Abstract
Signaling through β-arrestins is a recently appreciated mechanism used by seven-transmembrane receptors. Because G protein-coupled receptor kinase (GRK) phosphorylation of such receptors is generally a prerequisite for β-arrestin binding, we studied the roles of different GRKs in promoting β-arrestin-mediated extracellular signal-regulated kinase (ERK) activation by a typical seven-transmembrane receptor, the Gs-coupled V2 vasopressin receptor. Gs- and β-arrestin-mediated pathways to ERK activation could be distinguished with H89, an inhibitor of protein kinase A, and β-arrestin 2 small interfering RNA, respectively. The roles of GRK2, -3, -5, and -6 were assessed by suppressing their expression with specific small interfering RNA sequences. By using this approach, we demonstrated that GRK2 and -3 are responsible for most of the agonist-dependent receptor phosphorylation, desensitization, and recruitment of β-arrestins. In contrast, GRK5 and -6 mediated much less receptor phosphorylation and β-arrestin recruitment, but yet appeared exclusively to support β-arrestin 2-mediated ERK activation. GRK2 suppression actually increased β-arrestin-stimulated ERK activation. These results suggest that β-arrestin recruited in response to receptor phosphorylation by different GRKs has distinct functional potentials.
Keywords: extracellular signal-regulated kinase, phosphorylation, desensitization, small interfering RNA
Seven-transmembrane (7TM) receptors traditionally are thought to signal by means of activation of heterotrimeric G proteins and then to be desensitized by G protein-coupled receptor kinase (GRK)-mediated phosphorylation and β-arrestin binding (1, 2). Only recently has it been appreciated that β-arrestins also can serve as signaling intermediates in their own right, connecting the receptors to effectors such as MAP kinases (3–5), tyrosine kinases (6), and others (7–11). In particular, the 7TM V2 vasopressin receptor (V2R), which regulates water homeostasis in the renal tubules (12), has been shown to form a complex with β-arrestin 2 upon agonist stimulation. In that complex, β-arrestin 2 scaffolds components of the extracellular signal-regulated kinase (ERK) cascade and sequesters the activated ERK into endocytic vesicles in the cytoplasm (13). Recent studies have shown that the angiotensin II type 1A receptor (AT1AR) activates ERK signaling via two distinct pathways, dependent on either G protein or β-arrestin 2 (14). In addition, these two pathways have remarkably different kinetic and spatial activation patterns, making them readily distinguishable from each other. The G protein-dependent activated pool of ERK is stimulated rapidly, is transient (t1/2 ≈ 2 min) and translocates to the nucleus, whereas the β-arrestin 2-dependent ERK activation is slower (maximum at 10 min) but sustained and is restricted to the cytoplasm (15).
For most 7TM receptors, phosphorylation by one or more GRKs is a prerequisite for β-arrestin binding. The family of GRKs consists of seven members. Two of these, GRKs, 1 and 7, are confined to retinal rods and cones, respectively, whereas GRK4 has very limited expression in the cerebellum, testis, and kidney (16). In contrast, GRKs 2, 3, 5, and 6 are widely expressed in mammalian tissues (17, 18). Although a few studies have indicated preferential phosphorylation and desensitization of one or another 7TM receptor by a particular GRK, specialized functions of these enzymes have not been clearly defined. Accordingly, in the present study we set out to answer the following important question: Once a 7TM receptor is phosphorylated by a GRK, is an invariant program of signaling events set in motion, or do different GRKs, perhaps by phosphorylating distinct sites on a receptor, engender distinct programs of regulatory activity? To approach this question, we have used small interfering RNA (siRNA) directed against GRKs 2, 3, 5, and 6 to study the ability of these enzymes to regulate the signaling properties of a typical 7TM receptor, Gs-coupled V2R.
Materials and Methods
Materials. Radiolabeled [3H]Arg-vasopressin, [3H]cAMP, and [32P]Pi were obtained from PerkinElmer. H89 was purchased from Calbiochem. GeneSilencer transfection reagent was from Gene Therapy Systems (San Diego). Horseradish peroxidase-conjugated secondary antibody was from Amersham Pharmacia. All other reagents were purchased from Sigma unless indicated.
Synthesis of siRNAs. GRK-specific siRNA duplexes were rationally designed according to several published criteria (19, 20). A scrambled RNA duplex was used as a control sequence (5′-AAUUCUCCGAACGUGUCACGU-3′). Chemically synthesized, double-stranded siRNAs, with 19-nt duplex RNA and 2-nt 3′-dTdT overhangs, were purchased from Dharmacon Research (Lafayette, CO) or Xeragon (Germantown, MD) in deprotected and desalted form.
Cell Culture and siRNA Transfection. Human embryonic kidney (HEK) 293 cells were maintained as described in ref. 21. First, 30–40% confluent, slow-growing early passage (<10) cells in 100-mm dishes were transfected simultaneously with 200 ng of FLAG-V2R and 20 μg of siRNA by using the GeneSilencer Transfection reagent (21). After 48 h, cells were divided into poly(d-lysine)-coated 12-well plates (Becton Dickinson Lab-ware) for receptor binding assay and regular 6-well plates or 100-mm dishes to prepare cellular extracts. The V2R expression was 400–600 fmol/mg of protein in all experiments except receptor phosphorylation (≈1 pmol/mg) as determined by radioligand binding assays (22). When necessary, the amount of plasmid transfected was adjusted to obtain equivalent receptor expression levels in all of the experimental conditions. All assays were performed at least 3 days after siRNA transfection. Cells were serum-starved for at least 6 h before stimulation.
Immunoblotting. After stimulation, cells were solubilized in 2× SDS sample buffer (pH 6.8) and sonicated. Equivalent amounts of proteins were separated by SDS/PAGE on Tris·glycine polyacrylamide gels (Invitrogen), transferred to nitrocellulose, and immunoblotted with rabbit polyclonal antibodies. An anti-GRK2 polyclonal antibody was raised against bovine GRK2 C-terminal residues (483–690). Anti-GRK3, -GRK5, and -GRK6 polyclonal antibodies were purchased from Santa Cruz Biotechnology. The phospho (1:2,000 dilution) and total (1:6,000 dilution) ERK antibodies were from Cell Signaling Technology (Beverly, MA) and Upstate (Charlottesville, VA), respectively. Immunoblots were quantified by densitometry with a Fluor-S MultiImager (Bio-Rad).
Receptor Phosphorylation. Three days after transfection, HEK 293 cells plated in 100-mm dishes were incubated at 37°C for 60 min in phosphate-free MEM containing [32P]Pi (100 μCi/ml; 1 Ci = 37 GBq). The amount of [32P]Pi in the medium was increased to 200 μCi/ml in the GRK6 siRNA-tranfected cells to normalize the 50% decrease in the uptake, which we consistently found in these cells. After agonist stimulation, the lysates were normalized according to the relative receptor expression levels. Immunoprecipitation was carried out to determine phosphorylation as described in ref. 23.
cAMP Assays. Arginine vasopressin (AVP)-stimulated cAMP accumulation in cells was measured, and values were normalized to forskolin-stimulated cAMP (22).
Coimmunoprecipitation. After 3 days of transfection with the FLAG-V2R and the different siRNAs, 100-mm plates were incubated in 4.0 ml of Dulbecco's PBS plus 10 mM Hepes for 1 h at 37°C and were subsequently stimulated. Cells then were subjected to cross-linking by using dithiobis(succinimidylpropionate) from Pierce (6). Immunoprecipitates were analyzed by immunoblotting with the A1CT polyclonal antibody (1:3,000 dilution), which detects β-arrestin 1 and 2 (24).
Statistical Analysis. Statistical significance was determined by using one-way ANOVA (Bonferroni's multiple comparison tests) to compare samples, or two-way ANOVA was used to compare curves (prism software).
Results
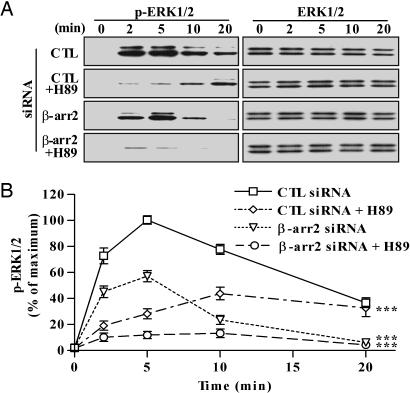
We first determined the temporal characteristics of V2R-mediated ERK activation (Fig. 1). To isolate a potential G protein-dependent pathway, in analogy with the AT1AR system (21), we used siRNA to deplete β-arrestin 2 in HEK 293 cells transiently expressing V2R. In control siRNA-transfected cells, ERK activation reached maximum (≈16-fold over basal level) within 5 min of stimulation with a selective V2R agonist 1-deamine-4-valine-d-arginine vasopressin (dvdAVP) (Fig. 1), was stable for up to 10 min and decreased to ≈40% of maximal levels after 20 min. Depletion of β-arrestin 2 had a modest effect at 2 min of stimulation but led to a substantial inhibition of the ERK phosphorylation signal at longer stimulation times. In contrast, in analogy with previous findings for AT1AR (25), suppression of β-arrestin 1 led to increased V2R-mediated ERK activation (data not shown).
Fig. 1.
Effects of siRNA-mediated suppression of β-arrestin 2 (β-arr2) expression and protein kinase A inhibitor H89 on the kinetics of ERK activation by the V2R. (A) Cells were transfected simultaneously with the FLAG-V2R-encoding plasmid and the indicated siRNAs. Cells were preincubated for 15 min with DMSO or 20 μM H89 and stimulated with 100 nM dvdAVP at 37°C for the indicated periods. Equal amounts of protein from each sample were used to visualize the phosphorylated ERK (Left) or total ERK (Right) by immunoblotting. (B) Signals were quantified by densitometry and expressed as percentage of the maximal phosphorylated ERK obtained at 5 min of stimulation in control (CTL) siRNA-transfected cells. Each data point represents the mean ± SEM from at least four independent experiments. ***, P < 0.001 compared with the entire control curve.
We next sought to isolate a β-arrestin 2 dependent pathway for ERK activation by using a protein kinase A inhibitor (H89) to inhibit Gs-dependent ERK activation. Pretreatment of the cells with H89 resulted in a dramatic and statistically significant decrease (>75%) in ERK activation at the early time points (2 and 5 min) after agonist stimulation, whereas no significant inhibition was observed at later time points (Fig. 1). Moreover, the rapid and transient ERK activation elicited by the agonist in β-arrestin 2-depleted cells was abolished by pretreatment with H89. Taken together, these data demonstrate that V2R activates ERK by two distinct pathways dependent on either Gs or β-arrestin 2 with different temporal patterns; the majority of early activity (<5 min) is elicited via the G protein-dependent pathway, whereas late activity (>10 min) is predominantly mediated by the β-arrestin 2 pathway.
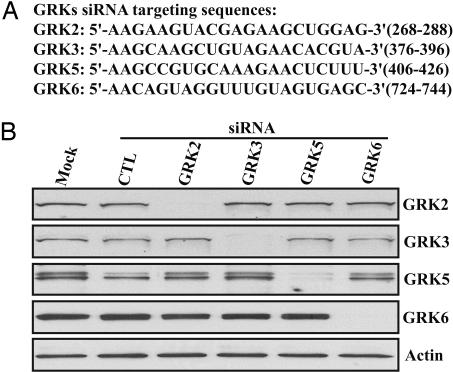
Having established that β-arrestin 2 plays an important role in V2R-mediated activation of ERK, we sought to evaluate the role of different GRKs in V2R phosphorylation, β-arrestin binding, and ERK activation. To address these roles, we first determined that HEK 293 cells expressed detectable GRK2, -3, -5, and -6 levels. Then, we generated siRNAs specifically targeted to human GRK2, -3, -5, and -6. Two to six different siRNAs were evaluated for each GRK, and the most efficient ones were selected for further studies (Fig. 2A). The siRNAs were transfected into HEK 293 cells, and expression levels of the different GRKs were measured (Fig. 2B). The expression level of each GRK was reduced by >95%. Scrambled control siRNA, siRNA against other GRKs, or mock transfection, had no effect on the targeted GRK expression levels (Fig. 2B). Thus, these GRK siRNAs can be used to specifically deplete expression of GRK2, -3, -5, and -6 in HEK 293 cells.
Fig. 2.
siRNA-mediated inhibition of GRK2, -3, -5, or -6 endogenously expressed in HEK 293 cells. (A) Targeting sequences of the optimal siRNA selected. All of the numbers indicate the position of targeting sequences and are relative to the start codon respectively. (B) Cells were transfected with either control (CTL) or the indicated GRK-specific siRNAs. Three days after transfection, cells were lysed and analyzed by immunoblotting using GRK-specific antibodies. Actin was used as a loading control.
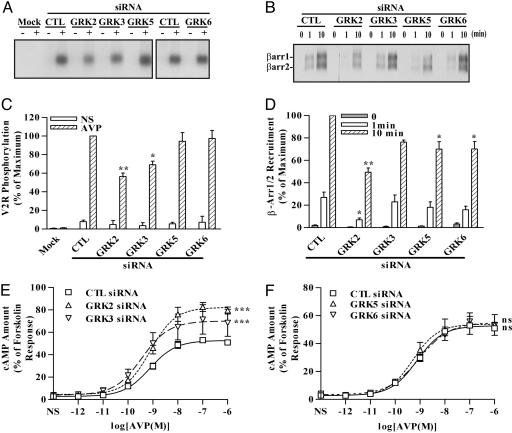
We used these specific siRNAs to determine the contribution of each GRK to the agonist-induced phosphorylation of the V2R. In FLAG-V2R-transfected cells, agonist-promoted phosphorylation of the receptor occurred upon agonist exposure for 5 min (Fig. 3A). Agonist-induced phosphorylation of the V2R was reduced significantly by suppression of GRK2 or -3 expression (45% and 26% decrease, respectively), whereas inhibition of GRK5 or -6 expression had minimal effect on agonist-induced receptor phosphorylation (Fig. 3 A and C). These results suggest that GRK2 and -3 are responsible for the majority of agonist-induced V2R phosphorylation.
Fig. 3.
Effects of siRNA-mediated inhibition of GRK2, -3, -5, or -6 expression on agonist-induced V2R phosphorylation, β-arrestin recruitment, and cAMP production. Cells were transfected with the FLAG-V2R-encoding plasmid and the indicated siRNAs. (A and C) Cells were loaded with [32P]Pi and subsequently stimulated for 5 min with 100 nM of dvdAVP. Equal amounts of receptors were used for immunoprecipitation, and their phosphorylation levels were visualized (A). V2R phosphorylation was quantified and expressed as percentage of the maximal phosphorylation in stimulated control (CTL) siRNA-transfected cells (C). NS, nonstimulated. (B and D) Cells were stimulated for the indicated periods, incubated in the presence of a cross-linker, and immunoprecipitated by using FLAG beads. The coimmunoprecipitated endogenous β-arrestins (β-arr1 and -2) were detected by immunoblotting (B). Signals were quantified by densitometry and expressed as percentage of the level in control cells stimulated for 10 min (D). (E and F) cAMP produced upon stimulation with increasing doses of AVP. The cAMP values were normalized according to the forskolin-induced levels. Data correspond to the mean ± SEM from at least four independent experiments. ns, not significant; *, P < 0.05; **, P < 0.01 compared with the equivalent control values; ***, P < 0.001 compared with the control curve obtained from control cells.
We next explored the specificity of the different GRKs in regulating agonist-dependent β-arrestin recruitment to the V2R. As determined by coimmunoprecipitation, both endogenous β-arrestin 1 and 2 were recruited to the V2R upon agonist stimulation for 1 or 10 min. Virtually no signal was detected in the absence of agonist (Fig. 3 B and D). The ability of β-arrestin to bind to the activated V2R was impaired significantly (≈50%) with GRK2 depletion compared with control siRNA-transfected cells. Suppression of GRK3, -5, or -6 expression more modestly inhibited the amount of β-arrestin recruited to the V2R (Fig. 3 B and D). These results correlate with the specific contribution of each GRK to V2R phosphorylation presented above.
Next, we examined the capacity of GRKs to modulate V2R-mediated cAMP production. Stimulation of the V2R for 5 min with agonist led to maximal cAMP accumulation (data not shown). We found that cAMP accumulation was significantly elevated in cells transfected with GRK2 or -3 siRNA, whereas GRK5 or -6 siRNA transfection induced no change relative to control (Fig. 3 E and F). This finding is consistent with previous reports showing the ability of GRK2 and/or GRK3 to elicit agonist-induced desensitization of numerous other receptors (2).
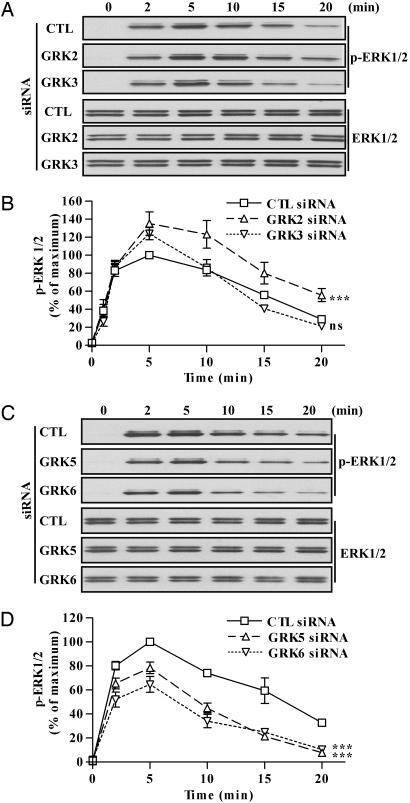
The results presented above establish that β-arrestin 2 is involved in V2R-mediated ERK activation and that there is specificity in the control of V2R phosphorylation, β-arrestin recruitment, and cAMP response by the GRKs (Figs. 1 and 3). Accordingly, we speculated that different GRKs also might specifically modulate V2R-mediated ERK activation. Unexpectedly, suppression of GRK2, the most effective GRK for receptor phosphorylation and β-arrestin recruitment, actually increased the β-arrestin 2-mediated component of ERK activation (from 5 to 20 min of agonist stimulation) compared with control siRNA-transfected cells (Fig. 4 A and B). In GRK3 siRNA-transfected cells, the kinetic of ERK activation remained unchanged (Fig. 4 A and B). In either GRK5 or -6 siRNA-transfected cells, the activated ERK level remained close to control levels during the first 5 min of agonist treatment, but, upon longer stimulation, ERK activation was profoundly inhibited (Fig. 4 C and D), mimicking the effect of β-arrestin 2 siRNA transfection on V2R-mediated ERK activation. Moreover, the different siRNAs used had no effect on epidermal growth factor-stimulated ERK activation (data not shown).
Fig. 4.
Effects of siRNA-mediated inhibition of GRK2, -3, -5, or -6 expression on the kinetics of ERK activation by the V2R. (A and C) Cells were transfected with the FLAG-V2R-encoding plasmid and the indicated siRNAs. Cells were stimulated with 100 nM of dvdAVP at 37°C for the indicated periods. The levels of phosphorylated ERK (upper three blots) and total ERK (lower three blots) were visualized by immunoblotting. (B and D) Signals were quantified by densitometry and expressed as percentage of the maximum phosphorylated ERK obtained at 5 min of stimulation in control (CTL) siRNA-transfected cells. Data represent the mean ± SEM from at least four independent experiments. ns, not significant; ***, P < 0.001 compared with control curve.
To validate the biological significance of these siRNA results, we demonstrated that similar effects on ERK signaling were observed by using at least two different target sequences for GRK5 and -6 and that the effect of GRK6 siRNA could be substantially rescued (≈60%) by cotransfecting a “wobble” mutant construct that encodes the wild-type kinase but is resistant to siRNA targeting (data not shown). In the accompanying paper by Kim et al. (26), rescue of the effects of GRK5 siRNA on AT1AR-mediated ERK activation are similarly demonstrated.
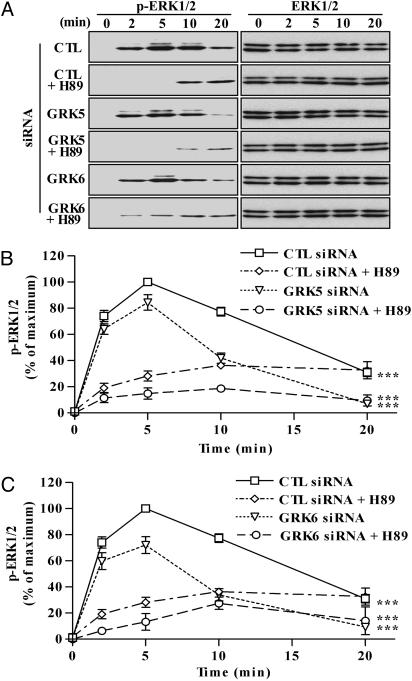
To further characterize the nature of the GRK5 and -6 effects, we determined whether ERK activation occurring during the first 5 min of agonist treatment in the absence of GRK5 or -6 was sensitive to H89. In GRK5 and -6 siRNA-transfected cells, V2R-mediated ERK activation was almost completely abolished by H89 at 2 and 5 min of agonist stimulation and did not affect the prolonged ERK signaling (Fig. 5). These data confirm that GRK5 and -6 play a specific role in regulating β-arrestin 2 but not Gs-dependent ERK signaling by the V2R.
Fig. 5.
Combined effects of GRK5 or -6 siRNA and H89-mediated inhibition on the kinetics of ERK activation by the V2R. (A) Cells were transfected with the FLAG-V2R-encoding plasmid and the indicated siRNAs. Cells were preincubated for 15 min with DMSO or 20 μM H89 and stimulated with 100 nM dvdAVP at 37°C for the indicated periods. Equal amounts of protein from each sample were used to visualize the phosphorylation of ERK (Left) and total ERK (Right) by immunoblotting. (B and C) Signals were quantified by densitometry and expressed as percentage of the maximal phosphorylated ERK obtained at 5 min of stimulation in control (CTL) siRNA-transfected cells. Each data point represents the mean ± SEM from at least four independent experiments. ***, P < 0.001 compared with entire control curve.
Discussion
Of the seven mammalian GRKs, only four, GRK2, -3, -5, and -6, are widely expressed (16, 17). GRK2 and -3 on the one hand, and GRK4, -5, and -6 on the other, are members of structurally distinct subfamilies of GRKs. Given the very limited number of GRKs and the large number of 7TM receptors, individual GRKs must have broad and possibly overlapping receptor substrate specificities. However, specialized functions of these enzymes have not been clearly defined. To address this issue, we developed specific siRNA against GRK2, -3, -5, and -6 and used this approach to investigate the specificity of these GRKs in the regulation of a typical 7TM receptor, the Gs-coupled V2R. We evaluated the contribution of each individual GRK to the following four agonist-induced parameters: (i) receptor phosphorylation, (ii) β-arrestin recruitment, (iii) cAMP production, and (iv) ERK 1/2 activation. Analogous to the situation recently described for the Gq-coupled AT1AR (14) and the Gs-coupled β2-adrenergic receptor (27), we found that the V2R triggers ERK activation via two pathways dependent on either Gs or β-arrestin 2. Gs-dependent ERK activation is rapid and transient whereas the β-arrestin 2 pathway for ERK signaling is slower and more sustained.
Our data reveal a previously unappreciated specialization of the different GRKs in the regulation of receptor signaling. Most of the GRK-mediated phosphorylation of the V2R, and consequent β-arrestin recruitment, are due to GRK2 and -3, whereas GRK5 and -6 make only minor contributions to these two parameters. Consistent with these findings, GRK2 and -3, but not -5 and -6, dampen the agonist-induced Gs-mediated cAMP response. These data are similar to the recent finding that human H1 histamine receptor desensitization is mediated by GRK2 but not GRK5 (28). Interestingly, GRK2 and -3 translocate to the plasma membrane and phosphorylate the activated receptor by interacting with free Gβγ subunits after they dissociate from the heterotrimeric G protein complex upon agonist stimulation (29). In striking contrast, both GRK5 and -6, which are constitutively associated with the plasma membrane, are required to enable β-arrestin 2-mediated ERK activation; GRK2 actually inhibits ERK activation. In the accompanying paper, Kim et al. report a virtually identical GRK specificity for the Gq-coupled AT1AR (26).
The remarkable similarity in the temporal patterns of V2R-mediated ERK activation in β-arrestin 2, GRK5, and GRK6 siRNA-transfected cells strongly suggests that all three components act in the same pathway. That the protein kinase A inhibitor H89 abolishes the residual early ERK response observed when expression of GRK5 or -6 or β-arrestin 2 are inhibited reinforces this interpretation. However, the average GRK5 and -6 siRNA-mediated inhibition is of lower magnitude than that achieved in the presence of β-arrestin 2 siRNA. This finding may simply reflect some redundancy in the actions of the two kinases. Alternatively, the explanation for this result may lie in the fact that GRKs are capable of enzymatic amplification, whereas β-arrestin 2 acts by nonenzymatic scaffolding of proteins. Therefore, greater siRNA-mediated inhibition of catalytically active GRKs may be required to elicit similar effects. In agreement with that notion, we consistently have observed in the course of this study that >90% inhibition of the different GRKs is a prerequisite to detect measurable effects on the ERK pathway. Moreover, the amplitudes of the effects we observed always correlated with the degree of siRNA-mediated inhibition.
The increased β-arrestin 2-mediated ERK activation observed in the presence of GRK2 siRNA suggests that a competition might exist between GRK2 and GRK5/6. Thus, when there is less GRK2, GRK-5 and/or -6 are more active, leading to increased ERK activation via the β-arrestin 2 pathway. This result also demonstrates that there is not a simple relationship between the amount of β-arrestin 2 recruited to the receptor and the activation level of the β-arrestin 2-dependent ERK pathway. Indeed, when GRK2 expression is inhibited, much less β-arrestin 2 interacts with the V2R, but the ERK phosphorylation is consistently higher than in controls. Reciprocally, in the presence of GRK5 or -6 siRNA, ERK activation by the β-arrestin 2 pathway was profoundly inhibited, whereas more modest differences were seen in β-arrestin 2 recruitment. Thus, these findings suggest that GRK5 and -6 actions are able to generate some qualitative, rather than quantitative, changes in the receptor-bound β-arrestin 2. Changes in the ratio of β-arrestin 1/2 recruited also could contribute because β-arrestin 1 has been shown to exert a reciprocal inhibitory effect on the β-arrestin 2-dependent ERK activation by the AT1AR (25). However, the technique used in the current study does not allow us to carefully test this hypothesis.
It is well established that β-arrestin is a scaffold molecule regulating 7TM receptor desensitization, endocytosis, and signaling (30). To exert these complex functions, β-arrestins bind to multiple proteins (7). The interaction with GRK-phosphorylated receptors is in general a prerequisite for β-arrestin functions. This interaction presumably induces conformational changes in the β-arrestins, thereby regulating its subsequent actions. In support of this hypothesis is a recent in vitro study showing that a synthetic phosphorylated peptide derived from the C terminus of the V2R binds to and induces conformational changes in β-arrestin 2 (31). Furthermore, clathrin binding to β-arrestin 2 was enhanced 10-fold when compared with the nonphosphorylated peptide (31). Moreover, we speculate that β-arrestins recruited to receptors phosphorylated by different GRKs might adopt unique conformations. Our results suggest that GRK2- and/or GRK3-phosphorylated V2R have very high binding affinity for β-arrestins. In addition, GRK2/3 action might induce a particular conformational change in the recruited β-arrestin that ultimately favors receptor uncoupling from its cognate G protein. Conversely, GRK5- and/or GRK6-phosphorylated V2R might adopt the proper conformation to induce ERK activation via the β-arrestin 2-dependent pathway. The validation of this theory will require identification of the specific sites phosphorylated by each of the GRKs and comparison of the conformational changes induced in β-arrestins by peptides phosphorylated on different positions according to the determined GRK-specific signatures.
Another interesting observation is that GRK5 and -6 seem to act in concert to induce the β-arrestin 2 ERK pathway. The inhibition of either one of these two GRKs is sufficient to virtually block the β-arrestin 2-dependent pathway for ERK activation, granted that the efficiency of siRNA-mediated inhibition is optimal. This finding suggests that GRK5 and -6 phosphorylate distinct sites on the receptor and that both contributions are required to impart the ERK signaling competent conformation to β-arrestin 2. Such a mechanism would impose tight control on β-arrestin 2-dependent signaling because the concerted action of two different GRKs would be required for the activation of this pathway.
Recent data from studies of gene knockout mice indicate that GRK6 and β-arrestin 2, but not GRK5, play positive roles in mediating the chemotactic responses of T and B lymphocytes to CXCL12 (32). Moreover, GRK5 and -6 are implicated not only in β-arrestin 2-mediated signaling, but also in numerous examples of receptor desensitization in vivo and/or in vitro (33–37). Depending on the receptors or the tissues considered, the two kinases can act either cooperatively or independently of each other and either inhibit or facilitate signaling, thus emphasizing the remarkable versatility and sophistication of the GRK regulatory system.
In conclusion, this study together with the accompanying paper on the AT1AR by Kim et al. (26) provide strong evidence for a previously unsuspected level of GRK specialization in the regulation of 7TM receptors. Individual GRKs regulate different aspects of receptor signaling. It is speculated that the agonist-activated receptor might be phosphorylated on distinct sites by different GRKs, thus delineating different active conformations of β-arrestins and ultimately divergent functional outcomes. These specialized actions of the different GRKs suggest the possibility of developing novel drugs, which might target a limited subset of 7TM receptor signaling capabilities. Such drugs should have unique biological actions and display more limited side effects.
Acknowledgments
We thank Donna Addison and Elizabeth Hall for excellent secretarial assistance and Richard Premont and Chris Nelson for helpful discussion. This work was supported by National Institutes of Health Grants R01 HL16037 and HL 70631 (to R.J.L.). R.J.L. is an Investigator of the Howard Hughes Medical Institute. E.R. is a Researcher from Institut National de la Recherche Agronomique.
Abbreviations: AT1AR, angiotensin II type 1A receptor; AVP, arginine vasopressin; dvdAVP, 1-deamine-4-valine-d-arginine vasopressin; ERK, extracellular signal-regulated kinase; GRK, G protein-coupled receptor kinase; HEK, human embryonic kidney; 7TM, seven-transmembrane; siRNA, small interfering RNA; V2R, V2 vasopressin receptor.
References
- 1.Lefkowitz, R. J. (1998) J. Biol. Chem. 273, 18677-18680. [DOI] [PubMed] [Google Scholar]
- 2.Kohout, T. A. & Lefkowitz, R. J. (2003) Mol. Pharmacol. 63, 9-18. [DOI] [PubMed] [Google Scholar]
- 3.Luttrell, L. M., Roudabush, F. L., Choy, E. W., Miller, W. E., Field, M. E., Pierce, K. L. & Lefkowitz, R. J. (2001) Proc. Natl. Acad. Sci. USA 98, 2449-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McDonald, P. H., Chow, C. W., Miller, W. E., Laporte, S. A., Field, M. E., Lin, F. T., Davis, R. J. & Lefkowitz, R. J. (2000) Science 290, 1574-1577. [DOI] [PubMed] [Google Scholar]
- 5.DeFea, K. A., Zalevsky, J., Thoma, M. S., Dery, O., Mullins, R. D. & Bunnett, N. W. (2000) J. Cell Biol. 148, 1267-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luttrell, L. M., Ferguson, S. S., Daaka, Y., Miller, W. E., Maudsley, S., Della Rocca, G. J., Lin, F., Kawakatsu, H., Owada, K., Luttrell, D. K., et al. (1999) Science 283, 655-661. [DOI] [PubMed] [Google Scholar]
- 7.Shenoy, S. K. & Lefkowitz, R. J. (2003) Biochem. J. 375, 503-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Witherow, D. S., Garrison, T. R., Miller, W. E. & Lefkowitz, R. J. (2004) Proc. Natl. Acad. Sci. USA 101, 8603-8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao, H., Sun, Y., Wu, Y., Luan, B., Wang, Y., Qu, B. & Pei, G. (2004) Mol. Cell 14, 303-317. [DOI] [PubMed] [Google Scholar]
- 10.Imamura, T., Huang, J., Dalle, S., Ugi, S., Usui, I., Luttrell, L. M., Miller, W. E., Lefkowitz, R. J. & Olefsky, J. M. (2001) J. Biol. Chem. 276, 43663-43667. [DOI] [PubMed] [Google Scholar]
- 11.Hupfeld, C. J., Resnik, J. L., Ugi, S. & Olefsky, J. M. (November 1, 2004) J. Biol. Chem., www.jbc.org/cgi/reprint/M403674200v1. [DOI] [PubMed]
- 12.Thibonnier, M., Coles, P., Thibonnier, A. & Shoham, M. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 175-202. [DOI] [PubMed] [Google Scholar]
- 13.Tohgo, A., Choy, E. W., Gesty-Palmer, D., Pierce, K. L., Laporte, S., Oakley, R. H., Caron, M. G., Lefkowitz, R. J. & Luttrell, L. M. (2003) J. Biol. Chem. 278, 6258-6267. [DOI] [PubMed] [Google Scholar]
- 14.Wei, H., Ahn, S., Shenoy, S. K., Karnik, S. S., Hunyady, L., Luttrell, L. M. & Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. USA 100, 10782-10787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahn, S., Shenoy, S. K., Wei, H. & Lefkowitz, R. J. (2004) J. Biol. Chem. 279, 35518-35525. [DOI] [PubMed] [Google Scholar]
- 16.Premont, R. T., Macrae, A. D., Stoffel, R. H., Chung, N., Pitcher, J. A., Ambrose, C., Inglese, J., MacDonald, M. E. & Lefkowitz, R. J. (1996) J. Biol. Chem. 271, 6403-6410. [DOI] [PubMed] [Google Scholar]
- 17.Pitcher, J. A., Freedman, N. J. & Lefkowitz, R. J. (1998) Annu. Rev. Biochem. 67, 653-692. [DOI] [PubMed] [Google Scholar]
- 18.Willets, J. M., Challiss, R. A. & Nahorski, S. R. (2003) Trends Pharmacol. Sci. 24, 626-633. [DOI] [PubMed] [Google Scholar]
- 19.Elbashir, S. M., Harborth, J., Lendeckel, W., Yalcin, A., Weber, K. & Tuschl, T. (2001) Nature 411, 494-498. [DOI] [PubMed] [Google Scholar]
- 20.Reynolds, A., Leake, D., Boese, Q., Scaringe, S., Marshall, W. S. & Khvorova, A. (2004) Nat. Biotechnol. 22, 326-330. [DOI] [PubMed] [Google Scholar]
- 21.Ahn, S., Nelson, C. D., Garrison, T. R., Miller, W. E. & Lefkowitz, R. J. (2003) Proc. Natl. Acad. Sci. USA 100, 1740-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin, N. P., Lefkowitz, R. J. & Shenoy, S. K. (2003) J. Biol. Chem. 278, 45954-45959. [DOI] [PubMed] [Google Scholar]
- 23.Oppermann, M., Freedman, N. J., Alexander, R. W. & Lefkowitz, R. J. (1996) J. Biol. Chem. 271, 13266-13272. [DOI] [PubMed] [Google Scholar]
- 24.Attramadal, H., Arriza, J. L., Aoki, C., Dawson, T. M., Codina, J., Kwatra, M. M., Snyder, S. H., Caron, M. G. & Lefkowitz, R. J. (1992) J. Biol. Chem. 267, 17882-17890. [PubMed] [Google Scholar]
- 25.Ahn, S., Wei, H., Garrison, T. R. & Lefkowitz, R. J. (2004) J. Biol. Chem. 279, 7807-7811. [DOI] [PubMed] [Google Scholar]
- 26.Kim, J., Ahn, S., Ren, X.-R., Whalen, E. J., Reiter, E., Wei, H. & Lefkowitz, R. J. (2005) Proc. Natl. Acad. Sci. USA 102, 1442-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azzi, M., Charest, P. G., Angers, S., Rousseau, G., Kohout, T., Bouvier, M. & Pineyro, G. (2003) Proc. Natl. Acad. Sci. USA 100, 11406-11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iwata, K., Luo, J., Penn, R. B. & Benovic, J. L. (November 12, 2004) J. Biol. Chem., www.jbc.org/cgi/reprint/M408834200v1.
- 29.Pitcher, J. A., Inglese, J., Higgins, J. B., Arriza, J. L., Casey, P. J., Kim, C., Benovic, J. L., Kwatra, M. M., Caron, M. G. & Lefkowitz, R. J. (1992) Science 257, 1264-1267. [DOI] [PubMed] [Google Scholar]
- 30.Luttrell, L. M. & Lefkowitz, R. J. (2002) J. Cell Sci. 115, 455-465. [DOI] [PubMed] [Google Scholar]
- 31.Xiao, K., Shenoy, S. K., Nobles, K. & Lefkowitz, R. J. (2004) J. Biol. Chem., 279, 55744-55753. [DOI] [PubMed] [Google Scholar]
- 32.Fong, A. M., Premont, R. T., Richardson, R. M., Yu, Y. R., Lefkowitz, R. J. & Patel, D. D. (2002) Proc. Natl. Acad. Sci. USA 99, 7478-7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gainetdinov, R. R., Bohn, L. M., Walker, J. K., Laporte, S. A., Macrae, A. D., Caron, M. G., Lefkowitz, R. J. & Premont, R. T. (1999) Neuron 24, 1029-1036. [DOI] [PubMed] [Google Scholar]
- 34.Gainetdinov, R. R., Bohn, L. M., Sotnikova, T. D., Cyr, M., Laakso, A., Macrae, A. D., Torres, G. E., Kim, K. M., Lefkowitz, R. J., Caron, M. G. & Premont, R. T. (2003) Neuron 38, 291-303. [DOI] [PubMed] [Google Scholar]
- 35.Tiberi, M., Nash, S. R., Bertrand, L., Lefkowitz, R. J. & Caron, M. G. (1996) J. Biol. Chem. 271, 3771-3778. [DOI] [PubMed] [Google Scholar]
- 36.Aiyar, N., Disa, J., Dang, K., Pronin, A. N., Benovic, J. L. & Nambi, P. (2000) Eur. J. Pharmacol. 403, 1-7. [DOI] [PubMed] [Google Scholar]
- 37.Willets, J. M., Challiss, R. A. & Nahorski, S. R. (2002) J. Biol. Chem. 277, 15523-15529. [DOI] [PubMed] [Google Scholar]