Abstract
Cardiovascular disorders are the leading cause of mortality worldwide. Berberis vulgaris (B. vulgaris) is a commonly used plant in traditional medicine. In recent studies, B. vulgaris showed antiarrhythmic, antihypertensive, anticholinergic, and cardioprotective effects. We reviewed the literature to explore the possible prophylactic and therapeutic roles of B. vulgaris in cardiovascular medicine. A computer literature search was conducted to identify all relevant studies that have investigated the role of B. vulgaris in prevention or treatment of cardiovascular diseases. We also searched the citations of the retrieved articles. Using a systematic approach, we conducted a scoping review that included a total of 37 articles. Twelve studies examined the antihypertensive effects of B. vulgaris, seven studies investigated its antiarrhythmic effects, while its inotropic and cardioprotective effects were evaluated in four and eight studies, respectively. B. vulgaris showed a beneficial effect in reducing blood pressure, enhancing cardiac contractility, and protection from reperfusion injury. However, the mechanisms of these effects are still under investigation. Moreover, it could modify major risk factors for cardiovascular disorders, such as oxidative stress, hyperglycemia, and hyperlipidemia. Further studies are needed to translate these findings into effective cardiovascular medications.
Keywords: Antiarrhythmic, Antihypertensive, Berberis vulgaris, Cardioprotective Cardiovascular
Introduction
Cardiovascular disorders are the leading cause of morbidity and mortality worldwide (1). According to the American heart association (AHA), about 16.5% of the global mortality can be attributed to hypertension, of which 45% are caused by coronary heart disease (2). Most of these disorders are untreatable and the current pharmacological strategies only aim at disease control (3). Adding to this, various biochemical compounds, especially those used in the treatment of arrhythmia and heart failure, have serious adverse events. Therefore, there is a growing trend towards using medicinal plants in health care generally and cardiovascular medicine, in particular (4).
Berberis vulgaris (B. vulgaris) is a shrub of the plant family Berberidaceae that grows in northwest Africa, western Asia, central, and southern Europe. Its fruit is an oblong red berry that ripens in late summer and autumn. It has obovate leaves and pendulous yellow flowers (5). It contains multi- ple phenolic compounds, organic acids, flavonoids (anthocyanins), protoberberines, and alkaloids, to which its biological activities are attributed (Figure 1) (4).
Figure 1.
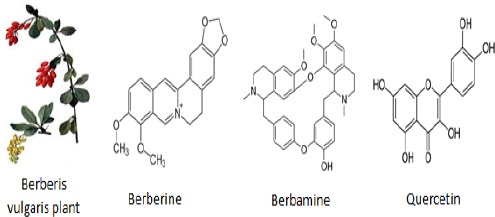
Berberis vulgaris plant and chemical structure of its main active ingredients
For 3000 years in traditional medicine, B. vulgaris has been used to stop chronic bleeding, relieve arthralgia, fight infections, and treat urinary stones due to its diuretic effect (6). Therapeutic uses of B. vulgaris have been a focus for experimental research recently. Both animal and human trials have concluded that its extracts may have antioxidant (7, 8), anti-inflammatory (9), anti-mutagenic, antimicrobial, and anti-parasitic activities (10). It has also been tested for the management of chronic cholecystitis, non-alcoholic fatty liver disease (11), osteoarthritis, and other rheumatoid disorders (12). Its fruit is safe for human intake and has been approved by the FDA (13).
In cardiovascular medicine, B. vulgaris and its active constituents showed antiarrhythmic, anti-hypertensive, anticholinergic, anti-inflammatory, and cardioprotective effects from ischemia/reperfusion (I/R) injury (14, 15). Despite its numerous applications, the mechanism of action for most of its effects is not yet clear (4). This review explores the potential role of B. vulgaris in prevention or treatment of cardiovascular disorders.
Materials and Methods
Data sources
We performed a computer literature search of the following authentic databases: PubMed, Persian Electronic Scientific Information Database (SID), Iran medex, and Natural Medicines Comprehensive Database for all preclinical and human studies of b. vulgaris in cardiovascular medicine, using the search terms “Barbery”, “Berberis vulgaris”, “Berberine”, “Berbamine”, “Cardiovascular”, “Heart”, and “Hypertension”. We also searched the reference list of retrieved records for any relevant studies.
Study selection
We included original studies of all designs, including preclinical (in vivo and in vitro) and human studies provided that they evaluated the prophylactic or therapeutic roles of B. vulgaris or its active constituents in cardiovascular medicine. We excluded secondary reports (literature reviews, systematic reviews, or meta-analyses) and non-English articles. Two authors independently reviewed the titles and abstracts of the search results and if the abstract was not conclusive, the full text was obtained to make a cut-off decision.
Data extraction
For each included study, two independent authors extracted the following data: first author’s name, year of publication, used active constituent, animal/cellular model, findings, and possible mechanisms.
Results
Our search strategy retrieved 721 records. After abstract and full-text screening, we included 33 full-text articles. An additional four articles were retrieved by screening the reference list of included articles. Details of our literature search and screening process are illustrated in Figure 2.
Figure 2.
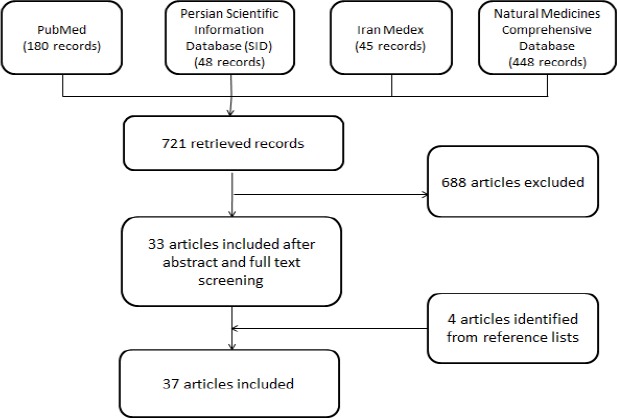
A summary of the search and article selection process
Antihypertensive effects
Several in vitro and animal studies have shown an antihypertensive effect for B. vulgaris. However, the mechanisms and vascular sites for this effect are still debatable. Fatehi et al reported that the immediate reduction of blood pressure after B. vulgaris intake can be attributed to modulation of both cardiac and vascular contractility (16, 17). In vitro studies have shown that pretreatment of isolated aortic rings and mesenteric vascular beds with B. vulgaris extract decreases their contractile response to phenyl-ephrine administration. This effect may be attributed to alpha-adrenoreceptor antagonism (18). Moreover, the augmentation of K+ currents through activation of 4-aminopyridine-sensitive K+ channels and the inhibition of intracellular Ca+2 release from caffeine-sensitive pools can initiate relaxation of vascular smooth muscles and vasodilatation (19-22). Animal studies have also suggested a central nervous mechanism for B. vulgaris antihypertensive effect, based on the observation of parallel reduction of blood pressure and heart rate (17). Other mechanisms have been suggested as inhibition of angiotensin-converting enzyme and direct release of NO/cGMP from rat aortic rings (19) and the potentiation of acetylcholine (23).
Table 1.
Summary of the findings of preclinical and clinical studies that investigated the antihypertensive effects of Berberis vulgaris extracts
| Study ID | Active constituent | Study design/ animal model | Findings |
|---|---|---|---|
| Mahdavi et al 2016 (24) | B. vulgaris crude extracts | Rats (in vivo) | B. vulgaris crude extract (200 mg) or sildenafil significantly reduced the right ventricular systolic pressure, right ventricular hypertrophy (stimulated by monocrotaline-induced pulmonary hypertension). However, none of them had a significant effect on the plasma level of endothelin-1 or the lung tissue levels of glutathione peroxidase and malondialdehyde |
| Lee et al 2006 (25) | Berberine | Rats (in vivo and in vitro) | Berberine inhibited angiotensin II and heparin-binding epidermal growth factor (HB-EGF) mediated vascular smooth muscle cell migration and proliferation in vitro. It also improved neointima formation in vivo. This effect is mostly mediated through suppression of the Akt (protein kinase B) pathway |
| Fatehi et al 2005 a, b (16,17) | B. vulgaris crude extracts | Rats (in vivo and in vitro) | B. vulgaris crude extract reduced the heart rate and the mean arterial pressure in hypertensive rats in a dose-dependent manner. Adding its extracts to isolated rat aortic rings and mesenteric beds reduced the contractile response, induced by phenylephrine |
| Kang et al 2002 (19) | Berberine | Rats (in vitro) | Berberine-induced vasodilatation of rat aorta, most probably through angiotensin converting enzyme-inhibitory activity and direct release of NO/cGMP from rat aortic rings |
| Ko et al 2000 (21) | Berberine | Rats (in vitro) | Berberine reduced the contractile response of vascular smooth muscle to phenylephrine. Interestingly, removal of the endothelium attenuated the berberine-induced effect. This suggests that the vasodilator effect of berberine can be mediated through both endothelial and smooth muscle cells |
| Marin-Neto et al 1988 (26) | Berberine | Human trial | In a clinical trial on 12 patients with congestive heart failure, berberine could reduce pulmonary and peripheral vascular resistance, increase left ventricular ejection fraction, and improve cardiac performance as measured by echocardiography |
| Wong 1998 (27) | Berberine | Rats (in vitro) | The vasodilator effect of low concentrations of berberine is solely endothelium-dependent, while high concentrations of berberine cause vasodilatation, irrespective of the endothelium state |
| Olmez and Ilhan 1992 (18) | Berberine | Rats and rabbits (in vitro) | Berberine-induced vasodilatation of rat and rabbit aorta, most probably through α1-adrenoceptor antagonism. It also inhibited the contractile response of the aorta to norepinephrine and phenylephrine exposure |
| Bova et al 1992 (28) | Berberine | Guinea pig (in vitro) | Berberine inhibited the aortic contractile response to norepinephrine and histamine, but this effect is unlikely to be mediated through voltage-gated calcium channels |
| Chiou et al 1991 (29) | Berberine | Rats (in vitro) | Berberine-induced vasodilatation of the rat mesenteric artery [Directly by inhibiting Ca+2 release from internal stores and indirectly by releasing endothelial derived relaxing factor (EDRF)] |
| Chun et al 1979 (23) | Berberine | Rats (in vivo) | Berberine intravenous infusion lowered the blood pressure and the heart rate of rats, most probably through inhibition of true cholinesterase and potentiation of acetylcholine levels |
Although its effects were widely studied in systemic hypertension, few studies have investigated the possible therapeutic effect of B. vulgaris extract in pulmonary hypertension. Mahdavi et al showed that B. vulgaris extract can be as effective as sildenafil in reducing right ventricular systolic pressure and right ventricular hypertrophy in monocrotaline-induced pulmonary hypertension (24).
Antiarrhythmic effects
Several animal studies have shown that the active compounds from B. vulgaris extracts can have anti-arrhythmic properties, such as prolonging the action potential duration (APD), increasing the atrial refractory period, and suppression of delayed afterdepolarization (30, 31). Berberine has been suggested to act as a class IA or III antiarrhythmic agent. In vitro studies reported different possible mechanisms for the observed effects of berberine, such as K+ channels blockade (32, 33), decreasing Na+ influx (30), and increase of [Ca+2]i (34). Berberine can indirectly supplement anti-arrhythmic drugs by its inhibitory effect on the human CYP3A4 enzyme, through which many of these drugs are metabolized, increasing their potency. However, this interaction may require adjusting the anti-arrhythmic dosage and should be prescribed by a specialist (35).
In a clinical study by Zeng and Li, in patients with congestive heart failure (CHF), berberine reduced the frequency of ventricular premature complexes and increased the left ventricular ejection fraction (EF) (36). Other pharmacological studies have shown that it can prevent the development of ventricular fibrillation (VF) due to its inhibitory effect on K+ channels (32, 33). Although berberine has not been tested in large-scale clinical studies, the existing body of literature shows a great potential for this compound as a future anti-arrhythmic drug.
Table 2.
Summary of the findings of preclinical and clinical studies that investigated the antiarrhythmic effects of Berberis. vulgaris extracts
| Study ID | Active constituent | Animal models | Findings |
|---|---|---|---|
| Li et al 2001 (33) | Berberine | Rats (in vitro) | Berberine prolonged action potential duration (APD) and blocked the inward rectifier K+ current and the outward delayed rectifier K+ current. Therefore, the antiarrhythmic mechanism of berberine is related to its inhibitory effects on inward and outward K+ currents |
| Xu et al 1997 (22) | Berberine | Guinea pig (in vitro) | Berberine inhibited both L-type and T-type calcium channels in isolated guinea pig ventricular myocytes in a concentration-dependent manner |
| Li and Wang. 1997 (37) | Berberine | Rats (in vitro) | Berberine (10 to 400 mumol.L-1) has a positive inotropic effect by increasing the resting [Ca+2]i, an effect that was not suppressed by atropine, phentolamine, propranolol, or tetrodotoxin, but was inhibited by verapamil. The increased [Ca+2]i is mostly mediated via Ca+2 influx and intracellular Ca+2 release |
| Wang and Zheng 1997 (34) | Berberine | Guinea pig (in vitro) | Berberine increased the APD in a concentration-dependent manner in isolated guinea pig ventricular myocytes. Berberine prolonged the repolarization phase of AP by inhibiting the delayed rectifier K+ currents and increasing the L-type Ca+2 currents (ICa) |
| Chi et al 1996 (31) | 8-oxybereberine (JKL1073A) | Canine (in vitro) | Berberine increased the APD in canine Purkinje and ventricular muscle in a concentration-dependent manner (3 to 30 μm), without influencing other parameters of AP. It also reduced the sinoatrial spontaneous frequency. This means that berberine exerted class III antiarrhythmic and proarrhythmic actions in the cardiac muscle of dogs in vitro |
| Wang et al 1994 (30) | Berberine | Rats (in vitro) | Berberine possesses an antiarrhythmic activity via suppression of delayed afterdepolarizations (suppressed amplitude at 3 μm and suppressed frequency at 1 mg/kg), which is likely due to the reduction of Na+ influx |
| Shaffer 1985 (38) | Berberine | Rats (in vitro) | In spontaneously beating right atria, berberine (1 x 105-3 x 104 M) caused bradycardia, which was not prevented by atropine. It also had a positive inotropic effect by enhancing both the force-velocity relationship and the duration of the active state. The mechanisms for these actions may include an alteration in the trans-sarcolemmal flux of calcium and inhibition of intracellular calcium sequestration system |
Cardioprotective effects
Ischemic preconditioning (IPC) is a powerful adaptive response to protect the heart from subsequent ischemic damage (39). Ischemia/Reperfusion injury is thought to be mediated through intracellular Ca+2 overload due to the reduced activity of sarcoplasmic reticulum Ca+2 ATPase, leading to contractile dysfunction and activation of lytic proteases, such as calpain, which digests cytoskeleton and myofilament proteins including troponin I and T (40, 41).
Several studies have discussed the cardioprotective effects of berbamine; however, its mechanism is still under investigation (4). It is suggested that berbamine inhibits calpain by maintaining Ca+2 homeostasis.
Another suggested mechanism is the activation of phosphoinositide-3 kinase and protein kinase B
enzymes, leading to inhibition of the glycogen synthetase kinase-3β (GSK 3β) and opening the mitochondrial ATP-sensitive K channels (mitoKATP) (42). Zhang et al noticed that low concentrations (10-100 nmol/l) of berbamine improve post-ischemic cardiac function in a concentration-dependent manner. However, that effect diminished with higher concentrations of 300 nmol/l in Langendroff-perfused rat hearts and 100 nmol/l in isolated cardiac cells (32).
Other studies have shown that berberine can also prevent cardiac hypertrophy in rats that were exposed to high doses of L-thyroxine or underwent surgical binding of the aorta. Possible mechanisms are elevating cardiac NO content, Na+/K+ ATPase and Ca+2 ATPase activities, as well as decreasing plasma levels of norepinephrine and controlling the sympathetic tone (43, 44).
Table 3.
Summary of the findings of preclinical studies that investigated the cardioprotective effects of Berberine vulgaris
| Study ID | Active constituent | Animal/ cellular models | Findings |
|---|---|---|---|
| Tanabe et al 2005 (45) | Berberine | Rats (in vivo) | Berberine and coptisine showed antiproliferative effects against vascular smooth muscle cells (VSMCs) by blocking the cell cycle at G1 and G2/M phases. This effect was mediated through a selective reduction in the cyclin D1 protein through accelerated proteolysis |
| Yang et al 2004 (43) | Berberine | Rats (in vivo) | Berberine prevented cardiac hypertrophy, induced by L-thyroxine, in rats through elevating cardiac NO content, Na+/K+ ATPase activity, and Ca+2 ATPase activity |
| Hong et al 2003 (44) | Berberine | Rats (in vivo) | In rats with cardiac hypertrophy, berberine decreased the plasma levels of norepinephrine, controlled the total sympathetic tone, and inhibited the progress of cardiac hypertrophy |
| Zeng et al 2003 (46) | Berberine | Rats (in vivo) | Berberine alleviated ischemia/reperfusion (I/R) injury and attenuated apoptosis in rat neonatal myocytes that were exposed to I/R. Berberine pretreatment of myocytes reduced lactate dehydrogenase (LDH) release and methylenedioxyamphetamine (MDA) formation in I/R groups, and inhibited apoptosis in ischemia and reperfusion groups |
| Zhou et al 2001 (47) | Berberine | Rats (in vitro) | Berberine pretreatment significantly reduced the degree of verapamil-induced heart failure in the experimental group, compared to the control group. |
| Zhang et al 1992 (32) | Berbamine | Rats (in vitro) | Berbamine alleviated myocardial I/R injury through preservation of Na+-K+ ATPase activity, attenuation of ischemia-induced Na+ overload and reperfusion-induced Ca+2 overload. Also, it reduced free radicals generation during reperfusion. |
| Li et al 1991 (48) | Berberine | Rabbits (in vitro) | Berberine (1 mumol.L-1) reduced the myocardial I/R damages and restored all parameters to the level of preischemia within 10 min of reperfusion |
| Ren et al 1988 (49) | Berberine | Human aortic intimal cell culture | Administration of 30 to 100 μg/ml of berberine to a culture of human aortic intimal cells decreased intracellular cholesterol by 41% and decreased the proliferative activity of these cells |
Table 4.
Summary of the findings of preclinical studies that investigated the inotropic effects of Berberis vulgaris extracts
| Study ID | Active constituent | Study Design/ Animal model | Findings |
|---|---|---|---|
| Zhang et al 2011 (50) | Berbamine | Rats (in vitro) | Berbamine increased myocardial contractility by increasing intracellular Ca+2concentrations. It also enhanced myofilament Ca+2 sensitivity by increasing cytosolic protein kinase C (PKCɛ) |
| Zeng and Li 2001 (36) | Berberine | Human trials | Berberine reduced the frequency of ventricular premature complexes and increased the left ventricular ejection fraction (EF) in patients with congestive heart failure |
| Hu et al 1992 (52) | Berbamine compound E6 | Rats (in vitro) | Berbamine compound E6 inhibited calmodulin-dependent myosin light chain kinase (MLCK) in a dose-dependent manner, an effect which was antagonized by the addition of more calmodulin. This suggests that berbamine has an inotropic effect by competitive antagonism with calmodulin |
| Xu 1986 (51) | O-(4-ethoxyl-butyl) berbamine (EBB) | Human trial | EBB had a more potent calmodulin antagonist activity than berbamine. This antagonism is competitive because it can be reversed by adding higher doses of calmodulin |
Inotropic effects
Berberine is used in the East to treat CHF (46). Several studies have investigated the underlying mechanisms of the positive inotropic effect (PIE) of B. vulgaris extracts. Zhang et al showed that the PIE of berbamine can be attributed to increasing myofilament Ca+2 sensitivity, thus avoiding the adverse events of several cardiotonic agents that improve cardiac contractility through increasing intracellular Ca+2 concentration (50). Improving myofilament Ca+2 responsiveness can be explained by increasing cytosolic protein kinase C (an enzyme family that regulates cardiac contraction through controlling Ca+2 transients and myofilament Ca+2 sensitivity).
They also revealed that high concentrations of berbamine (300 nM) can be associated with a negative inotropic effect (NIE) through suppression of Ca+2 transients and cellular shortening, thus suggesting a biphasic concentration-dependent regulation of cardiac function (50). In another study by Li et al (48), high concentrations of berbamine resulted in inhibition of cardiac muscle contraction in the isolated rabbit hearts. Moreover, berbamine and its active derivatives were proven to inhibit calmodulin at micromole concentrations (51, 52).
Antiplatelet effect
Few studies have reported a possible antiplatelet effect of B. vulgaris extracts and berberine, in particular. In a clinical study by Huang et al, using berberine increased thrombolysis, induced by plasminogen activators (53). Feng et al reported that berberine could be more efficacious than aspirin in preventing platelet aggregation in patients with atherosclerotic cerebral infarction (54). In another study by Fukuda et al, berberine could directly inhibit different stages of platelet-dependent inflammatory processes in a dose-dependent manner (55).
Several mechanisms have been proposed for the observed antiplatelet effect of berberine, such as inhibition of arachidonic acid metabolism, reduction of thromboxane A2 release from platelets (56), delinea-tion of the calcium influx (57) and the partial agonist effect on platelet α2-adrenoceptors (53, 58). However, the exact mechanism has not been fully uncovered. Further studies are warranted to understand the molecular mechanism behind this effect before translation into large randomized clinical trials.
Discussion
Our study adds to the literature by outlining the effects and mechanisms of B. vulgaris in cardiovascular medicine, making it a strong candidate for further clinical trials and human applications. The current literature suggests that B. vulgaris extracts can directly influence the cardiovascular system through their cardioprotective, inotropic, and antihypertensive effects. However, some of the mechanisms, underlying these effects, are still largely unknown and have not been tested in clinical trials due to lack of preclinical evidence. Figure 3 summarizes the mechanisms of B. vulgaris cardiovascular actions.
Figure 3.
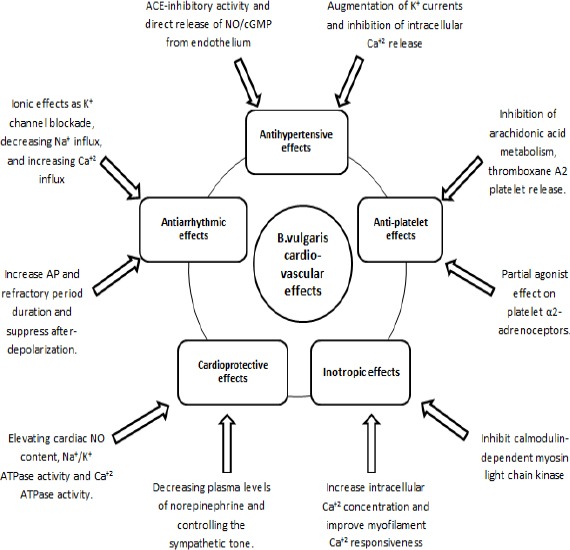
Summary of the mechanisms of Berberis vulgaris cardiovascular actions
Moreover, B. vulgaris extracts can protect against cardiovascular pathologies indirectly through targeting the associated risk factors. They exert a potent antioxidant activity through decreasing the formation of cellular thiobarbituric acid reactive species formation (TBARS) and nitric oxide (NO), as well as increasing the activity of glutathione peroxidase (GPx) and superoxide dismutase (SOD) (7, 8). There is a wealth of evidence regarding the hypoglycemic and hypolipemic properties of berberine. The hypoglycemic effect of berberine can be attributed to improving insulin sensitivity, inhibiting glucogenic enzymes, delaying carbohydrate absorption, and increasing cellular glucose uptake (59-61), while the hypolipemic effects are related to inhibiting adipogenesis and increasing LDL-C uptake by increasing its cellular receptors (62, 63). These effects can act in synergism with the direct cardiovascular effects of B. vulgaris.
The proven effects of berberine can have numerous applications, such as controlling stenosis after balloon angioplasty (25), replacing or supplementing antihypertensive and antiarrhythmic drugs, being an ideal drug for CHF due to its vasodilator, inotropic, and lusitropic effects (64). However, lack of evidence from randomized clinical trials limits the acceptability of the results from small clinical studies and further use in clinical practice (65).
Dosage: The therapeutic dose of B. vulgaris, used in most clinical situations, is about 200 mg for three to four times daily. Its extracts are standardized to contain 8% to 12% isoquinoline alkaloids (4). B. vulgaris extracts are widely available in the form of capsules, solutions, and topical preparations, such as ointments and tinctures. It can be used as a supplement for food and tea (66).
Safety: No toxic effects have been reported at doses used for clinical purposes. However, overdosage has been linked to vertigo, convulsion, nasal bleeding, kidney failure, skin and eye inflammation, and hypoglycemia (67). According to an animal study by Peychev, B. vulgaris is moderately toxic (LD50= 2.6± 0.22 g/kg body weight in mice). It is not recommended to exceed a daily dose of 500 mg (68).
According to a study by Arayne et al, B. vulgaris should not be used during pregnancy because it can cause uterine constriction, leading to miscarriage. Furthermore, large doses of berberine are warranted to have a teratogenic effect (69). B. vulgaris fruits contain dihydro-palimitinum hydroxide, which exerts anti-estrogen activities, causing endometrial atrophy and fetal malnourishment. Moreover, it should be avoided in jaundiced infants because it has bilirubin displacement properties (70). Despite the lack of clinical evidence, it is not recommended for use in infants below 2 years of age and elders over 65 years, as well as breastfeeding women (4). Individuals who suffer any organ dysfunction should only use it after medical consultation.
Recommendations: The mechanism of action and the bioactive components of these extracts need further exploration. Moreover, further information regarding dosage, duration, and pharmacokinetics are needed to optimize its pharmacological use. Larger clinical trials with longer follow-up periods are necessary to detect rare adverse events and investigate the long-term safety of B. vulgaris extracts. Randomized data about cost-effectiveness and quality of life, in comparison to the standard drugs of cardiovascular disorders, would be beneficial. Integrated use of B. vulgaris extracts with the current cardiovascular drugs should be considered and the possible interaction between these agents should be characterized. It is also essential to increase awareness of alternative medicine strategies among health care professionals to enable them to provide alternative treatments for their patients who are not responding or intolerant to current medications (71).
Conclusion
B. vulgaris showed a beneficial effect in reducing blood pressure, enhancing cardiac contractility, and protection from reperfusion injury. However, the mechanisms of these effects are still under investigation. Moreover, it can modify major risk factors for cardiovascular disorders, such as oxidative stress, hyperglycemia, and hyperlipidemia. Further trials are needed to translate these findings into effective cardiovascular medications.
References
- 1.Hoyert DL, Xu J. Deaths:preliminary data for 2011. Natl Vital Stat Rep. 2012;61:1–51. [PubMed] [Google Scholar]
- 2.Pagidipati NJ, Gaziano TA. Estimating deaths from cardiovascular disease:a review of global methodologies of mortality measurement. Circulation. 2013;127:749–756. doi: 10.1161/CIRCULATIONAHA.112.128413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Canto JG, Kiefe CI, Greenland P. Coronary heart disease risk factors and mortality—reply. JAMA. 2012;307:1137–1138. doi: 10.1001/jama.2012.324. [DOI] [PubMed] [Google Scholar]
- 4.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 5.Abd El-Wahab AE, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine:antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarei A, Changizi-Ashtiyani S, Taheri S, Ramezani M. A quick overview on some aspects of endocrinological and therapeutic effects of Berberis vulgaris L. Avicenna J Phytomed. 2015;5:485–497. [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng F, Wang Y, Li J, Su C, Wu F, Xia W-H, et al. Berberine improves endothelial function by reducing endothelial microparticles-mediated oxidative stress in humans. Int J Cardiol. 2013;167:936–942. doi: 10.1016/j.ijcard.2012.03.090. [DOI] [PubMed] [Google Scholar]
- 8.Mohammadi A, Sahebkar A, Kermani T, Zhilaee M, Tavallaie S, Ghayour Mobarhan M. Barberry administration and pro-oxidant-antioxidant balance in patients with metabolic syndrome. Iran Red Crescent Med J. 2014;16:e16786. doi: 10.5812/ircmj.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi B, Ahn I, Kim Y, Park J, Lee S, Hyun C, et al. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp Mol Med. 2006;38:599. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- 10.Lee HY, He X, Ahn J. Enhancement of antimicrobial and antimutagenic activities of Korean barberry (Berberis koreana Palib.) by the combined process of high-pressure extraction with probiotic fermentation. J Sci Food Agric. 2010;90:2399–2404. doi: 10.1002/jsfa.4098. [DOI] [PubMed] [Google Scholar]
- 11.Iloon Kashkooli R, Najafi SS, Sharif F, Hamedi A, Hoseini Asl MK, Najafi Kalyani M, et al. The effect of Berberis vulgaris extract on transaminase activities in non-alcoholic Fatty liver disease. Hepat Mon. 2015;15:e25067. doi: 10.5812/hepatmon.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenbaum CC, O’Mathuna DP, Chavez M, Shields K. Antioxidants and antiinflammatory dietary supplements for osteoarthritis and rheumatoid arthritis. Altern Ther Health Med. 2010;16:32–40. [PubMed] [Google Scholar]
- 13.Kermanshahi H, Riasi A. Effect of dietary dried Berberis vulgaris fruit and enzyme on some blood parameters of laying hens fed wheat-soybean based diets. Int J Poult Sci. 2006;5:89–93. [Google Scholar]
- 14.Guo ZB, Fu JG. [Progress of cardiovascular pharmacologic study on berbamine] Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:765–768. [PubMed] [Google Scholar]
- 15.Manolov P, Nikolov N, Markov M, Toneva M. [Experimental research on Berberis vulgaris] Eksp Med Morfol. 1985;24:41–45. [PubMed] [Google Scholar]
- 16.Fatehi M, Saleh TM, Fatehi-Hassanabad Z, Farrokhfal K, Jafarzadeh M, Davodi S. A pharmacological study on Berberis vulgaris fruit extract. J Ethnopharmacol. 2005;102:46–52. doi: 10.1016/j.jep.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 17.Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res. 2005;19:222–225. doi: 10.1002/ptr.1661. [DOI] [PubMed] [Google Scholar]
- 18.Olmez E, Ilhan M. Evaluation of the alpha-adrenoceptor antagonistic action of berberine in isolated organs. Arzneimittelforschung. 1992;42:1095–1097. [PubMed] [Google Scholar]
- 19.Kang DG, Sohn EJ, Kwon EK, Han JH, Oh H, Lee HS. Effects of berberine on angiotensin-converting enzyme and NO/cGMP system in vessels. Vasc Pharmacol. 2002;39:281–286. doi: 10.1016/s1537-1891(03)00005-3. [DOI] [PubMed] [Google Scholar]
- 20.Wen-Fei C, Mao-Hsiung Y, Chieh-Fu C. Mechanism of vasodilatory effect of berberine in rat mesenteric artery. Eur J Pharmacol. 1991;204:35–40. doi: 10.1016/0014-2999(91)90832-b. [DOI] [PubMed] [Google Scholar]
- 21.Ko WH, Yao XQ, Lau CW, Law WI, Chen ZY, Kwok W, et al. Vasorelaxant and antiproliferative effects of berberine. Eur J Pharmacol. 2000;399:187–196. doi: 10.1016/s0014-2999(00)00339-3. [DOI] [PubMed] [Google Scholar]
- 22.Xu SZ, Zhang Y, Ren JY, Zhou ZN. Effects of berberine of L-and T-type calcium channels in guinea pig ventricular myocytes. Zhongguo Yao Li Xue Bao. 1997;18:515–518. [PubMed] [Google Scholar]
- 23.Chun YT, Yip TT, Lau KL, Kong YC, Sankawa U. A biochemical study on the hypotensive effect of berberine in rats. Gen Pharmacol. 1979;10:177–182. doi: 10.1016/0306-3623(79)90085-5. [DOI] [PubMed] [Google Scholar]
- 24.Mahdavi N, Joukar S, Najafipour H, Asadi-Shekaari M. The promising effect of barberry (Zereshk) extract against experimental pulmonary microvascular remodeling and hypertension:A comparison with sildenafil. Pharm Biol. 2016;54:509–515. doi: 10.3109/13880209.2015.1050676. [DOI] [PubMed] [Google Scholar]
- 25.Lee S, Lim HJ, Park HY, Lee KS, Park JH, Jang Y. Berberine inhibits rat vascular smooth muscle cell proliferation and migration in vitro and improves neointima formation after balloon injury in vivo berberine improves neointima formation in a rat model. Atherosclerosis. 2006;186:29–37. doi: 10.1016/j.atherosclerosis.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 26.Marin Neto JA, Maciel BC, Secches AL, Gallo L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11:253–260. doi: 10.1002/clc.4960110411. [DOI] [PubMed] [Google Scholar]
- 27.Wong KK. Mechanism of the aortic relaxation induced by low concentrations of berberine. Planta Med. 1998;64:756–757. doi: 10.1055/s-2006-957575. [DOI] [PubMed] [Google Scholar]
- 28.Bova S, Padrini R, Goldman WF, Berman DM, Cargnelli G. On the mechanism of vasodilating action of berberine:possible role of inositol lipid signaling system. J Pharmacol Exp Ther. 1992;261:318–323. [PubMed] [Google Scholar]
- 29.Wen-Fei C, Mao-Hsiung Y, Chieh-Fu C. Mechanism of vasodilatory effect of berberine in rat mesenteric artery. Eur J Pharmacol. 1991;204:35–40. doi: 10.1016/0014-2999(91)90832-b. [DOI] [PubMed] [Google Scholar]
- 30.Wang YX, Yao XJ, Tan YH. Effects of berberine on delayed afterdepolarizations in ventricular muscles in vitro and in vivo. J Cardiovasc Pharmacol. 1994;23:716–722. doi: 10.1097/00005344-199405000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Chi J, Chu S, Lee C, Chou N, Su M. Mechanical and electrophysiological effects of 8-oxoberberine (JKL1073A) on atrial tissue. Bri J Pharm. 1996;118:503–512. doi: 10.1111/j.1476-5381.1996.tb15431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang W, Chen SG, Ju HS, Zhao SH, Zou CM, Hao JM, et al. Mechanisms of protective effects of berbamine on ischemia/reperfusion injury in isolated rat heart. Methods Find Exp Clin Pharm. 1992;14:677–684. [PubMed] [Google Scholar]
- 33.Li BX, Yang BF, Zhou J, Xu CQ, Li YR. Inhibitory effects of berberine on IK1, IK, and HERG channels of cardiac myocytes. Acta Pharm Sin. 2001;22:125–131. [PubMed] [Google Scholar]
- 34.Wang YX, Zheng YM. Ionic mechanism responsible for prolongation of cardiac action-potential duration by berberine. J Cardiovasc Pharmacol. 1997;30:214–222. doi: 10.1097/00005344-199708000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Guo Y, Chen Y, Tan Z, Klaassen CD, Zhou H. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur J Clin Harmacol. 2012;68:213–217. doi: 10.1007/s00228-011-1108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng XH, Li YY. Clinical observations of the effect of berberine for congestive heart failure. US Chinese J Angiocardiomyopathy. 2001;6:308–311. [Google Scholar]
- 37.Li XT, Wang YL. [Effect of berberine on cytosolic free calcium of rat myocardial cells in vitro] Yao Xue Xue Bao. 1997;32:721–725. [PubMed] [Google Scholar]
- 38.Shaffer JE. Inotropic and chronotropic activity of berberine on isolated guinea pig atria. J Cardiovasc Pharmacol. 1985;7:307–315. doi: 10.1097/00005344-198503000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia:a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 40.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 41.Gao H, Chen L, Yang HT. Activation of α1B-adrenoceptors alleviates ischemia/reperfusion injury by limitation of mitochondrial Ca2+overload in cardiomyocytes. Cardiovasc Res. 2007;75:584–595. doi: 10.1016/j.cardiores.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Zhang CM, Gao L, Zheng YJ, Yang HT. Berbamine protects the heart from ischemia/reperfusion injury by maintaining cytosolic Ca(2+) homeostasis and preventing calpain activation. Circ J. 2012;76:1993–2002. doi: 10.1253/circj.cj-11-1431. [DOI] [PubMed] [Google Scholar]
- 43.Yang J, Zhou ZY, Xu JG. [Protective effect of berberine on cardiac hypertrophy induced by L-thyroxine in rats] Sichuan Da Xue Xue Bao. 2004;35:223–225. [PubMed] [Google Scholar]
- 44.Hong Y, Hui SS, Chan BT, Hou J. Effect of berberine on catecholamine levels in rats with experimental cardiac hypertrophy. Life Sci. 2003;72:2499–2507. doi: 10.1016/s0024-3205(03)00144-9. [DOI] [PubMed] [Google Scholar]
- 45.Tanabe H, Suzuki H, Mizukami H, Inoue M. Double blockade of cell cycle progression by coptisine in vascular smooth muscle cells. Biochem Pharmacol. 2005;70:1176–1184. doi: 10.1016/j.bcp.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 46.Zeng XH, Zeng XJ, Li YY. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. The Am J Cardiol. 2003;92:173–176. doi: 10.1016/s0002-9149(03)00533-2. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Z, Xu J, Lan T. [Protective effect of berberine on isolated perfused heart in heart failure] Hua Xi Yi Ke da Xue Xue Bao. 2001;32:417–418. [PubMed] [Google Scholar]
- 48.Li BY, Yang BF, Li WH. Effects of berbamine on hemodynamics and myocardial reperfusion injury in isolated working rabbit hearts. Zhongguo Yao Li Xue Bao. 1991;12:48–52. [PubMed] [Google Scholar]
- 49.Ren LH, Vasil’ev AV, Orkehov AN, Tertov VV, Tutel’ian VA. [Evaluation of the antiatherosclerotic properties of natural compounds of plant origin on cell cultures of the human aortic intima] Farmakol Toksikol. 1988;52:44–46. [PubMed] [Google Scholar]
- 50.Zhang CM, Gao L, Zheng YJ, Yang HT. Berbamine increases myocardial contractility via a Ca2+-independent mechanism. J Cardiovasc Pharmacol. 2011;58:40–48. doi: 10.1097/FJC.0b013e31821b70d1. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Zhang S. A derivative of bisbenzylisoquinoline alkaloid is a new and potential calmodulin antagonist. Biochem Biophys Res Commun. 1986;140:461–467. doi: 10.1016/0006-291x(86)91113-7. [DOI] [PubMed] [Google Scholar]
- 52.Zhuo-Yi H, Yue-Shong G, Wen-Long H. Interaction of berbamine compound E 6 and calmodulin-dependent myosin light chain kinase. Biochem Pharmacol. 1992;44:1543–1547. doi: 10.1016/0006-2952(92)90470-4. [DOI] [PubMed] [Google Scholar]
- 53.Huang CG, Chu ZL, Wei SJ, Jiang H, Jiao BH. Effect of berberine on arachidonic acid metabolism in rabbit platelets and endothelial cells. Thromb Res. 2002;106:223–227. doi: 10.1016/s0049-3848(02)00133-0. [DOI] [PubMed] [Google Scholar]
- 54.Feng CL, Liu SX, Feng QY, Yin JJ, Zhao L. A comparative study of antiplatelet aggregation by berberine hydrochloride with low dose aspirin. Shandong Med. 1996;36:11–12. [Google Scholar]
- 55.Fukuda K, Hibiya Y, Mutoh M, Koshiji M, Akao S, Fujiwara H. Inhibition by berberine of cyclooxygenase-2 transcriptional activity in human colon cancer cells. J Ethnopharmacol. 1999;66:227–233. doi: 10.1016/s0378-8741(98)00162-7. [DOI] [PubMed] [Google Scholar]
- 56.Wu JF, Liu TP. Effects of berberine on platelet aggregation and plasma levels of TXB2 and 6-keto- PGFla in rats withreversible middle cerebral artery occlusion. Acta Pharm Sin. 1995;2 [PubMed] [Google Scholar]
- 57.Huang CG, Chu ZL, Yang ZM. Effects of berberine on cytoplasmic cAMP and calcium influx of rabbit platelets. Acad J Sec Mil Med Univ. 1991;12:320–322. [Google Scholar]
- 58.Hui KK, Jun LY, Chan WFA, Tse E. Interaction of berberine with human platelet α2 adrenoceptors. Life sciences. 1991;49:315–324. doi: 10.1016/0024-3205(91)90019-8. [DOI] [PubMed] [Google Scholar]
- 59.Moazezi Z, Qujeq D. Berberis Fruit Extract and Biochemical Parameters in Patients With Type II Diabetes. Jundishapur J Nat Pharm Prod. 2014;9:e13490. doi: 10.17795/jjnpp-13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kong WJ, Zhang H, Song DQ, Xue R, Zhao W, Wei J, et al. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism. 2009;58:109–119. doi: 10.1016/j.metabol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 61.Shidfar F, Keshavarz A, Hosseyni S, Ameri A, Yarahmadi S. Effects of omega-3 fatty acid supplements on serum lipids, apolipoproteins and malondialdehyde in type 2 diabetes patients. East Mediterran Health J. 2008;14:305–315. [PubMed] [Google Scholar]
- 62.Tang LQ, Wei W, Chen L-M, Liu S. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006;108:109–115. doi: 10.1016/j.jep.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Shidfar F, Ebrahimi SS, Hosseini S, Heydari I, Shidfar S, Hajhassani G. The effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, homocysteine, glycemic control and total antioxidant capacity in Type 2 diabetic patients. Iran J Pharm Res. 2012;11:643–652. [PMC free article] [PubMed] [Google Scholar]
- 64.Grossman W. Diastolic function and heart failure:an overview. Eur Heart J. 1990;11:2–7. doi: 10.1093/eurheartj/11.suppl_c.2. [DOI] [PubMed] [Google Scholar]
- 65.Kanmanthareddy A, Reddy M, Ponnaganti G, Sanjani HP, Koripalli S, Adabala N, et al. Alternative medicine in atrial fibrillation treatment-Yoga, acupuncture, biofeedback and more. J Thorac Dis. 2015;7:185–92. doi: 10.3978/j.issn.2072-1439.2015.01.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palamarchuk AS, Bondarenko VE, Grazhevich Iu V, Guletskaia VN. [Biochemical composition of the fruits of the common barberry] Farm Zh. 1973;28:84–85. [PubMed] [Google Scholar]
- 67.Shamsa F, Ahmadiani A, Khosrokhavar R. Antihistaminic and anticholinergic activity of barberry fruit (Berberis vulgaris) in the guinea-pig ileum. J Ethnopharmacol. 1999;64:161–166. doi: 10.1016/s0378-8741(98)00122-6. [DOI] [PubMed] [Google Scholar]
- 68.Peychev L. Pharmacological investigation on the cardiovascular effects of Berberis vulgaris on tested animals. Pharmacia. 2005;52:118–121. [Google Scholar]
- 69.Arayne MS, Sultana N, Bahadur SS. The berberis story Berberis vulgaris in therapeutics. Pak J Pharm Sci. 2007;20:83–92. [PubMed] [Google Scholar]
- 70.Chan E. Displacement of bilirubin from albumin by berberine. Neonatology. 1993;63:201–208. doi: 10.1159/000243932. [DOI] [PubMed] [Google Scholar]
- 71.Abushouk AI, Negida A, Ahmed H, Abdel-Daim MM. Neuroprotective mechanisms of plant extracts against MPTP induced neurotoxicity:Future applications in Parkinson’s disease. Biomed Pharmacother. 2017;85:635–645. doi: 10.1016/j.biopha.2016.11.074. [DOI] [PubMed] [Google Scholar]


