Abstract
Objective(s):
Lung cancer is the main leading cause of cancer death worldwide. Angiogenesis is the main step in proliferation and spreading of tumor cells. Targeting vascular endothelial growth factor (VEGF) is an effective approach for inhibition of cancer angiogenesis. Nanobodies (NBs) are a novel class of antibodies derived from the camel. Unique characteristics of Nbs like their small size and good penetration to tumor tissues makes them promising tools in drug development. Development of NBs targeting both human and mouse VEGF is required for understanding their in vivo functions. Therefore, development of cross-species reactive anti-VEGF Nbs for immunotherapy of lung cancer was the main aim of the current study.
Materials and Methods:
Here we developed NBs from Camelus dromedarius library with high specificity and binding affinity to both human and mouse VEGF. In vitro and In vivo function of developed NB was evaluated on human endothelial cells and lung epithelial tumor cells (TC-1).
Results:
A nanobody showed the highest affinity to human and mouse VEGF and potently inhibited VEGF in the ELISA experiment. Anti-VEGF NBs significantly inhibited in vitro human endothelial cell migration through blockade of VEGF (P=0.045). Anti-VEGF NBs also significantly inhibited in vivo TC-1 growth in a dose-dependent manner (P=0.001) and resulted in higher survival rate in the nanobody treated group
Conclusion:
These findings demonstrate the potential of anti-VEGF NBs in tumor growth inhibition and are promising as novel cancer therapeutic candidate.
Keywords: Angiogenesis Immunotherapy, Lung cancer, Nanobody, Vascular endothelial - growth factor
Introduction
Lung cancer is the leading cause of cancer death around the world (1). The incidence of lung cancer is 4.7-9.2 per 100,000 people in Iran (2). Lung cancer ranked as second and third leading causes of cancer-related death in men and women, respectively (2). The advancement in knowledge of angiogenesis and its important role in tumor progression and metastasis resulted in the development of anti-angiogenesis therapies for cancer treatment (3). Anti-angiogenesis treatments are based on inhibition of neovascularization by blocking the interaction between VEGF and its receptors, VEGFR1 and VEGFR2 (4). Targeting vascular endothelial growth factor (VEGF) is an effective approach for inhibition of cancer angiogenesis. Tumor cells need new blood vessel formation or angiogenesis for growth and invasion (5). VEGF has two main receptors, VEGFR1 (Flt-1) and VEGFR2 (Flk-1/KDR) on the endothelial cell surface. Interaction of VEGF with its receptors
initiates the intracellular signaling cascade which results in proliferation, migration and tube formation of endothelial cells. These events lead to the growth, invasion, and metastasis of tumor cells (6). VEGF is a key regulator of tumor angiogenesis and therefore blockade of VEGF leads to inhibition of tumor angiogenesis (5). Due to the importance of VEGF in cancer development, several VEGF inhibitors have recently been developed. These inhibitors including, anti-VEGF or VEGFR (VEGF receptor) antibody, soluble receptors targeting VEGF, and tyrosine kinase inhibitors (7-9). Bevacizumab (Avastin®, Genentech) is anti-VEGF humanized monoclonal antibody and was approved by the Food and Drug Administration (FDA) in 2004 as a first anti-VEGF therapy for treatment of metastatic colorectal cancer (10) and subsequently for the treatment of metastatic breast cancer (11). VEGF-TrapR1R2 (Aflibercept; Regeneron Inc.), is a soluble receptor which blockades circulating VEGF.
VEGF-TrapR1R2 currently is in clinical trials and has shown anti-tumor activities (12). It has been shown that anti-VEGF antibodies are effective in tumor treatment through targeting angiogenesis (13). But there are some drawbacks (large size, immuno-genicity, and expense of production of purification) which restrict the use of anti-VEGF antibodies (14). Thus, many studies attempt to develop a new format of antibodies with reduced size and cost. Nanobody or VHH is a novel class of heavy chain-only antibodies. Nanobodies (NBs) naturally exist in the serum of Camelidae and have many advantages: small size (about 15 kDa), low-cost bacterial production and purification, low immunogenicity, and high thermal stability (15). The small size of NBs allows them to detect epitopes that are usually not detected by conventional monoclonal antibodies. Special pro-perties make NBs promising candidates in cancer therapy (16). It has been proven that bevacizumab only blocks human VEGF-A and not mouse VEGF (17). Therefore, the disease model of human VEGF is required for analysis of clinical effects of bevacizumab. To overcome this problem many studies have focused on the development of anti-VEGF antibodies that cross-react with human and mouse VEGF (17, 18). Given the importance of VEGF in cancer angiogenesis promotion, and the need to develop NBs targeting both human and mouse VEGF for understanding their in vivo functions, this study for the first time aimed to develop NBs cross-reacting with human and mouse VEGF and evaluate their function in tumor treatment of mouse model.
Materials and Methods
Materials
Tumor cell line TC-1, derived from primary TC-1 of C57BL/6 mice (ATCC: CRL-2785) was purchased from the National Cell Bank of Iran (Pasteur Institute of Iran). Human Umbilical Vein Endothelial Cells (HUVECs) were isolated from umbilical cord veins and cultured in EBM-2 medium (Lonza, Switzerland) supplemented with FBS and EGM-2 BulletKit (Lonza, Switzerland), and used just for 4 passages (19). Human and mouse vascular endothelial growth factor (VEGF-A165), human bFGF (basic fibroblast growth factor), vascular endothelial growth factor receptor 2 (VEGFR2, Flk-1/KDR), epidermal growth factor (EGF), and bovine serum albumin (BSA) were purchased from R&D, Minneapolis, USA. Bevacizumab was from Roche, Switzerland.
Isolation, expression, and purification of cross-reactive VEGF-specific NBs
A cDNA library of nanobody genes was constructed from camels immunized by human and mouse VEGF. Phages displaying VEGF specific NBs were isolated through four consecutive rounds of biopanning on immobilized VEGF in our previous work (19). Briefly, after biopanning, four clones carrying VEGF-specific NBs were selected and sequenced. The sequences of selected NBs were aligned using the MEGA-5 multiple sequence alignment program (19). The framework and CDR regions of NBs were numbered according to IMGT database. Unique VEGF-specific nanobody genes were re-cloned into the pHEN-6C expression vector (19). Positive transformants were selected by colony-PCR and then confirmed by DNA sequencing. Expression of NBs was induced by adding 1 mM IPTG (isopropyl D-1-thiogalactopyranoside) (Sigma, Germany) to exponentially growing Escherichia coli Wk6 cells. The NBs were expressed as c-terminus His-tag fusion and purified using nickel affinity chromatography (Ni+-NTA) (Qiagen, Germany). The purified NBs were dialyzed against 10 mM phosphate buffer (PBS, pH 7.2). Final protein yield was determined by UV absorption at 280 nm (19).
Cross-reactivity analysis
For analysis the cross-reactivity of VEGF-specific NBs, human VEGF, mouse VEGF, and BSA were assessed. One hundred microliters of proteins (1 µg/ml) in bicarbonate buffer (pH 9) was coated in a 96-well plate (Nunc, Denmark) overnight at 4 °C. The next day, the plate was blocked with 2% skim milk and incubated for 1 hr at room temperature (RT). The plate was rinsed four times with PBST (0.05% (V/V) Tween 20 in PBS). One hundred microliters of 1 µg/ml of each nanobody were added to the wells and incubated at 37 °C for 1 hr. After washing, 100 microliters of anti-His HRP-conjugated (1:500) were added to the wells and the plate was incubated for 1 hr at 37 °C. The wells were washed and developed by TMB (3, 3’, 5, 5’-tetramethylbenzidine). Finally, the reaction was stopped with 50 microliters 2N H2SO4 and signal value was measured at 450 nm.
Competition inhibition enzyme-linked immuno -sorbent assay
Competition inhibition ELISA was designed to determine that VEGF-specific NBs are able to detect VEGF in the solution phase and inhibit its binding to immobilized VEGF-antibodies. ELISA assay was set using the avidin-biotin-complex (ABC). For competition inhibition analysis, a microtiter well was coated with 1 µg/ml of anti-hVEGF or goat anti-mVEGF antibody. The wells were blocked with BSA 2% and incubated at RT for 1 hr. Fifty µl of hVEGF (500 ng/ml) or mVEGF (500 ng/ml) were mixed with 50 µl of anti-VEGF NB (0 and 10 µg/ml), bevacizumab (0 and 10 µg/ml), or the anti-mVEGF antibody (0 and 10 µg/ml) and added to the wells coated with anti-hVEGF or the anti-mVEGF antibody, respectively and incubated for 1 hr at 37°C. The wells were emptied and washed 4 times with PBST. One hundred µl of biotinylated polyclonal anti-VEGF (R&D) (1:100) were added to each well and the plate was incubated for 1 hr at 37 °C. After washing, wells were incubated with 100 µl of streptavidin HRP conjugated antibody (R&D, Minneapolis) (1:5000) for 1 hr at 37 °C. The peroxide activity was detected using TMB and subsequently, absorption was measured at a wavelength of 450 nm. Maximal signal value refers to 0% inhibition and minimal signal refers to 100 inhibitions.
Inhibition ELISA assay
In inhibition, ELISA assay NBs and VEGF were incubated at 37 °C for 1 hr. The difference between competition inhibition and inhibition ELISA is that in competition inhibition ELISA, competition and inhibition happen at the same time. Nbs bind to VEGF in the solution phase and inhibit its binding to immobilized NBs. However, in inhibition ELISA, NBs were pre-incubated with VEGF at 37 °C for 1 hr to determine whether temperature and pre-incubation could affect nanobody binding to VEGF or not. In fact with incubating of NBs and VEGF, we allow them to react in the solution in presence of temperature. One µg/ml of anti-hVEGF or anti-mVEGF antibody was coated in a microtiter well. In microtube 500 ng/ml of hVEGF or mVEGF was mixed with NBs (10 µg/ml) or bevacizumab (10 µg/ml) or anti-mVEGF antibody (10 µg/ml) and incubated at 37 °C for 1 hr. A microtube also was incubated at the same condition with 500 ng/ml of hVEGF or mVEGF as control. Then the mixture was added to each well and incubation was performed for a further 1 hr at the same condition. The remaining steps were performed as mentioned above.
Affinity analysis
Affinity was calculated according to the Beatty ELISA-based method (20). Briefly, two concentrations of hVEGF and mVEGF (1 and 10 µg/ml) were chosen and incubated overnight at 4 °C. Next day, various concentrations of NBs (0-50 nM) were added to the wells and the plate was incubated at 37 °C for 1 hr. Binding detection was performed by anti-His HRP conjugated (1:500).
Western blot analysis
Western blot assay was performed to confirm binding of selected NBs to human and mouse VEGF. The protein bands of hVEGF and mVEGF from 15% SDS-PAGE gel (reduced condition) were electro-phoretically transferred to PDVF membranes (Bio-Rad, USA). The membranes were blocked with skim milk 2% and incubated overnight at 4°C. The next day, membranes were washed with PBST and incubated with 10 µg/ml of selected cross-reacted anti-mouse and human VEGF NBs for 2 hr at RT. Then membranes were washed and incubation was performed with anti-His HRP (1:500) for an additional 2 hr at RT. Protein bands were detected by adding 4-choloro-1-naftol (4-CN) (Sigma, Germany) as substrate.
In vitro functional assay
Migration assay was performed to show if anti-VEGF NBs could inhibit VEGF-stimulated HUVEC migration. A 24-well Boyden chambers with 8-µm pores (Costar, USA) was used for migration analysis. The cells were grown overnight in starving media (EBM-2 and 0.1% FBS) until 80% plate confluency. The next day, cells were trypsinized and counted. About 2.5×104 cells in endothelial basal medium (EBM-2) without supplementation were seeded into the upper chamber of the plate. Then, 50 ng/ml of VEGF were incubated at 37 °C for 2 hr in presence or absence of anti-VEGF NBs (10 µg/ml), bevacizumab (10 µg/ml), or H39NB (10 µg/ml) and added to the lower chamber of the plate containing EBM-2 in a final volume of 500 µl. The plate was incubated at 37 °C and 5% CO2 for 6-12 hr to allow HUVECs to migrate from the upper chamber to the source of growth factor (VEGF) in the lower chamber. The assay was performed in triplicate and five microscopic fields were pictured (with 10x magnification) and the number of cells migrated to the lower chamber was counted (21).
MTT assay was performed for analyzing the inhibitory effect of anti-VEGF NBs on HUVECs proliferation(19). Briefly, HUVECs were cultured at the density of 104 cells in a 96-well plate and incubated at 37°C and 5% CO2 for 14-16 hr. Various concentrations (0-10 µg/ml) of anti-VEGF NBs, bevacizumab (positive control), or H39NB (anti-scorpion nanobody as negative control) (22) were preincubated for 2 hr with 50 ng/ml of VEGF before adding to the wells. The cells were incubated for 48 hr at the same condition. The wells were washed with PBS and then 20 µl of MTT (5 mg/ml) (Sigma, Germany) were added to the wells and incubation was performed for 4 hr at 37 °C under dark conditions. Dye was solubilized by adding 100 µl of dimethylsulfoxide (DMSO) (Sinaclon, Bioscience). The intensity of signal value was measured at 570 nm. Suppression of proliferation was calculated according to the following formula: 1- the proliferation of HUVEC cells in the treated well ×100 /proliferation of HUVEC cells in the control well.
In vivo assay
Mouse and tumor model development
Six to eight weeks old female C57BL/6 mice were purchased from animal facility of Pasteur Institute of Iran. Six mice per cage were housed in standard ventilated cages containing food and water and were maintained according to the laboratory animal care protocol of Pasteur Institute of Iran in 12 hr light/12 hr dark and the ethical committee license. TC-1 cells were cultured in RPMI1640 supplemented by 10% FBS. Cells were trypsinized and twice washed with PBS. About 106 TC-1 cells resuspended in 200 µl of PBS and immediately subcutaneously injected to the shaved right flank of C57BL/6 mice. Seven to ten days after tumor cell inoculation, tumor size reached 5-8 mm in diameter and 4-7 mm in thickness. During these times tumor growth was monitored by palpation every two days. Tumor size was monitored three times a week using a caliper according to the equation(23): V=L×W2×0.52 where V: volume, L: length, and W: width. Tumor volume change was monitored according to relative tumor volume (RTV) formula: tumor volume in day X / tumor volume on day 0.
Treatment study
The TC-1 tumor-bearing C57BL/6 mice were randomly divided into four groups (n=6). All mice were injected subcutaneously adjacent to tumor site three times a week. Treatment started when tumor volume reached 100-150 mm3 and continued for 60 days. The control group (G1) received 100 µl PBS; the control nanobody group (G2) received 300 µg H39NB. Test groups G3 and G4 received 100 µg and 300 µg endotoxin-free (endotoxin removed by triton X-114, and level of endotoxin was <0.01 EU per 1 μg of the nanobody by the LAL method) anti-VEGF NBs, respectively (Table 1). The final volume for injection was 100 µl. Treatment was continued for 60 days and the rate of mortality was monitored during this period.
Table 1.
Treatment plan of groups. Mice divided into 4 groups (n=6)
| Groups | Treatments |
|---|---|
| G1 | PBS |
| G2 | H39NB(negative control NB) |
| G3 | 100 µg/mouse Anti-VEGF NB |
| G4 | 300 µg/mouse Anti-VEGF NB |
Statistical analysis
Statistical analysis was performed using the GraphPad PRISM software (version 5.0). For comparison between groups, one-way ANOVA followed by Tukey was performed. Unpaired Student’s t-test also was performed to compare each data. Survival rate was evaluated by Kaplan-Meier analysis. Statistical significance was at P<0.05.
Results
Characterization of anti-VEGF nanobodies
Anti-VEGF nanobodies were isolated from the hyper immune Camelus dromedaries nanobody library through four consecutive rounds of biopanning. Progress of biopanning was monitored by polyclonal phage-ELISA on immobilized VEGF and the highest signal value was observed in third and fourth round. Over than 90 clones was screened for specific binding to VEGF in ELISA experiment. Four positive binders were selected according to their highest binding to VEGF. Specificity of selected nanobody to human and mouse VEGF was determined using ELISA. Results indicate, anti-VEGF nanobody was able to react with both human and mouse VEGF (Figure 1). However, bevacizumab only detected human VEGF. According to ELSA experiment the concentration of 500 ng/ml of VEGF was chosen as saturating concentration.
Figure 1.
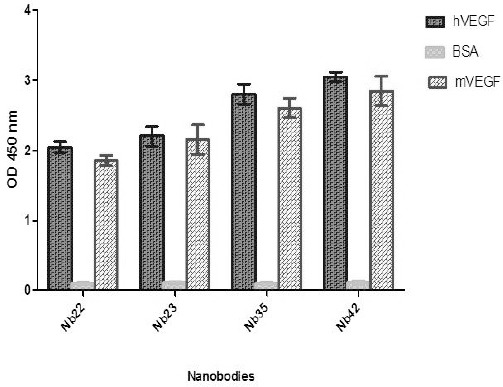
Binding cross-reactivity analysis. Anti-VEGF NB detected human and mouse VEGF in the ELISA experiment. The data expressed as the mean of three experiments±SD
Competition inhibition analysis revealed that concentration 10 µg/ml of anti-VEGF NB resulted in 44% and 42% inhibition of hVEGF and mVEGF binding, respectively. However 10 µg/ml concentration of bevacizumab 47%inhibited hVEGF binding. Anti-mVEGF antibody also 48% inhibited mVEGF binding in concentration 10 µg/ml (data not shown). The potency of Anti- VEGF NB in inhibition of hVEGF and mVEGF binding approximately was identical to potency of bevacizumab and anti-mVEGF antibody, respectivly. Inhibition ELISA results showed that 10 µg/ml of anti-VEGF NB 76% and 72% of hVEGF and mVEGF binding, respectively. Furthermore, 10 µg/ml bevacizumab inhibited 86% hVEGF binding (The observed potency of bevacizumab was higher than anti-VEGF NB). Anti-mVEGF antibody also inhibited 80% mVEGF binding (The observed potency of anti-mVEGF antibody was higher than anti-VEGF NB). The evaluated affinity of NB was 109 M-1 to hVEGF and mVEGF.
Western blot analysis
In the next step, western blot was performed to confirm cross reactivity of anti-VEGF NB to human and mouse VEGF. Detection of nanobody in western blot was performed with anti-His HRP conjugated. As shown in Figure 2 anti-VEGF NB detected both hVEGF (22kDa) and mVEGF (20) in western blot analysis.
Figure 2.
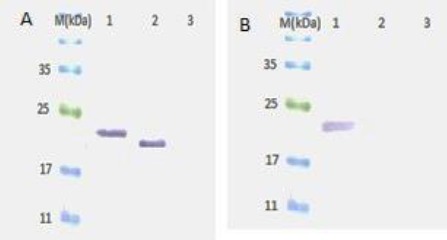
Western blots analysis. SDS-PAGE was performed under reducing conditions and protein bands transferred to PDVF membrane. Western blots results of anti-VEGF NB, M; protein marker (Sinaclon, Bioscience), 1; hVEGF, 2; mVEGF, 3; negative control. As shown in the Figure anti-VEGF NB detected both hVEGF (22kDa) and mVEGF (20) in Western blot analysis
HUVECs proliferation and migration assay results
Migration assay results revealed that migration of HUVECs to the VEGF source (lower chamber) was started within 1 hr and progressively increased during 12 hr. As can be seen in Figure 1A, anti-VEGF NB significantly inhibited HUVECs migration as compared with control well (well without nanobody) (t-test, P value =0.045). Migrated cells were 27% and 36 % in case of bevacizumab and anti-VEGF NB, respectively (Figure 3A and B). MTT results revealed that anti-VEGF NB significantly inhibited HUVEC proliferation in dose-dependent manner (P=0.035). This result indicates that the inhibitory effect of nanobody on HUVECs proliferation is dose-dependent (Figure 3C). According to results, nanobody and bevacizumab at concentration of 10 µg/ml, inhibited 65% and 77% of VEGF stimulated HUVEC proliferation, respectively. However HUVECs proliferation was observed in cells stimulated by VEGF. The negative control nanobody (H39NB) didn’t inhibit HUVECs proliferation.
Figure 3.

HUVEC inhibition assay results. (A) HUVEC migration assay results. HUVEC migration assay was performed using Boyden chamber with 8 µm pores. HUVEC in EBM-2 seeded into the upper chamber. VEGF preincubated with or without anti-VEGF NB, bevacizumab, or H39NB (negative control nanobody) and added into the lower chamber. A; Control well (absence of NB), B; negative NB (H39NB), C; anti-VEGF NB, D; Bevacizumab. (B) HUVECs migrated to the lower chamber counted in five different fields under invert microscope with 10x magnification. Migrated HUVECs into lower chamber were reduced to 27% and 36% in case of bevacizumab and anti-VEGF NB, respectively (t-test, P=0.045 *). (C) MTT assay demonstrated the inhibitory effect of anti-VEGF NB on VEGF-stimulated HUVECs proliferation. The bar represents mean of three replicates ±SD
Successful in vivo treatment of tumor
For tumor therapy study, twenty four C57BL/6 mouse fall into four groups (Table 1). Treatment plans were administrated to C57BL/6 mouse after TC-1 tumor development. Therapeutic effect of anti-VEGF NB is shown in Figure 4A. In G3 group, 100 µg/ mouse of anti-VEGF NB significantly inhibited tumor growth in compare with control groups (G3 Vs G1) (P=0.0237). According to tumor volume results in G3 group until day 14 of anti-VEGF NB administration, approximately tumor growth was not observed. But from day 14 to 35 a low increasing trend in tumor volume was observed. Also from day 35 to 60 the increasing trend in tumor volume is faster. However, in the end of treatment, there was a significant difference between tumor volume of G3 and G1 or G2 group. Furthermore tumor volume in day 60 in G3 group is approximately identical with tumor volume of G1 or G2 group in day 28. The higher dose (G4: 300 µg/ mouse) of Anti-VEGF NB showed higher effect in tumor growth (P=0.001). In G4 group until day 21 tumor growths completely inhibited and between days 21 to 42 the slow rate in tumor growth was observed. However, from day 42 to 60 significant increasing in tumor volume was not observed. And tumor volume in day 60 is identical to day 28 in G1 or G2 group. Furthermore, mouse in anti-VEGF NB group had higher survival rate than those in PBS or H39NB groups. In G1 and G2 all six mouse died in the end of treatment but in G3 and G4 only three mice were died. According to results, NB treatment in G3 and G4 group resulted in 50% increased survival rate than G1 and G2 group after 60 days (Figure 4 B).
Figure 4.
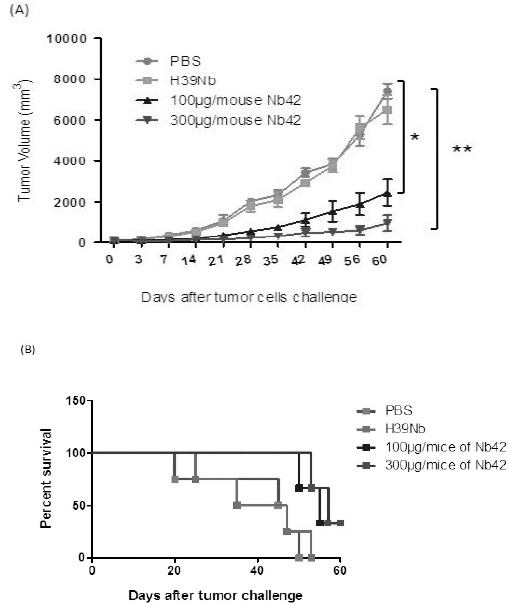
In vivo treatment study results. Female C57BL/6 mice (6 mice per group) were injected with TC-1 cells (106 cells resuspended in 200 µl of PBS). (A) The mice were challenged 10 days after TC-1 inoculation. Tumor volume was monitored three times a week. Tumor increasingly grew in the control group (G1; PBS). While in treatment groups (G3; 100 µg/mouse of anti-VEGF NB and G4; 300 µg/mouse of anti-VEGF NB) tumor growth was significantly inhibited. The P-value for groups were as follows: G3 vs. G1 on days 42, 49, 56, and 60 (P=0.0237 *), G4 vs. G1 on days 42, 49, 56, and 60 (P=0.001 **). On the contrary, H39NB had no effect on tumor inhibition. Data are expressed as mean±SD. (B) Survival analysis. Survival curve after tumor challenge of each group was performed using Kaplan-Meier
Discussion
The small size (15 kDa) and single domain nature of NB causes good penetration into inner region of tumor tissue. It has been shown that in solid tumors only 3 of 10 mAbs that approved by FDA can reach to tumor tissue. In fact the large size of mAbs (150 kDa) inhibit their penetration into deeper region of tumor tissue (24). NBs are able to detect unique epitopes on antigen which usually are not detected y mAbs. NBs expressed in bacterial system with 2-3 fold lower cost of production in compare to mAbs that need to mammalian expression systems (16). Untill now there is no any report indicating the immunogenicity of NBs, because of homology between NBs and human VH sequences (known as VH3 gene family). It is evaluated that NBs saturate tumor cells 10 time faster than conventional mAbs (25). Unique features of NBs, like: high solubility and stability, resistance to proteolysis as well as extremes of pH, non-injectable routes of administration and appropriable biochemical and biophysical properties make them potential candidate for drug development (16).
Here we isolated NB cross-reacting with human and mouse VEGF from Camel-antibody library by phage display. Development of high affinity and specificity antibodies are required for ideal immunotherapy of cancer. The developed NB showed high specificity and affinity to human and mouse VEGF. Detection of both human and mouse VEGF by Anti-VEGF NB was confirmed using western blot analysis. For in vitro functional experiment of Anti-VEGF NB, we used human umbilical vein endothelial cells (HUVECs) proliferation and migration assay (19). It has been demonstrated that presence of VEGF, initiate signaling pathway through binding to its receptors on surface of endothelial cell. These signals cause endothelial cell proliferation, migration and tube formation (26). Blockade of VEGF and its interaction with its receptors can inhibit tumor cell proliferation, migration and tube formation (27, 28). Our results demonstrated that Anti-VEGF NB significantly inhibited VEGF-stimulated HUVEC proliferation and migration. The observation indicates that Anti-VEGF NB blocked interaction of VEGF with its receptors on HUVECs cells. We next evaluated Anti-VEGF NB function in in vivo tumor growth inhibition. Anti-VEGF NB significantly inhibited implanted lung epithelial tumor (TC-1) cells in C57BL/6 mouse. It is the first report of inhibition of tumor growth in animal model of mouse by NB cross-reacting human and mouse VEGF. Our observation indicates that Anti-VEGF NB blocked VEGF and consequent resulted in inhibition of tumor growth. Indeed this result confirmed achieved in vitro results. Treatment plans that start in early stage of tumorigenesis leads to the successful inhibition of tumor progression (29). Thus we started our treatment plan when tumor volume reached to about 100 mm3. In G3 group (100 µg/mouse of Anti-VEGF NB) tumor growth was started from day 14 but in G4 group (300 µg/mouse of Anti-VEGF NB) tumor growth was observed since day 21. One week delay in tumor growth of G4 group indicates the efficiency of high dose administration of Anti-VEGF NB in tumor inhibition. High dose administration of Anti-VEGF NB (G4: 300 µg/mouse) resulted in higher tumor growth inhibition (P=0.001). Our hypothesis was that the high dose administration of NB sufficiently could neutralize the VEGF in tumor cells and thus inhibits tumor growth and angiogenesis. However, low dose administration of Anti-VEGF NB significantly inhibited tumor growth (P=0.0237). In low dose administration of Anti-VEGF NB, tumor growth was slowly and increasing trend in tumor volume was observed. Indicating may be such low dose is not sufficient for neutralizing of whole VEGF in tumor cells. Our achievement was in consistent with the study of Liang et al (29) which showed inhibition of VEGF by anti-VEGF antibody is sufficient for tumor growth and angiogenesis inhibition. It has been shown in various studies that NB has short half-life in circulation (30, 31), therefore mouse injected with NB three times a week and also injection was performed into adjacent of tumor site for better response. In other study demonstrated that knocking out of VEGF in tumor cells potently inhibited tumor growth (32). These finding represents the potential of anti-VEGF molecules in VEGF blockade and tumor growth and angiogenesis inhibition in both in vitro and in vivo experiments.
Conclusion
Since it has been shown that VEGF blockade significantly inhibited tumor development in patient (33). It is necessary to establish preclinical models to study the effect of VEGF inhibitor in tumor progression. The developed cross-reactive NB showed high specificity and binding affinity in nanomolar range to both human and mouse VEGF. Anti-VEGF NB potently inhibited human endothelial cells migration (P=0.045). Anti-VEGF NB, significan-tly inhibited tumor growth in tumor-bearing mouse (P=0.001). The results indicate that developed NB can be a promising candidate in cancer drug development.
Acknowledgment
We thank with deep respect from Pasteur Institute of Iran for funding this work.
References
- 1.Cheng T-YD, Cramb SM, Baade PD, Youlden DR, Nwogu C, Reid ME. The international epidemiology of lung cancer:latest trends, disparities, and tumor characteristics. J Thorac Oncol. 2016;11:1653–1671. doi: 10.1016/j.jtho.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adnan K, Zahra EM, Sharareh S, Shirin K, Habib E, Kian K. Clinicopathological characteristics of Iranian patients with lung cancer:a single institute experience. Asian Pac J Cancer Prev. 2016;17:3815–3820. [PubMed] [Google Scholar]
- 3.Tulotta C, He S, van der Ent W, Chen L, Groenewoud A, Spaink H, et al. Imaging cancer angiogenesis and metastasis in a zebrafish embryo model. Cancer and Zebrafish:Springer. 2016:239–263. doi: 10.1007/978-3-319-30654-4_11. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell RK, Falcon B, Hanson J, Goldstein WE, Perruzzi C, Rafii S, et al. VEGF-A/VEGFR inhibition restores hematopoietic homeostasis in the bone marrow and attenuates tumor growth. Cancer Res. 2016;76:517–524. doi: 10.1158/0008-5472.CAN-14-3023. [DOI] [PubMed] [Google Scholar]
- 5.Shojaei F. Anti-angiogenesis therapy in cancer:Current challenges and future perspectives. Cancer Lett. 2012;320:130–137. doi: 10.1016/j.canlet.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Bhattacharya R, Xia L, Fan F, Wang R, Boulbes D, Ye X-C, et al. Inhibition of intracrine VEGF signaling prevents colorectal cancer cell migration and invasion. Cancer Res. 2016;76:3255. [Google Scholar]
- 7.Gille H, Hülsmeyer M, Trentmann S, Matschiner G, Christian HJ, Meyer T, et al. Functional characteri-zation of a VEGF-A-targeting Anticalin, prototype of a novel therapeutic human protein class. Angiogenesis. 2016;19:79–94. doi: 10.1007/s10456-015-9490-5. [DOI] [PubMed] [Google Scholar]
- 8.Cosmai L, Gallieni M, Liguigli W, Porta C. Renal toxicity of anticancer agents targeting vascular endothelial growth factor (VEGF) and its receptors (VEGFRs) J Nephrol. 2016;30:171–180. doi: 10.1007/s40620-016-0311-8. [DOI] [PubMed] [Google Scholar]
- 9.Hamilton JL, Nagao M, Levine BR, Chen D, Olsen BR, Im HJ. Targeting VEGF and its receptors for the treatment of osteoarthritis and associated pain. J Bone Miner Res. 2016;31:911–924. doi: 10.1002/jbmr.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 11.Miller K, Wang M, Gralow J, Dickler M, Cobleigh M, Perez EA, Shenkier T, Cella D, Davidson NE. Paclitaxel plus bevacizumab versus paclitaxel alonefor metastatic breast cancer. N Engld J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 12.Ellis LM, Hicklin DJ. Pathways mediating resistance to vascular endothelial growth factor-targeted therapy. Clin Cancer Res. 2008;14:6371–6375. doi: 10.1158/1078-0432.CCR-07-5287. [DOI] [PubMed] [Google Scholar]
- 13.Huang T, Wang H, Chen NG, Frentzen A, Minev B, Szalay AA. Expression of anti-VEGF antibody together with anti-EGFR or anti-FAP enhances tumor regression as a result of vaccinia virotherapy. Mol Ther Oncol. 2015;2:15003. doi: 10.1038/mto.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senturk B, Cubuk MO, Ozmen MC, Aydin B, Guler MO, Tekinay AB. Inhibition of VEGF mediated corneal neovascularization by anti-angiogenic peptide nanofibers. Biomaterials. 2016;107:124–132. doi: 10.1016/j.biomaterials.2016.08.045. [DOI] [PubMed] [Google Scholar]
- 15.Behdani M, Zeinali S, Khanahmad H, Karimipour M, Asadzadeh N, et al. Generation and characteri-zation of a functional Nanobody against the vascular endothelial growth factor receptor-2;angiogenesis cell receptor. Mol Immunol. 2012;50:35–41. doi: 10.1016/j.molimm.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 16.Kolkman JA, Law DA. Nanobodies-from llamas to therapeutic proteins. Drug Discov Today Technol. 2010;7:139–146. doi: 10.1016/j.ddtec.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Gerber HP, Wu X, Yu L, Wiesmann C, Liang XH, Lee CV, et al. Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. Proc Natl Acad Sci U S A. 2007;104:3478–3483. doi: 10.1073/pnas.0611492104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heist RS, Duda DG, Sahani DV, Ancukiewicz M, Fidias P, Sequist LV, et al. Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer. Proc Natl Acad Sci. 2015;112:1547–1552. doi: 10.1073/pnas.1424024112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazemi-Lomedasht F, Behdani M, Pooshang Bagheri K, Habibi-Anbouhi M, Abolhassani M, Arezumand R, Shahbazzadeh D, Mirzahoseini H. Inhibition of angiogenesis in human endothelial cell using VEGF specific nanobody. Mol Immunol. 2015;65:58–67. doi: 10.1016/j.molimm.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Beatty JD, Beatty BG, Vlahos WG. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J Immunol Methods. 1987;100:173–139. doi: 10.1016/0022-1759(87)90187-6. [DOI] [PubMed] [Google Scholar]
- 21.Han M, Wang H, Zhang H-T, Han Z. Expression of Tax-interacting protein 1 (TIP-1) facilitates angiogenesis and tumor formation of human glioblastoma cells in nude mice. Cancer Lett. 2013;328:55–64. doi: 10.1016/j.canlet.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yardehnavi N, Behdani M, Pooshang Bagheri K, Mahmoodzadeh A, Khanahmad H, Shahbazzadeh D, et al. A camelid antibody candidate for development of a therapeutic agent against Hemiscorpius lepturus envenomation. FASEB J. 2014;28:4004–4014. doi: 10.1096/fj.13-247478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xanthopoulos JM, Romano AE, Majumdar SK. Response of mouse breast cancer cells to anastrozole, tamoxifen, and the combination. J Biomed Biotechnol. 2005;1:10–19. doi: 10.1155/S111072430440504X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jamnani FR, Rahbarizadeh F, Shokrgozar MA, Ahmadvand D, Mahboudi F, Sharifzadeh Z. Targeting high affinity and epitope-distinct oligoclonal nanobodies to HER2 over-expressing tumor cells. Exp Cell Res. 2012;318:1112–1124. doi: 10.1016/j.yexcr.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Muyldermans S. Single domain camel antibodies:current status. J Biotechnol. 2001;74:277–302. doi: 10.1016/s1389-0352(01)00021-6. [DOI] [PubMed] [Google Scholar]
- 26.Zhou R, Curry J, Roy L, Grover P, Haider J, Moore L, et al. A novel association of neuropilin-1 and MUC1 in pancreatic ductal adenocarcinoma:role in induction of VEGF signaling and angiogenesis. Oncogene. 2016;35:5608–5618. doi: 10.1038/onc.2015.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simons M, Gordon E, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016;17:611–625. doi: 10.1038/nrm.2016.87. [DOI] [PubMed] [Google Scholar]
- 28.García-Caballero M, Blacher S, Paupert J, Quesada A, Medina M, Noël A. Novel application assigned to toluquinol:inhibition of lymphangiogenesis by interfering with VEGF-C/VEGFR-3 signalling pathway. Br J Pharmacol. 2016;173:1966–1987. doi: 10.1111/bph.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang WC, Wu X, Peale FV, Lee CV, Meng YG, Gutierrez J, et al. Cross-species vascular endothelial growth factor(VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem. 2006;281:951–961. doi: 10.1074/jbc.M508199200. [DOI] [PubMed] [Google Scholar]
- 30.McMurphy T, Xiao R, Magee D, Slater A, Zabeau L, Tavernier J, et al. The anti-tumor activity of a neutralizing nanobody targeting leptin receptor in a mouse model of melanoma. PLoS One. 2014;9:e89895. doi: 10.1371/journal.pone.0089895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vosjan MJ, Vercammen J, Kolkman JA, Stigter-van Walsum M, Revets H, van Dongen GA. Nanobodies targeting the hepatocyte growth factor:potential new drugs for molecular cancer therapy. Mol Cancer Ther. 2012;11:1017–1025. doi: 10.1158/1535-7163.MCT-11-0891. [DOI] [PubMed] [Google Scholar]
- 32.Inoue M, Hager JH, Ferrara N, Gerber HP, Hanahan D. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]


