Abstract
Berberis vulgaris L (B. vulgaris) and its main constituent berberine have been used in traditional medicine for a long time. This medicinal plant and berberine have many properties that have attracted the attention of researchers over the time. According to several studies, B. vulgaris and berberine exhibited anti-inflammatory, antioxidant, anticonvulsant, antidepressant, anti-Alzheimer, anti-cancer, anti-arrhythmic, antiviral, antibacterial and anti-diabetic effects in both in vitro and in vivo experiments. In regard to many reports on protective effects of B. vulgaris and berberine on natural and chemical toxins, in the current review article, the inhibitory effects of these compounds against natural, industrial, environmental and chemical toxicities with focus on cellular mechanism have been categorized. It has been mentioned that berberine could ameliorate toxicity of chemical toxins in brain, heart, kidney, liver and lung in part through antioxidant, anti-inflammatory, anti-apoptotic, modulation of mitogen-activated protein kinase (MAPK) and nuclear factor-κB (NF-κB) signaling pathways.
Keywords: Antidote, Anti-inflammation, Antioxidant, Barberry, Berberine, Berberis vulgaris, Chemical toxin, Natural toxin
Introduction
Berberis vulgaris L. (B. vulgaris) is a well-known medicinal plant which belongs to Berberidaceae family that is cultivated in Asia and Europe. The phytochemical investigations of various species of Berberis have led to the isolation of alkaloids, tannins, phenolic compounds, sterols and triterpenes (1). Berberine, the main compound of berberis, is an isoquinoline alkaloid and produced by many plants, such as Coptis japonica Makino, Coptis, Berberis petiolaris and B. vulgaris (2). The chemical structure of berberine has been shown in Figure 1.
Figure 1.

Chemical structure of berberine
It was shown that oral bioavailability of berberine is below 1% (3). Some factors such as first-pass effect in the intestine, interaction with P-glycoprotein (P-gp) pumps and high extraction and distribution in the liver are involved to its poor oral bioavailability (4). Permeation enhancers, P-gp inhibitors and lipid micro-particle delivery system can improve the bioavailability of berberine (3).
B. vulgaris and berberine have a long history in traditional remedy as anti-bacterial, anti-pyretic, anti-
pruritic, anti-arrhythmic, anti-inflammatory, laxative, anti-cholinergic, anti-leishmaniasis, anti-malaria and sedative agents (1, 4-6). Different pharmacological effects of B. vulgaris and berberine including anticonvul-sant (4), antidepressant (4, 7), anti-Alzheimer (7), anti-arrhythmic (4), anti-inflammatory (4), antiviral (8), antibacterial (8), antineoplastic (6) and anti-diabetic (9, 10) properties have been reported in both in vitro and in vivo studies. Additionally, B. vulgaris and berberine can be effective against natural (11) and chemical toxins (12). Interestingly, the protective effects of these compounds in different organs such as brain (13) liver (14), kidney (15), heart (16) and lung (17) have been reported in many studies.
Different studies revealed that berberine has very low toxicity and only limited adverse reactions such as gastrointestinal effects, transient elevation in serum bilirubin level, disruption of sex-hormone synthesis pathway, prothrombotic effects and suppression of both cellular and humoral immune functions has been seen in humans (3, 4).
Potent effects against natural and chemical toxicities have been reported following administration of medicinal plants and their main constituents (18, 19). Regarding to the lack of comprehensive review on protective properties of B. vulgaris and berberine, in this review, we investigated the antidotal properties of mentioned compounds against toxic agents with the special focus on suggested mechanisms in selected organs. For this purpose, studies in scientific databases including Scopus, MEDLINE, Web of Science databases and local references have been discussed, which introduced the antidotal properties of B. vulgaris and berberine under in vitro and in vivo studies.
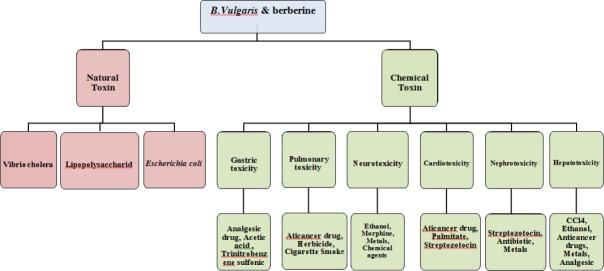
Natural toxins
Based on different documents, B. vulgaris and its main constituent berberine exhibited antidotal effects against some natural toxins including lipopolysaccharides (LPS) and cholera toxin. These effects in part might be due to their anti-inflammatory and antimicrobial properties (8, 20, 21).
Lipopolysaccharides (LPS)
Studies have been shown, berberine could suppress several LPS-(endotoxin derived from Gram negative bacteria) induced diseases including lung injury in mice and rats (11, 22, 23), endometritis in mice (24), intestinal injury in rats (25, 26), extracellular matrix accumulation and inflammation in rat mesangial cells (27), osteolysis in mice (28), dyslipidemia in mice (29), inflammation in mice (30) and LPS-stimulated RAW 264.7 macrophages (31). It also increased survival in endotoxemic mice (32).
Dyslipidemia
Berberine inhibited dyslipidemia in C57BL/6 mice with LPS-induced inflammation by regulating PCSK9-LDLR (Proprotein convertase subtilisin/kexin type-9- LDL receptor) pathway (29).
Endometritis
The inhibition of inflammatory cell infiltration, reduction of tumor necrosis factor alpha (TNF-α) and interleukin-1β (IL-1β) levels and also activating nuclear factor-κB (NF-κB) signaling pathway are involved in anti-inflammatory properties of berberine hydrochloride in the mouse endometritis model (24).
Intestinal injury
The low dose of berberine (30 mg/kg) recovered the intestinal oxidative damage through elevating the activities of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), reducing the levels of malondialdehyde (MDA) and nitrite oxide (NO), suppressing the expression of toll-like receptor 4 (TLR4) and NF-κB in ileum (25). In another study, pretreatment with berberine improved intestinal recovery and reduced the impairment of glutamine transport and glutaminase activity in rat sepsis (26). Also, berberine decreased lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms (33).
Osteolysis
Administration of berberine (10 mg/kg) blocked LPS-induced osteoclast recruitment and bone resorption in the mouse calvarial model. It also could inhibit biofilm formation by the attenuation of bacterial adhesion and proliferation (28).
Lung injury
Zhang et al (2007) showed that the possible protective mechanism of berberine (50 mg/kg, orally) against acute lung injury (ALI) induced by LPS in BALB/c mice, primarily could be mediated via the inhibition of cPLA2 phosphorylation and reduction of TNF-α production (11). Furthermore, pretreatment with berberine and tetrahydroberberrubine (THBru), a berberine derivative, inhibited LPS-induced tissue factor (TF) activity and down regulated NF-κB, protein kinase B (AKT) and mitogen-activated protein kinases (MAPK)/c-Jun N-terminal protein kinase (JNK)/P38/ extracellular signal-regulated kinases (ERK) pathways in THP-1 cells and mice, respectively (23, 34).
According to another report, berberine attenuated leukocyte adhesion to LPS-stimulated endothelial cells and vascular cell adhesion molecule-1 (VCAM-1) expression both in in vivo and in vitro models (22).
Vibrio cholera and enterotoxigenic Escherichia coli (ETEC)
According to reports, berberine reduced the cholera toxin-induced secretion of water, Na+, Cl+ and calculated residual ions in the dose-dependent manner (35). This compound also markedly inhibited the secretory response of E. coli heat-stable enterotoxin in the infant mouse model (36). Another study revealed berberine could be as effective as chloramphenicol or tetracycline in controlling experimental cholera (37).
Effects of berberine against natural toxins have been summarized in Table 1.
Table 1.
Antidotal effects of berberine against natural toxins
| Toxin | In vitro/in vivo | Constituents | Results | Ref. |
|---|---|---|---|---|
| Cholera toxin | Baby rabbit | Breberine sulphate (10, 20, 30 mg) | Effective as chloramphenicol or tetracycline in controlling experimental cholera | (37) |
| Cholera toxin | Infant mouse | Berberine sulphate | Inhibition of secretory response of E. coli heat-stable enterotoxin | (36) |
| Enterotoxigenic Escherichis coli | Adult men who Had watery diarrhea | Berberine sulphate (400 mg, single dose) | Reducing of the mean stool Volumes | (20) |
| Lipopolysaccharide | Male kumming Strain mice | Neutral sulfate berberine (50 mg/kg/day, 5 days) | Reduction of plasma TNF-α, IFN-γ and NO levels | (32) |
| Lipopolysaccharide | Female BALB/c mice | Berberine hydrochloride (2.5, 5 and 10 mg/kg) | Reduction of neutrophil infiltration, NO, TNF-α And IL-1β production.inhibiting of NF-κB signaling pathway activation | (24) |
| Lipopolysaccharide | Mouse calvarial model | Berberine (10 mg/kg single dose) | Blocking LPS-induced osteoclast recruitment and bone resorption | (28) |
| Lipopolysaccharide | Male Spraque Dawley rats | Berberine (30 mg/kg and 120 mg/kg) | Reduction of the intestinal damage by elevating the activities of SOD and GSH-Px and suppressing the activation of TLR4 and NF-κB in ileum | (25) |
| Lipopolysaccharide | Male Spraque-Dawley rats | Berberine (50 mg/kg) | Improving of intestinal recovery | (26) |
| Lipopolysaccharide | Male BALB/c mice | Berberine (20 mg/kg) | Inhibiting cytosolic phospholipase A2 and TNF-α production | (11) |
| Lipopolysaccharide | THP-1 cells | Berberine (0.01-1.0 μM) | Inhibitng of TF-activity and expression Down- regulating of NF-κB, AKT and MAPK/JNK/P38/ERK pathways | (34) |
| Lipopolysaccharide | Male Sprague-Daley rats | Berberine | Inhibiting of the nuclear translocation and DNA binding activity of LPS-induced NF-κB | (22) |
| Lipopolysaccharide | Male ICR mice | THBru (2,10 and 50 mg/kg) | Decreasing of the lung wet to weight (W/D) ratio | (23) |
| Lipopolysaccharide | Mouse primary splenocytes from female BALB/c mice | Berberine (0.8-3.3 μM) | Down-regulation of the Th1/Th2 cytokine gene expression | (21) |
| Lipopolysaccharide | Rat Mesenchymal Stem Cell (MSCs) | Berberine (10-90 μM) | Attenuation of extracelluar matrix accumulation and inflammation | (27) |
| Lipopolysaccharide | RAW 264.7 macrophages | 13-methylberberine (13-MB) and 13-ethylberberine (13-EB) (0.1–10 μM) | Inhibition of iNOS protein expression | (31) |
| Lipopolysaccharide | Pathogen-free BALB/C mice | Berberine (1-10 μM) | Up-regulation of the heme oxygen-ase(HO)-1 level | (30) |
Chemical-induced toxicity
Protective effect against chemical-induced gastric toxicity
B. vulgaris and its active compound possess protective effects against gastric toxicity induced by some chemical agents including aspirin (a non-steroidal anti-inflammatory drug) (38), acetic acid (39) and trinitrobenzene sulfonic acid (a nitroaryl oxidizing acid) (40) through antioxidant and anti-inflammatory properties (38), inhibition of lipoxygenase and IL-8 production (40).
Aspirin
Aspirin damages the gastric mucosa by suppressing the synthesis of prostaglandins (PGs). This drug non-selectively blocks both cyclooxygenase (COX) 1 and 2 that are present in gastric mucosal membranes. Aspirin causes the dose-dependent reduction of PGs especially PGE2 and PGI2, which are responsible for gastric abrasion and gastric mucosal damage (41).
A study indicated that B. vulgaris (300, 600 and 900 mg/kg, orally) significantly alleviated the tissue proliferation, infiltration of cells and sloughing induced by aspirin in male adult albino mice. Additionally, the effect of B. vulgaris (900 mg/kg) was really similar to effect of omeprazole (20 mg/kg), an antiulcer drug. Consequently, it is concluded that B. vulgaris is effective in reduction the gastric toxicity mainly via its antioxidant activity (38).
Acetic acid
Minaiya et al (2010) compared the effects of B. vulgaris fruit extract (BFE) and berberine chloride (BEC) on acetic acid-induced colitis in male Wistar rats. Results indicated that BFE in doses of 750, 1500 mg/kg as well as BEC in dose of 10 mg/kg were effective in protection against colonic damage and these effects might be due to its anthocyanin constituents (39).
Trinitrobenzene sulfonic acid (TNBS)
In 2000, Zhou et al showed that berberine has a beneficial effect on the mucosal healing process, possibly by inhibition of IL-8 production, in trinitrobenzene sulfonic acid (TNBS)-induced colitis in rats both using in vivo and in vitro models (40). Also, the synergistic effects of the three-alkaloid combination regimen containing berberine, skimmia-nine and hypaconitine against TNBS-induced colitis in rats have been reported (42).
Protective effects against chemical-induced pulmonary toxicity
Bleomycin
Pulmonary fibrosis is a progressive and lethal lung disorder with high mortality rate (43). It occurs due to the adverse toxic effects of anti-neoplastic drugs such as bleomycin. Apart from this, cigarette smoking and breathing in mineral dusts/asbestos are additional factors responsible for its pathogenesis (44). Bleomycin induced oxidative stress and caused a notable reduction in antioxidant status. Also, the expression of TNF-α and transforming growth factor beta 1 (TGF-β1) were significantly increased in bleomycin-induced toxicity.
Chitra et al (2013) demonstrated berberine (200 mg/kg, 14 days, IP) decreased bleomycin-induced pulmonary toxicity and fibrosis in male Wistar albino rats. It also increased the antioxidant status by up-regulating the redox sensing transcription factor nuclear factor E2-related factor2 (Nrf2) and curb down bleomycin- induced oxidative stress, histological alteration and collagen deposition. In contrast with bleomycin irritation, berberine significantly inhibited NF-κB dependent pro-inflammatory and pro-fibrotic mediators production such as inducible nitric oxide synthase (iNOS), TNF-α and TGF-β1 (17).
Another study revealed a single intratracheal instillation of bleomycin (2.5 U/Kg) caused activation of focal adhesion kinase (FAK), phosphoinositide 3-kinase (PI3K) /AKT cascade, the mechanistic target of rapamycin (mTOR) and smad 2/3. It also increased Smad 7 in rat lungs. Administration of berberine (200 mg/Kg/IP/day) markedly attenuated the pulmonary toxic effects of bleomycin (45).
Paraquat (PQ)
Paraquat (PQ) is a potent herbicide. It is highly toxic when swallowed orally by people and there are no specific medical treatment available (46). A study by Javad Mousavi et al (2016) exhibited B. vulgaris fruit extract (100, 200, 400 mg/kg/day, 4 weeks, orally) has advantageous effects in rat pulmonary fibrosis induced by PQ (intratracheal instillation 20 mg/kg) in a dose-dependent manner, probably through antioxidant and anti-iflammatory properties (47).
Cigarette smoke (CS)
It is proved that pretreatment with berberine (50 mg/kg, orally) profoundly diminished cigarette smoke (CS)-induced lung inflammation in C57BL/6 mice. The reduction of secretion of macrophage inflammatory protein 2, TNF-α, IL-6, and monocyte chemotactics protein-1 in bronchoalveor lavage fluid (BALF) has been mentioned as involved mechanisms (48).
Protective effects against chemical-induced neurotoxicity
Ethanol
Chronic ethanol consumption is the most common cause of neurotoxicity (49). Ethanol increases oxidative stress through the production of oxygen free radicals, lipid peroxidation (LPO) and reduction of endogenous antioxidants such as glutathione (GSH) and vitamin E (50, 51). Also, stopping of long-term ethanol intake results in a withdrawal syndrome, consisting tremor and hyperexcitability (52).
Patil et al (2015) evaluated berberine effect against ethanol-induced cognitive dysfunction in Wistar rats using Morris water maze paradigm and showed that chronic treatment with berberine (25-100 mg/kg, orally once a day for 45 days) improved learning and memory through inhibition of oxidative stress and cholinesterase activity (53).
Based on another report, acute administration of berberine (2.5, 5, and 10 mg/kg, IP) dose dependently attenuated locomotor stimulant and rewarding effect of ethanol, via modulation of various neurotransmitters (54). Moreover, berberine (10 and 20 mg/kg, IP) from day 1 to day 10, markedly reduced the ethanol withdrawal-induced hyperexitability signs in adult male C57BL/6J mice (52).
Scopolamine (SCP)
Scopolamine (SCP), a blocker of muscarinic Ach receptors, can impair learning and memory in humans and animals (55, 56). Treatment with berberine (0.1 and 0.5 g/kg/day, orally) for 7 or 14 days, increased the release of epinephrine via blocking α2-adrenoreceptor and improved the SCP-induced amnesia in rats (57). A study by Lee et al (2012) showed that daily administration of berberine (20 mg/kg, IP) 30 min before SCP injection (2 mg/kg, IP) prevented cholinergic dysfunction and dementia through reduction of the expression of proinflammatory cytokines including, IL-1β, TNF-α and COX-2 mRNA in the hippocampus of rats (58). Different mechanisms which are involved in protective effect of berberine against Alzheimer’s disease have been shown in Figure 2.
Figure 2.
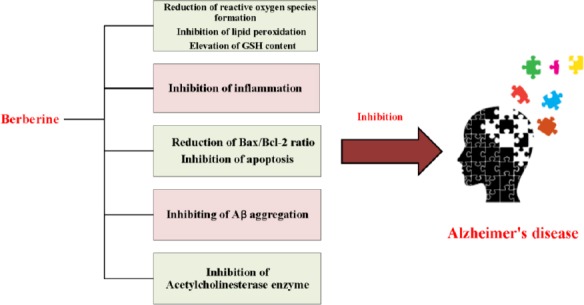
Different mechanisms which are involved in protective effect of berberine against Alzheimer’s disease
Mercury (Hg)
Mercury (Hg) is a heavy metal with a well- known toxicity which reported in both human and mammalian models (12, 59). The mechanism of Hg-induced neurotoxicity is still unclear. It can increase lipid peroxidation, nitrite/nitrate (NO) and reactive oxygen species (ROS) generation and decrease GSH content. In 2015, Moneim et al reported berberine (100 mg/kg, orally for 7 days) had beneficial effects on Hg-induced oxidative stress, apoptosis and inflammation in rat brain. The reduction of LPO, elevation of GSH content and regeneration of the activities of antioxidant enzymes were involved in inhibition of oxidative stress. The anti-inflammatory effects of berberine were mediated through attenuation of NO production and decreasing TNF-α level. Additionally, berberine diminished apoptotic neuronal cell death by reduction the Bax/Bcl-2 ratio and the level of cleaved caspase-3 (59). Moreover, berberine could inhibit apoptosis by activation of the PI3K/AKT signaling pathway which subsequently improved the survival rate of neuron (12).
Ibotenic acid
Treatment with berberine (5 mg/kg, IP for 7 days) increased hippocampal cells about 2.7 fold in the pyramidal layer of CA1 region and about 2 fold in the dentate gyrus in the memory deficient model in rat which induced by stereotaxic injection of ibotenic acid into entorhinal cortex (Ibo model). Blocking the retrograde cell death and elevating the cell survival of endogenous neural stem cells were mentioned mechanisms for berberine neuroprotection. In addition, it could promote neuronal differentiation of hippocampal precursor cells in the Ibo model (60).
1-methyl-4-phenyl-1, 2, 3, 6- tetrahedropyridine/ probenecid (MPTP/P)
Kim et al (2013) showed the neuroprotective effect of berberine in Parkinson model in rats which induced by MPTP/P. The administration of MPTP/P enhanced the number of cleaved caspase-3-positive cells and TUNEL-positive cells in the hippocampal dentate gyrus in mice while treatment with berberine (50 mg/kg/day, orally, for 5 weeks) markedly attenuated the numbers of apoptotic cells. Moreover, berberine recovered coordination and motor balance via inhibition of the dopaminergic neuronal damage (61).
Morphine
Morphine, an opioid pain medication, is common-ly used to treat moderate to severe pain, but the use of morphine is limited by its adverse effects, which include drug craving, tolerance to opiate analgesia, withdrawal syndrome and addiction (62). Dopaminergic (DA) system and the N-methyl-D-aspartate (NMDA) receptor are involved in morphine addiction and analgesic tolerance. It was reported that berberine (1 and 2 mg/kg, orally for 6 days) inhibited both morphine-induced locomotor sensitization and analgesic tolerance through modulation of D1 and NMDA receptors in ICR mice (63). In another study, pretreatment with berberine (10, 20 and 50 mg/kg, IP for 10 days) significantly attenuated depression and anxiety-like behaviors induced by development of morphine dependence, likely via reduction of hypothalamic corticotrophin-releasing factors (CRF) expression and tyrosine hydroxylase expression in the locus coeruleus (LC) of rats (64). Also, B. vulgaris extract (100 and 200 mg/kg, IP) ameliorated the acquisition and reinstatement of morphine-induced conditioned place preference in mice (65).
Aluminum and Aluminium-Maltol
Berberine chloride (50 mg/kg, orally, for 14 days) protected CNS cells in Alzheimer’s disease (AD) induced by aluminium-maltol in rabbit. Different mechanisms including the inhibition of β-secretase and acetylcholine esterase activity, activation of microgolia, clearing senile plaque were involved in ameliorating spatial memory impairment in animals (66).
The repeated intragastrically exposure of aluminum induced neurodegenerative disease via increasing the monoamine oxidase B (MAO-B) mRNA and protein expression and activity. Acetylcholinesterase (AchE) and SOD activities, the enhancement of MAO-B expression and activity, the elevation of MDA content were significantly reduced following exposure to berberine (100 mg/kg, orally, 4 hr after the administration of aluminum) (67).
Nicotine
It was shown that repeated nicotine exposure elevated locomotor activity and the expression of immediate-early gene, c-fos, in the central dopaminer-gic areas. Administration of berberine (100 mg/kg, IP, twice daily for 7 days) attenuated nicotine-induced behavioural activity and it also modulated the central dopaminergic system in rats (68).
Amyloid β
Deposition of β-amyloid (a protein fragment snipped from an amyloid precursor protein) and tangle in the brain play an important role in AD (69, 70). Zhu and Qian (2006) exhibited berberine chloride (50 m/kg/day, intragastric, for 14 days) improved the spatial memory impairment and augmented the expression of iNOS and IL-1β in the rat model of AD induced by Aβ (1-40) (71). Furthermore, berberine had the therapeutic effects against Aβ-induced neurotoxicity by balancing effect on the Ca+2 entry and inhibition of Aβ aggregation (70, 72).
Harmaline
Harmaline (a tremorgenic alkaloid), induced a nonspecific tremor through enhancing glutamate discharge in climbing fibers, consequently Purkinje cell demolition and changes in the olivocerebellar pathway (73-76). Medicinal plants and their main constituents have been shown protective effects in harmaline-induced tremor in animal models (77, 78). Administration of berberine (20 mg/kg, IP, 15 min before harmaline injection) ameliorated harmaline-induced tremor and recovered gait disturbance and mobility duration in rats by blocking NMDA receptors or regulating neurotransmitter release in different areas of the brain involved in motor and balance function (77).
MK-801
Mk-801 is a noncompetitive antagonist of NMDA receptors. Lee et al (2010) indicated that treatment with berberine (20 mg/kg, IP, for 5 days) could attenuate the cell death induced by MK-801 and promote activity dependent cell survival in the brain of rats. Berberine probably was effective through blocking potassium current or lowering the threshold of the action potential (13).
Kainic acid (KA)
A study showed that injection of kianic acid (KA) (35 mg/kg, IP.) induced tonic-clonic seizures in albino mice. Pretreatment with berberine (10 and 20 mg/kg, IP, 30 min before injection of KA) reduced the percent of mortality (79) possibly by decreasing NMDA receptor binding (63).
Pilocarpine
It was proved that berberine (25, 50 and 100 mg/kg, orally for 7 days) could protect against pilocarpine (a potent muscarinic cholinergic agonist) -induced convulsions in rats by moderating the oxidative stress burden (80, 81). Moreover, berberine reduced memory impairment and the number of fluoro-jade B-positive cells in the hippo-campal CA1 region (80).
Streptozotocin (STZ)
Streptozotocin (STZ), a naturally occurring chemical, derives from Streptomyces achromogenes. STZ-induced diabetic rat by pancreatic β cells damage (82). In 2011, Bhutada et al revealed the effect of berberine on memory dysfunction in rat model of STZ-induced diabetes. Chronic treatment with berberine (25-100 mg/kg, orally, twice daily, 30 days) improved cognitive deficits, oxidative stress and choline esterase activity. Probably, the modulation of glucagon-like peptide-1 (GLP-1) played important role in these effects (83). According to another study treatment with berberine (100 mg/kg, for 24 weeks) protected against STZ-induced diabetic neuropathy, which are presumed to be associated with decreasing the mRNA and protein expression of neuritin, p38, and JNK (84). In addition, chronic administration of berberine (100 mg/kg/day, orally) could improve learning and memory by restoring synaptic dysfunction and anti-apoptotic properties in STZ-diabetic rats (85). In another study, cognitive dysfunction was induced in rats by intracerebroventricular injection of (3 mg/kg) STZ. Then rats were treated with berberine (25 and 50 mg/kg, orally for 21 days). Berberine decreased TNF-α and MDA levels and restored catalase, GSH and SOD activity in both hippocampus and frontal cortex (86). Berberine (10 and 20 mg/kg) produced antiallodynic effects against STZ-induced diabetic neuropathy in rats by its antioxidant effects (87).
Pentylenetetrazol (PTZ)
Pentylenetetrazol (PTZ) is a chemoconvulsant agent that used for assessment of antiepileptic drugs (AEDs) (88). Injection of high dose of berberine (400 mg/kg, 30 min prior to PTZ) enhanced minimal clonic seizures (MCS) and generalized tonic-clonic seizures (GTCS) latencies and protected against PTZ-induced epileptic seizures in rats (89). In another study, administration of B. integerrima extract exhibited significant anticonvul-sant properties in PTZ-induced seizure model (90).
Reserpine
Berberine reversed the immobility period induced by reserpine. Kulkarni et al (2008) reported adminis-tration of berberine chloride (5 and 10 mg/kg, IP, 60 min before the forced swim test) significantly attenuated the immobility period in mice which received reserpine. The anti-depressant mechanism of berberine was possibly due to regulating the biogenic amines, sigma receptor pathway and L-arginine-nitric oxide-cyclic guanosine monophosphate pathway (91).
Protective effects against chemical-induced cardio-toxicity
Doxorubicin (DOX)
Doxorubicin (DOX) is a chemotherapeutic drug that is used for the treatment of various types of human neoplastic disease, such as solid tumor, leukemia, lymphomas and breast cancer (92). However, the clinical usefulness of DOX has been greatly hampered due to its cardiotoxicity. Recently, Hao et al demonstrated administration of berberine for 2 weeks could improve the DOX-induced cardiac dysfunction in rats by decreasing the activity of myocardial enzymes, such as creatine kinase (CK), aspartate aminotransferase (AST), CK isoenzyme (CK-MB), lactate dehydrogenase (LDH). Additionally, the metabolism of DOX in the cytoplasm of heart and accumulation of doxorubicinol (the major metabolite of DOX) were inhibited by berberine (93). In another report, berberine (60 mg/kg, IP, 1 hr before injection of DOX, for 14 days) significantly attenuated prolongation of QRS in mice (94). Also, berberine reduced myocardial apoptosis, AMP-activated protein kinase (AMPK) phosphoryla-tiona, p53 phosphorylation, caspase-3 activation and improved Bcl-2 expression in an acute DOX-treated rat model (16).
Palmitate
Palmitate, a saturated fatty acid, induces apopto-sis in cardiomyocyte (95). In 2015, Chang et al revealed berberine decreased hypertrophy, beta-myosin heavy chain (β-MHC) expression, glycogen synthase kinase 3 beta (GSK3β) activation and increased alpha-myosin heavy chain (α-MHC) expression, AMPK and AKT activation in palmitate-incubated H9c2 cells (rat cardiac myoblasts) (96).
Streptozotocin (STZ)
Administration of berberine (100 mg/kg, orally for 16 weeks) improved cardiac function and ameliorated cardiac hypertrophy and fibrosis in high fat diet and streptozotocin induced-type 2 diabetic rats. Also, treatment of diabetic animals with berberine increased AMPK and AKT activation and reduced glycogen synthase kinase 3 beta (GSK3β) activation in comparison to control (96).
Protective effects against chemical-induced nephrotoxicity
Streptozotocin (STZ)
Based on evidences, berberine ameliorated the renal damage and decreased several parameters such as: urine total protein to urine creatinine (UTP/C), serum creatinine (SCr), blood urea nitrogen (BUN), fasting blood glucose (FBG) in STZ-diabetic nephropathy (DN) animal model (97, 98).
PGE-2 and prostaglandin E2 receptor 1 (EP-1) probably are involved in diabetic progression (99). Ni et al (2016) reported that treatment with berberine (100 mg/kg/day, orally, 8 weeks) improved renal functional via modulation of PGE2-EP1-Gαq- Ca2+ in glomerular mesangial cells of diabetic rats (100).
Several studies revealed that the renoprotective effects of berberine were associated with improving EP-4, Gαs, cyclic adenosine monophosphate (cAMP) (98), β-arrestins (101) and serum SOD activity (97). Also, reducing intercellular adhesion molecule 1 (ICAM-1), VCAM-1 levels (101) and aldose reductase (AR) activity (97), inhibition of renal advanced glycation endproducts (AGEs) accumulation (102) were mentioned as other mechanisms. In addition, berberine could normalize the proteins expression of renal nephrine, podocin (102) and G protein-coupled receptor kinases (GRKs) in STZ-induced diabetic nephropathy rats (103).
Oxidative stress and inflammation play critical roles in renal fibrosis and diabetic nephropathy (104, 105). Therefore, in 2015, Sun et al induced diabetic nephropathy in rats by high-fat diet-fed and low-dose of STZ injection (25 mg/kg, IP) and demonstrated treatment with berberine (25 mg/kg, orally, for 20 weeks) attenuated renal inflammation and histological injuries. The underlying molecular mechanism might be related to blocking the up-regulation of pro-inflammatory cytokines (TNF-α, IL-1β), monocyte chemotactic molecule-1 (MCP-1), collagen I, collagen IV, and fibronectin in the renal tissue of rats (106). Moreover, berberine activated Nrf2 pathway (107) and inhibited NF-κB and TGF-β/Smad3/Epithelial-to-mesenchymal transition (EMT) signaling activity (106, 107).
Gentamicin (GM)
Gentamicin (GM) is an antibiotic which is widely used in the treatment of gram-negative infection. However, nephropathy is a major side effect of GM. One study indicated that administration of berberine (20 and 40 mg/kg, orally) reduced GM-induced nephrotoxicity via antioxidant, anti-inflammatory and anti-apoptosis activities. Furthermore, berberine decreased the mRNA expression of kidney injury molecule-1(KIM-1), neutrophil gelatinase-associated lipocalin (NGAL), and NF-κB. Also, berberine inhibited the apoptotic effect of GM by increasing the expression of Bcl-2 mRNA in kidney rat (108).
Mercury (Hg)
Othman et al (2014) showed administration of HgCl2 (0.4 mg/g, for 7 days) induced hepatorenal toxicity in rats. Treatment with berberine (100 mg/kg, for 7 days, orally) showed significant therapeutic effects against Hg-induced kidney injury probably through inhibition of LPO and NO production. It also restored activities of antioxidants enzymes including SOD, catalase, glutathione peroxidase and glutathione content (109).
Cisplatin
Cisplatin is a chemotherapy agent used to treat various types of cancer. Nephrotoxicity is the important side effect of cisplatin. Oxidative/nitrosative pathway was blocked by the improving 4-hydroxynonenal (4-HNE), 3-nitrotyrosine (3-NT), and cytochrome p450 E1 (CYP2E1) and heme oxygenase (HO-1) in kidneys following treatment with berberine (1, 2, and 3 mg/kg, Orally). In addition, berberine inhibited the expression of iNOS, NF-κB, TNF-α and COX-2. It also ameliorated apoptotic cell death that related to P53 (15).
Cyclophosphamide (CTX)
Cyclophosphamde (CTX) is an antitumor drug which can cause haemorrhagic cystitis as a side effect (110). It is reported that berberine could protect against urotoxicity induced by CTX. Pretreatment with a single dose of berberine (200 mg/kg, IP) or two doses of berberine (100 and 200 mg/kg, IP) was able to reduce CTX-induced bladder edema and haemorrhagic cystitis (111).
Alloxan
Studies showed that berberine (300 mg/kg, orally for 12 weeks) has protective effects against alloxan-induced renal injury in C57BL/6 mice (112, 113). It down regulated the protein levels of intercellular adhesion molecule-1, TGF-β1 and fibronectin (112). Berberine also suppressed sphingosine kinase (SphK)/Sphingosine 1-phosphate (S1P) (113) and NF-κB signaling pathways (112).
Protective effects against chemical-induced hepatotoxicity
The protective effects of berberine have been shown against carbon tetrachloride (CCl4) (14, 114-118), ethanol (115, 119), lead acetate (120), CTX (121), tert-butyl hydroperoxide (t-BHP) (122), DOX (123) and acetaminophen (124) induced hepatotoxicity in previous studies by several mechanism including suppression of oxidative/nitrosative stress (114-116, 118-122), LPO (115, 118, 120), inflammatory response (116) and hepatic CYPs (124), modulation of the pro-inflammatory cytokines (121), activation of AMPK, decreasing NADPH oxidase 4 (Nox4) and AKT expression levels (14).
Carbon tetrachloride (CCl4)
CCl4-induced hepatotoxicity is characterized by elevation of LPO, increase the levels of serum transaminase and biotransformation of free radical derivatives (125).
Studies showed the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) (115, 117), alkaline phosphatase (ALP) (118), hepatic content of MDA and hepatic hydroxyproline (Hyp) (115), the expression of smooth muscle actin (α-SMA), the marker of activated hepatic stellate cell (14, 115), TNF-α (114, 116), TGF-β1 (114, 115), COX-2 and iNOS (116) were attenuated by berberine treatment. In addition, administration of berberine enhanced the GSH (118) levels and activity of hepatic SOD (115, 117).
In 2013, Domitrovic et al revealed berberine (9 mg/kg/day, IP, for 2 weeks) ameliorated liver fibrosis in CCl4-intoxicated mice through suppression of fibrogenic potential and activation of matrix metalloproteinase (MMP)-2 (114). Another study provided direct evidence that berberine (50 mg/kg/day, for 6 weeks, orally) could prevent CCl4-induced hepatotoxicity in mice which probably mediated through the activation of AMPK and blocking of the NADPH oxidase/AKT signaling pathway (14).
Doxorubicin (DOX)
Zhao et al (2012) demonstrated that berberine (60 mg/kg, IP) reduced DOX-induced hepatocellular degeneration and necrosis in mice. Also, berberine attenuated the serum levels of AST and ALT. However, the mechanism of this action was not clear yet (123).
Cyclophosphamide (CTX)
CTX generated ROS through its toxic metabolites, phosphoramide mustard and acrolein (126, 127).
Treatment with berberine (50 mg/kg/day, orally, for 11 days) following administration of a single dose of CTX (200 mg/kg) showed a curative effect on liver function in mice. Furthermore berberine significantly reduced the expression of hepatic TNF-α and COX-2 (121).
Ethanol
Alcohol exposure induces liver damage. In 2014, Zhang et al showed berberine (200 and 300 mg/kg, orally for 10 days) protected liver from ethanol-induced toxicity in mice through anti-oxidant activity. It also could decreased glutathione exhaust and inhibited LPO (119).
Tert-butyl hydroperoxide (T-BHP)
T-BHP, an organic hydroperoxide, can induce hepatotoxicity by initiation of LPO (128, 129). A study indicated pretreatment with berberine (0.5 and 5 mg/kg IP, for 5 days) had hepatoprotective effects on t-BHP-induced oxidative damage in rat liver (122).
Acetaminophen
Acetaminophen is an analgesic and antipyretic drug (130). The ingestion of overdoses of acetaminophen can produce hepatic necrosis. This analgesic agent is bioactivated by CYP metabolism to form a reactive metabolite which depletes glutathione content (130, 131). Janbaz and Gilani (2000) reported that pretreatment (4 mg/kg; orally twice daily for 2 days) and post treatment (4 mg/kg every 6 h for three doses) of rats with berberine attenuated acetaminophen-induced hepatotoxicity via suppressing of hepatic CYPs (124).
Lead acetate
Lead is a toxic metal which may induce hepatotoxicity (132). Administration of berberine (50 mg/kg/day, orally, for 8 weeks) reduced the serum ALT, AST and ALP levels. In addition, berberine protected against lead-induced liver damage through inhibition of LPO in rats (120). In Figure 3 protective effects of berberine and B. vulgaris against natural and chemical toxin have been shown.
Figure 3.
The schematic of protective effects of berberine and B. vulgaris against natural and chemical toxin
Conclusion
In the current review article, the antidotal effects of B. vulgaris and its main constituent berberine against natural and chemical toxins under in vitro and in vivo experiments were discussed. Based on evidences, B. vulgaris and berberine significantly inhibited LPS and cholera toxin-induced toxicity mainly through anti-inflammatory and antimicrobial properties. Additionally, the protective effects of B. vulgaris and berberine against industrial or environmental toxins including heavy metals (Hg, Al, Pb), pesticides (paraquat), cigarette smoke and CCl4 have been investigated. Interestingly, berberine markedly prevented toxicity of antitumors drugs such as (cisplatin, cyclophosphamide, doxorubicin and bleomycin (, antibiotics (gentamycin), analgesics (acetaminophen, aspirin) in different tissues. The inhibition of oxidative/nitrosative stress, reduction of inflammatory cytokines, modulation of MAPK, NF-κB signaling pathway and inhibition of apoptotic cell death have been mentioned as different mechanisms which are responsible for protective properties of berberine in brain, heart, lung, liver and kidney. Finally, regarding to valuable effects of berberine in experimental studies it is suggested to verify these effects in human under clinical trials.
References
- 1.Mokhber-Dezfuli N, Saeidnia S, Gohari AR, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of berberis species. Pharmacogn Rev. 2014;8:8–15. doi: 10.4103/0973-7847.125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh IP, Mahajan S. Berberine and its derivatives:a patent review (2009–2012) Expert Opin Ther Pat. 2013;23:215–231. doi: 10.1517/13543776.2013.746314. [DOI] [PubMed] [Google Scholar]
- 3.Liu C-S, Zheng Y-R, Zhang Y-F, Long X-Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia. 2016;109:274–282. doi: 10.1016/j.fitote.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Imenshahidi M, Hosseinzadeh H. Berberis vulgaris and berberine:an update review. Phytotherapy Research. 2016;3:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 5.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 6.Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y. Berberine and Coptidis rhizoma as novel antineoplastic agents:A review of traditional use and biomedical investigations. Journal of Ethnopharmacol. 2009;126:5–17. doi: 10.1016/j.jep.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Kulkarni SK, Dhir A. Berberine:a plant alkaloid with therapeutic potential for central nervous system disorders. Phytotherapy Research. 2010;24:317–324. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]
- 8.Siow YL, Sarna L, O K. Redox regulation in health and disease —Therapeutic potential of berberine. Food Research Inte. 2011;44:2409–2417. [Google Scholar]
- 9.Yin J, Zhang H, Ye J. Traditional Chinese medicine in treatment of metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tabeshpour J, Imenshahidi M, Hosseinzadeh H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci. 2017;20 doi: 10.22038/IJBMS.2017.8682. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H-q, Wang H-d, Lu D-x, Qi R-b, Wang Y-p, Yan Y-x, et al. Berberine inhibits cytosolic phospholipase A2 and protects against LPS-induced lung injury and lethality independent of the α2-adrenergic receptor in mice. Shock. 2008;29:617–622. doi: 10.1097/SHK.0b013e318157ea14. [DOI] [PubMed] [Google Scholar]
- 12.Moneim AEA. Mercury-induced neurotoxicity and neuroprotective effects of berberine. Neural Regen Res. 2015;10:881–882. doi: 10.4103/1673-5374.158336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee T, Heo H, Kim Kwon Y. Effect of berberine on cell survival in the developing rat brain damaged by MK-801. Exp Neurobiol. 2010;19:140–145. doi: 10.5607/en.2010.19.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Pan Y, Kan M, Xiao X, Wang Y, Guan F, et al. Hepatoprotective effects of berberine on liver fibrosis via activation of AMP-activated protein kinase. Life Sci. 2014;98:24–30. doi: 10.1016/j.lfs.2013.12.211. [DOI] [PubMed] [Google Scholar]
- 15.Domitrović R, Cvijanović O, Pernjak-Pugel E, Škoda M, Mikelić L, Crnčević-Orlić Ž. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Lv X, Yu X, Wang Y, Wang F, Li H, Wang Y, et al. Berberine inhibits doxorubicin-triggered cardiomyocyte apoptosis via attenuating mitochondrial dysfunction and increasing Bcl-2 expression. PLoS One. 2012;7:e47351. doi: 10.1371/journal.pone.0047351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-κB dependant TGF-βactivation:A biphasic experimental study. Toxicol Lett. 2013;219:178–193. doi: 10.1016/j.toxlet.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Tavakkoli A, Ahmadi A, Razavi BM, Hosseinzadeh H. Black seed (Nigella sativa) and its constituent thymoquinone as an antidote or a protective agent against natural or chemical toxicities. Iran J Pharm Res. 2017;16:2–23. [PMC free article] [PubMed] [Google Scholar]
- 19.Razavi BM, Hosseinzadeh H. Saffron as an antidote or a protective agent against natural or chemical toxicities. Daru. 2015;23:31. doi: 10.1186/s40199-015-0112-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabbani G, Butler T, Knight J, Sanyal S, Alam K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholerae. J Infect Dis. 1987;155:979–984. doi: 10.1093/infdis/155.5.979. [DOI] [PubMed] [Google Scholar]
- 21.Lin W-C, Lin J-Y. Berberine down-regulates the Th1/Th2 cytokine gene expression ratio in mouse primary splenocytes in the absence or presence of lipopolysaccharide in a preventive manner. Int Immunopharmacol. 2011;11:1984–1990. doi: 10.1016/j.intimp.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Wu Y, Chuang S, Hong W, Lai Y, Chang G, Pang JS. Berberine reduces leukocyte adhesion to LPS-stimulated endothelial cells and VCAM-1 expression both in vivo and in vitro. Int J Immunopathol Pharmacol. 2012;25:741–750. doi: 10.1177/039463201202500320. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Yu S, Chen L, Liu H, Zhang J, Ge H, et al. Tetrahydroberberrubine attenuates lipopolysaccharide-induced acute lung injury by down-regulating MAPK, AKT, and NF-κB signaling pathways. Biomed Pharmacother. 2016;82:489–497. doi: 10.1016/j.biopha.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Fu K, Lv X, Li W, Wang Y, Li H, Tian W, et al. Berberine hydrochloride attenuates lipopolysaccharide-induced endometritis in mice by suppressing activation of NF-κB signal pathway. Int Immunopharmacol. 2015;24:128–132. doi: 10.1016/j.intimp.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Q, Piao X-L, Piao X-S, Lu T, Wang D, Kim SW. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol. 2011;49:61–69. doi: 10.1016/j.fct.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 26.Niu L, Qiao W, Hu Z, Li N, Huang Q, Gong J, et al. Berberine attenuates lipopolysaccharide-induced impairments of intestinal glutamine transport and glutaminase activity in rat. Fitoterapia. 2011;82:323–330. doi: 10.1016/j.fitote.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, et al. Berberine attenuates lipopolysaccharide-induced extracelluar matrix accumulation and inflammation in rat mesangial cells:involvement of NF-κB signaling pathway. Mol Cell Endocrinol. 2011;331:34–40. doi: 10.1016/j.mce.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Zhang C, Wang X, An B, Zhang P, Zhu Za. Berberine inhibits lipopolysaccharide-and polyethylene particle-induced mouse calvarial osteolysis in vivo. J Surg Res. 2012;173:e47–e52. doi: 10.1016/j.jss.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Xiao H-B, Sun Z-L, Zhang H-B, Zhang D-S. Berberine inhibits dyslipidemia in C57BL/6 mice with lipopolysaccharide induced inflammation. Pharmacol Rep. 2012;64:889–895. doi: 10.1016/s1734-1140(12)70883-6. [DOI] [PubMed] [Google Scholar]
- 30.Lee D, Bae J, Kim YK, Gil M, Lee J-Y, Park C-S, et al. Inhibitory effects of berberine on lipopolysaccharide-induced inducible nitric oxide synthase and the high-mobility group box 1 release in macrophages. Biochem Biophys Res Commun. 2013;431:506–511. doi: 10.1016/j.bbrc.2012.12.143. [DOI] [PubMed] [Google Scholar]
- 31.Lee D-U, Kang YJ, Park MK, Lee YS, Seo HG, Kim TS, et al. Effects of 13-alkyl-substituted berberine alkaloids on the expression of COX-II, TNF-α, iNOS, and IL-12 production in LPS-stimulated macrophages. Life Sci. 2003;73:1401–1412. doi: 10.1016/s0024-3205(03)00435-1. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Wang H-d, Lu D-x, Wang Y-p, Qi R-b, Fu Y-m, et al. Neutral sulfate berberine modulates cytokine secretion and increases survival in endotoxemic mice. Acta Pharmacologica sinica. 2006;27:1199–1205. doi: 10.1111/j.1745-7254.2006.00368.x. [DOI] [PubMed] [Google Scholar]
- 33.Li H-m, Wang Y-y, Wang H-d, Cao W-j, Yu X-h, Lu D-x, et al. Berberine protects against lipopolysaccharide-induced intestinal injury in mice via alpha 2 adrenoceptor-independent mechanisms. Acta Pharmacol Sin. 2011;32:1364–1372. doi: 10.1038/aps.2011.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao M-y, Chen L, Yang L, Yu X, Kou J-p, Yu B-y. Berberine inhibits LPS-induced TF procoagulant activity and expression through NF-κB/p65, Akt and MAPK pathway in THP-1 cells. Pharmacol Rep. 2014;66:480–484. doi: 10.1016/j.pharep.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Swabb EA, Tai Y-H, Jordan L. Reversal of cholera toxin-induced secretion in rat ileum by luminal berberine. Am J Physiol Gastrointest Liver Physiol. 1981;241:G248–G252. doi: 10.1152/ajpgi.1981.241.3.G248. [DOI] [PubMed] [Google Scholar]
- 36.Sack RB, Froehlich JL. Berberine inhibits intestinal secretory response of Vibrio cholerae and Escherichia coli enterotoxins. Infect Immun. 1982;35:471–475. doi: 10.1128/iai.35.2.471-475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutta N, Marker P, Rao N. Berberine in toxin-induced experimental cholera. Br J Pharmacol. 1972;44:153–159. doi: 10.1111/j.1476-5381.1972.tb07247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majeed W, Aslam B, Javed I, Khaliq T, Muhammad F, Ali A, et al. Histopathological evaluation of gastro protective effect of Berberis vulgaris(Zereshk) seeds against aspirin induced ulcer in albino mice. Pak J Pharm Sci. 2015;1:1953–1958. [PubMed] [Google Scholar]
- 39.Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative study of Berberis vulgaris fruit extract and berberine chloride effects on acetic acid-induced colitis in rats. Iran J Pharm Res. 2011:97–104. [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou H, Mineshita S. The effect of berberine chloride on experimental colitis in rats in vivo and in vitro. J Pharm Exp Ther. 2000;294:822–829. [PubMed] [Google Scholar]
- 41.Sathish R, Sahu A, Natarajan K. Antiulcer and antioxidant activity of ethanolic extract of Passiflora foetida L. Indian J Pharmacol. 2011;43:336. doi: 10.4103/0253-7613.81501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang M, Long Y, Sun Y, Wang Y, Li Q, Wu H, et al. Evidence for the complementary and synergistic effects of the three-alkaloid combination regimen containing berberine, hypaconitine and skimmianine on the ulcerative colitis rats induced by trinitrobenzene-sulfonic acid. Eur J Pharmacol. 2011;651:187–196. doi: 10.1016/j.ejphar.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med. 2001;345:517–525. doi: 10.1056/NEJMra003200. [DOI] [PubMed] [Google Scholar]
- 44.Green FH. Overview of pulmonary fibrosis. Chest. 2002;122:334S–339S. doi: 10.1378/chest.122.6_suppl.334s. [DOI] [PubMed] [Google Scholar]
- 45.Chitra P, Saiprasad G, Manikandan R, Sudhandiran G. Berberine inhibits Smad and non-Smad signaling cascades and enhances autophagy against pulmonary fibrosis. J Mol Med. 2015;93:1015–1031. doi: 10.1007/s00109-015-1283-1. [DOI] [PubMed] [Google Scholar]
- 46.Sun B, Chen Y. Advances in the mechanism of paraquat-induced pulmonary injury. Eur Rev Med Pharmacol Sci. 2016;20:1597–1602. [PubMed] [Google Scholar]
- 47.Javad-Mousavi SA, Hemmati AA, Mehrzadi S, Hosseinzadeh A, Houshmand G, Nooshabadi MRR, et al. Protective effect of Berberis vulgaris fruit extract against paraquat-induced pulmonary fibrosis in rats. Biomed Pharmacother. 2016;81:329–336. doi: 10.1016/j.biopha.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Lin K, Liu S, Shen Y, Li Q. Berberine attenuates cigarette smoke-induced acute lung inflammation. Inflammation. 2013;36:1079–1086. doi: 10.1007/s10753-013-9640-0. [DOI] [PubMed] [Google Scholar]
- 49.Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry. 1998;55:905–912. doi: 10.1001/archpsyc.55.10.905. [DOI] [PubMed] [Google Scholar]
- 50.Mantle D, Preedy V. Free radicals as mediators of alcohol toxicity. Adverse Drug React Toxicol Rev. 1999;18:235–252. [PubMed] [Google Scholar]
- 51.Nordmann R, Ribiere C, Rouach H. Ethanol-induced lipid peroxidation and oxidative stress in extrahepatic tissues. Alcohol Alcohol. 1990;25:231–237. doi: 10.1093/oxfordjournals.alcalc.a044996. [DOI] [PubMed] [Google Scholar]
- 52.Bhutada P, Mundhada Y, Bansod K, Hiware R, Rathod S, Dixit P, et al. Berberine protects C57BL/6J mice against ethanol withdrawal-induced hyperexcitability. Phytother Res. 2011;25:302–307. doi: 10.1002/ptr.3272. [DOI] [PubMed] [Google Scholar]
- 53.Patil S, Tawari S, Mundhada D, Nadeem S. Protective effect of berberine, an isoquinoline alkaloid ameliorates ethanol-induced oxidative stress and memory dysfunction in rats. Pharmacol Biochem Behav. 2015;136:13–20. doi: 10.1016/j.pbb.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Bhutada P, Mundhada Y, Bansod K, Rathod S, Hiware R, Dixit P, et al. Inhibitory effect of berberine on the motivational effects of ethanol in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:1472–1479. doi: 10.1016/j.pnpbp.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Klinkenberg I, Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment:a review of animal behavioral studies. Neurosci Biobehav Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 56.Elrod K, Buccafusco JJ. An evaluation of the mechanism of scopolamine-induced impairment in two passive avoidance protocols. Pharmacol Biochem Behav. 1988;29:15–21. doi: 10.1016/0091-3057(88)90267-5. [DOI] [PubMed] [Google Scholar]
- 57.Peng W-H, Hsieh M-T, Wu C-R. Effect of Long-Term Administration of berberine on scopolamine induced amnesia in rats. Jpn J Pharmacol. 1997;74:261–266. doi: 10.1254/jjp.74.261. [DOI] [PubMed] [Google Scholar]
- 58.Lee B, Sur B, Shim I, Lee H, Hahm D-H. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J Physiol Pharmacol. 2012;16:79–89. doi: 10.4196/kjpp.2012.16.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moneim AEA. The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis. 2015;30:935–942. doi: 10.1007/s11011-015-9652-6. [DOI] [PubMed] [Google Scholar]
- 60.Lim JS, Kim H, Choi Y, Kwon H, Shin KS, Joung I, et al. Neuroprotective effects of berberine in neurodegeneration model rats induced by ibotenic acid. Animal Cells Syst. 2008;12:203–209. [Google Scholar]
- 61.Kim M, Cho K-H, Shin M-S, Lee J-M, Cho H-S, Kim C-J, et al. Berberine prevents nigrostriatal dopaminergic neuronal loss and suppresses hippocampal apoptosis in mice with Parkinson’s disease. Int J Mol Med. 2014;33:870–878. doi: 10.3892/ijmm.2014.1656. [DOI] [PubMed] [Google Scholar]
- 62.Zhou W, Zhang F, Liu H, Tang S, Lai M, Zhu H, et al. Effects of training and withdrawal periods on heroin seeking induced by conditioned cue in an animal of model of relapse. Psychopharmacology. 2009;203:677–684. doi: 10.1007/s00213-008-1414-2. [DOI] [PubMed] [Google Scholar]
- 63.Yoo J-H, Yang E-M, Cho J-H, Lee J-H, Jeong S, Nah S-Y, et al. Inhibitory effects of berberine against morphine-induced locomotor sensitization and analgesic tolerance in mice. Neuroscience. 2006;142:953–961. doi: 10.1016/j.neuroscience.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Lee B, Sur B, Yeom M, Shim I, Lee H, Hahm D-H. Effect of berberine on depression-and anxiety-like behaviors and activation of the noradrenergic system induced by development of morphine dependence in rats. Korean J Physiol Pharmacol. 2012;16:379–386. doi: 10.4196/kjpp.2012.16.6.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Imenshahidi M, Qaredashi R, Hashemzaei M, Hosseinzadeh H. Inhibitory Effect of Berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur J Nat Pharm Prod. 2014;9:e16145. doi: 10.17795/jjnpp-16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Panahi N, Mahmoudian M, Mortazavi P, Hashjin GS. Effects of berberine on β-secretase activity in a rabbit model of Alzheimer’s disease. Arch Med Sci. 2013;9:146–150. doi: 10.5114/aoms.2013.33354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang J, Yang J-Q, He B-C, Zhou Q-X, Yu H-R, Tang Y, et al. Berberine and total base from rhizoma coptis chinensis attenuate brain injury in an aluminum-induced rat model of neurodegenerative disease. Saudi Med J. 2009;30:760–766. [PubMed] [Google Scholar]
- 68.Lee B, Yang CH, Hahm DH, Lee HJ, Choe ES, Pyun KH, et al. Coptidis Rhizoma attenuates repeated nicotine-induced behavioural sensitization in the rat. J Pharm Pharmacol. 2007;59:1663–1669. doi: 10.1211/jpp.59.12.0008. [DOI] [PubMed] [Google Scholar]
- 69.Agostinho P, Pliássova A, Oliveira CR, Cunha RA. Localization and trafficking of amyloid-βprotein precursor and secretases:impact on Alzheimer’s disease. J Alzheimers Dis. 2015;45:329–347. doi: 10.3233/JAD-142730. [DOI] [PubMed] [Google Scholar]
- 70.Jiang H, Wang X, Huang L, Luo Z, Su T, Ding K, et al. Benzenediol-berberine hybrids:multifunctional agents for Alzheimer’s disease. Bioorg Med Chem. 2011;19:7228–7235. doi: 10.1016/j.bmc.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 71.Zhu F, Qian C. Berberine chloride can ameliorate the spatial memory impairment and increase the expression of interleukin-1beta and inducible nitric oxide synthase in the rat model of Alzheimer’s disease. BMC neuroscience. 2006;7:1. doi: 10.1186/1471-2202-7-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Haghani M, Shabani M, Tondar M. The therapeutic potential of berberine against the altered intrinsic properties of the CA1 neurons induced by Aβneurotoxicity. Eur J Pharmacol. 2015;758:82–88. doi: 10.1016/j.ejphar.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 73.Handforth A. Harmaline tremor:underlying mechanisms in a potential animal model of essential tremor. Tremor Other Hyperkinet Mov. 2012;2:769. doi: 10.7916/D8TD9W2P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miwa H. Rodent models of tremor Cerebellum. 2007;6:66–72. doi: 10.1080/14734220601016080. [DOI] [PubMed] [Google Scholar]
- 75.O’Hearn E, Molliver ME. The olivocerebellar projection mediates ibogaine-induced degeneration of Purkinje cells:a model of indirect, trans-synaptic excitotoxicity. J Neurosci. 1997;17:8828–8841. doi: 10.1523/JNEUROSCI.17-22-08828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Llinas R, Volkind R. The olivo-cerebellar system:functional properties as revealed by harmaline-induced tremor. Expe Brain Res. 1973;18:69–87. doi: 10.1007/BF00236557. [DOI] [PubMed] [Google Scholar]
- 77.Vaziri Z, Abbassian H, Sheibani V, Haghani M, Nazeri M, Aghaei I, et al. The therapeutic potential of Berberine chloride hydrate against harmaline-induced motor impairments in a rat model of tremor. Neurosci Lett. 2015;590:84–90. doi: 10.1016/j.neulet.2015.01.078. [DOI] [PubMed] [Google Scholar]
- 78.Amin B, Malekzadeh M, Heidari MR, Hosseinzadeh H. Effect of Crocus sativus extracts and its active constituent safranal on the harmaline-induced tremor in mice. Iran J Basic Med Sci. 2015;18:449–458. [PMC free article] [PubMed] [Google Scholar]
- 79.Bhutada P, Mundhada Y, Bansod K, Dixit P, Umathe S, Mundhada D. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav. 2010;18:207–210. doi: 10.1016/j.yebeh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Gao F, Gao Y, Liu Y-f, Wang L, Li Y-j. Berberine exerts an anticonvulsant effect and ameliorates memory impairment and oxidative stress in a pilocarpine-induced epilepsy model in the rat. Neuropsychiatr Dis Treat. 2014;10:2139. doi: 10.2147/NDT.S73210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Graham ML, Janecek JL, Kittredge JA, Hering BJ, Schuurman H-J. The streptozotocin-induced diabetic nude mouse model:differences between animals from different sources. Comp Med. 2011;61:356–360. [PMC free article] [PubMed] [Google Scholar]
- 83.Bhutada P, Mundhada Y, Bansod K, Tawari S, Patil S, Dixit P, et al. Protection of cholinergic and antioxidant system contributes to the effect of berberine ameliorating memory dysfunction in rat model of streptozotocin-induced diabetes. Behav Brain Res. 2011;220:30–41. doi: 10.1016/j.bbr.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 84.Zhou J, Du X, Long M, Zhang Z, Zhou S, Zhou J, et al. Neuroprotective effect of berberine is mediated by MAPK signaling pathway in experimental diabetic neuropathy in rats. Eur J Pharmacol. 2016;774:87–94. doi: 10.1016/j.ejphar.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 85.Kalalian-Moghaddam H, Baluchnejadmojarad T, Roghani M, Goshadrou F, Ronaghi A. Hippocampal synaptic plasticity restoration and anti-apoptotic effect underlie berberine improvement of learning and memory in streptozotocin-diabetic rats. Eur J Pharmacol. 2013;698:259–266. doi: 10.1016/j.ejphar.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 86.Kumar A, Mishra J, Chopra K, Dhull DK. Possible role of P-glycoprotein in the neuroprotective mechanism of berberine in intracerebroventricular streptozotocin-induced cognitive dysfunction. Psychopharmacology. 2016;233:137–152. doi: 10.1007/s00213-015-4095-7. [DOI] [PubMed] [Google Scholar]
- 87.Kim SO, Kim HJ. Berberine ameliorates cold and mechanical allodynia in a rat model of diabetic neuropathy. J Med Food. 2013;16:511–517. doi: 10.1089/jmf.2012.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hosseinzadeh H, Sadeghnia H. Protective effect of safranal on pentylenetetrazol-induced seizures in the rat:involvement of GABAergic and opioids systems. Phytomedicine. 2007;14:256–262. doi: 10.1016/j.phymed.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Sadeghnia HR, Darbarpanah S, Hosseini SM. Effect of berberine on pentylenetetrazol-induced seizures in rats. Avicenna J Phytomed. 2011;1:78–82. [Google Scholar]
- 90.Hosseinzadeh H, Ramezani M, Shafaei H, Taghiabadi E. Anticonvulsant effect of Berberis integerrima L. root extracts in mice. J Acupunct Meridian Stud. 2013;6:12–17. doi: 10.1016/j.jams.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 91.Kulkarni SK, Dhir A. On the mechanism of antidepressant-like action of berberine chloride. Eur J Pharmacol. 2008;589:163–172. doi: 10.1016/j.ejphar.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 92.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyo-pathy:from molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Hao G, Yu Y, Gu B, Xing Y, Xue M. Protective effects of berberine against doxorubicin-induced cardiotoxicity in rats by inhibiting metabolism of doxorubicin. Xenobiotica. 2015;45:1024–1029. doi: 10.3109/00498254.2015.1034223. [DOI] [PubMed] [Google Scholar]
- 94.Zhao X, Zhang J, Tong N, Liao X, Wang E, Li Z, et al. Berberine attenuates doxorubicin-induced cardiotoxicity in mice. J Int Med Res. 2011;39:1720–1727. doi: 10.1177/147323001103900514. [DOI] [PubMed] [Google Scholar]
- 95.Ying Y, Zhu H, Liang Z, Ma X, Li S. GLP1 protects cardiomyocytes from palmitate-induced apoptosis via Akt/GSK3b/b-catenin pathway. J Mol Endocrinol. 2015;55:245–262. doi: 10.1530/JME-15-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chang W, Zhang M, Meng Z, Yu Y, Yao F, Hatch GM, et al. Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5′-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur J Pharmacol. 2015;769:55–63. doi: 10.1016/j.ejphar.2015.10.043. [DOI] [PubMed] [Google Scholar]
- 97.Liu W-h, Hei Z-q, Nie H, Tang F-t, Huang H-q, Li X-j, et al. Berberine ameliorates renal injury in streptozotocin-induced diabetic rats by suppression of both oxidative stress and aldose reductase. Chin Med J. 2008;121:706–712. [PubMed] [Google Scholar]
- 98.Yang Y, Ni W, Cai M, Tang L, Wei W. The renoprotective effects of berberine via the EP4-Gαs-cAMP signaling pathway in different stages of diabetes in rats. J Recept Signal Transduct Res. 2014;34:445–455. doi: 10.3109/10799893.2014.917324. [DOI] [PubMed] [Google Scholar]
- 99.Kennedy CR, Xiong H, Rahal S, Vanderluit J, Slack RS, Zhang Y, et al. Urine concentrating defect in prostaglandin EP1-deficient mice. Am J Physiol Renal Physiol. 2007;292:868–875. doi: 10.1152/ajprenal.00183.2005. [DOI] [PubMed] [Google Scholar]
- 100.Ni WJ, Tang LQ, Zhou H, Ding HH, Qiu YY. Renoprotective effect of berberine via regulating the PGE2-EP1-Gαq-Ca2+signalling pathway in glomerular mesangial cells of diabetic rats. J Cell Mol Med. 2016;20:1491–1502. doi: 10.1111/jcmm.12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tang LQ, Ni WJ, Cai M, Ding HH, Liu S, Zhang ST. Renoprotective effects of berberine and its potential effect on the expression of β-arrestins and intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in streptozocin-diabetic nephropathy rats. J Diabetes. 2016;8:693–700. doi: 10.1111/1753-0407.12349. [DOI] [PubMed] [Google Scholar]
- 102.Wu D, Wen W, Qi C-L, Zhao R-X, L J-H, Zhong C-Y, et al. Ameliorative effect of berberine on renal damage in rats with diabetes induced by high-fat diet and streptozotocin. Phytomedicine. 2012;19:712–718. doi: 10.1016/j.phymed.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 103.Wang FL, Tang LQ, Yang F, Zhu LN, Cai M, Wei W. Renoprotective effects of berberine and its possible molecular mechanisms in combination of high-fat diet and low-dose streptozotocin-induced diabetic rats. Mol Biol Rep. 2013;40:2405–2418. doi: 10.1007/s11033-012-2321-5. [DOI] [PubMed] [Google Scholar]
- 104.Su J, Zhang P, Zhang J-J, Qi X-M, Wu Y-G, Shen J-J. Effects of total glucosides of paeony on oxidative stress in the kidney from diabetic rats. Phytomedicine. 2010;17:254–260. doi: 10.1016/j.phymed.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 105.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediat Inflamm. 2012;2012:146–154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun SF, Zhao TT, Zhang HJ, Huang XR, Zhang WK, Zhang L, et al. Renoprotective effect of berberine on type 2 diabetic nephropathy in rats. Clin Exp Pharmacol Physiol. 2015;42:662–670. doi: 10.1111/1440-1681.12402. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, He H, Liang D, Jiang Y, Liang W, Chi Z-H, et al. Protective effects of berberine on renal injury in streptozotocin (STZ)-induced diabetic mice. Int J Mol Sci. 2016;17:1327. doi: 10.3390/ijms17081327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Adil M, Kandhare AD, Dalvi G, Ghosh P, Venkata S, Raygude KS, et al. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Renal Fail. 2016;38:996–1006. doi: 10.3109/0886022X.2016.1165120. [DOI] [PubMed] [Google Scholar]
- 109.Othman MS, Safwat G, Aboulkhair M, Moneim AEA. The potential effect of berberine in mercury-induced hepatorenal toxicity in albino rats. Food Chem Toxicol. 2014;69:175–181. doi: 10.1016/j.fct.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 110.Philips FS, Sternberg SS, Cronin AP, Vidal PM. Cyclophosphamide and urinary bladder toxicity. Cancer Res. 1961;21:1577–1589. [PubMed] [Google Scholar]
- 111.Xu X, Malavé A. Protective Effect of Berberine on Cyclophosphamide-Induced Haemorrhagic Cystitis in Rats. Pharmacol Toxicol. 2001;88:232–237. doi: 10.1034/j.1600-0773.2001.d01-109.x. [DOI] [PubMed] [Google Scholar]
- 112.Liu W, Zhang X, Liu P, Shen X, Lan T, Li W, et al. Effects of berberine on matrix accumulation and NF-kappa B signal pathway in alloxan-induced diabetic mice with renal injury. Eur J Pharmacol. 2010;638:150–155. doi: 10.1016/j.ejphar.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 113.Lan T, Shen X, Liu P, Liu W, Xu S, Xie X, et al. Berberine ameliorates renal injury in diabetic C57BL/6 mice:Involvement of suppression of SphK–S1P signaling pathway. Arch Biochem Biophys. 2010;502:112–120. doi: 10.1016/j.abb.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 114.Domitrović R, Jakovac H, Marchesi VV, Blažeković B. Resolution of liver fibrosis by isoquinoline alkaloid berberine in CCl4-intoxicated mice is mediated by suppression of oxidative stress and upregulation of MMP-2 expression. J Med Food. 2013;16:518–528. doi: 10.1089/jmf.2012.0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang BJ, Xu D, Guo Y, Ping J, Chen Lb, Wang H. Protevtion by and anti-oxidant mechanism of berberine against rat liver fibrosis induced by multiple hepatotoxic factors. Clin Exp Pharmacol Physiol. 2008;35:303–309. doi: 10.1111/j.1440-1681.2007.04819.x. [DOI] [PubMed] [Google Scholar]
- 116.Domitrović R, Jakovac H, Blagojević G. Hepatoprotective activity of berberine is mediated by inhibition of TNF-α, COX-2, and iNOS expression in CCl 4-intoxicated mice. Toxicology. 2011;280:33–43. doi: 10.1016/j.tox.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 117.Feng Y, Siu K-Y, Ye X, Wang N, Yuen M-F, Leung C-H, et al. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin Med. 2010;5:1. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sarhadynejad Z, Sharififar F, Pardakhty A, Nematollahi M-H, Sattaie-Mokhtari S, Mandegary A. Pharmacological safety evaluation of a traditional herbal medicine “Zereshk-e-Saghir𠇍 and assessment of its hepatoprotective effects on carbon tetrachloride induced hepatic damage in rats. J Ethnopharmacol. 2016;22:387–395. doi: 10.1016/j.jep.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 119.Zhang P, Ma D, Wang Y, Zhang M, Qiang X, Liao M, et al. Berberine protects liver from ethanol-induced oxidative stress and steatosis in mice. Food Chem Toxicol. 2014;74:225–232. doi: 10.1016/j.fct.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 120.Hasanein P, Ghafari-Vahed M, Khodadadi I. Effects of isoquinoline alkaloid berberine on lipid peroxidation, antioxidant defense system, and liver damage induced by lead acetate in rats. Redox Report. 2016:1–9. doi: 10.1080/13510002.2016.1140406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Germoush MO, Mahmoud AM. Berberine mitigates cyclophosphamide-induced hepatotoxicity by modula-ting antioxidant status and inflammatory cytokines. J Cancer Res Clin Oncol. 2014;140:1103–1109. doi: 10.1007/s00432-014-1665-8. [DOI] [PubMed] [Google Scholar]
- 122.Hwang J-M, Wang C-J, Chou F-P, Tseng T-H, Hsieh Y-S, Lin W-L, et al. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch Toxicol. 2002;76:664–670. doi: 10.1007/s00204-002-0351-9. [DOI] [PubMed] [Google Scholar]
- 123.Zhao X, Zhang J, Tong N, Chen Y, Luo Y. Protective effects of berberine on doxorubicin-induced hepato-toxicity in mice. Biol Pharm Bull. 2012;35:796–800. doi: 10.1248/bpb.35.796. [DOI] [PubMed] [Google Scholar]
- 124.Janbaz K, Gilani A. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71:25–33. doi: 10.1016/s0367-326x(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 125.Recknagel RO, Glende EA, Dolak JA, Waller RL. Mechanisms of carbon tetrachloride toxicity. Pharmacol Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 126.Sudharsan PT, Mythili Y, Selvakumar E, Varalakshmi P. Lupeol and its ester exhibit protective role against cyclophosphamide-induced cardiac mitochondrial toxicity. J Cardiovasc Pharmacol. 2006;47:205–210. doi: 10.1097/01.fjc.0000200658.89629.ba. [DOI] [PubMed] [Google Scholar]
- 127.Lindley C, Hamilton G, McCune JS, Faucette S, Shord SS, Hawke RL, et al. The effect of cyclophosphamide with and without dexamethasone on cytochrome P450 3A4 and 2B6 in human hepatocytes. Drug Metab Dispos. 2002;30:814–822. doi: 10.1124/dmd.30.7.814. [DOI] [PubMed] [Google Scholar]
- 128.Joyeux M, Rolland A, Fleurentin J, Mortier F, Dorfman P. tert-Butyl hydroperoxide-induced injury in isolated rat hepatocytes:a model for studying anti-hepatotoxic crude drugs. Planta Med. 1990;56:171–174. doi: 10.1055/s-2006-960918. [DOI] [PubMed] [Google Scholar]
- 129.Högberg J, Orrenius S, O’brien PJ. Further studies onlipid-peroxide formation in isolated hepatocytes. Eur J Biochem. 1975;59:449–455. doi: 10.1111/j.1432-1033.1975.tb02473.x. [DOI] [PubMed] [Google Scholar]
- 130.Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- 131.Hinson JA, Roberts DW, James LP. Mechanisms of acetaminophen-induced liver necrosis. Handb Exp Pharmacol. 2010;196:369–405. doi: 10.1007/978-3-642-00663-0_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang J, Yang Z, Lin L, Zhao Z, Liu Z, Liu X. Protective effect of naringenin against lead-induced oxidative stress in rats. Biol Trace Elem Res. 2012;146:354–359. doi: 10.1007/s12011-011-9268-6. [DOI] [PubMed] [Google Scholar]