-1647. [DOI] [PubMed] [Google Scholar]
- 18.Weihua, Z., Lathe, R., Warner, M. & Gustafsson, J.-Å. (2002) Proc. Natl. Acad. Sci. USA 99, 13589-13594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuiper, G. G., Carlsson, B., Grandien, K., Enmark, E., Haggblad, J., Nilsson, S. & Gustafsson, J.-Å. (1997) Endocrinology 138, 863-870. [DOI] [PubMed] [Google Scholar]
- 20.Risbridger, G. P., Wang, H., Frydenberg, M. & Cunha, G. (2001) Endocrinology 142, 2443-2450. [DOI] [PubMed] [Google Scholar]
- 21.Prins, G. S. & Birch, L. (1997) Endocrinology 138, 1801-1809. [DOI] [PubMed] [Google Scholar]
- 22.Yamashita, S. (2004) Anat. Rec. 279A, 768-778. [Google Scholar]
- 23.Asano, K., Maruyama, S., Usui, T. & Fujimoto, N. (2003) Endocr. J. 50, 281-287. [DOI] [PubMed] [Google Scholar]
- 24.Sugimura, Y., Cunha, G. R. & Donjacour, A. A. (1986) Biol. Reprod. 34, 961-971. [DOI] [PubMed] [Google Scholar]
- 25.Mueller, S. O., Clark, J. A., Myers, P. H. & Korach, K. S. (2002) Endocrinology 143, 2357-2365. [DOI] [PubMed] [Google Scholar]
- 26.Weihua, Z., Makela, S., Andersson, L. C., Salmi, S., Saji, S., Webster, J. I., Jensen, E. V., Nilsson, S., Warner, M. & Gustafsson, J.-Å. (2001) Proc. Natl. Acad. Sci. USA 98, 6330-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Shaughnessy, P. J., Willerton, L. & Baker, P. J. (2002) Biol. Reprod. 66, 966-975. [DOI] [PubMed] [Google Scholar]
- 28.Luu-The, V. (2001) J. Steroid Biochem. Mol. Biol. 76, 143-151. [DOI] [PubMed] [Google Scholar]
- 29.Panet-Raymond, V., Gottlieb, B., Beitel, L. K., Pinsky, L. & Trifiro, M. A. (2000) Mol. Cell. Endocrinol. 167, 139-150. [DOI] [PubMed] [Google Scholar]