Abstract
Background/Aims
This study assessed the efficacy and safety of high-dose multimatrix mesalazine once-daily (QD) compared to another form of high-dose mesalazine.
Methods
In this multicenter, randomized, double-blind study, 280 patients with mildly to moderately active ulcerative colitis (UC) received multimatrix mesalazine 4.8 g/day QD or pH-dependent-release mesalazine 3.6 g/day three times daily for 8 weeks. The primary endpoint was the change in the UC-Disease Activity Index (UC-DAI) at the end of the treatment period.
Results
The change in the UC-DAI (mean±standard deviation) in the per-protocol set was −2.6±2.47 in the multimatrix mesalazine 4.8 g/day group (n=134) and −1.8±2.64 in the pH-dependent-release mesalazine 3.6 g/day group (n=129). The difference in the mean change between the 2 groups was −0.7 (two-sided 95% confidence interval, −1.3 to −0.1). The noninferiority of multimatrix mesalazine 4.8 g/day to pH-dependent-release mesalazine 3.6 g/day was verified within the noninferiority margin (1.1). The superiority of multimatrix mesalazine 4.8 g/day to pH-dependent-release mesalazine 3.6 g/day was also investigated and confirmed in the full analysis set, according to the study protocol. In subgroup analyses, the effectiveness of multimatrix mesalazine 4.8 g/day was consistent in all subgroups. There was no difference in safety between the 2 treatment groups.
Conclusions
Multimatrix mesalazine 4.8 g/day has higher efficacy and shows no difference in safety in mildly to moderately active UC, in comparison with pH-dependent-release mesalazine 3.6 g/day.
Keywords: Ulcerative colitis, Mesalazine, High-dose
INTRODUCTION
It has been reported that the therapeutic effect of oral mesalazine (5-aminosalicylic acid [5-ASA]) correlates with its concentration in the colonic mucosa.1 A Cochrane report indicates that high-dose mesalazine is effective for treating mildly to moderately active UC.2 Several drug delivery system formulations have been developed to efficiently deliver mesalazine to the colon.3 However, there has been no active control study to directly compare the efficacy among different formulations of high-dose mesalazine at doses exceeding 3.0 g/day.
Multimatrix mesalazine (Lialda® in the United States; Mezavant™ XL in the United Kingdom, Ireland, and Malta; Mezavant™ elsewhere in the European Union) is a once-daily (QD) oral formulation of 5-ASA. The core of the tablet consists of a multimatrix structure with an inner lipophilic matrix in which mesalazine is dispersed, and an outer hydrophilic matrix.4 The tablet is coated with a gastro-resistant polymer film that dissolves at a pH of approximately 7.5 This formulation design enables multimatrix mesalazine to deliver mesalazine specifically to the colon and to release the drug continuously, throughout the colon.4 The efficacy of multimatrix mesalazine 2.4 g/day QD and 4.8 g/day QD in active UC has been demonstrated in 2 placebo-controlled studies.6,7
This study was conducted in patients with mildly to moderately active UC with pH-dependent-release mesalazine 3.6 g/day (pH-3.6 g/day) in divided doses administered 3 times daily (TID) to compare the efficacy of high-dose multimatrix mesalazine 4.8 g/day (multimatrix-4.8 g/day) QD with another formulation of high-dose mesalazine.
METHODS
1. Patients
Eligible participants were outpatients diagnosed with UC and aged ≥16 years at the time of informed consent, with a UC-Disease Activity Index (UC-DAI) score ≥3 and ≤8 at randomization assignment, sigmoidoscopy score ≥1, rectal bleeding score ≥1, and physician's global assessment (PGA) score ≤2. The UC-DAI consists of the following 4 variables: stool frequency, rectal bleeding, sigmoidoscopic findings regarding the mucosal appearance, and PGA. The UC-DAI employs a 4-point scoring scale from 0 to 3 to evaluate each variable, and the evaluation index score is the total of the 4 variables (Table 1).8
Table 1. UC-Disease Activity Index.
| Subscale | Score |
|---|---|
| Stool frequency score | 0: Normal (normal indicates healthy state or maintained remission state of the subject) |
| 1: 1–2 Stools >normal | |
| 2: 3–4 Stools >normal | |
| 3: ≥5 Stools >normal | |
| Rectal bleeding score | 0: None |
| 1: Streaks of blood in stool | |
| 2: Obvious blood in stool | |
| 3: Mostly blood in stool | |
| Sigmoidoscopy score | 0: Normal |
| 1: Mild (erythema, reduced vascular pattern, mild friability) | |
| 2: Moderate (marked erythema, lack of vascular pattern, friability, erosion) | |
| 3: Severe (spontaneous bleeding, ulceration) | |
| PGA score | 0: Normal |
| 1: Mild | |
| 2: Moderate | |
| 3: Severe |
The mean score of daily stool frequency and rectal bleeding for 3 days before each visit were calculated.
PGA, physician's global assessment.
Patients were excluded from the study if they had any of the following: history of hypersensitivity to mesalazine and salicylic acid; severe UC; chronic continuous or acute fulminating UC; use of topical mesalazine, topical salazosulfapyridine, adrenal corticosteroid (oral, enema, suppository, medication for hemorrhoidal diseases, and injection), or cytapheresis therapy within 2 weeks before randomization assignment; treatment with an immune-regulating drug (oral, injection) or anti-tumor necrosis factor-α antibody within 12 weeks before randomization assignment; previous colonic resection (excluding appendectomy); moderate to severe renal or liver disorders; serious complications including diseases of the blood, respiratory, gastrointestinal, or cardiovascular organs; neuropsychiatric disease; metabolic/electrolyte imbalance or hypersensitivity; or malignant tumor. The following patients were also excluded: women who were pregnant, breastfeeding, or possibly pregnant; female patients who were planning to get pregnant during the study period; and patients who used other investigational products within the 4 months before study enrollment.
2. Study Drugs
Multimatrix mesalazine (Lialda®; Shire US Inc., Wayne, PA, USA) tablets were red-brown, oval, film-coated tablets containing 1.2 g of mesalazine per tablet. pH-dependent-release mesalazine (Asacol®; Zeria Pharmaceutical Co., Ltd., Tokyo, Japan) tablets, the comparator, were red-brown to brown film-coated tablets containing 400 mg of mesalazine per tablet. The study adopted a double-dummy trial design. Adherence to each study drug was measured based on patient diaries.
3. Study Design
This was a multicenter, randomized, double-blind, active-controlled, and parallel-group study with an 8-week treatment period that was conducted at 77 study centers in Japan from April 2014 to September 2015 (Japan Pharmaceutical Information Center clinical trial registration number: Japic CTI-142475). After informed consent was obtained, eligibility was evaluated based on the inclusion/exclusion criteria. The study drugs were administered to eligible patients.
The following 2 groups were given the study drugs: the multimatrix-4.8 g/day group and the pH-3.6 g/day group. Patients were randomized to 1 of the 2 groups in a 1:1 ratio by the permuted block method, with each study center as 1 block. A double-dummy design was adopted to maintain blinding of the investigators and study participants, and the multimatrix-4.8 g/day group received 4 tablets of multimatrix mesalazine 1.2 g QD, after breakfast, and 3 tablets of pH-dependent-release mesalazine placebo TID, after each meal. The pH-3.6 g/day group received 4 tablets of multimatrix mesalazine placebo QD, after breakfast, and 3 tablets of pH-dependent-release mesalazine 400 mg TID, after each meal.
4. Efficacy and Safety Evaluation
During the study, patients were required to visit the study center at weeks 2, 4, and 8, or at the time of discontinuation. At each visit, efficacy and safety were evaluated by the investigator. The mean scores of daily stool frequency and rectal bleeding for 3 days before each visit were calculated, based on the patient diary in which patients recorded stool frequency and rectal bleeding status. Colonoscopy was performed at the start of the treatment period and at week 8 or at the time of discontinuation, and the sigmoidoscopy score was assessed by the same investigator, with reference to the Atlas of endoscopy in IBD.9 The PGA score was evaluated based on the clinical symptoms and endoscopic findings at the beginning of the treatment period and at week 8 or at the time of discontinuation.
Adverse events (AEs) and vital signs were evaluated at each visit. Body weight and clinical laboratory test results were evaluated every 4 weeks or at the time of discontinuation. AEs were summarized by the preferred term using the Medical Dictionary for Regulatory Activities version 18.0 (http://www.meddra.org/).
5. Objective/Endpoints
The primary objective of this study was to verify the non-inferiority of multimatrix-4.8 g/day QD to pH-3.6 g/day TID based on the primary efficacy endpoint of change in the UC-DAI score from baseline to the end of the treatment period. The primary safety objective was to evaluate treatment with multimatrix-4.8 g/day QD in comparison with pH-3.6 g/day TID in relation to AEs that occurred during the treatment period.
Secondary efficacy endpoints were remission (UC-DAI score ≤2 and rectal bleeding score=0 at the end of the treatment period), clinical remission (rectal bleeding score=0 and stool frequency score=0 at the end of the treatment period), endoscopic remission (sigmoidoscopy score=0 at the end of the treatment period), improvement (from baseline, an improvement of at least 2 points in the UC-DAI score at the end of the treatment period), and the change in the score of each variable of the UC-DAI (score at the end of the treatment period − score at baseline). Secondary safety endpoints were AEs during the screening or follow-up periods and adverse drug reactions during the treatment or follow-up periods.
6. Statistical Analysis
The full analysis set (FAS) consisted of enrolled patients who received the study drug at least once and had at least one efficacy evaluation. The per protocol set (PPS) was the primary analysis set for the efficacy analysis, which consisted of patients in the FAS who satisfied the inclusion criteria, did not meet any exclusion criteria, did not receive prohibited concomitant drugs or therapy, received the study drugs for at least 18 days, and whose drug adherence was ≥75%. The safety analysis set consisted of patients who received the study drug at least once and underwent safety assessment.
For primary analysis of the efficacy, analysis of covariance using the baseline UC-DAI score as a covariate, was performed on the change in the UC-DAI score as the primary endpoint, and the two-sided 95% CI of the difference between the treatment groups was calculated to evaluate noninferiority. The noninferiority margin was set at 1.1 with respect to the change in the UC-DAI score based on the effect size of pH-3.6 g/day TID in a previous study.10 Under the condition that noninferiority was met, the superiority in the FAS was also investigated based on the two-sided 95% CI in the same manner as the noninferiority analysis with a closed procedure. With remission, clinical remission, endoscopic remission, and improvement as secondary endpoints, the proportion of patients who achieved each endpoint was compared between the treatment groups, along with the two-sided 95% CI of the difference. The changes in each variable of the UC-DAI were analyzed in the same manner as the primary endpoint. Subgroup analyses were performed to support the primary endpoint. For the safety endpoint, the incidence of AEs was compared between the treatment groups.
SAS version 9.2 (SAS Institute Inc., Cary, NC, USA) was used for statistical analysis. Assuming that the difference in the UC-DAI score between the 2 groups was 0.0 and the SD was 2.6 in both groups, we set the number of patients to demonstrate noninferiority at 125 patients per group with a one-sided type 1 error (α) of 2.5%, more than 90% power and the noninferiority margin of 1.1.
7. Ethical Considerations
The study was conducted in accordance with ethical principles that have their origin in the Declaration of Helsinki, the Ministerial Ordinance on Good Clinical Practice for Drugs, and other relevant laws, regulations, and standards. Written informed consent was obtained from each patient. Prior to conducting the study, the study protocol, a sample patient information sheet, and informed consent were approved by the Institutional Review Board at each study center; the appropriateness of the conduct of the clinical trial was also approved.
RESULTS
1. Demographic Background of Patients
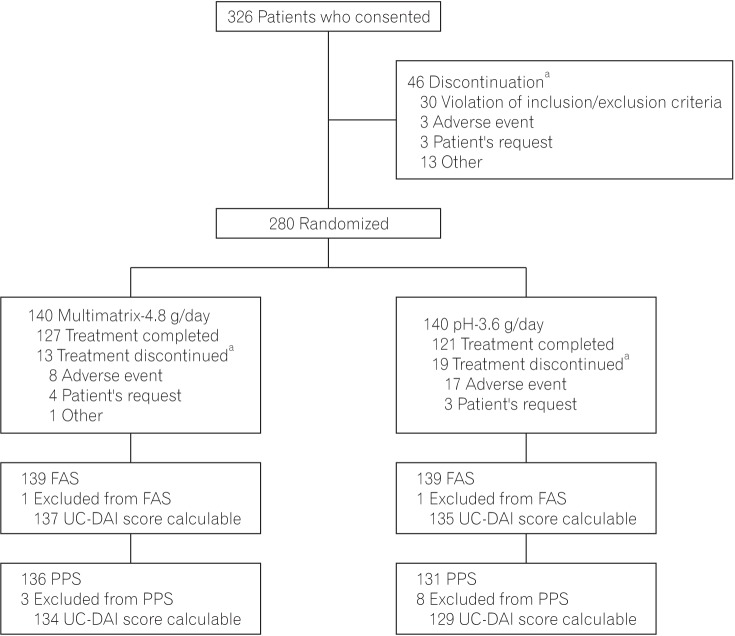
Consent was obtained from 326 patients, and 280 received study drugs (multimatrix-4.8 g/day group, 140; pH-3.6 g/day group, 140). Forty-six patients withdrew from the study before the randomization assignment. Of the 280 patients who received the study drugs, 248 patients completed treatment with the study drugs, and 32 patients (multimatrix-4.8 g/day group, 13; pH-3.6 g/day group, 19) discontinued the study drugs during the treatment period. The main reason for discontinuation in all treatment groups was AEs (multimatrix-4.8 g/day group, 8; pH-3.6 g/day group, 17) (Fig. 1).
Fig. 1. Patient disposition. aMultiple options are allowed as reasons for discontinuation. FAS, full analysis set; UC-DAI, UC-Disease Activity Index; PPS, per protocol set.
The disposition of the FAS and PPS is shown in Fig. 1. Two patients were excluded from the FAS (multimatrix-4.8 g/day group, 1; pH-3.6 g/day group, 1) because they received no efficacy evaluation after the start of the treatment period. Eleven patients were excluded from the PPS (multimatrix-4.8 g/day group, 3; pH-3.6 g/day group, 8). The most common reason for exclusion from the PPS was failure to achieve at least 18 days of use of the study drugs. Six patients in the FAS had a change in the UC-DAI score that was not calculable (multimatrix-4.8 g/day group, 2; pH-3.6 g/day group, 4); the corresponding number was 4 in the PPS (multimatrix-4.8 g/day group, 2; pH-3.6 g/day group, 2). The mean rates of drug adherence in the PPS were not less than 95% in both treatment groups. There were no intergroup differences in terms of patient background factors in the FAS (Table 2).
Table 2. Patient Demographics (Full Analysis Set).
| Variable | Multimatrix-4.8 g/day (n=139) | pH-3.6 g/day (n=139) |
|---|---|---|
| Sex | ||
| Male | 85 (61.2) | 72 (51.8) |
| Female | 54 (38.8) | 67 (48.2) |
| Age (yr) | 41.20±11.38 | 42.50±12.99 |
| 16–19 | 1 (0.7) | 3 (2.2) |
| 20–29 | 21 (15.1) | 19 (13.7) |
| 30–39 | 45 (32.4) | 34 (24.5) |
| 40–49 | 40 (28.8) | 49 (35.3) |
| 50–59 | 22 (15.8) | 16 (11.5) |
| 60–64 | 4 (2.9) | 7 (5.0) |
| ≥65 | 6 (4.3) | 11 (7.9) |
| Height (cm) | 165.730±8.830 | 164.050±9.482 |
| Body weight (kg) | 62.560±12.078 | 60.660±11.465 |
| Disease course | ||
| First attack | 27 (19.4) | 25 (18.0) |
| Relapsing-remitting | 112 (80.6) | 114 (82.0) |
| Extent of disease | ||
| Proctitis | 51 (36.7) | 56 (40.3) |
| Left-sided colitis | 65 (46.8) | 65 (46.8) |
| Pancolitis | 22 (15.8) | 16 (11.5) |
| Segmental colitis | 1 (0.7) | 2 (1.4) |
| UC-DAI score at baseline | 5.90±1.59 | 5.90±1.52 |
Values are presented as number (%) or mean±SD.
UC-DAI, UC-Disease Activity Index.
2. Efficacy
1) Primary Endpoint
In the PPS, the change in the UC-DAI score at the end of the treatment period was −2.60±2.47 (mean±SD) in the multimatrix-4.8 g/day group and −1.80±2.64 in the pH-3.6 g/day group. The difference in the mean change in the UC-DAI score (adjusted) between the multimatrix-4.8 g/day group and the pH-3.6 g/day group was −0.7 (two-sided 95% CI, −1.3 to −0.1). The upper limit of the two-sided 95% CI of the difference between the treatment groups was lower than the noninferiority margin (1.1), indicating that the noninferiority of multimatrix-4.8 g/day to pH-3.6 g/day was met (Table 3). The result in the FAS is also shown in Table 3, in which the robustness of the result is confirmed. Since the primary analysis demonstrated the noninferiority of multimatrix-4.8 g/day to pH-3.6 g/day, the superiority of multimatrix-4.8 g/day to pH-3.6 g/day was also investigated according to the study protocol. In the FAS, the change in the UC-DAI score at the end of the treatment period was −2.50±2.51 in the multimatrix-4.8 g/day group and −1.70±2.73 in the pH-3.6 g/day group. The difference in the mean change in the UC-DAI score (adjusted) between the treatment groups was −0.8. The two-sided 95% CI of this parameter was −1.4 to −0.2. The upper limit of the two-sided 95% CI of the difference between the treatment groups was lower than 0, and the superiority of multimatrix-4.8 g/day to pH-3.6 g/day was demonstrated.
Table 3. Change in UC-Disease Activity Index Score at the End of Treatment.
| Variable | Multimatrix-4.8 g/day | pH-3.6 g/day |
|---|---|---|
| Per protocol set | ||
| No. of patients | 134 | 129 |
| Mean±SD | −2.60±2.47 | −1.80±2.64 |
| Differences between groupsa (95% CI) | −0.7 (−1.3 to −0.1) | - |
| P-value | 0.017 | - |
| Full analysis set | ||
| No. of patients | 137 | 135 |
| Mean±SD | −2.50±2.51 | −1.70±2.73 |
| Differences between groupsa (95% CI) | −0.8 (−1.4 to −0.2) | - |
| P-value | 0.009 | - |
Change in UC-DAI score=UC-DAI score at the end of treatment − UC-DAI score at baseline.
aDifferences in mean change in UC-DAI scores between groups, adjusted according to UC-DAI score at baseline (multimatrix-4.8 g/day–pH-3.6 g/day).
UC-DAI, UC-Disease Activity Index.
2) Secondary Endpoints
The proportions of patients who achieved remission, clinical remission, endoscopic remission, and improvement are shown in Fig. 2. The remission rate was 43.4% (59/136) in the multimatrix-4.8 g/day group and 30.5% (40/131) in the pH-3.6 g/day group. The difference in the remission rate between the multimatrix-4.8 g/day group and the pH-3.6 g/day group was 12.8% (two-sided 95% CI, 1.4%–24.3%). The lower limit of the 95% CI did not fall below 0 (Fig. 2). The change in each variable of the UC-DAI was generally comparable or higher in the multimatrix-4.8 g/day group than in the pH-3.6 g/day group (Table 4). The difference in the change in the rectal bleeding score between the treatment groups was −0.2 at week 2 (two-sided 95% CI, −0.4 to −0.02). The upper limit of the 95% CI did not exceed 0. These results obtained for individual secondary endpoints supported the results for the primary endpoint.
Fig. 2. The proportion of patients who achieved remission, clinical remission, endoscopic remission, and improvement at the end of the treatment period in the per protocol set. The differences in each endpoint between the multimatrix mesalazine 4.8 g/day group and pH-dependent−release mesalazine 3.6 g/day group were 12.8% (two-sided 95% CI, 1.4% to 24.3%) for remission, 10.6% (two-sided 95% CI, −0.8% to 22.1%) for clinical remission, 5.4% (two-sided 95% CI, −3.5% to 14.2%) for endoscopic remission, and 9.0% (two-sided 95% CI, −2.7% to 20.8%) for improvement. aThe difference in the remission rate between the treatment groups was statistically significant.
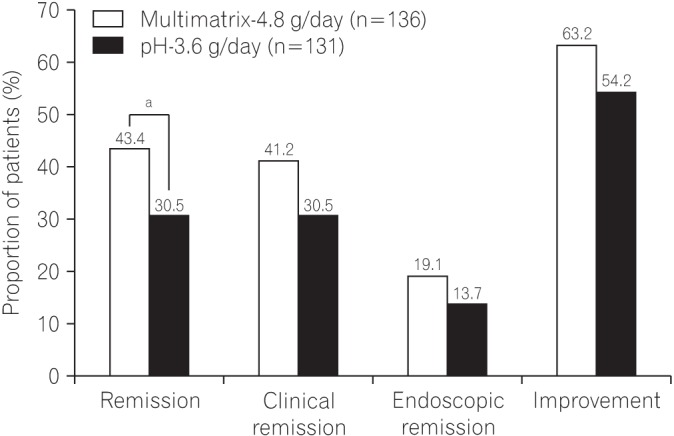
Table 4. Change in UC-Disease Activity Index Score Variables (Per Protocol Set).
| Variable | Multimatrix-4.8 g/day | pH-3.6 g/day |
|---|---|---|
| Stool frequency score | ||
| Week 2 | ||
| No. of patients | 136 | 131 |
| Mean±SD | −0.30±0.84 | −0.10±0.73 |
| Difference between groupsa (95% CI) | −0.2 (−0.3 to 0.0) | - |
| Week 4 | ||
| No. of patients | 135 | 129 |
| Mean±SD | −0.40±0.92 | −0.40±0.92 |
| Difference between groupsa (95% CI) | 0.0 (−0.2 to 0.2) | - |
| Week 8 | ||
| No. of patients | 127 | 121 |
| Mean±SD | −0.50±0.91 | −0.40±0.98 |
| Difference between groupsa (95% CI) | −0.1 (−0.3 to 0.1) | - |
| End of treatment | ||
| No. of patients | 136 | 131 |
| Mean±SD | −0.50±0.93 | −0.30±1.02 |
| Difference between groupsa (95% CI) | −0.2 (−0.4 to 0.0) | - |
| Rectal bleeding score | ||
| Week 2 | ||
| No. of patients | 136 | 131 |
| Mean±SD | −0.70±0.79 | −0.40±0.69 |
| Difference between groupsa (95% CI) | −0.2 (−0.4 to <0.0) | - |
| Week 4 | ||
| No. of patients | 135 | 129 |
| Mean±SD | −0.90±0.87 | −0.50±0.77 |
| Difference between groupsa (95% CI) | −0.3 (−0.5 to −0.1) | - |
| Week 8 | ||
| No. of patients | 127 | 121 |
| Mean±SD | −1.10±0.80 | −0.70±0.79 |
| Difference between groupsa (95% CI) | −0.3 (−0.5 to −0.1) | - |
| End of treatment | ||
| No. of patients | 136 | 131 |
| Mean±SD | −1.00±0.85 | −0.60±0.82 |
| Difference between groupsa (95% CI) | −0.3 (−0.5 to −0.2) | - |
| Sigmoidoscopy score | ||
| End of treatment | ||
| No. of patients | 134 | 129 |
| Mean±SD | −0.50±0.74 | −0.40±0.81 |
| Difference between groupsa (95% CI) | −0.1 (−0.3 to 0.1) | - |
| Physician’s global assessment score | ||
| End of treatment | ||
| No. of patients | 134 | 129 |
| Mean±SD | −0.60±0.68 | −0.50±0.71 |
| Difference between groupsa (95% CI) | −0.1 (−0.3 to 0.0) | - |
Change in each score=score at each evaluation point − score at baseline.
aDifferences in mean change in scores between groups, adjusted according to score at baseline (multimatrix-4.8 g/day − pH-3.6 g/day).
3) Subgroup Analysis
The results of subgroup analyses for the primary endpoint are shown in Table 5. The main UC categories were classification on the basis of disease course, extent of disease, and UC-DAI score at baseline. The difference in the mean change in the UC-DAI score (adjusted) between the multimatrix-4.8 g/day group and the pH-3.6 g/day group at the end of the treatment period, with classification according to disease course, was −0.8 (two-sided 95% CI, −1.9 to 0.3) among patients classified as having a “first attack,” and −0.7 (two-sided 95% CI, −1.4 to −0.1) among patients classified as having a “relapsing-remitting” course. With classification by the extent of disease, the difference was −1.9 in patients with “proctitis” (two-sided 95% CI, −2.8 to −1.1), 0.1 in patients with “left-sided colitis” (two-sided 95% CI, −0.8 to 1.0), and −0.6 in patients with “pancolitis” (two-sided 95% CI, −2.6 to 1.4). When patients were classified by UC-DAI score at baseline, the difference was −0.1 in those with scores of “3–5” (two-sided 95% CI, −0.9 to 0.6), and −1.1 in those with scores of “6–8” (two-sided 95% CI, −2.0 to −0.3).
Table 5. Subgroup Analysis of Change in UC-Disease Activity Index Score (Per Protocol Set).
| Variable | Multimatrix-4.8 g/day | pH-3.6 g/day |
|---|---|---|
| Disease course | ||
| First attack | ||
| No. of patients | 24 | 22 |
| Mean±SD | −3.60±1.77 | −3.10±2.59 |
| Differences between groupsa (95% CI) | −0.8 (−1.9 to 0.3) | - |
| Relapsing-remitting | ||
| No. of patients | 110 | 107 |
| Mean±SD | −2.30±2.55 | −1.60±2.59 |
| Differences between groupsa (95% CI) | −0.7 (−1.4 to −0.1) | - |
| Extent of disease | ||
| Proctitis | ||
| No. of patients | 50 | 53 |
| Mean±SD | −2.70±2.64 | −0.70±1.86 |
| Differences between groupsa (95% CI) | −1.9 (−2.8 to −1.1) | - |
| Left-sided colitis | ||
| No. of patients | 64 | 60 |
| Mean±SD | −2.40±2.32 | −2.50±2.77 |
| Differences between groupsa (95% CI) | 0.1 (−0.8 to 1.0) | - |
| Pancolitis | ||
| No. of patients | 19 | 14 |
| Mean±SD | −2.80±2.50 | −3.00±3.09 |
| Differences between groupsa (95% CI) | −0.6 (−2.6 to 1.4) | - |
| Segmental colitis | ||
| No. of patients | 1 | 2 |
| Mean±SD | 1.0±0 | −3.50±3.54 |
| Differences between groupsa (95% CI) | 4.5 (−50.5 to 59.5) | - |
| UC-DAI score at baseline | ||
| 3–5 | ||
| No. of patients | 56 | 52 |
| Mean±SD | −1.60±2.23 | −1.50±1.83 |
| Differences between groupsa (95% CI) | −0.1 (−0.9 to 0.6) | - |
| 6–8 | ||
| No. of patients | 78 | 77 |
| Mean±SD | −3.30±2.40 | −2.10±3.06 |
| Differences between groupsa (95% CI) | −1.1 (−2.0 to −0.3) | - |
Change in UC-DAI score=UC-DAI score at the end of treatment − UC-DAI score at baseline.
aDifferences in mean change in UC-DAI scores between groups, adjusted according to UC-DAI score at baseline (multimatrix-4.8 g/day − pH-3.6 g/day).
UC-DAI, UC-Disease Activity Index.
3. Safety
The incidence of AEs (proportion of patients who experienced at least 1 AE) during the treatment period was 50.0% (70/140) in the multimatrix-4.8 g/day group, and 55.7% (78/140) in the pH-3.6 g/day group. The most common AEs in both treatment groups were nasopharyngitis, increase in N-acetyl-beta-D-glucosaminidase, and aggravation of UC. The incidence of these AEs in individual treatment groups is shown in Table 6. The incidence of adverse drug reactions (side effects) during the treatment period was 26.4% (37/140) in the multimatrix-4.8 g/day group and 22.9% (32/140) in the pH-3.6 g/day group. All AEs observed with multimatrix-4.8 g/day treatment were mild or moderate in severity.
Table 6. Incidence of Adverse Events.
| Characteristic | Multimatrix-4.8 g/day (n=140) | pH-3.6 g/day (n=140) |
|---|---|---|
| Total | 70 (50.0) | 78 (55.7) |
| Nasopharyngitis | 11 (7.9) | 15 (10.7) |
| N-acetyl-β-D-glucosaminidase increased | 9 (6.4) | 10 (7.1) |
| Aggravation of UC | 7 (5.0) | 17 (12.1) |
| Blood bilirubin increased | 7 (5.0) | 2 (1.4) |
| Amylase increased | 4 (2.9) | 1 (0.7) |
| Headache | 3 (2.1) | 8 (5.7) |
| Oropharyngeal pain | 3 (2.1) | 1 (0.7) |
| Anemia | 1 (0.7) | 3 (2.1) |
| Pharyngitis | 1 (0.7) | 3 (2.1) |
| Rash | 1 (0.7) | 3 (2.1) |
| Frequent bowel movement | 0 | 4 (2.9) |
| Nausea | 0 | 3 (2.1) |
Values are presented as number (%). Adverse events reported by at least 2% of subjects in any treatment group during the treatment period are listed.
There were no deaths during the course of the study. Concerning serious AEs during the treatment period, interstitial lung disease, organizing pneumonia, and aggravation of UC occurred in 1 patient each in the multimatrix-4.8 g/day group, while bladder cancer and myocarditis occurred in 1 patient each, and aggravation of UC occurred in 2 patients in the pH-3.6 g/day group. With the exception of the aggravation of UC experienced by the patient in the multimatrix-4.8 g/day group and the bladder cancer experienced by the patient in the pH-3.6 g/day group, all of these events were adverse drug reactions. In terms of serious AEs occurring during the follow-up period, depression occurred in 1 patient in the multimatrix-4.8 g/day group. No causal relationship was found with the study drugs.
DISCUSSION
In the treatment of active UC, the effectiveness of high-dose oral mesalazine has been recognized. However, there have been no reports directly comparing the efficacy among different formulations of mesalazine administered in doses exceeding 3.0 g/day. Thus, the clinical positioning of high-dose mesalazine among formulations has not been clearly defined.
In a previous study (JAPIC clinical trial registration number: Japic CTI-101380), it was demonstrated that multimatrix-4.8 g/day QD was more effective than time-dependent−release mesalazine 2.25 g/day TID for all evaluation items and subgroups, but the noninferiority of multimatrix-2.4 g/day QD to time-dependent−release mesalazine 2.25 g/day TID was not shown (unpublished data). In the present study, the noninferiority of multimatrix-4.8 g/day QD to pH-3.6 g/day TID was demonstrated in the primary efficacy endpoint. In addition, the superiority of multimatrix-4.8 g/day QD to pH-3.6 g/day TID was also investigated and confirmed in accordance with the predefined procedure in the study protocol. A double-blind study comparing the efficacy of pH-dependent-release mesalazine between 3.6 g/day and 4.8 g/day reported no apparent benefit with the highest dose versus the lowest dose in patients with active UC.11 In the present study, the benefit of multimatrix-4.8 g/day QD was demonstrated in comparison with pH-3.6 g/day TID, coupled with no specific differences in safety.
It is worth noting that pH-3.6 g/day TID was selected as a comparator because in Japan, where this study was conducted, the highest approved dose of pH-dependent-release mesalazine was limited to 3.6 g/day, and the highest dose of time-dependent−release mesalazine 4.0 g/day was indicated only for patients with moderate and relapsing-remitting active UC.
The results for the secondary endpoints (i.e., remission, clinical remission, endoscopic remission, improvement, and change in each variable of the UC-DAI) supported the result obtained for the primary endpoint. The difference in the remission rate (UC-DAI score ≤2 and rectal bleeding score=0 at the end of the treatment period) between the multimatrix-4.8 g/day group and the pH-3.6 g/day group was statistically significant (12.8%; two-sided 95% CI, 1.4%–24.3%). In addition, the endoscopic remission rate of the multimatrix-4.8 g/day group was numerically greater than that of the pH-3.6 g/day group by 5.4% (two-sided 95% CI, −3.5% to 14.2%), under the strict definition of endoscopic remission as a sigmoidoscopy score of 0. Recently, the importance of mucosal healing has been emphasized. It has been reported that remission of UC is maintained for a longer period when the mucosal healing is achieved by induction therapy.12,13,14 Furthermore, in the present study, the difference in the change in the rectal bleeding score, the main symptom of UC, was statistically significant between the 2 groups at week 2, as well as at subsequent visits.
Although this study did not assess the benefits of extending high-dose induction treatment beyond 8 weeks, a previous study of multimatrix mesalazine suggested the benefit of high-dose induction therapy beyond this time period. An open-label study showed that among patients who failed to achieve remission after treatment with multimatrix mesalazine 2.4 g/day or 4.8 g/day, or pH-dependent, delayed-release mesalazine 2.4 g/day for 8 weeks, more than 60% achieved clinical and endoscopic remission after a further 8 weeks of treatment with multimatrix mesalazine 4.8 g/day.15
Multimatrix-4.8 g/day QD demonstrated consistent efficacy in all subgroups, including findings with respect to the disease course, the extent of disease, and the severity of UC. Mean changes in UC-DAI scores with multimatrix-4.8 g/day were similar in subgroups of patients regardless of the extent of disease. Since the lesions spread from the rectum, and rectal inflammation tends to heal poorly in UC, a topical formulation of mesalazine used alone or in combination with an oral mesalazine formulation is the recommended treatment for proctitis.3 However, topical preparations require complicated administration procedures and may not be acceptable to all patients because of issues of tolerability and compliance. Taking these factors into consideration, it is of great clinical significance that the efficacy of multimatrix-4.8 g/day was observed in proctitis as well as left-sided colitis, pancolitis, and segmental colitis in the current study. These results are in keeping with the fact that 5-ASA is released specifically and continuously into the colon, based on the characteristics of the multimatrix mesalazine formulation.4 However, this study was not designed to identify whether the difference in the efficacy between multimatrix-4.8 g/day QD and pH-3.6 g/day TID is a result of the difference in the dose or formulation, or a combination of these factors. Regarding the severity of UC, the difference in the mean change in the UC-DAI score between the 2 groups was −1.1 in the patients with UC-DAI scores of 6 to 8 at baseline, whereas it was −0.1 in the patients with scores of 3 to 5. Apparently, the benefit of the 1.2 g higher dose per day of multimatrix-4.8 g/day compared to pH-3.6 g/day was not clear in the patients with mild UC. However, in the patients with proctitis and UC-DAI scores of 3 to 5, the mean change in the UC-DAI score was −1.9 in the multimatrix-4.8 g/day group and −1.0 in the pH-3.6 g/day group in the same subgroup analysis (data not shown), suggesting that there might be some patients with mild UC who could receive greater benefit from multimatrix-4.8 g/day QD than from pH-3.6 g/day TID.
As discussed previously, multimatrix-4.8 g/day QD is expected to contribute to the control of symptoms of various forms and categories of severity of active UC, and it has been shown to be superior to pH-3.6 g/day TID with respect to change in UC-DAI scores in patients with mildly or moderately active UC.
ACKNOWLEDGEMENTS
The authors would like to thank all patients and the following investigators who participated in this study. The affiliation of each investigator described here is that which existed at the time the study was conducted.
Investigators: Satoshi Motoya, Sapporo-Kosei General Hospital; Shinji Kumagai, Nakajima Hospital; Yoshinori Sakai, Tsuchiura Kyodo General Hospital; Mitsuhide Goto, Ibaraki Prefectural Central Hospital; Kenichi Iizuka, Maebashi Red Cross Hospital; Toshihide Ohmori, Ohmori Toshihide Gastro-intestinal Clinic; Hiroyuki Kurihara, Tokorozawa Proctologic Hospital; Morio Takahashi, Morio Clinic; Yukihiro Hamahata, Tsujinaka Hospital Kashiwanoha; Takehiro Arai, Tokatsu-Tsujinaka Hospital; Yasuo Suzuki, Toho University Sakura Medical Center; Toru Isono, Isono Clinic; Ryuzo Murai, Onaka Clinic; Hitoshi Kaneko, Kaneko Clinic; Hiroyuki Kimura, Kimura Medical Clinic; Nobuyuki Matsuhashi, NTT Medical Center Tokyo; Masakazu Nagahori, Medical Hospital of Tokyo Medical and Dental University; Yukiya Yoshida, Mishuku Hospital; Naoki Yoshimura, Japan Community Health Care Organization, Tokyo Yamate Medical Center; Toshiaki Terada, Terada Hospital; Taku Kobayashi, Kitasato Institute Hospital, Kitasato University; Tadashi Teramoto, Machida Gastrointestinal Hospital; Yasuo Nakajima, ART Shimbashi Clinic; Asako Yamada, IVY Clinic; Mitsuhiro Tsukui, Tsukui-naika-onaka Clinic; Osamu Noguchi, Ome Municipal General Hospital; Atsushi Hayashi, Hino Municipal Hospital; Hidenori Kurakata, Inoue Gastrointestinal Internal Medicine Clinic; Kiyonori Kobayashi, Kitasato University East Hospital; Ryoichi Suzuki, Kannai Suzuki Clinic; Haruo Nishino, Matsushima Clinic; Seiji Otsuka, Kokan Clinic; Masao Araki, Sagamihara Kyodo Hospital; Yutaka Endo, Ofuna Chuo Hospital; Yuichiro Kojima, Yamanashi Prefectural Central Hospital; Akihiko Ohta, Ieda Hospital; Tadashi Yokoyama, Yokoyama Gastrointestinal Hospital; Yasuhisa Yokoyama, Yokoyama Gastrointestinal Hospital; Masahiro Yamada, Toyohashi Municipal Hospital; Yuji Nishio, Meitetsu Hospital; Mitsuki Miyata, Miyata Clinic; Hitoshi Hongo, Fujita Gastroenterological Division Hospital; Tatsuhiko Usui, Usui Internal Medicine/Gastroenterology Department Clinic; Soken Sai, Sai Clinic; Kentaro Tsuji, Tsuji Kentaro Clinic; Hirohisa Tanimura, Osaka Kaisei Hospital; Shiro Nakamura, Hyogo College of Medicine Hospital; Toshio Nakajima, Nakajima Clinic; Shusaku Yoshikawa, Dongo Hospital; Masaki Kunihiro, Hiroshima City Hiroshima Citizens Hospital; Masaaki Sumioka, Hiroshima Prefectural Hospital; Keiichi Mitsuyama, Kurume University Hospital; Toshiaki Ochiai, Saiseikai Fukuoka General Hospital; Takashi Hisabe, Fukuoka University Chikushi Hospital; Hideki Kitada, Kumamoto Red Cross Hospital; Naoto Ishikawa, Ishikawa Clinic; Hironori Masuyama, Masuyama Gastrointestinal Clinic; Masahiko Ohata, Maruyama Memorial General Hospital; Akihiko Tsuchiya, Ageo Central General Hospital; Akifumi Akai, Tokai Memorial Hospital; Hirokazu Yamagami, Osaka City University Hospital; Shunichi Yoshida, Akashi Medical Center; Mitsunari Yamamoto, Kinki Central Hospital; Tomoyuki Ninomiya, Ehime Prefectural Central Hospital; Kazuya Akahosi, Iizuka Hospital; Shinichi Ogata, Saga Prefectural Medical Centre Koseikan; Toru Niihara, Nanpuh Hospital; Mineo Kudo, Sapporo Hokuyu Hospital; Tomohiro Tada, Tada Tomohiro Clinic; Hirozumi Obata, Obata Medical Clinic; Mitsuyuki Murano, Murano Clinic; Yukinori Sameshima, Sameshima Hospital; Mitsushige Shibatouge, Takamatsu Red Cross Hospital; Yoshiyuki Shiwaku, Crystal Bldg Clinic; Hisamitsu Hidaka, Hidaka Clinic; Nobusada Koike, Hachioji Digestive Disease Hospital; Takumi Fukuchi, Osakafu Saiseikai Nakatsu Hospital.
Multimatrix mesalazine was kindly donated by Shire US Inc., Wayne, PA, USA, and pH-dependent-release mesalazine was kindly provided by Zeria Pharmaceutical Co., Ltd., Tokyo, Japan. We would also like to thank Shire US Inc. and Zeria Pharmaceutical Co., Ltd.
Footnotes
Financial support: The study was supported by Mochida Pharmaceutical Co., Ltd. Multimatrix mesalazine was kindly donated by Shire US Inc., Wayne, PA, USA. Mochida Pharmaceutical Co., Ltd. provided funding to support the provision of pH-dependent-release mesalazine (Zeria Pharmaceutical Co., Ltd., Tokyo, Japan).
Conflict of interest: Haruhiko Ogata has received consulting, grant, or lecture fees from Mochida Pharmaceutical, JIMRO, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma, Kyorin Pharmaceutical, Otsuka Pharmaceutical, Astellas Pharma, Eisai, Zeria Pharmaceutical, AbbVie G.K., EA Pharma, and Boston Scientific Japan K.K. Seiichi Mizushima and Atsushi Hagino are employees of Mochida Pharmaceutical. Toshifumi Hibi is editor-in-chief and has received consulting, grant, lecture, or manuscript preparation fees from Mochida Pharmaceutical, AbbVie G.K., EA Pharma, AstraZeneca K.K., JIMRO, Mitsubishi Tanabe Pharma, Eisai, Takeda Pharmaceutical, Zeria Pharmaceutical, Janssen Pharmaceutical K.K., Astellas Pharma, and Otsuka Pharmaceutical.
References
- 1.Frieri G, Giacomelli R, Pimpo M, et al. Mucosal 5-aminosalicylic acid concentration inversely correlates with severity of colonic inflammation in patients with ulcerative colitis. Gut. 2000;47:410–414. doi: 10.1136/gut.47.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feagan BG, Macdonald JK. Oral 5-aminosalicylic acid for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD000544. doi: 10.1002/14651858.CD000544.pub3. [DOI] [PubMed] [Google Scholar]
- 3.Dignass A, Lindsay JO, Sturm A, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030. doi: 10.1016/j.crohns.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 4.Brunner M, Assandri R, Kletter K, et al. Gastrointestinal transit and 5-ASA release from a new mesalazine extended-release formulation. Aliment Pharmacol Ther. 2003;17:395–402. doi: 10.1046/j.1365-2036.2003.01445.x. [DOI] [PubMed] [Google Scholar]
- 5.Tenjarla S, Abinusawa A. In-vitro characterization of 5-aminosalicylic acid release from MMX mesalamine tablets and determination of tablet coating thickness. Adv Ther. 2011;28:62–72. doi: 10.1007/s12325-010-0087-5. [DOI] [PubMed] [Google Scholar]
- 6.Lichtenstein GR, Kamm MA, Boddu P, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5:95–102. doi: 10.1016/j.cgh.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 7.Kamm MA, Sandborn WJ, Gassull M, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132:66–75. doi: 10.1053/j.gastro.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland LR, Martin F, Greer S, et al. 5-Aminosalicylic acid enema in the treatment of distal ulcerative colitis, proctosigmoiditis, and proctitis. Gastroenterology. 1987;92:1894–1898. doi: 10.1016/0016-5085(87)90621-4. [DOI] [PubMed] [Google Scholar]
- 9.Hibi T Ministry of Health, Labour and Welfare; Research Group for Intractable Inflammatory Bowel Disease (Watanabe Group), editors . Health and labour science research grants from the Japanese Ministry of Health, Labour and Welfare and research on measures for intractable disease: 2007 research report. Tokyo: Ministry of Health, Labour and Welfare; 2008. Atlas of endoscopy in inflammatory bowel disease; pp. 423–457. [Google Scholar]
- 10.Ito H, Iida M, Matsumoto T, et al. Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double-blind, randomized study. Inflamm Bowel Dis. 2010;16:1567–1574. doi: 10.1002/ibd.21193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki Y, Iida M, Ito H, Saida I, Hibi T. Efficacy and safety of two pH-dependent-release mesalamine doses in moderately active ulcerative colitis: a multicenter, randomized, double-blind, parallel-group study. Intest Res. 2016;14:50–59. doi: 10.5217/ir.2016.14.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laharie D, Filippi J, Roblin X, et al. Impact of mucosal healing on long-term outcomes in ulcerative colitis treated with infliximab: a multicenter experience. Aliment Pharmacol Ther. 2013;37:998–1004. doi: 10.1111/apt.12289. [DOI] [PubMed] [Google Scholar]
- 13.Rubin DT, Bradette M, Gabalec L, et al. Ulcerative colitis remission status after induction with mesalazine predicts maintenance outcomes: the MOMENTUM trial. J Crohns Colitis. 2016;10:925–933. doi: 10.1093/ecco-jcc/jjw049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yokoyama K, Kobayashi K, Mukae M, Sada M, Koizumi W. Clinical study of the relation between mucosal healing and longterm outcomes in ulcerative colitis. Gastroenterol Res Pract. 2013;2013:192794. doi: 10.1155/2013/192794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamm MA, Lichtenstein GR, Sandborn WJ, et al. Effect of extended MMX mesalamine therapy for acute, mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2009;15:1–8. doi: 10.1002/ibd.20580. [DOI] [PubMed] [Google Scholar]