Abstract
Berberis vulgaris and berberine, its main component, traditionally have been used for treatment of various disorders. The pharmacological properties of them have been investigated using different in vivo and in vitro models. In spite of beneficial effects of B. vulgaris on different cell lines, there are documents have revealed negative impacts of it on animal and human. In this regards, the determination of its toxicity in a scientific view is necessary. In current report, we provide classified information about the toxicity of B. vulgaris and berberine in different conditions consist of acute, sub-acute, sub-chronic and chronic state. Besides, it discusses the cytotoxicity, genotoxicity, mutagenicity, and carcinogenicity of B. vulgaris and berberine as well as developmental toxicity and clinical studies. Data from the present study indicate that their toxicity is depending on the route and duration of administration. According to present study, they could induce GI upset and ulceration, immunotoxicity, phototoxicity, neurotoxicity, cardiotoxicity and jaundice in a dose dependent manner. They should be used with caution in pregnancy, neonatal and G6PD deficiency. Besides, consideration should be taken in co-administration of berberine with drugs that are metabolized with CYP enzymes due their inhibitory effects on these enzymes. Furthermore, they evoke cytotoxicity on both normal and cancer cell line which is time and concentration dependent.
Keywords: Acute toxicity, Berberine, Berberis vulgaris, Cancer cell, Chronic toxicity, CYP enzyme, Developmental toxicity, Pregnancy
Introduction
The genus Berberis with more than 500 species belongs to Berberidaceae family (1). Berberis vulgaris which is known as barberry, common or European barberry (2) is an evergreen shrub which possesses yellow, spiny, angled or sulcated bark, oblong, obovate, or elliptic leaves, yellow flowers and red, oblong fruits (3). It grows in Asia and Europe and is a well-known herb in Iran (4). The main isolated compounds from B. vulgaris are tannins, phenolic compounds, triterpenes (lupeol, oleanolic acid), sterols (stigmasterol, stigmasterol glucoside) and alkaloids (berberamine, palmatine, berberine, oxyberberine, columbamine, isocorydine, lambertinea and magniflo-rine). Besides, bisbenzlisoquinolines (oxycanthine), N-(p-trans- coumaroyl) tyramine, cannabisin G and (±)-lyoniresinol have been isolated from this plant (5).
Berberine, an isoquinoline alkaloid, is a member of naturally occurring protoberberines class (Figure 1). This alkaloid is present in plants of Berberidaceae, Papaveraceae and Ranunculaceae families including Arcangelisia flava (menispermaceae), B. vulgaris (barberry), B. aristata (tree turmeric), B. aquifolium (Oregon prape), B. lyceum, B. crataegina, Hydrastis canadensis (goldenseal) and Coptis chinensis (Chinese goldthread). Berberine widely consumed in Ayurvedic and Chinese medicine. (4, 6, 7). Berberine has been isolated from various parts of these species such as root, stem, bark, fruit and rhizome (6).
Figure 1.
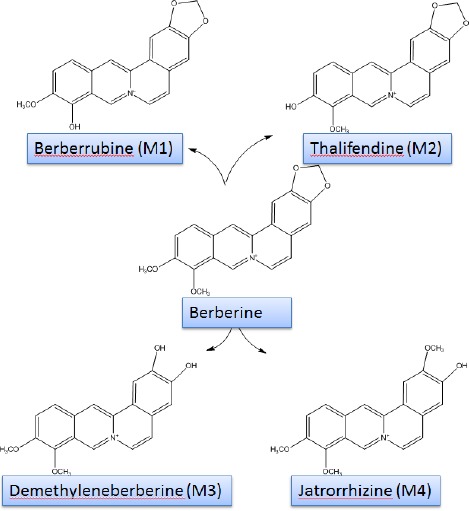
Chemical structure of berberine and its metabolites
Traditional use of root, bark, leaf and fruits of barberry as an immunemodulator and anti-microbial agent as well as a treatment for central nervous system, cardiovascular, gastrointestinal, endocrine, and renal problems have been proved with so many pharmacological studies (4, 8). Recently published articles demonstrated that barberry and berberine (its main constituents) have anti-oxidant (9), anti-inflammatory (10), anti-tumor (11), anti-mutagenic (12) and anti-diabetic (9) effects. Their hypoglycemic and cholesterol lowering properties (13), neuroprotective (14), and hepatoprotective (15-17), effects have scientifically been proved by numerous studies. B. vulgaris may possesses preventive effects in relapse of morphine consumption in addicted individuals (18). It has been demonstrated that B. vulgaris and berberine induces inhibitory effects on Leishmania species (19-21). Also, it has been reported as an anti-fungal compound (20). There are evidences in vivo and in vitro that berberine hydrochloride has beneficial effects on colitis (22).
Berberine could be absorbed from the gastrointes-tinal (GI) however; its oral bioavailability and its plasma level are very low. It should be noticed that berberine converted to ionized form in the physiolo-gical conditions and self-aggregated in low pH conditions. Self-aggregation decreases its solubility in the GI track and its permeability. The other barriers of berberine oral bioavailability are P-glycoprotein (P-gp) mediated efflux, hepatobiliary re-extraction and metabolization by CYP2D6 and CYP3A4 in the intestine (23). It has been shown that berberine is converted to dihydroberberine form by gut flora, which has higher intestine-absorbable rate in comparison berberine (24). Berberine is distributed in the liver, kidneys, muscle, lungs, brain, heart, pancreas and fat. Strikingly, tissue concentration of berberine and its metabolites is higher than plasma concentration (25). Berberine is metabolized in the liver by oxidative demethylation and glucuronidation to berberrubine (M1), thalifendine (M2), demethyleneberberine (M3), and jatrorrhizine (M4) and their glucoronide forms (Figure 1). CYP2D6, 1A2, 3A4, 2E1 and CYP2C19 are the main CYPs in berberine metabolisms (26). Finally, berberine metabolites are excreted through feces, urine, and bile (Figure 2). It is important to notice that there are some pharmacokinetic interactions in co-adminstration of berberine with metformin, ketoconazole, digoxin and cyclosporine A (8).
Figure 2.
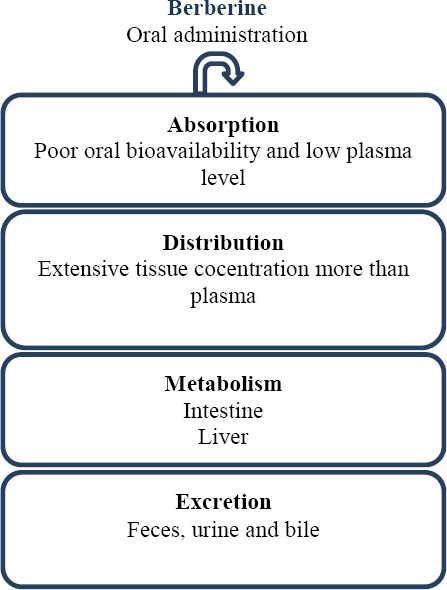
Schematic diagram of berberine pharmacokinetic
In spite of the panoramic use of barberry in different folklores as an herbal medicine, there is no comprehensive study which categorized the toxic effects of barberry in animal models and human studies. Hence, this review discusses on the toxic effects of barberry and berberine, as its main derivative, in terms of acute, sub-acute, sub-chronic, chronic toxicity with a mention on their develop-mental and mutagenic toxicity as evidenced from the scientific literature in animal and human assays.
Methods
A literature search was conducted and the available information on toxicology and pharmacological proper-ties of B. vulgaris and berberine was collected via relevant keywords including B. vulgaris, barberry, berberine, acute toxicity, sub-acute toxicity, sub-chronic toxicity, chronic toxicity, mutagenic, developmental, miscarriage, cancer, and clinical trial in the following databases: PubMed, Scopus, Google Scholar and Web of Science. All kinds of relevant articles, abstracts or books were included. Furthermore, the reference lists of key papers for further leads were searched. No time limitation was considered in this review. Besides, no constraint was accounted about including all kinds of berberine containing herbs. Both in vivo and in vitro studies were included to this investigation. The toxic effects of barberry and berberine were categorized in following main headings: toxicological findings, developmental toxicity, cytotoxicity, genotoxicity, mutagenicity, carcinogenicity and clinical toxicity (Figure 3).
Figure 3.
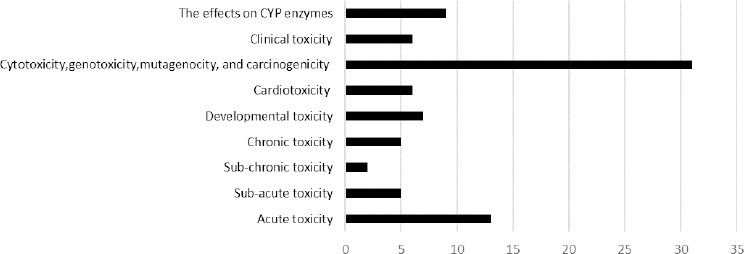
Number of published articles which are cited in each field
Toxicological findings
Acute toxicity
Acute toxicity test is the first toxicity assessment which is examined during 14 days and determined from the administration of a single exposure. It estimates approximate lethal dose or concentration (e.g., LD50 or LC50) as an intrinsic toxicity of substance. The most often used species are mouse and rat (27).
B. vulgaris is moderately toxic. The oral LD50 value for powdered root of B. vulgaris in mice is 2600 mg/kg (28). In “The Essential Guide to Herbal Safety”, according the former studies, the following LD50 values have been reported: the oral LD50 value for barberry root extract fraction 520 mg/kg (mice) and 1280 mg/kg (rats), for berberine 23 mg/kg (mice, IP) and for its sulphated form in oral administration have been reported >1 g/kg (rat) (29). Besides, in the earlier studies, the oral LD50 values of berberine in mice was reported 329 mg/kg (30).
It has been found that berberine sulphate LD50 value (isolated from Berberis aristata) after intraperitoneal administration in rats was 205 mg/kg. However, in 50 mg/kg, it evokes diarrhea in 4 out of 10 rats which has been attributed to its direct effects on the gastrointestinal track (31). In the early studies, oral LD50 value of berberine sulfate in rats and mice was reported more than 1000 mg/kg and 329 mg/kg (32). Oral doses of 100 mg/kg berberine sulfate to cats induced vomiting for 6 to 8 hr followed by death of all animals 8 to 10 days, while it was well tolerated by rats. Following an oral administration of 2.75 g berberine (base or salt not stated) to dogs (weight unspecified) nausea, emesis, salivation, diarrhea, muscular tremor and paralysis appeared (33).
Acute toxicity of berberine hydrochloride in mice indicated the following LD50 values: 9.0386 mg/kg (IV) and 57.6103 mg/kg (IP). However, berberine LD50 value by intragastric administration couldn’t be evaluated which attributed to its low gastrointestinal absorption. Increasing berberine dosage from 20.8 to 41.6 g/kg (orally) evokes the elevation of berberine blood concentration from 0.168 μg/ml to 0.432 μg/ml and leads to 30% mortality rate. The safety dosage of berberine in oral administration in mice is 20.8 g/kg and in human would be 2.97 g/kg which is 100 times above the typical prescribed doses in clinical trials studies (34).
The oral LD50 of isolated berberine from Rhizoma coptidis is 713.58 mg/kg in mice that is classified in slight toxicity rank (35, 36). The comparison of fibrous root of Rhizoma coptidis (FRC) with Rhizoma coptidis (RC) revealed LD50 value of FRC and RC were greater than 7 and 4.89 g/kg in mice, respectively. Since no acute toxicity effect was observed with FRC, it classified in lowest toxicity class. The content of berberine in FRC and RC measured by HPLC was 1.20 and 5.61%, respectively. So, the lower toxicity effects of FRC can be attributed to lower content of its berberine (37).
In mice acute toxicity test, it has been shown that the mortality of berberine in 409, 512, 640, 800 and 1000 mg/kg, orally, is 10, 20, 40, 60, and 75%, separately (35).
Isoquinoline alkaloids from the roots of Turkish Berberis species manifested that administration of berberine in acute doses (200 mg/kg, orally) induces gastric lesions in 4 out of 6 mice which attributed to the inhibition of cyclooxygenese-2 transcription by berberine (38). Parallel to the consequences of acute toxicity, it has been revealed that berberine induces gastric damages dose dependently. After 24 hr of single therapy with berberine (104 and 209 mg/kg, orally) the ratio of ulceration were 1/6 and 3/6, respectively in mice. Mortality rate was 5/6 for 418 mg/kg while for 104 and 209 mg/kg there was no death (39).
The LD50 values of B. vulgaris and berberine are summarized in Table 1.
Table 1.
The median lethal dose (LD50) values of barberry and berberine
| Compound | Animals | Route of administration | LD50 value | Ref. |
|---|---|---|---|---|
| B. vulgaris (powdered root) | Mice | Oral | 2600 mg/kg | (28) |
| B. vulgaris (root extract fraction) | Rat | Oral | 1280 mg/kg | (29) |
| Mice | 520 mg/kg | |||
| Berberine | Mice | IP | 23 mg/kg | (29) |
| Mice | Oral | 329 mg/kg | (30) | |
| Berberine (rhizoma coptidis) | Mice | Oral | 713.58 mg/kg | (35,36) |
| Berberine sulphate | Rat | Oral | > 1 g/kg | (29) |
| Berberine sulphate (Berberis aristata) | Rat | IP | 205 mg/kg | (31) |
| Berberine sulphate | Rat | Oral | 1000 mg/kg | (32) |
| Mice | 329 mg/kg | |||
| Berberine hydrochloride | Mice | IV | 9.0386 mg/kg | (34) |
| IP | 57.6103 mg/kg | |||
| Rhizoma coptidis | Mice | Oral | 4890 mg/kg | (37) |
| Rhizoma coptidis (fibrous root) | Mice | Oral | > 7000 mg/kg |
Sub-acute toxicity
In sub-acute toxicity test (repeated-dose study) a special substance is administrated in 3 to 4 different dosages to the animals during one month or less and its biochemical and pathologic effects are evaluated following 14-28 days of exposure. Results of this study provide a good information about the toxicity of the substance after treatment with consecutive dosages and it offers a guideline to determine doses for sub-chronic studies (27).
Oral sub-acute toxicity of derived alkaloids from the roots of Turkish Berberis species including berberine, berbemine, palmatine, oxyacanthine, magnoflorine and columbamine (25, 50 and 200 mg/kg) was evaluated in mice. The results showed that all alkaloids in 25 mg/kg for 7 days induces different degree of gastric ulcers (38). A sub-acute toxicity evaluation on the root extract of B. crataegina represented that in Freund’s complete adjuvant-induced chronic arthritis, administration of active ethanol, buthanol, CHCl3- ethanol and H2O-I extract and fractions (300, 642, 472, 614 mg/kg, respectively) to rats for 21 days was safe without any toxicity and death. However, liver and kidney enlargement was observed with ethanol extract (21.3% and 9.7%, respectively), buthanol fraction (14.6% and 4.2%, respectively) and CHCl3-ethanol fraction (7.2% and 2.8%, respectively). Body weight was increased up to 30% with H2O-I fraction, 27% with buthanol fraction, 17.8% with ethanol extract and 11.3% with CHCl3-ethanol fraction (39). Furthermore, it has been reported that intraperitoneal injection of berberine (10 and 20 mg/kg/day, for 1 week) decreases bilirubin protein binding in adult rats. Clinically it should be noticed that this substance could increase the risk of kernicterus in risky patients (36).
Mahmoudi et al (2016) in their study investigated the immunotoxic effects of the berberine (5 and 10 mg/kg/day, IP, for 14 days) in BALB/c mice. Administration of berberine (10 mg/kg) decreased spleen weight, blood cell count including the number of leukocytes, neutrophils and lymphocytes and diminished generation/differentiation of B- and T-cells and splenic CD19+ B-cells, CD4+ and CD8+ T-cells. Totally, berberine in 10 mg/kg suppressed both cellular and humoral immune functions while in 5 mg/kg just influenced proliferation of lymphocytes and delayed-type hypersensitivity response (40).
Oral administration of berberine sulphate 50 or 100 mg/kg but not 25 mg/kg for 10 days to cats evoked hemorrhagic inflammatory problems in both small and large intestine (33).
Sub-chronic toxicity
The sub-chronic test occurring in repeatedly administration of a chemical for 30 to 90 days and its main goals are to determine “no observed adverse effect level” (NOAEL), “lowest observed adverse effect level” (LOAEL) and identifying the organs that are affected by chemical after consecutive administration. This type of study conducted in two species (10-20 rodents and 4-6 dogs), orally, for at least 3 doses as follow: a low dose without toxic effects, a high dose that causes toxic effects with less than 10% fatalities and an intermediate dose. At the end of the 90th day, biochemical and hematological parameters, body weight, food consumption and other factors are measured (27).
Berberine is the main alkaloid in FRC. It has been shown that the extract of RC with 1.88 g/kg in Sprague Dawley rats has no adverse effects without any effects on blood biochemical factors. In 3.76 g/kg, damages to lung and liver have been reported where it increases alanine aminotransferase (ALT) and aspartate aminotransferase (AST), significantly (37). Another study indicated administration of RC alkaloids in 156 mg/kg, orally, for 90 days to Sprague Dawley rats has no poisoning symptoms, without any effects on the body weight and mortality (35).
Chronic toxicity
The duration of study in chronic test is longer than 90 days which depends on the species types. For example, chronic exposure in rodents is about 180 to 720 days and for non-rodent animals usually is 360 days or longer. The evaluation of maximum tolerable dose and the urinary metabolite indexes, carcinogenicity, physiological and pharmacokinetic aspects of chemicals are the main goal of the chronic toxicity studies (27).
The phototoxic effect of berberine concomitant with UVA radiation on mosquito larvae (Aedes atropatpus) has been assessed. Larvae were treated with 10 ppm berberine in exposure to 0.4 W/m2 UV for 24 hr, then they were transferred to the clean jars and observations were made to their adult stage during 4-week development. Berberine showed chronic toxicity and markedly increased cumulative mortality. The effects were further in pupil and adult stage. The underlying mechanism of berberine may be attributed to the production of singlet O2 by bounded berberine to DNA (41).
Treating rats with 50 mg/kg berberine demonstrated that berberine has no obvious toxic effect on kidney and liver (13). Parallel to these results, in other study has been revealed that berberine at concentrations >50, 100 and 150 mg/kg after 16 weeks induces liver tissue damages in diabetic rats but not in healthy rats (42). In another study, chronic treatment with berberine (5 mg/kg/day for 15 weeks, IP) evoked atherosclerosis in apoE-/- mice (43).
Developmental toxicity
Developmental toxicity is the study of adverse effects of a substance on the development of the organism resulting from exposure to chemical or physical agents pre-natal or post-natal until the time of puberty. Reproductive toxicity and teratogenicity also, assessed. Reproductive toxicity is a part of developmental toxicity, in which the adverse effects of the substances on male and female reproductive system are assessed. The substances that cause developmental toxicity from the time of conception till birth are called teratogen (27).
Administration of berberine (97.5 mg/kg/day, orally) and goldthread rhizome (0.6 g/kg/day, orally) to pregnant mice and rats (6th to 15th day of gestation) had no effects on induction of vaginal bleeding, abortion, neonates malformation and fetal toxicity (44). However, it has been reported that berberine evokes uterine contraction and could be a potent maternal toxic with teratogenic effects. The maternal LOAEL, developmental LOAEL and maternal NOAEL for mated rats treated with berberine chloride dehydrate during gestation period (day 6th to 20th) have been reported with doses 531, 1313 and 282 mg/kg/day, respectively (45).
Berberine chloride dihydrate administration to rats (282, 531 and 1313 mg/kg/day, orally) and mice (569, 841 and 1155 mg/kg/day, orally) reduces maternal weight and fetal body weight, respectively. The maternal toxicity LOAEL related is 7250 ppm (531 mg/kg/day) and 5250 ppm (841mg/kg/day) in rats and mice, respectively. One thousand mg/kg/day has been reported as the developmental toxicity NOAEL in rats and developmental toxicity LOAEL in mice (46). These levels would be lower for berberine chloride due the hydration in berberine chloride dihydrate (2).
Although plants rich in berberine traditionally has been used for the treatment of jaundiced neonates, recently it has been proved that berberine may induce jaundice and haemolysis in susceptible people. Bilirubin-displacing capacity of berberine has been proved recently. In vitro studies showed that berberine has displacement effects on the bilirubin from its binding sites on the human serum albumin. Furthermore, chronic administration of berberine to rats (10 and 20 µg/g, IP for 7 days) increased serum bilirubin concentration without any change in the total albumin concentration in the serum (47). Oral treatment or in vitro exposing of glucose-6-phosphate dehydrogenase (G6PD) deficient rat erythrocytes with excessively high doses of berberine increased the red blood cell osmotic fragilities and induced hemolysis (48). Nevertheless, there are records that demonstrated berberine did not induce jaundice and has no effects on G6PD performance during pregnancy (44, 49). Following administration of 200 mg/kg goldthread rhizome and 32.5 mg/kg berberine to neonate rats (subcutaneous), no effect on the G6PD activity and jaundice induction have been observed (44). Altogether, B. vulgaris and berberine-containing plants are organized in category C during pregnancy and should be avoided in lactation (2).
Cardiotoxicity
Some documents have mentioned on cardiotoxicity of berberine. Intravenous administration of berberine to dogs induced cardiac depression due to the dilation of splanchnic vessels (50). Also, the reduction of mitochon-drial respiration and Ca2+ influx capacity has been reported (51). It has been shown that berberine induces inhibitory effects on L type voltage dependent Ca2+ channels but in higher concentration (100 µM) stimulates the release of Ca2+ from intracellular stores (52). Previous studies demonstrate that berberine (10-100 µM) can reversibly block human ether-a-go-go (hERG) channels in Xenopus oocytes (53) by directly binding to F656V (54). This inhibitory concentration was higher than the effective concentration in clinical application (53). The IC50 for berberine on hERG in HEK-293 cells and Xenopus oocytes has been reported 3.1±0.5 and 80±5 µM, respectively (55). Besides, it was shown that berberine by inhibition of inward rectifier potassium current, delayed rectifier potassium current and hERG channels increases the action potential which could have both anti-arrhythmic (53) and arrhythmogenic properties (54). Myotropic effects of B. vulgaris induces hypotensive effects (28).
Cytotoxicity, genotoxicity, mutagenicity, and carcinogenicity
Several studies have been reported that berberine has anti-tumor activity both in vitro and in vivo that could be mentioned as a worth anticancer drug. It decreases cell viability in different type of cancer cells such as nasopharyngeal (56), hepatocellular (57), breast (58), and cervical cancer cells (59) in a dose- and time-dependent manner. Berberine has some degree of toxicity in normal cells that its underlying mechanism is the inhibition of adenine nucleotide translocase followed by decrease in energy production (51). Treating cerebellar granule neurons (CGN) and hippocampal neurons (HCN) with berberine (10 nM to 10 mM) for 6 hr indicated that berberine at concentration less than 0.3 mM not only had no effects on the gross structure of axon and dendrite of CGN, but also in 0.1 mM could moderately increases cell viability. However, berberine in higher concentrations (more than 1mM) disturbs neural integrity and increases condensation of nuclei. Berberine in 10 mM induces the most toxic potential and after 2 hr from treatment, it increases oxidative stress and decreases neuronal viability (60).
It has been shown that berberine hydrochloride (extracted from RC) decreases proliferation, inhibits migration and induces apoptosis in CNE-1 nasopharyngeal carcinoma cells in a time- and dose-dependent manner. Treating CNE-1 cells with berberine hydrochloride for 48 hr inhibits invasion and migration (10 µg/ml) and their cell growth (40 µg/ml) by activating apoptosis pathway (56).
Other study proved the anti-cancer effects of berberine on MCF-7 and MDA-MB-231 breast cancer cells. The results have indicated that treating MCF-7 and MDA-MB-231 cells by berberine (10 to 100 µM
for 48 or 72 hr) inhibits cell viability in a dose and time-dependent manner. The morphology of MCF-7 and MDA-MB-231 cells is changed in response to berberine (50 µM). Besides, treating with berberine, for 24-72 hr, elevates the ratio of apoptotic proteins and caspase-3 activity. This cytotoxicity has been attributed to reactive oxygen species (ROS) formation. It has been shown that berberine with 50 µM increases the ROS production after 6 hr (58). It has been suggested that apoptosis and specially necrosis play a main role in berberine-induced cytotoxicity on prostate cancer LNCaP cells, too (61).
Parallel to the consequences mentioned above, it has been reported that berberine at the concentration of 20 µM significantly inhibits the invasion ability of cervical cancer cells and decreases their motility (59). The IC50 values of berberine in the HL-7702 normal hepatic cells is 838.4 µM whereas in HepG2, SMMC-7721 and Bel-7402 cells are 34.5 µM, 25.2 µM and 53.6 µM, respectively (57). Acute cytotoxicy of berberine on Ehrlich ascites carcinoma (EAC) cells has been observed in 10 µg/ml that markedly induces apoptosis and in 50 and 100 µg/ml that suppresses DNA synthesis, changes the structure of dsDNA and induces death. The IC50 value of berberine on EAC cells has been reported less than 1 µg/ml (62).
Berberine has cytotoxicity effects on L929 Murine Fibroblast cells. Berberine in the concentrations from 0.0025 mg/ml to 0.025 mg/ml has no cytotoxic effects, whereas DNA damages in concentration more than 0.025 mg/ml has been observed. Berberine in concentrations higher than 0.1 mg/ml changes the structure of L929 cells and induces ROS formation and apoptosis. Cytotoxic effects of berberine significantly manifests when these cells are treated with 0.2 mg/ml (63). The morphology of C6 rat glioma cells is altered after treating with berberine (>50 µM). Besides, this alkaloid induces cell death in a time- and dose-dependent manner. Following treatment with berberine (100 µM, for 12, 24, 48 and 72 hr), G2/M cell cycle phases are arrested and significantly apoptotic cell death is observed when it promotes the activity of caspase-3, -8 and -9 time-dependently (64). Berberine iodide and acetoneberberine are more toxic against human oral squamous cell carcinoma and human promyelocytic leukemia than against normal human oral tissue-drived cells. Respsctively, the IC50 values on HL-60 have been reported 18, 22 µM, on HSC-2, HSC-3, HSC-4 and NA cells 47, 88 µM, and on CA9-22 cells 132, 136 µM, where for HGF >400, 293 µM, HPC 235, 219 µM and HPLF 245, 189 µM (65).
Several lines of evidences have been conducted on the effect of berberine in normal and cancerous cells in vivo. Li and colleagues (2015) in their study evaluated the effect of berberine on azoxymethane and dextran sulfate sodium (AOM/DSS) promoted colorectal carcinogenesis mouse model. They observed a reduction in tumor multiplicity when 40 mg/kg of berberine was given to mice for 10 weeks (66). In the other study SiHs cells were injected subcutaneously or into tail vein of mice for evaluating the effects of berberine (20 mg/kg/day, oral) on the tumor growth and lung metastasis, respectively. According gathered data, berberine treatment decreased tumor size and angiogenesis. Besides, it reduced pulmonary weight and lung metastasis (59). In murine 4T1 breast cancer model, berberine moderately inhibited growth and weight of tumor. Its combination with anti-DR5 increased synergically their anti-tumor effects and reduced the incidence of lung metastatic (73). Furthermore, the growth and weight of tumors that were induced by implanication of S 180 sarcoma tumor cells to Kunming mice (74), prostate cancer PC-3 and LNCaP cells to BALB/c athymic nude mice (67), Lewis lung carcinoma cell line (LLC) to C57BL/6 mice (69) and Dalton’s lymphoma ascites cells (DLA) to Swiss albino mice (68) were inhibited by berberin treatment. However, berberine but not NAX014 compounds (berberine derivatives) had no suppressive effects in spontaneous mammary tumors in HER-2/neu transgenic mice (75).
The effects of B. vulgaris or berberine on normal and cancer cell line under in vitro and in vivo conditions are summarized in Tables 2 and 3, respectively.
Table 2.
The effect of Berberis vulgaris or berberine on normal and cancer cell line under in vitro conditions
| Type | Dose | Toxic responses | Ref. | |
|---|---|---|---|---|
| Normal | HL-7702 | Berberine: 3.125, 6.25, 12.5, 25, 50 and 100 µM | No changes in cell viability (IC50: 838.4 µM) | (57) |
| HUVEC cells | Berberine: 40µM | Decreased the cell viability by 87.6% The cell invasion decreased significantly | (59) | |
| L929 | Berberine: 2.5-200 µg/mL | Did not show any cytotoxic effects (at 2.5 µg/ml to 25 µg/ml) The morphology of cells changed, intracellular ROS formation increased, apoptotic cell death induced (at higher than 100 µg/ml) and cell viability decreased (at 50, 100 and 200 µg/ml) | (63) | |
| Gingival fibroblast HGF, pulp cell HPC, periodontal ligament fibroblast HPLF | Berberine iodide and acetoneberberine: 10, 20 and 80 µM | Tumor-specific cytotoxic effect of berberine iodide and acetoneberberine that LC50 values in HGF, HPC and HPLF were >400, 293; 235, 219; and 245, 189 µM, respectively | (65) | |
| HEK293 | Berberine: 0-100 µM | Did not show any growth inhibitory effect | (66) | |
| Normal human prostate epithelial PWR-1E cells | Berberine: 5-50µ M | NO growth inhibition in normal prostate cells observed | (67) | |
| L929 | Berberine hydrochloride: 10-100 µg/ml | In a concentration-dependent manner berberine induced cytotoxic effects (IC50: 40 µg/ml) | (68) | |
| Cancerous | CNE-1 | Berberine hydrochloride: 2.5, 5, 10, 20 and 40 µg/ml | Decreased Cell viability Activated caspase-3, decreased twist protein levels and increased apoptotic proteins Inhibited migration and invasion of cells | (56) |
| HepG2, SMMC-7721 and Bel-7402 | Berberine: 3.125, 6.25, 12.5, 25, 50 and 100 µM | Decreased the cell viability in a time- and dose-dependent manner (the IC50 value in HepG2, SMMC-7721 and Bel-7402: 34.5 µM, 25.2 µM and 53.6 µM, respectively) | (57) | |
| MCF-7 and MDA-MB-231 | Berberine: 10– 100 µM | Dose- and time dependent inhibitory effect Increased apoptotic ratio, caspase-3 activity and alteration in cell morphology Increased the ROS generation and accumulation | (58) | |
| CsSki, SiHa, and HeLa | Berberine: 20µM | In a dose-dependent manner, inhibited the invasion of CsSki, HeLa and SiHa cells Inhibited the migration of CsSki, SiHa, and HeLa cells Decreased the SiHa cell motility | (59) | |
| LNCaP and PC-82 | Berberine: 1-100 µM | Dose-dependently decreased the cell viability and induced programmed necrosis and apoptosis | (61) | |
| EAC | Berberine: 10, 50 and 100 µg/ml | Increased apoptotic cells (at 10 µg/ml) Inhibited DNA synthesis, changed the morphology of dsDNA and induced cell death (at 50 and 100 µg/ml) (IC50: below 1µg/ml) | (62) | |
| C6 rat glioma | Berberine: 100µM | In a time- and dose-dependent manner, altered the cell morphology, promoted the caspase-3, -8 and -9 activity, increased the production of ROS and induced apoptotic cell death | (64) | |
| Human oral squamous cell carcinoma: HSC-2, HSC-3, HSC-4, NA, CA9-22 and human promyelocytic leukemia: HL-60 | Berberine iodide and acetoneberberine: 10, 20 and 80 µM | Increased apoptotic cells, DNA fragmentation, caspase-3, -8 and -9 and pro-apoptotic BAD protein. (did not elevate BAD protein in HSC-2 cells) IC50 for Berberine iodide and acetoneberberine, in sequence: HL-60 (18, 22 µM), HSC-2 (59, 65 µM), HSC-3 (83, 47 µM), HSC-4 (54, 69 µM) and NA (88, 53 µM) and CA 9-22 cells (136, 132 µM) | (65) | |
| HCT116, SW480 and LOVO colorectal carcinoma cells | Berberine: 0-100 µM for 24, 48 and 72 hr | In a concentration- and time-dependent manner, inhibited the growth of cancer cells via programmed death | (66) | |
| Human prostate cancer LNCaP cells, PC-3 cells | Berberine: 0, 5, 10, 20, 50, and 100 µM | Inhibited cell growth and proliferation in cancer cells in a time- and concentration-dependent manner (IC50 value in LNCap cells: 60µM and in PC-3 cells: ≥100µM) Induced apoptotic cell death | (67) | |
| DLA | Berberine hydrochloride: 100- 1000 mg/ml | Berberine showed cytotoxic effect by 44% at 1 mg/ml At lower concentrations, it caused a dose-dependent cytotoxicity in DLA cells | (68) | |
| A549 | Berberine:2.5-40 µM | Did not show cytotoxic effect on the cells (up to 24 hr) Showed slight cytotoxicity after 48 hr (20 and 40 µM) | (69) | |
| Human esophageal cancer cell line YES-2 | Berberine: 8-32 µM | Reduced cell viability and proliferation, inhibited production of interleukin-6 dose- and time- dependently | (70) | |
| Oral cancer cell line OC2 and KB cells | Berberine: 1, 10, and 100 µM for 2-12 hr | Inhibited activator protein 1, reduced the production of cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) and showed anti-inflammatory effect Berberine did not show irritation of stomach and kidney toxicity | (71) | |
| K1735-M2 Mouse Melanoma Cells | Berberine: 0, 10, 25, 50, 75, and 100 µM | Dose- and time-dependent inhibitory effect on cell proliferation (50% of growth inhibition occurred for 72 and 96 hr since drug exposure) | (72) | |
Table 3.
The effect of Berberis vulgaris or berberine on cancer cell line under in vivo conditions
| Type | Dose | Toxic responses | Ref. |
|---|---|---|---|
| SiHa treated | Berberine 20 mg/kg | Reduced angiogenesis, tumor growth properties and pulmonary colonization of SiHa cells | (59) |
| Azoxymethane initiated and dextran sulfate sodium promoted colorectal carcinogenesis | Berberine: 40 mg/kg, orally, mice, for 10 weeks | Berberine reduced tumor multiplicity compare with control group and significantly decreased COX-2 synthesis | (66) |
| Prostate cancer PC-3 and LNCaP cells | Berberine: 5, 10 mg/kg, IP, for 4 weeks twice per week | Tumor size and growth was decresed | (67) |
| DLA cells to induce ascites tumour and solid tumour, separately | Berberine hydrochloride (10, 2.5 and 0.5 mg/kg/day), IP for 10 days | Increased life span and reduced tumore size | (68) |
| Berberine hydrochloride (25, 5 and 1 mg/kg), orally for 10 days | |||
| LLC | Berberine: 1, 2 mg/kg, IP | Berberine with infrared radiation significantly decreased tumor volume Berberine treatment alone has promising effects on suppressing tumor growth | (69) |
| 4T1 treated | Berberine chloride n-hydrate: 100 mg/kg/day, Orally, one month | Moderately inhibited tumor growth in 4T1 cells | (73) |
| Murine sarcoma S180 | Berberine 30 mg/kg, IV | Decreased tumor weight | (74) |
According to chromosomal aberration analysis, berberine stimulates double strand breaks in Rev3 deficient cells (76). The comet assay test shows that berberine and goldenseal (rich in berberine) induces DNA damage in HeLa cells (77) and HepG2 cells by inhibition of topoisomerase 1 in a dose and time dependent manner (78). This DNA damage is accompanied with increasing the level of histone H2A.X phosphorylation at Serine139 (γ-H2A.X), cell cycle arrest and activating checkpoints related protein (78). Former, SOS chromo test has been reported negative for berberine chloride. It had no mutagenic effects (induction of frameshift, point mutation and cytoplasmatic PETITE mutation) in haploid yeast cells under nongrowth conditions, but in dividing cells elevated the frequency of HOM3 frameshift revertants and cytoplasmatic PETITE mutation, dose dependently. Furthermore, berberine treatment increased induction of crossing over in diploid yeast cells during growth condition but not in nongrowth cells (79). Recently, it has been reported that berberine, time and concentration dependently, evokes oxidative stress and DNA damage both in mouse marrow cells (80) and mouse heart cells (81). In Ames test, berberine hydrochloride was weakly mutagenic to strain TA98 (82), but no mutagenic activity of tetrahydroberberine (82) and FRC has been observed in histidine-requiring Salmonella typhimurium mutant strains (37). Besides, mouse micronucleus test and mouse sperm abnormality test was negative for FRC (37).
Phototoxicity could be considered as genotoxicity of berberine. The use of berberine in eye drops and lotions results ocular photodamage and raises the possibility of lens epithelial cells disorders (83). Berberine (50 µM) in exposure to UVA radiation causes cell death in human HaCaT keratinocytes (80% decrease in cell viability) and DNA damage in the form of single strand breaks. Also, the berberine phototoxicity is interestingly dependent on its solvent. Radiation of berberine generates both O2 and radical species in nonpolar solvents such as CH2Cl2 but not in aqueous solutions. UVA protection should be considered when berberine topical preparation is used (84).
Clinical toxicity
In a clinical study, 34.5% patients with type-2 diabetes that treated with berberine (500 mg three times/day) for 13 weeks have shown transient GI side effects diarrhea, constipation, flatulence and abdominal complaint. However, no obvious change in liver enzymes and creatinine was observed (85).
Infusion of berberine, 0.2 mg/kg/min for 30 min, in patients (n=12) with refractory cardiac heart failure improved cardiac performance probably due to peripheral vasodilator and inotropic effects. However, in 4 patients 1-20 hr after the infusion of berberine ventricular tachycardia with torsade de pointes appeared (86).
There are evidences that revealed berberine could cause kernicterus in infants with G6PD deficiency (87) and the bilirubin displacement from binding proteins (88). Numerous endogenous and exogenous substances by interfering with the binding of bilirubin to albumin may cause the risk of jaundice. In a clinical study, administration of RC and Cortex phellodendri (chinese herbs containing beberine) 3 and 9 g/day, respectively for 24 weeks to 3 thalasemic patients, increased bilirubin in serum (89). It is proposed that berberine usage should be avoided in pregnancy, breastfeeding period and G6PD deficient neonates (6, 87). Nevertheless, in a cohort study, the extract of RC and C. phelledendri (rich in berberine) were administrated to chronic cytopenic haematologic patients for 24 weeks. The results illuminated that berberine was safe without any clinical deterioration and influence in creatinine level and liver function (89).
The effects on CYP enzymes
An important subject for notification is the effects of B. vulgaris and berberine on the CYP enzymes that is important in coadministration with narrow therapeutic index drugs such as cyclosporine (54). Evaluation of berberine IC50 values for CYPs and its constituents may be useful for prediction of possible herb-drug interactions which may have either positive or negative therapeutic effects. The effect of berberine on these enzymes are summarized in Table 4.
Table 4.
Berberine IC50 values for cytochrome P450 enzymes (CYPs)
| Type of CYPs | Model | Berberine IC50 | Ref. |
|---|---|---|---|
| CYP1A1 | A recombinant enzyme system | 1.38 ± 0.12 µM | (90) |
| HepG2 cells | 2.5 µM | (91) | |
| CYP1A2 | A recombinant enzyme system | 60 µM< | (90) |
| CYP1A2 over expressing Huh-7 cells | 10 µM | (92) | |
| Human liver microsomes | >100 µM | (93) | |
| Supersomes system | 73.2 ± 5.5 µM< (higher than human blood concentration) | (94) | |
| CYP3A4 | Human liver microsomes | >100 µM | (93) |
| Supersomal system | 48.9 ± 9 µM | (94) | |
| Human liver microsomes | 400 µM | (95) | |
| CYP1B1 | A recombinant enzyme system | 94 ± 8 nM (possible potential for clinical inhibition) | (90) |
| CYP2C9 | Human liver microsomes | >100 µM | (93) |
| CYP2C8 | Human liver microsomes | >100 µM | (93) |
| CYP2C19 | Human liver microsomes | >100 µM | (93) |
| CYP2D6 | Human liver microsomes | 49.4 µM | (93) |
| Supersomal system | 7.40 ± 0.36µM (possible potential for clinical inhibition) | (94) | |
| Human liver microsomes | 45 µM | (95) |
In a two-phase randomized-crossover clinical study on 70 healthy Chinese male volunteers [(mean±SD) age, 21.6 ±1.5 years; weight, 61.35±3.89 kg], the effect of berberine on CYP enzymes activities in vivo were accessed. Midazolam, omeprazole, dextromethorphan, losartan, and caffeine were used as CYP450 probe drugs to evaluate enzyme activities of CYP3A4, 2C19, 2D6, 2C9, and CYP1A2. According to data, repeated administration of berberine (300 mg, T.I.D., orally, 2 weeks) to volunteers decreased CYP2D6, 2C9, and 3A4 activities (96).
In another randomized, controlled clinical trial two groups of renal transplant patients on cyclosporine A were included. Berberine treated group: 52 renal transplant recipients both male and female (31/21) volunteers [(mean±SD) age, 42.5 ±10.8 years; weight, 55.3±10.2 kg] that received cyclosporine A (319.2±90.7 mg/day), azathioprine (43.4±17.4 mg/day), prednisone (12±4.1 mg/day) and berberine hydrochloride tablets orally (200 mg, t.i.d.) for 3 months and berberine free group: 52 renal transplant recipients both male and female (40/12) volunteers [(mean±SD) age, 39.6±11.9 years; weight, 56.9±8.5 kg] that received cyclosporine A (309.3±62.9 mg/day), azathioprine (47.3±5.3 mg/day) and prednisone (12.4±4.1 mg/day). This study showed that berberine markedly increases the blood concentration of cyclosporine A in renal-transplant recipients which could be attributed to inhibition of CYP3A4 by berberine in the liver and/or small intestine (97).
Conclusion
Huge numbers of published articles on the medi-cal worth of barberry and its constituents have shown that they have a great potential to develop as a useful drug especially in the area of cancer, cardiovascular and metabolic disorders. However, till now no drug has been introduced in the market using pure barberry, berberine or the other barberry components. This might be resulted from low clinical data. Certain information about barberry/its main secondary metabolites toxicity needs to be addressed before they can be used as approved therapeutic drugs in human.
In the present study, we summarized a variety of scientific articles that evaluated the toxicity of B. vulgaris and berberine. By considering the LD50 values, it is clear that their toxicity depends on the experimental animal species, dose and rout of administration as well as the herb source. The risk of toxicity in oral route is less than IP and IV injections.
Results in animal and in vitro studies have proved the potential of berberine to induce GI upset and ulceration, immunotoxicity, phototoxicity, neurotoxicity and cardiotoxicity in a dose dependent manner (Figure 4). Besides, there are controversial data in using berberine and its derivative during pregnancy, so the use of them should be cautioned in pregnancy and neonatal. Berberine by inhibiting adenine nucleotide translocase, promoting ROS formation, apoptosis and necrosis pathways induces its toxicity on both normal and cancer cell in a time and concentration dependent manner. Finally, the inhibitory effects of berberine on CYP enzymes should be mentioned which could evoke indirect toxicity. This interaction is important in co-administration with narrow therapeutic index drugs which may increase the drug plasma concentration and toxicity.
Figure 4.
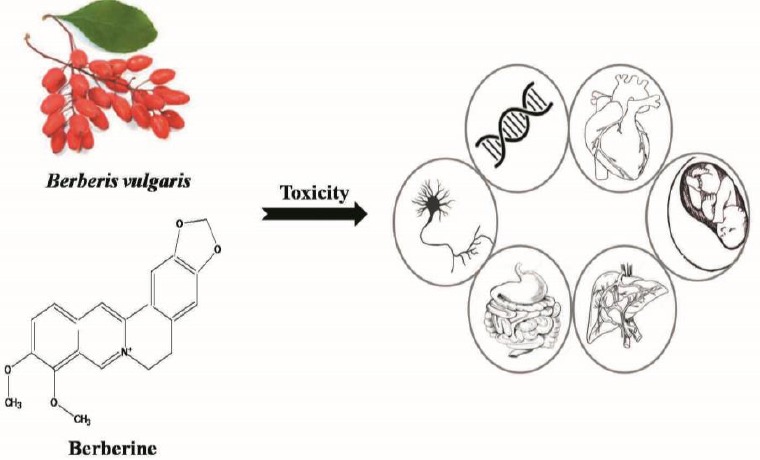
Schematic diagram showing toxic effects of Berberis vulgaris and berberine in different animal toxicity tests
Nevertheless, as mentioned, these data mostly have been gathered from animal studies and there are differences between animals and human body, so more clinical studies in future could reveal the concealed toxicity of berberine and its derivatives.
References
- 1.Rounsaville TJ, Ranney TG. Ploidy levels and genome sizes of Berberis L. and Mahonia Nutt. species, hybrids, and cultivars. HortScience. 2010;45:1029–1033. [Google Scholar]
- 2.Bone K, Mills S. Materia medica. Principles and practice of phytotherapy modern herbal medicine. 2st ed. USA: Churchill livingstone; 2013. pp. 399–418. [Google Scholar]
- 3.Ahrendt LWA. Berberis and Mahonia:a taxonomic revision. Bot J Linn Soc. 1961;57:1–410. [Google Scholar]
- 4.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 5.Mokhber-Dezfuli N, Saeidnia S, Gohari A, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of Berberis species. Pharmacogn Rev. 2014;8:8–15. doi: 10.4103/0973-7847.125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh IP, Mahajan S. Berberine and its derivatives:a patent review (2009-2012) Expert Opin Ther Pat. 2013;23:215–231. doi: 10.1517/13543776.2013.746314. [DOI] [PubMed] [Google Scholar]
- 7.Smolinske SC. Herbal product contamination and toxicity. J Pharm Pract. 2005;18:188–208. [Google Scholar]
- 8.Imenshahidi M, Hosseinzadeh H. Berberis Vulgaris and berberine:an update review. Phytother Res. 2016;30:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 9.Abd El-Wahab AE, Ghareeb DA, Sarhan EE, Abu-Serie MM, El Demellawy MA. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine:antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218–229. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin K, Liu S, Shen Y, Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013;36:1079–1086. doi: 10.1007/s10753-013-9640-0. [DOI] [PubMed] [Google Scholar]
- 11.Yu FS, Yang JS, Lin HJ, Yu CS, Tan TW, Lin YT, et al. Berberine inhibits WEHI-3 leukemia cells in vivo. In Vivo. 2007;21:407–412. [PubMed] [Google Scholar]
- 12.Cernakova M, Kost’alova D, Kettmann V, Plodova M, Toth J, Drimal J. Potential antimutagenic activity of berberine, a constituent of Mahonia aquifolium. BMC Complement Altern Med. 2002;2:2. doi: 10.1186/1472-6882-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang Z, Liu F, Ong ES, Li SFY. Metabolic profile associated with glucose and cholesterol lowering effects of berberine in Sprague–Dawley rats. Metabolomics. 2012;8:1052–1068. [Google Scholar]
- 14.Kulkarni SK, Dhir A. Berberine:a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res. 2010;24:317–324. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]
- 15.Domitrovic R, Jakovac H, Blagojevic G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-a, COX-2, and iNOS expression in CCl(4)-intoxicated mice. Toxicology. 2011;280:33–43. doi: 10.1016/j.tox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Siu KY, Ye X, Wang N, Yuen MF, Leung CH, et al. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin Med. 2010;5:33. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hermenean A PC, Ardelean A, Stan M, Hadaruga N, Mihali CV, Costache M, Dinischiotu A. Hepatoprotective effects of Berberis vulgaris L.extract/βcyclodextrin on carbon tetrachloride–induced acute toxicity in mice. Int J Mol Sci. 2012;13:9014–9034. doi: 10.3390/ijms13079014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imenshahidi M, Qaredashi R, Hashemzaei M, Hosseinzadeh H. Inhibitory effect of Berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur J Nat Pharm Prod. 2014;9:e16145. doi: 10.17795/jjnpp-16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoudvand H, Sharififar F, Sharifi I, Ezatpour B, Fasihi Harandi M, Makki MS, et al. In vitro Inhibitory effect of Berberis vulgaris (berberidaceae) and its main component, berberine against different leishmania species. Iran J Parasitol. 2014;9:28–36. [PMC free article] [PubMed] [Google Scholar]
- 20.Mahmoudvand H, Ayatollahi Mousavi SA, Sepahvand A, Sharififar F, Ezatpour B, Gorohi F, et al. Antifungal, antileishmanial, and cytotoxicity activities of various extracts of Berberis vulgaris (berberidaceae) and its active principle berberine. ISRN Pharmacol. 2014;2014 doi: 10.1155/2014/602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salehabadi A KM, Farzad MH, Namaei MH. Effect of root bark extract of Berberis vulgaris L. on leishmania major on BALB/c mice. Parasitol Res. 2014;113:953–957. doi: 10.1007/s00436-013-3727-2. [DOI] [PubMed] [Google Scholar]
- 22.Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative study of Berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran J Pharm Res. 2011:97–104. [PMC free article] [PubMed] [Google Scholar]
- 23.Liu CS, Zheng YR, Zhang YF, Long XY. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282. doi: 10.1016/j.fitote.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Feng R, Shou JW, Zhao ZX, He CY, Ma C, Huang M, et al. Transforming berberine into its intestine-absorbable form by the gut microbiota. Sci Rep. 2015;5:12155. doi: 10.1038/srep12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan XS, Ma JY, Feng R, Ma C, Chen WJ, Sun YP, et al. Tissue distribution of berberine and its metabolites after oral administration in rats. PloS One. 2013;8 doi: 10.1371/journal.pone.0077969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo Y, Li F, Ma X, Cheng X, Zhou H, Klaassen CD. CYP2D plays a major role in berberine metabolism in liver of mice and humans. Xenobiotica. 2011;41:996–1005. doi: 10.3109/00498254.2011.597456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eaton DL, Gilbert SG. Principles of Toxicology. In: D. Kilassen C, editor. Casarett &Doull’s Toxicology the basic science of poisons. 8st ed. New York: MC Grow Hi education; 2013. pp. 34–37. [Google Scholar]
- 28.Peychev L. Pharmacological investigation on the cardiovascular effects of Berberis vulgaris on tested animals. Pharmacia. 2005;52:118–121. [Google Scholar]
- 29.Morgan M, Bone K, Mills S, McMillan J. Safety monographs. In: Mills S, Bone K, editors. The Essential Guide to Herbal Safety. USA: Elsevier Health Sciences; 2005. pp. 255–261. [Google Scholar]
- 30.Haginiwa J, Harada M. Pharmacological studies on crude drugs. V. Comparison of berberine type alkaloid-containing plants on their components and several pharmacological actions. Yakugaku Zasshi. 1962;82:726–731. [PubMed] [Google Scholar]
- 31.Kulkarni SK, Dandiya PC, Varandani NL. Pharmacological investigations of berberine sulphate. Jpn J Pharmacol. 1972;22:11–16. doi: 10.1254/jjp.22.11. [DOI] [PubMed] [Google Scholar]
- 32.Gardner Z, McGuffin M. American Herbal Products Association’s Botanical Safety Handbook. 2nd ed. New York: CRC Press; 2013. pp. 130–132. [Google Scholar]
- 33.Lampe KF. Berberine. In: Smet PAGMD, Keller K, Hansel R, Chandler R.F, editors. Adverse effects of herbal drugs. Vol. 1. New York: Springer-Verlag; 1992. pp. 97–104. [Google Scholar]
- 34.Kheir MM, Wang Y, Hua L, Hu J, Li L, Lei F, et al. Acute toxicity of berberine and its correlation with the blood concentration in mice. Food Chem Toxicol. 2010;48:1105–1110. doi: 10.1016/j.fct.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 35.Yi J, Ye X, Wang D, He K, Yang Y, Liu X, et al. Safety evaluation of main alkaloids from rhizoma coptidis. J Ethnopharmacol. 2013;145:303–310. doi: 10.1016/j.jep.2012.10.062. [DOI] [PubMed] [Google Scholar]
- 36.Ho CE, Goh YL, Zhang C. From prejudice to evidence:the case of rhizoma coptidis in singapore. Evid Based Complement Alternat Med. 2014;871720:25. doi: 10.1155/2014/871720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ning N, Wang YZ, Zou ZY, Zhang DZ, Wang DZ, Li XG. Pharmacological and safety evaluation of fibrous root of rhizoma coptidis. Environ Toxicol Pharmacol. 2015;39:53–69. doi: 10.1016/j.etap.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Kupeli E, Kosar M, Yesilada E, Husnu K, Baser C. A comparative study on the anti-inflammatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the roots of turkish berberis species. Life Sci. 2002;72:645–657. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 39.Yesilada E, Kupeli E. Berberis crataegina DC. root exhibits potent anti-inflammatory, analgesic and febrifuge effects in mice and rats. J Ethnopharmacol. 2002;79:237–248. doi: 10.1016/s0378-8741(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 40.Mahmoudi M, Zamani Taghizadeh Rabe S, Balali-Mood M, Karimi G, Memar B, Rahnama M, et al. Immunotoxicity induced in mice by subacute exposure to berberine. J Immunotoxicol. 2016;13:255–262. doi: 10.3109/1547691X.2015.1058306. [DOI] [PubMed] [Google Scholar]
- 41.Philogene BJ, Arnason JT, Towers GH, Abramowski Z, Campos F, Champagne D, et al. Berberine:a naturally occurring phototoxic alkaloid. J Chem Ecol. 1984;10:115–123. doi: 10.1007/BF00987648. [DOI] [PubMed] [Google Scholar]
- 42.Zhou JY, Zhou SW, Zhang KB, Tang JL, Guang LX, Ying Y, et al. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol Pharm Bull. 2008;31:1169–1176. doi: 10.1248/bpb.31.1169. [DOI] [PubMed] [Google Scholar]
- 43.Li K, Yao W, Zheng X, Liao K. Berberine promotes the development of atherosclerosis and foam cell formation by inducing scavenger receptor A expression in macrophage. Cell Res. 2009;19:1006–1017. doi: 10.1038/cr.2009.76. [DOI] [PubMed] [Google Scholar]
- 44.Shouye Y, Xuhua W. A research on the erupted fetal diseases caused by traditional Chinese drugs—discussion from the issue that chinese goldthread rhizome is prohibited in Singapore. J Tradit Chin Med. 2008;28:235–240. doi: 10.1016/s0254-6272(08)60055-2. [DOI] [PubMed] [Google Scholar]
- 45.Bone K. Berberine-containing herbs and pregnancy. Townsend Letter: The Examiner of Alternative Medicine; 2007. pp. 59–61. [Google Scholar]
- 46.Jahnke GD, Price CJ, Marr MC, Myers CB, George JD. Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Res B Dev Reprod Toxicol. 2006;77:195–206. doi: 10.1002/bdrb.20075. [DOI] [PubMed] [Google Scholar]
- 47.Chan E. Displacement of bilirubin from albumin by berberine. Neonatology. 1993;63:201–208. doi: 10.1159/000243932. [DOI] [PubMed] [Google Scholar]
- 48.Lin N, Gao X, Li J, Zhu J. Influence of huanglian and berberine on the erythrocytic osmotic fragilitas of experimental glucose-6-phosphate dehydrogenase deficiency in rats. Zhongguo Zhong Yao Za Zhi. 1998;23:562–564. [PubMed] [Google Scholar]
- 49.Chen C YZ, Li Y, Fichna J, Storr M. Effects of berberine in the gastrointestinal tract—a review of actions and therapeutic implications. Am J Chin Med. 2014;42:1053–1070. doi: 10.1142/S0192415X14500669. [DOI] [PubMed] [Google Scholar]
- 50.Chopra RN IC. Indigenous Drugs of India. Calcutta: Academic Publishers; 1933. p. 294. [Google Scholar]
- 51.Pereira CV, Machado NG, Oliveira PJ. Mechanisms of berberine (natural yellow 18)-induced mitochondrial dysfunction:interaction with the adenine nucleotide translocator. Toxicol Sci. 2008;105:408–417. doi: 10.1093/toxsci/kfn131. [DOI] [PubMed] [Google Scholar]
- 52.Dong D, Sun J, Luo D, Chen Q, Wang F, He S, et al. Effect of berberine on the cytosolic calcium concentration in the single ventricular cell of guinea pig. Chin J Pharm Toxicol. 2000;14:128–130. [Google Scholar]
- 53.Li BX, Yang BF, Zhou J, Xu CQ, Li YR. Inhibitory effects of berberine on IK1, IK, and HERG channels of cardiac myocytes. Acta Pharmacol Sin. 2001;22:125–131. [PubMed] [Google Scholar]
- 54.Zhi D, Feng PF, Sun JL, Guo F, Zhang R, Zhao X, et al. The enhancement of cardiac toxicity by concomitant administration of berberine and macrolides. Eur J Pharm Sci. 2015;76:149–155. doi: 10.1016/j.ejps.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez-Menchaca A, Ferrer-Villada T, Lara J, Fernandez D, Navarro-Polanco RA, Sanchez-Chapula JA. Block of HERG channels by berberine:mechanisms of voltage- and state-dependence probed with site-directed mutant channels. J Cardiovasc Pharmacol. 2006;47:21–29. doi: 10.1097/01.fjc.0000191564.52242.00. [DOI] [PubMed] [Google Scholar]
- 56.Li CH, Wu DF, Ding H, Zhao Y, Zhou KY, Xu DF. Berberine hydrochloride impact on physiological processes and modulation of twist levels in nasopharyngeal carcinoma CNE-1 cells. Asian Pac J Cancer Prev. 2014;15:1851–1857. doi: 10.7314/apjcp.2014.15.4.1851. [DOI] [PubMed] [Google Scholar]
- 57.Yang X, Huang N. Berberine induces selective apoptosis through the AMPK-mediated mitochondrial/caspase pathway in hepatocellular carcinoma. Mol Med Rep. 2013;8:505–510. doi: 10.3892/mmr.2013.1506. [DOI] [PubMed] [Google Scholar]
- 58.Xie J, Xu Y, Huang X, Chen Y, Fu J, Xi M, et al. Berberine-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species generation and mitochondrial-related apoptotic pathway. Tumour Biol. 2015;36:1279–1288. doi: 10.1007/s13277-014-2754-7. [DOI] [PubMed] [Google Scholar]
- 59.Chu SC, Yu CC, Hsu LS, Chen KS, Su MY, Chen PN. Berberine reverses epithelial-to-mesenchymal transition and inhibits metastasis and tumor-induced angiogenesis in human cervical cancer cells. Mol Pharmacol. 2014;86:609–623. doi: 10.1124/mol.114.094037. [DOI] [PubMed] [Google Scholar]
- 60.Kysenius K, Brunello CA, Huttunen HJ. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. PloS One. 2014;9:e107129. doi: 10.1371/journal.pone.0107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang LY, Wu YL, Gao XH, Guo F. Mitochondrial protein cyclophilin-D-mediated programmed necrosis attributes to berberine-induced cytotoxicity in cultured prostate cancer cells. Biochem Biophys Res Commun. 2014;450:697–703. doi: 10.1016/j.bbrc.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 62.Letasiova S, Jantova S, Miko M, Ovadekova R, Horvathova M. Effect of berberine on proliferation, biosynthesis of macromolecules, cell cycle and induction of intercalation with DNA, dsDNA damage and apoptosis in Ehrlich ascites carcinoma cells. J Pharm Pharmacol. 2006;58:263–270. doi: 10.1211/jpp.58.2.0015. [DOI] [PubMed] [Google Scholar]
- 63.Gu M, Xu J, Han C, Kang Y, Liu T, He Y, et al. Effects of berberine on cell cycle, DNA, reactive oxygen species, and apoptosis in l929 murine fibroblast cells. Evid Based Complement Alternat Med. 2015;796306:13. doi: 10.1155/2015/796306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen TC, Lai KC, Yang JS, Liao CL, Hsia TC, Chen GW, et al. Involvement of reactive oxygen species and caspase-dependent pathway in berberine-induced cell cycle arrest and apoptosis in C6 rat glioma cells. Int J Oncol. 2009;34:1681–1690. doi: 10.3892/ijo_00000299. [DOI] [PubMed] [Google Scholar]
- 65.Inoue K, Kulsum U, Chowdhury SA, Fujisawa S, Ishihara M, Yokoe I, et al. Tumor-specific cytotoxicity and apoptosis-inducing activity of berberines. Anticancer Res. 2005;25:4053–4059. [PubMed] [Google Scholar]
- 66.Li W, Hua B, Saud SM, Lin H, Hou W, Matter MS, et al. Berberine regulates AMP-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol Carcinog. 2015;54:1096–1109. doi: 10.1002/mc.22179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi MS, Oh JH, Kim SM, Jung HY, Yoo HS, Lee YM, et al. Berberine inhibits p53-dependent cell growth through induction of apoptosis of prostate cancer cells. Int J Oncol. 2009;34:1221–1230. [PubMed] [Google Scholar]
- 68.Anis K, Kuttan G, Kuttan R. Role of berberine as an adjuvant response modifier during tumour therapy in mice. J Pharm Pharmacol. 1999;5:697–700. [Google Scholar]
- 69.Peng PL, Kuo WH, Tseng HC, Chou FP. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer:the contribution of autophagic cell death. Int J Radiat Oncol Biol Phys. 2008;70:529–542. doi: 10.1016/j.ijrobp.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 70.Iizuka N, Miyamoto K, Hazama S, Yoshino S, Yoshimura K, Okita K, et al. Anticachectic effects of coptidisrhizoma, an anti-inflammatory herb, on esophageal cancer cells that produce interleukin 6. Cancer Lett. 2000;158:35–41. doi: 10.1016/s0304-3835(00)00496-1. [DOI] [PubMed] [Google Scholar]
- 71.Kuo CL, Chi CW, Liu TY. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Pereira GC, Branco AF, Matos JA, Pereira SL, Parke D, Perkins EL, et al. Mitochondrially targeted effects of berberine [Natural Yellow 18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium] on K1735-M2 mouse melanoma cells:comparison with direct effects on isolated mitochondrial fractions. J Pharmacol Exp Ther. 2007;323:636–649. doi: 10.1124/jpet.107.128017. [DOI] [PubMed] [Google Scholar]
- 73.Refaat A, Abdelhamed S, Yagita H, Inoue H, Yokoyama S, Hayakawa Y, et al. Berberine enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast cancer. Oncol Lett. 2013;6:840–844. doi: 10.3892/ol.2013.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang L, Li J, Ma F, Yao S, Li N, Wang J, et al. Synthesis and cytotoxicity evaluation of 13-n-alkyl berberine and palmatine analogues as anticancer agents. Molecules. 2012;17:11294–11302. doi: 10.3390/molecules171011294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pierpaoli E, Damiani E, Orlando F, Lucarini G, Bartozzi B, Lombardi P, et al. Antiangiogenic and antitumor activities of berberine derivative NAX014 compound in a transgenic murine model of HER2/neu-positive mammary carcinoma. Carcinogenesis. 2015;36:1169–1179. doi: 10.1093/carcin/bgv103. [DOI] [PubMed] [Google Scholar]
- 76.Hu X, Wu X, Huang Y, Tong Q, Takeda S, Qing Y. Berberine induces double-strand DNA breaks in Rev3 deficient cells. Mol Med Rep. 2014;9:1883–1888. doi: 10.3892/mmr.2014.1999. [DOI] [PubMed] [Google Scholar]
- 77.Jagetia GC, Rao Isoquinoline alkaloid berberine exerts its antineoplastic activity by inducing molecular DNA damage in hela cells:a comet assay study. Biol Med. 2015;7:223. [Google Scholar]
- 78.Chen S, Wan L, Couch L, Lin H, Li Y, Dobrovolsky VN, et al. Mechanism study of goldenseal-associated DNA damage. Toxicol Lett. 2013;221:64–72. doi: 10.1016/j.toxlet.2013.05.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pasqual MS, Lauer CP, Moyna P, Henriques JAP. Genotoxicity of the isoquinoline alkaloid berberine in prokaryotic and eukaryotic organisms. Mutat Res. 1993;286:243–252. doi: 10.1016/0027-5107(93)90189-m. [DOI] [PubMed] [Google Scholar]
- 80.Xu C, Wang L, Hui X, Ma X, Ma F, Zhao Q, et al. DNA damage and effects on antioxidative enzymes in mouse marrow induced by berberine 2013 International Conference on Human Health and Medical Engineering. China: WIT Transactions on Biomedicine and Health; 2014. pp. 479–486. [Google Scholar]
- 81.Xu CB, Wang L, Hui XJ, Ma XP, Bi X, Zhao Q Genetic toxicity of berberine on mouse heart 2013 3rd International Conference on Biotechnology. Chemical and Materials Engineering. China: Adv Mat Res; 2014. pp. 634–637. [Google Scholar]
- 82.Nozaka T, Watanabe F, Tadaki Si, Ishino M, Morimoto I, Kunitomo Ji, et al. Mutagenicity of isoquinoline alkaloids, especially of the aporphine type. Mutat Res. 1990;240:267–279. doi: 10.1016/0165-1218(90)90077-f. [DOI] [PubMed] [Google Scholar]
- 83.Fu PP, Xia Q, Zhao Y, Wang S, Yu H, Chiang H-M. Phototoxicity of herbal plants and herbal products. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2013;31:213–255. doi: 10.1080/10590501.2013.824206. [DOI] [PubMed] [Google Scholar]
- 84.Inbaraj JJ, Kukielczak B, Bilski P, Sandvik S, Chignell C. Photochemistry and photocytotoxicity of alkaloids from goldenseal (Hydrastis canadensis L.). 1. berberine. Chem Res Toxicol. 2001;14:1529–1534. doi: 10.1021/tx0155247. [DOI] [PubMed] [Google Scholar]
- 85.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism. 2008;57:712–717. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marin-Neto JA, Maciel BC, Secches AL, Gallo Junior L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11:253–260. doi: 10.1002/clc.4960110411. [DOI] [PubMed] [Google Scholar]
- 87.Fung FY, Linn YC. Developing traditional chinese medicine in the era of evidence-based medicine:current evidences and challenges. Evid Based Complement Alternat Med. 2015;425037:8. doi: 10.1155/2015/425037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bateman J, Chapman RD, Simpson D. Possible toxicity of herbal remedies. Scott Med J. 1998;43:7–15. doi: 10.1177/003693309804300104. [DOI] [PubMed] [Google Scholar]
- 89.Linn YC, Lu J, Lim LC, Sun H, Sun J, Zhou Y, et al. Berberine-induced haemolysis revisited:safety of thizoma coptidis and cortex phellodendri in chronic haematological diseases. Phytother Res. 2012;26:682–686. doi: 10.1002/ptr.3617. [DOI] [PubMed] [Google Scholar]
- 90.Lo SN, Chang YP, Tsai KC, Chang CY, Wu TS, Ueng YF. Inhibition of CYP1 by berberine, palmatine, and jatrorrhizine:selectivity, kinetic characterization, and molecular modeling. Toxicol Appl Pharmacol. 2013;272:671–680. doi: 10.1016/j.taap.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Vrzal R, Zdařilová A, Ulrichová J, Bláha L, Giesy JP, Dvořák Z. Activation of the aryl hydrocarbon receptor by berberine in HepG2 and H4IIE cells:biphasic effect on CYP1A1. Biochem Pharmacol. 2005;70:925–936. doi: 10.1016/j.bcp.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 92.Chu CC, Pan KL, Yao HT, Hsu JT. Development of a whole-cell screening system for evaluation of the human CYP1A2-mediated metabolism. Biotechnol Bioeng. 2011;108:2932–2940. doi: 10.1002/bit.23256. [DOI] [PubMed] [Google Scholar]
- 93.Han Y-L, Yu H-L, Li D, Meng X-L, Zhou Z-Y, Yu Q, et al. In vitro Inhibition of huanglian [rhizoma coptidis (L.)] and its six active alkaloids on six cytochrome P450 isoforms in human liver microsomes. Phytother Res. 2011;25:1660–1665. doi: 10.1002/ptr.3475. [DOI] [PubMed] [Google Scholar]
- 94.Zhao Y, Hellum BH, Liang A, Nilsen OG. The in vitro inhibition of human CYP1A2, CYP2D6 and CYP3A4 by tetrahydropalmatine, neferine and berberine. Phytother Res. 2012;26:277–283. doi: 10.1002/ptr.3554. [DOI] [PubMed] [Google Scholar]
- 95.Chatterjee P, Franklin MR. Human cytochrome P450 inhibition and metabolic-intermediate complex formation by goldenseal extract and its methylenedioxyphenyl components. Drug Metab Dispos. 2003;31:1391–1397. doi: 10.1124/dmd.31.11.1391. [DOI] [PubMed] [Google Scholar]
- 96.Guo Y, Chen Y, Tan ZR, Klaassen CD, Zhou HH. Repeated administration of berberine inhibits cytochromes P450 in humans. Eur J Clin Pharmacol. 2012;68:213–217. doi: 10.1007/s00228-011-1108-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu X, Li Q, Xin H, Yu A, Zhong M. Effects of berberine on the blood concentration of cyclosporin A in renal transplanted recipients:clinical and pharmacokinetic study. Eur J Clin Pharmacol. 2005;61:567–572. doi: 10.1007/s00228-005-0952-3. [DOI] [PubMed] [Google Scholar]


