Abstract
Metabolic syndrome (MetS), characterized by a cluster of metabolic abnormalities including hypertension, obesity, type 2 diabetes mellitus (T2DM) and dyslipidemia, is a well-known cardiovascular risk factor (CVRF). Cardiovascular disease (CVD) represents a massive healthcare burden worldwide. In recent years, with regard to the adverse effects of synthetic drugs, increasing attention has been paid by researchers to herbal medicines for a variety of disorders such as CVD. A large body of literature supports different pharmacological actions of Berberis vulgaris (B. vulgaris) and its active component, berberine (BBR), such as antidiabetic, antiobesity, hypotensive and hypolipidemic properties that could be interesting in the management of MetS associated with high CVD risk. Numerous preclinical in vitro and in vivo studies support all these effects. In this review, we evaluated the most related original articles to discover the role of B. vulgaris on various constituents of MetS and CVRF comprising dyslipidemia, obesity, high blood pressure and high blood glucose. This review suggests a potential role of B. vulgaris and BBR in the managing of MetS; nevertheless more investigations, especially reliable clinical trials, need to be accomplished to evaluate their effectiveness.
Keywords: Berberis vulgaris, Berberine, Cardiovascular disease, Diabetes, Dyslipidemia, Hypertension, Metabolic syndrome
Introduction
Metabolic syndrome (MetS), also described as ’insulin resistance syndrome’ (1), ’syndrome X’ (2), ’hypertriglyceridemic waist’ (3) and ’the deadly quartet’ (4) characterized by a cluster of metabolic abnormali-ties including hypertension, obesity, insulin resistance and dyslipidemia, is a well-known risk factor for type 2 diabetes mellitus (T2DM) and cardiovascular disease (CVD) (5). On the word of the International Diabetes Federation (IDF) (6) and the American Heart Association/National Heart, Lung, and Blood Institute (7), manifestation of any 3 of 5 risk factors establishes a diagnosis of MetS (8). Criteria for clinical diagnosis of the MetS are shown in Table 1.
Table 1.
Criteria for clinical diagnosis of the metabolic syndrome according to International Diabetes Federation (IDF) and National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATPIII) (8)
| Measure | Cut points |
|---|---|
| Elevated waist circumference | Population and country-specific |
| Elevated triglycerides (or on therapy for hypertriglyceridemia) | ≥ 150 mg/dl |
| Reduced HDL-C*(or on therapy for reduced HDL-C) | < 40 mg/dl in males > 50 mg/dl in females |
| Elevated blood pressure (or on therapy with known history of hypertension) | SBP ≥ 130 and/or DBP ≥ 85 mm Hg |
| Elevated fasting glucose (or on therapy for hyperglycemia) | ≥ 100 mg/dl |
HDL-C= high-density lipoprotein cholesterol
Statistics show that 20-25% of adults are suffering from MetS in the world and its presence should be considered as an indicator of long-term risk (9). So, it is a life-threatening disease and seems to be of vital importance to reach an appropriate remedy. Present and future trends in CVD mortality will require growing attention in the 21st century. Therefore, preventive strategies should be considered to decrease the future CVD burden (10) and nowadays there is a rising demand for plant bio-resources in the management of
chronic diseases such as MetS, instead of synthetic drugs, to avoid adverse effects (11). Furthermore, traditional medicinal plants are often cheaper and more locally available (12). It is a fact that one quarter of all medical prescriptions are formulations based on plant-derived synthetic analogs, and as stated by the WHO, 80% of the world’s population; mainly those of developing countries; trust in plant-derived medicines for their healthcare (11). A number of plants and/or their constituents such as Vitis vinifera (Grapes) (13), Nigella sativa (14), rutin (15) and Allium sativum (Garlic) (16) have shown beneficial effects in the treatment of MetS.
In line with this, “zereshk” which is the Persian name for Berberis vulgaris (B. vulgaris), is extensively cultivated in Iran (typically in Birjand and Qaen cities), central and southern Europe and the northeastern of the United States (17-19). The fruit of B. vulgaris (Figure 1) has long scarlet colored berries (20) which are oblong, slightly curved, about 0.5 inches long and edible (21).
Figure 1.

Berberis vulguris plant: showing leaves, flowers and fruits
B. vulgaris has played an important role in herbal therapy for more than 2500 years. Russian therapists have utilized it for treatment of inflammations, high blood pressure, and abnormal uterine bleeding (21). Different parts of this plant including fruits, leaves and roots, have been used in traditional medicine for a long time (22). For instance its dried leaf and stem bark are utilized as antiedema and antihypertensive in varicose veins, respectively. In gastrointestinal system, dried root is used as choleretic, laxative, antidiarrheal, anti-hepatitis, anti-hemorrhoid, and also its fruit is used in treatment of painful menstruation, whooping cough, hepatic malfunctions (23). Numerous investigations have earlier indicated that B. vulgaris has further medicinal properties such as antihyperglycemic, hypolipidemic, antioxidant, anticholinergic, anticancer, antipyretic, antihistaminic, antimicrobial, hypnotic, jumping and locomotion decreasing activities, and also reduction in the acquisition and reinstatement of morphine-induced conditioned place preference (24-27), which confirm the traditional use of this plant in Ayurveda and Chinese medicine (28).
B. vulgaris contains isoquinoline alkaloids such as berberine (BBR) (29); acanthine, bargustanine,
berbamine, berlambine, palmatine and secondary metabolites such as aesculetin, ascorbic acid, caffeic acid, pectin and tannin (23, 30).
BBR, a type of isoquinoline alkaloid with a long history of medicinal application, is the major active component of B. vulgaris (31). Recent studies have proved that BBR exhibits several pharmacological activities such as antioxidant, anti-inflammatory, antidiarrheal, antimicrobial and anti-tumor activity (32-35). Furthermore, BBR has a broad range of therapeutic potential uses including lowering blood glucose, adjusting blood lipids, increasing insulin sensitivity and consequently ameliorating insulin resistance (23) (Figure 2).
Figure 2.
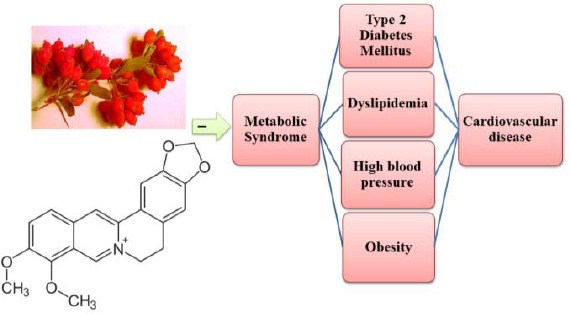
Therapeutic effects of Berberis vulgaris and berberine on metabolic syndrome
In this review, we illustrate the most related clinical trials, animal and in vitro studies to comprehend the role of B. vulgaris and its major active component, BBR,
in treatment of various constituents of MetS including dyslipidemia, hyperglycemia, hypertension and obesity. Although the reported biological results indicate that
this plant is a highly promising cardiovascular protective agent, more investigations need to be done to establish its effectiveness and in vivo cardioprotective effects, specifically in humans.
Search strategy
This review was carried out by means of the databases of Scopus, PubMed and Google Scholar. All the above databases were searched from the available date of inception until the latest issue up to May 2016. The search words contained MetS, CVD, dyslipidemia, obesity, hypertension, diabetes, B. vulgaris and BBR. All articles which included reports of the effect of B. vulgaris and BBR on MetS were taken into account. The reference lists of the included articles were also explored to identify additional studies.
Effects on lipid profile
Dyslipidemia is commonly known as an important cardiovascular risk factor (CVRF) with large randomized trials representing significant benefit of lipid modifying therapy in the management of CVD (36). Dyslipidemia is chiefly known as a triad of low HDL-C together with increases in triglycerides (TGs) and LDL (low-density lipoprotein) (37). In animal studies, hypercholesterolemic (HCh) rats which were fed by 30 mg/kg/day of BBR for six weeks showed reduced serum levels of TG and total cholesterol (TC). BBR caused significant increase in cardiac fatty acid transport protein-1, fatty acid transport proteins, fatty acid beta-oxidase, as well as peroxisome proliferator-activated receptor γ (PPARγ), but decrease in PPARα mRNA and protein expression (38). Treatment of hyperlipidemic (HL) hamsters with BBR reduced serum cholesterol by 40% and LDL-C by 42%, with a 3.5-fold rise in hepatic LDLR (low-density lipoprotein receptor) mRNA and a 2.6-fold rise in hepatic LDLR protein (39). In a study, it was indicated that because the mechanism of BBR (stabilization of the LDLR mRNA) differs from that of statins, the combination of BBR is more effective than that of the simvastatin or BBR monotherapy (40).
In another study HCh rats which received the alcoholic extract of B. vulgaris at minimum (75 mg/kg), moderate (150 mg/kg), and maximum (300 mg/kg) doses by intraperitoneal (IP) injection for three weeks, showed a decrease in serum levels of ALT (alanine transaminase) and ALP (alkaline phosphatase) through antioxidant properties of the plant (41). The mecha -nism of lowering blood cholesterol levels through inhibiting the intestinal absorption and further by interfering with intraluminal cholesterol micellariza-tion and decreasing enterocyte cholesterol uptake and secretion, was indicated in a study in which rats received 50, 100, and 150 mg/kg of BBR by gavage for 8 weeks (42). Furthermore, HFD rats treated with 200 mg/kg/d of BBR remarkably decreased the mRNA levels encoding carnitine palmitoyltransferase-1α (CPT-1α), microsomal triglyceride transfer protein (MTTP) and LDLR in the liver (43).
In vitro study also showed that BBR increased the LDLR mRNA and protein levels in a time- and dose-dependent manner and exhibits lipid-lowering effects by up-regulating LDLR expression in HepG2 cells (44).
Clinical studies also indicated the beneficial effects of this plant on dyslipidemia in human beings. Oral administration of BBR (0.5 g twice per day) in 32 HCh patients for 3 months reduced serum cholesterol by 29%, TGs by 35% and LDL-C by 25% compared to the control subjects (39). In another study, subjects on the BBR therapy were given 0.5 g of BBR hydrochloride orally twice a day for 3 months and it significantly lowered blood cholesterol, TG, LDL-C, ALT and AST, but increased HDL compared to the control subjects (45). Besides, decrease in TC (19.2%), TG (16.3%) and LDL-C (17.4%) compared to the control subjects was observed in a clinical study which BBR tablets (500 mg) were administered orally to HCh patients daily for 6 months (46). In several clinical studies, BBR has shown reduction in serum levels of TC, TG and LDL levels. Nevertheless, HDL level showed increase in some and no significant change in some other studies (47, 48).
The proposed mechanisms of protective effects of B. vulgaris and BBR on dyslipidemia may be associated with increase in hepatic LDLR mRNA and hepatic LDLR protein (39), a significant increase in adenosine monophosphate-activated protein kinase (AMPK) phosphorylation and activity (49) (Table 2).
Table 2.
Summary of the effects of Berberis vulgaris and berberine in metabolic syndrome
| Pharmacological effects | Results | Study protocol | Compound | References |
|---|---|---|---|---|
| ↓Cholesterol, TG, LDL ↑HDL | In vivo, rats (IP for 21 days) | B. vulgaris extracts (300 mg/kg) | (84) | |
| ↓TC, TG, LDL-C ↑HDL | Human, T2DM patients (p.o. for 2 months) | BBR (0.3 g t.i.d.) | (47) | |
| Hypolipidemic effects | ↓Serum cholesterol and dietary cholesterol absorption rate ↓cholesterol uptake and, gene and protein expressions of acyl-coenzyme A cholesterol acyltransferease-2 | In vivo, rats (p.o. for 8 weeks) in vitro, Caco-2 cells | BBR (50, 100, and 150 mg/kg twice a day) BBR (5, 10, and/or 15 μg/ml) | (42) |
| ↓Weight gain, TC, LDL-C, TG, ALT, AST, FI | In vivo, HFD rats (p.o. for 16 weeks) | BBR (200 mg/kg/d) | (43) | |
| ↓TC, TG, LDL-C ↑HDL | Human, HL and IGT patients (p.o. for 13 months) | BBR (0.2 g t.i.d.) | (48) | |
| ↓TC, TG, LDL-C ↑HDL | Human, HL patients (p.o. for 2 months) | BBR (0.4 g t.i.d.) | (85) | |
| ↓TC, TG, LDL-C ↑HDL | Human, T2DM patients (p.o. for 3 months) | BBR (0.5 g t.i.d.) | (86) | |
| ↓Serum cholesterol, TG, LDL-C ↓Serum cholesterol, LDL-C and ↑ hepatic LDLR mRNA, hepatic LDLR protein | Human, HCh patients (p.o. for 3 months) HL hamsters (p.o. for 10 days) | BBR (0.5 g twice a day) BBR (50 mg/kg/d) | (39) | |
| ↓Cholesterol, TG ↓LDL, TG, cholesterol, cholesteryl ester and hepatic fatty acid levels | In vitro, HepG2 cells in vivo, hamsters (p.o. for 10 days) | BBR (0-15 μg/ml) BBR (100 mg/kg/day) | (49) | |
| ↓TG, cholesterol, LDL-C ↑HDL-C | Human, HL patients (p.o. for 3 months) | BBR hydrochloride (0.5 g twice a day) | (45) | |
| ↓Body weight, TG, serum cholesterol, FBG, ALT, AST | Human, patients with non-alcoholic fatty liver disease (p.o. for 3 months) | Aqueous extracts of B. vulgaris (750 mg capsuls twice a day) | (53) | |
| ↓ALP, ALT, cholesterol ↑HDL-C | In vivo, HCh rats (IP for 3 weeks) | Alcoholic extract of B. vulgaris (75, 150 and 300 mg/kg) | (41) | |
| Antiobesity effects | ↑hepatic LDLR expression | In vitro, HepG2 cells | BBR (15 μM) | (44) |
| ↓Weight gain, food intake, serum glucose, TG, TC and PPARγ expression ↑GATA-3 expression | In vivo, mice (IP for 36 days) | BBR (0.75, 1.5, 3 mg/kg/day) | (52) | |
| ↓SBP, IL-6, IL-17, IL-23, angiotensin II and aldosterone | In vivo, HT rats (p.o.) | BBR (100 mg/kg/d) | (56) | |
| ↓ABP | In vivo, rats (IV for 1 week) | Aqueous extracts of B. vulgaris (17–33 mg/100 g) | (57) | |
| ↓SBP, DBP, HR | In vivo, rats (IV) | BBR (10 mg/kg in 1 hr) | (58) | |
| Hypotensive effects | ACE inhibition ↓MAP ↑NOx and cGMP | In vivo, rats (IV) | BBR (10, 100 μg/ml) | (59) |
| ↓SBP, DBP | Human, grade 1 essential HT patients (for 1 month) | BBR | (62) | |
| ↑Cardiac contractility and coronary flow | In vivo, dogs (IV) | BBR (2 to 4 μg/ml) | (87) | |
| Inhibition of Th17 differentiation by activating ERK1/2, inhibition of Th1 differentiation by inhibiting p38 MAPK and JNK activation | In vivo, NOD mice (p.o. for 2 weeks) in vitro, mouse CD4+ T cells | BBR (200 mg/kg) | (33) | |
| Antidiabetic effects | ↓HbA1c, FBG, PBG, TG, ↓HbA1c, FBG, PBG, TG, TC, LDL-C, | Human, newly diagnosed T2DM patients, poorly controlled type 2 diabetes (p.o. for 3 months) | BBR (0.5 g t.i.d..) | (32) |
| ↓Free fatty acids | Human, T2DM and DL patients (p.o. for 3 months) | BBR (1 g/d) | (64) | |
| ↓Serum TG, TC, LDL-C, apo B, glucose, and insulin | Human, T2DM patients (p.o. for 3 months) | B. vulgaris fruit extract (3 g/d) | (67) | |
| ↓FBG, serum insulin, HbA1c, TG, ALT | Human, T2DM patients (p.o. for 2 months) | BBR (1 g/d) | (68) | |
| ↑Insulin expression, β-cell regeneration and lipid peroxidation ↓lipid peroxidation | In vivo, HCh rats with T2DM (IP for 4 months) | BBR (150, 300 mg/kg) | (72) | |
| ↓FBG, MDA ↑SOD and CAT activity | In vivo, T2DM mice (for 2 weeks) | BBR (100 mg/kg/d) | (73) | |
| ↑Glucagon in plasma and intestine, and proglucagon mRNA expression | In vivo, STZ-diabetic rats (p.o. for 5 weeks) | BBR (120 mg/kg/d) | (75) | |
| ↑AMPK activity, glucose consumption and glucose uptake | In vitro, rat H9c2 cardiomyocyte cells (for up to 24 hr) | BBR (5–20 μM) | (77) | |
| ↑Insulin sensitizing activity and glucose-stimulated insulin secretion and proliferation ↓TG | In vitro, 3T3-L1 adipocytes | BBR (50μ M) | (78) | |
| ↓Sucrase, maltase and disaccharidase activity inhibition of α-glucosidase | In vitro, Caco-2 cells | BBR (0.02- 20 μg/mL) | (79) | |
| ↓FBG, insulin sensitivity ↑glucose consumption | In vitro, HepG2 cells in vivo, rats with T2DM (p.o.) in vivo, mice with T2DM (p.o. for 3 weeks) | BBR (7.5 μg/mL) BBR (75 or 150 mg/kg twice a day) BBR (100 or 200 mg/kg/d) | (88) | |
| ↓FBG, PBG, HOMA-IR ↑insulin sensitivity ↑glucose consumption, AMPK phosphorylation, | In vivo, obese rats (p.o. for 5 weeks) in vitro, mouse fibroblast 3T3-L1 preadipocytes, L6 rat skeletal myoblasts, C2C12 mouse myoblasts and rat hepatoma cell line H4IIE | BBR (125 mg/kg twice a day) BBR (5 and 20 μmol/l) | (81) | |
| ↓Body weight, adipose tissue mass and FBG ↓body weight and plasma TG ↓TG, free fatty acids and adipocyte differentiation | In vivo, obese and diabetic mice (IP for 3 weeks) in vivo, high-fat–fed rats (p.o. for 2 weeks) in vitro, 3T3-L1 cells | BBR (5 mg/kg) BBR (380 mg/kg) | (89) | |
| Antidiabetic and hypolipidemic effects | ↓Body weight, serum insulin and glucose, | In vivo, high fat diet rats (p.o. for 6 weeks) | B. vulgaris extract (0.2 g/Kg) | (51) |
| ↓FBG, TC, TG up-regulation of GLUT4, MAPK14, MAPK8, PPARα, UCP2 and HNF4α down-regulation of PPARγ, C/EBP, PGC1α and resistin | In vivo, obese and diabetic mice (IP for 4 weeks) | BBR (250 mg/kg/day) | (74) | |
| ↓Body weight, TC, TG, free fatty acid, LDL-C, MDA, TBARS, glucose and insulin levels ↑plasma superoxide dismutase activity and 4mRNA | In vivo, high glucose and high fat diet-induced diabetic hamsters (IP for 6 weeks) | BBR (50 and 100 mg/kg) | (90) | |
| ↓LDL-C, FBG and HbA1c | Human, T2DM and HCh patients (p.o. for 6 and 12 months) | BBR (588 mg/tablet) | (91) | |
| ↓FBG, TC, TG, LDL-C and Apo B | In vivo, STZ-diabetic mice (p.o. for 4 weeks) | Aqueous extracts of Rhizoma Coptidis (100 mg/kg/d) | (92) | |
| ↓SBP, DBP, HbA1c and FBG | Humans, HCh patients with T2DM (p.o. for 24 months) | BBR (0.1 g t.i.d.) | (93) | |
| Antidiabetic and hypotensive and antiobesity effects | ↑AMPK activation | In vitro, HUVE cells (for 1 hr) in vivo, rats | BBR (25 μM) | (94) |
| ↓expression of IL-1β, IL-6, TNFα, MCP-1, iNOS and COX-2, phosphorylation of p38, ERK and JNK ↓ TNFα | In vivo, obese db/db mice (IP for 3 weeks) in vitro, 264.7 macrophages and 3T3-L1 adipocytes | BBR (5 mg/kg/d) | (82) | |
| ↓DBP, FBG and TG | In vivo, HG and HCh rats (p.o. for 6 weeks) | BBR (30 mg/kg/day) | (38) | |
| Hypotensive and hypolipidemic effects | ↓SBP, PP, TC, LDL-C and TG | Human, grade 1 essential HT and HCh patients (p.o. daily for 6 months) | BBR (500 mg) | (46) |
↑= increase; ↓= decrease; FBG= fasting blood glucose; FI= fasting insulin; BMI= body mass index; TG= triglyceride; TC= total cholesterol; LDL-C= low-density lipoprotein cholesterol; LP (a) = lipoprotein (a); HOMA-IR= homeostatic model assessment; PPARγ= peroxisome proliferator-activated receptor γ; HbA1c= hemoglobin A1c; C/EBP= CCAAT enhancer-binding protein; GATA-3= globin transcription factor 3; STZ= streptozotocin; FPG= fasting plasma glucose; SBP= systolic blood pressure; DBP= diastolic blood pressure; MDA= malondialdehyde; TBARS= thiobarbituric acid reactive substances; t.i.d.= three times a day; PBG= postprandial blood glucose; ALT= alanine transaminase; AST= aspartate transaminase; ALP= alkaline phosphatase; p.o.= per os (orally); ABP= arterial blood pressure; SBP= systolic blood pressure, DBP= diastolic blood pressure; HR= heart rate; HUVECs= human umbilical vein endothelial cells; AMPK= adenosine monophosphate-activated protein kinase; ACE= angiotensin converting enzyme; MAP= mean arterial pressure; HT= hypertensiove; HCh= hypercholesterolemic; HG= hyperglycemic; HL= hyperlipidemic; PP= pulse pressure; Apo B= apolipoprotein B; IGT= impaired glucose tolerance; UCP2= uncoupling protein 2; HNF4α= hepatic nuclear factor 4α; PGC1α= PPARγ coactivator 1α; TNF-α= tumor necrosis factor; iNOS= inducible nitric oxide synthase; COX-2= cyclooxygenase-2; IV= intravenous; T2DM= type 2 diabetes mellitus; MCP-1= monocyte chemoattractant protein-
Effects on obesity
The increasing prevalence of obesity is associated with many diet-related chronic diseases including T2DM, CVD, stroke and hypertension (50). BBR successfully decreased body weight, fasting blood glucose (FBG), postprandial blood glucose (PBG), fasting insulin (FI) and homeostasis model assessment (HOMA IR) in obese rats fed on high fat diet (32). In a study, insulin resistance was developed by feeding the female rats a high fat diet (HFD) for six weeks, then treating them with B. vulgaris extract (0.2 g/Kg body weight) or vitamin A (12.8 μg/Kg/day) for two weeks. Co-administration of vitamin A and B. vulgaris extracts reduced body weight, blood glucose level, insulin, and retinol binding protein4 (RBP4) expression before, during and after HFD period (51). In another study when HFD-induced obese mice were treated with 0.75, 1.5 and 3 mg/kg/day (IP) of BBR for 36 days, it was demonstrated that BBR reduced mouse weight gain, food intake and serum glucose, TG and TC levels accompanied with a down-regulation of PPARγ expression and an up-regulation of globin transcription factor 3 (GATA-3) expression (52).
In a clinical trial which was conducted on 80 patients (case and control groups), a significant decrease was observed in weight, TG and cholesterol in patients who received two capsules (750 mg) containing B. vulgaris extract every day for 3 months (53).
Effects on high blood pressure
Hypertension is also one of the main CVRFs (54), which leads to increase in incidence of myocardial infarction and cerebrovascular disease, as well as heart failure and chronic renal disease (55).
Hypotensive effects of BBR have been observed in different animal models. In a study, the delay of the onset and attenuation of the severity of hypertension as well as amelioration of hypertension-induced renal damage were observed following the administration of BBR [per os (p.o.), 100 mg/kg/d body weight] to spontaneously hypertensive rats. Furthermore, BBR could inhibit the activities of renin-angiotensin system and pro-inflammatory cytokines interleukin 6 (IL-6), IL-17 and IL-23, which are involved in the pathophysiology of hypertension (56). Besides, intravenous (IV) administration of B. vulgaris (17-33 mg/100g) significantly reduced the rat arterial blood pressure (57). In another study, IV infusion of BBR (10 mg/kg in one hr) to rats lowered the blood pressure in a dose-dependent manner and a significant hypotensive effect was followed by bradycardia (58). The results of an experiment on rats also suggested that BBR has a hypotensive effect, via the inhibition of angiotensin-converting enzyme and direct release of NO/cGMP in the vascular tissues (59). In a dog ischemic heart failure model, IV administration of BBR, increased cardiac output; and decreased left ventricular end diastolic pressure and systemic vascular resistance (60).
Examination of hemodynamic parameters in humans revealed similar results with increased cardiac index and left ventricular ejection fraction, decreased systemic and pulmonary vascular resistance and left ventricular end-diastolic pressures (61). BBR also showed hypotensive effect in combination therapy. In a clinical study, a nutraceutical composition including policosanol, red yeast rice extract, BBR, folic acid and coenzyme Q10 with Orthosiphon stamineus was administered to grade I essential hypertensive patients for a month and an antihypertensive effect along with lipid lowering effect was revealed (62).
Effects on high glucose level
T2DM, a serious, complex metabolic disorder and one of the most common chronic diseases, is an independent CVRF (63-65). The antidiabetic and hypoglycemic effects of B. vulgaris and BBR have been described by several clinical, in vivo and in vitro scientific investigations (32, 33, 66). Many human studies have supported the antidiabetic effect of this plant. For example, in two different diabetic situation (the newly diagnosed T2DM and poorly controlled T2DM patients) who received 0.5 g/three times a day (t.i.d.) of BBR (orally for 3 months) showed decrease in HbA1c, FBG, PBG, TG, TC, LDL-C; and the authors presented BBR as a potent oral hypoglycemic agent with beneficial effects on lipid metabolism (32). In another clinical study on T2DM patients who were treated with B. vulgaris fruit extract (3 g/d) orally for 3 months, reduction in glucose, insulin, TG, TC, LDL-C and apolipoprotein B was indicated (67). In a clinical trial on T2DM patients, BBR was suggested as an ideal medicine with a mechanism different from metformin and rosiglitazone and demonstrated the effect of BBR on increasing insulin receptor mRNA in humans and its relationship with the glucose-lowering effect (68). In another human study, patients who received BBR (0.5 g/twice a day for three months), biomarkers such as TG, TC and LDL-C markedly decreased (69). Also it has been shown that consumption of 1 g/d of BBR in T2DM and DL (dyslipidemic) patients (for 3 months) reduced free fatty acids (64) that can be considered as a mechanism for the antidiabetic effect of BBR. Furthermore, in a meta-analysis, the effect and safety of BBR in the treatment of T2DM, hyperlipemia and hypertension on 2569 patients was investigated. This study indicated that BBR has comparable therapeutic effect on T2DM, hyperlipidemia and hypertension with no serious side effect (70).
Besides, in vivo studies have demonstrated this effect. In an animal study, 50 and 100 mg/kg of BBR was intraperitoneally injected to streptozotocin (STZ)-diabetic rats for 4 weeks and decrease in FBG, hepatic gluconeogenesis, glucose 6-phosphatase (G6Pase), weight gain, plasma TG and phospho-enolpyruvate carboxykinase was observed. But glucose tolerance and AMPK activity increased (71). This effect was also observed with a different mechanism in non-obese diabetic mice which were treated orally with BBR (200 mg/kg for 2 weeks) and inhibition of T helper17 (Th17) cell differentiation by activating extracellular signal-regulated kinase1/2 (ERK1/2), inhibition of Th1 cell differentiation by inhibiting p38 mitogen-activated protein kinase (MAPK) and c-jun N-terminal kinase (JNK) activation, was observed (33). In another animal study, BBR (150, 300 mg/kg for 4 months) was IP injected to HCh and T2DM rats and the results showed an increase in insulin expression, β-cell regeneration, antioxidant enzyme activity; and decrease in lipid peroxidation (72). Another mechanism which has been stated on the antidiabetic effect of this plant could be a reduction in FBG and malondialdehyde (MDA), and increase in superoxide dismutase (SOD) and catalase (CAT) activity by treating T2DM mice with BBR (100 mg/kg/d for 2 weeks) (73). The antidiabetic effect of BBR was also demonstrated in astudy on mice, accomplished by Zhang et al (74). In this study, up-regulated expression of glucose transporter 4 (GLUT4), MAPK14, MAPK8, PPARα, uncoupling protein 2, and hepatic nuclear factor 4α (HNF4α); and down-regulated expression of PPARγ, CCAAT/enhancer-binding protein (C/EBP), PPARγ coactivator 1α (PGC1α) and resistin (which moderates glucose and lipid metabolism), was observed (74). Besides, in adipose and muscle cell, several research projects demonstrated that BBR may stimulate glucose uptake into cell through up-regulating GLUT1 expression and inhibiting RBP4, which can act as an effective insulin sensitizing function (63). BBR treatment in STZ-diabetic rats significantly increases glucagon levels in plasma and intestine through promoting glucagon-like peptide-1 (GLP-1) secretion, accompanied with an increase of proglucagon mRNA expression. Furthermore, BBR raises insulin levels in plasma and pancreas as well as β-cell number in pancreas (75). On GLP-1 receptor activation, adenylyl cyclase (AC) is activated, and cyclic adenosine monophosphate (cAMP) is generat-ed, leading to activation of second messenger pathways and closure of ATP-dependent potassium channels. Increased intracellular potassium causes depolarization, and calcium influx through the voltage-dependent calcium channels occurs. This intracellular calcium increase stimulates the migration and exocytosis of the insulin granules and lowers the blood glucose (76).
In an experiment which was performed on rat H9c2 cardiomyocyte cell line, BBR (5-20 μM) could increase AMPK activity, glucose consumption and glucose uptake by these cells (77). In another experiment on MCF7, HepG2 and CACO-2 cell lines, the ethanolic extract of B. vulgaris and BBR caused the inhibition of α-glucosidase enzyme activity which verifies this effect (66). BBR (50μ M) also indicated an escalation of insulin sensitizing activity and glucose-stimulated insulin secretion and prolifera-tion; and a diminution of TG in 3T3-L1 adipocytes (78). A proposed mechanism for BBR (0.02- 20 μg/ml) could be a reduction in sucrase, maltase and disaccharidase activity; and the inhibition of α-glucosidase according to an experiment on Caco-2 cell line which lead to reduction in intestinal absorbtion of glucose (79). The stimulation of palmitate resulted in IL-6 and tumor necrosis factor-α (TNF-α) production in HepG2 cells, and antibody-neutralizing assay further confirms that IL-6 and TNF-α are involved in the development of insulin resistance. BBR effectively inhibits IL-6 and TNF-α production in a concentration-dependent manner, demonstrating its anti-inflammatory activity in hepatocytes (80). BBR augments glucose metabolism through stimulation of glycolysis, which is related to inhibition of glucose oxidation in mitochondria. BBR-induced AMPK activation is likely a result of mitochondria inhibition that escalates the AMP/ATP ratio (81). The improving effect of BBR on obesity, T2DM and dyslipidemia has been shown through stimulating of AMPK. BBR suppresses pro-inflammatory responses through AMPK activation in macrophages. In adipose tissue of obese db/db mice, BBR considerably down-regulated the expression of pro-inflammatory genes such as IL-1β, IL-6, TNFα, monocyte chemoattractant protein-1 (MCP-1), inducible nitric oxide synthase (iNOS) and cyclooxy-genase-2 (COX-2). Constantly, BBR inhibited lipopolysaccharide (LPS)-induced expression of pro-inflammatory genes including IL-1β, IL-6, iNOS, MCP-1, COX-2, and matrix metalloprotease-9 in peritoneal macrophages and RAW 264.7 cells. Upon various pro- inflammatory signals such as LPS, free fatty acids, and hydrogen peroxide, BBR suppressed the phospho-rylation of MAPKs, such as p38, ERK, and JNK, and the level of reactive oxygen species in macrophages (82). BBR also has significant antimicrobial activity against several microbes through inhibiting the assemblyfunction of filamenting temperature-sensitive mutant Z (FtsZ), an essential cell division protein, which results in the cessation of bacterial cell division. Acting topically in the gastrointestinal tract and poorly absorbed, BBR might modulate gut microbiota without systemic anti-infective activity (83), and this could be another mechanism for the antidiabetic effect of BBR (Figure 3).
Figure 3.
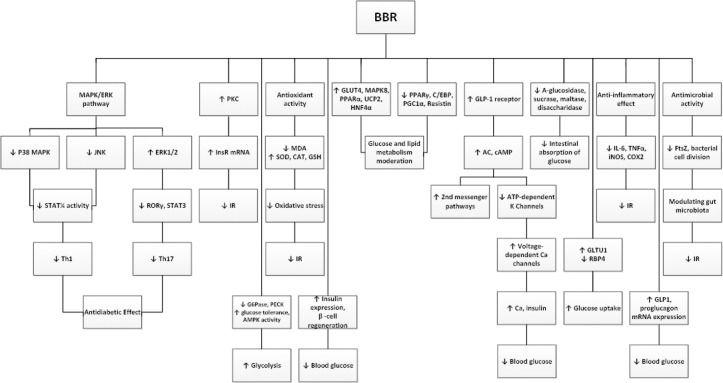
Main mechanisms of berberine on glucose metabolism
MAPK= mitogen-activated protein kinase; ERK= extracellular signal-regulated kinase; JNK= c-jun N-terminal kinase; STAT3= signal transducer and activator of transcription3; Th= T helper cells; PKC= protein kinase C; InsR= insulin receptor; IR= insulin resistance; MDA= malondialdehyde; SOD= superoxide dismutase; CAT= catalase; GSH= glutathione; G6Pase= glucose 6-phosphatase; AMPK= adenosine monophosphate-activated protein kinase; GLUT4= glucose transporter 4; PPARα= peroxisome proliferator-activated receptor α; UCP2= uncoupling protein 2; HNF4α= hepatic nuclear factor 4α; C/EBP= CCAAT enhancer-binding protein; PGC1α= PPARγ coactivator 1α; GLP-1= glucagon-like peptide-1; AC= adenylyl cyclase; cAMP= cyclic adenosine monophosphate; ATP= adenosine triphosphate; RBP4= retinol binding protein4; IL-6= interleukin 6; TNF-α= tumor necrosis factor; iNOS= inducible nitric oxide synthase; COX-2= cyclooxygenase-2; FtsZ= filamenting temperature-sensitive mutant Z; ROR= retinoic acid-related orphan receptors
Conclusion
The use of herbal drugs as complementary medicine is prevalent and gaining worldwide popularity. Many drugs are derived directly from plants; while the others are chemically modified natural products. The original research articles published so far have confirmed the pharmacological potential of B. vulgaris and its active component, BBR, which possess remarkable in vitro and in vivo (clinical studies, in lesser extent) antidiabetic, hypolipidemic, hypotensive and antiobesity effects against MetS. Based on the current review, it can be concluded that B. vulgaris may exhibit promise as a medication for modulating different constituents of MetS including T2DM, dyslipidemia, hypertension and obesity, and consequently CVD. However, more clinical trials need to be done for establishing the therapeutic effectiveness of B. vulgaris and BBR.
Conflict of interest
None to be declared.
References
- 1.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 3.Lemieux I, Pascot A, Couillard C, Lamarche Bt, Tchernof A, Alméras N, et al. Hypertriglyceridemic waist:a marker of the atherogenic metabolic triad (hyperinsulinemia;hyperapolipoprotein B;small, dense LDL) in men. Circulation. 2000;102:179–84. doi: 10.1161/01.cir.102.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan NM. The deadly quartet. Upper-body obesity, glucose intolerance, hypertriglyceridemia, and hypertension. Arch Intern Med. 1989;149:1514–1520. doi: 10.1001/archinte.149.7.1514. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Hansen B, Smith SC, Cleeman JI, Kahn RA. American Heart Association National Heart Lung and Blood Institute American Diabetes Association. Clinical management of metabolic syndrome:report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Arterioscler Thromb Vasc Biol. 2004;109:551–56. doi: 10.1161/01.ATV.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KGM, Zimmet P, Shaw J, Group IETFC. The metabolic syndrome—a new worldwide definition. The Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 7.Grundy S, Cleeman J, Daniels S, Donato K, Eckel R, Franklin B, et al. American Heart Association;National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome:an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome:a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention;National Heart, Lung, and Blood Institute;American Heart Association;World Heart Federation;International Atherosclerosis Society;and International Association for the Study of Obesity. Circulation. 2009;120:1640–45. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.Mamikutty N, Thent ZC, Sapri SR, Sahruddin NN, Mohd Yusof MR, Haji Suhaimi F. The establishment of metabolic syndrome model by induction of fructose drinking water in male wistar rats. Biomed Res Int. 2014;2014:263897. doi: 10.1155/2014/263897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Flaherty M, Buchan I, Capewell S. Contributions of treatment and lifestyle to declining CVD mortality:why have CVD mortality rates declined so much since the 1960s? Heart. 2013;99:159–162. doi: 10.1136/heartjnl-2012-302300. [DOI] [PubMed] [Google Scholar]
- 11.Gurib-Fakim A. Medicinal plants:Traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27:1–93. doi: 10.1016/j.mam.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Shrivastava R, Agrawal R, Parveen Z. A review on therapeutic applications of Nigella sativa. J Chem Chem Sci. 2011;1:241–248. [Google Scholar]
- 13.Akaberi M, Hosseinzadeh H. Grapes (Vitis vinifera) as a potential candidate for the therapy of the metabolic syndrome. Phytother Res. 2016;30:540–556. doi: 10.1002/ptr.5570. [DOI] [PubMed] [Google Scholar]
- 14.Razavi BM, Hosseinzadeh H. A review of the effects of Nigella sativa L and its constituent, thymoquinone, in metabolic syndrome. J Endocrinol Invest. 2014;37:1031–1040. doi: 10.1007/s40618-014-0150-1. [DOI] [PubMed] [Google Scholar]
- 15.Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 2014;37:783–788. doi: 10.1007/s40618-014-0096-3. [DOI] [PubMed] [Google Scholar]
- 16.Hosseini A, Hosseinzadeh H. A review on the effects of Allium sativum(Garlic) in metabolic syndrome. J Endocrinol Invest. 2015;38:1147–1157. doi: 10.1007/s40618-015-0313-8. [DOI] [PubMed] [Google Scholar]
- 17.Rounsaville TJ, Ranney TG. Ploidy levels and genome sizes of Berberis L. and Mahonia nutt. species, hybrids, and cultivars. HortScience. 2010;45:1029–1033. [Google Scholar]
- 18.Kafi M, Balendari A. Berberis Production and Processing. Mashhad, Iran: Ferdowsi University Press; 2004. p. 210. [Google Scholar]
- 19.Mokhber-Dezfuli N, Saeidnia S, Gohari AR, Kurepaz-Mahmoodabadi M. Phytochemistry and pharmacology of Berberis species. Pharmacogn Rev. 2014;8:8–15. doi: 10.4103/0973-7847.125517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drofler H, Roselt G. The dictionary of healing plants. New York: Blandford Press; 1989. [Google Scholar]
- 21.Arayne MS, Sultana N, Bahadur SS. The berberis story Berberis vulgaris in therapeutics. Pak J Pharm Sci. 2007;20:83–92. [PubMed] [Google Scholar]
- 22.Tomosaka H, Chin Y-W, Salim AA, Keller WJ, Chai H, Kinghorn AD. Antioxidant and cytoprotective compounds from Berberis vulgaris(barberry) Phytother Res. 2008;22:979–981. doi: 10.1002/ptr.2443. [DOI] [PubMed] [Google Scholar]
- 23.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 24.Hajzadeh M, Rajaei Z, Shafiee S, Alavinejhad A, Samarghandian S, Ahmadi M. Effect of barberry fruit (Berberis vulgaris) on serum glucose and lipids in streptozotocin-diabetic rats. Pharmacologyonline. 2011;1:809–817. [Google Scholar]
- 25.Potdar D, Hirwani RR, Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia. 2012;83:817–830. doi: 10.1016/j.fitote.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Nassiri-Asl M, Hosseinzadeh H, Mortazavi SR. Effects of Berberis vulgaris fruit extracts and its active component, berberine, on morphine dependence, hypnosis and locomotor activity in mice. Pharmacologyonline. 2007;1:190–202. [Google Scholar]
- 27.Imenshahidi M, Qaredashi R, Hashemzaei M, Hosseinzadeh H. Inhibitory effect of Berberis vulgaris aqueous extract on acquisition and reinstatement effects of morphine in conditioned place preferences (CPP) in mice. Jundishapur J Nat Pharm Prod. 2014;9:1–6.e16145. doi: 10.17795/jjnpp-16145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohammadi A, Sahebkar A, Kermani T, Zhilaee M, Tavallaie S, Ghayour Mobarhan M. Barberry administration and pro-oxidant–antioxidant balance in patients with metabolic syndrome. Iran Red Crescent Med J. 2014;16:e16786. doi: 10.5812/ircmj.16786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gorval LM, Grishkovets VI. Alkaloids of some species of the genus Berberis introduced into the Crimea. Chem Nat Compd. 1999;35:223–4. [Google Scholar]
- 30.Amin AH, Subbaiah TV, Abbasi KM. Berberine sulfate:antimicrobial activity, bioassay, and mode of action. Can J Microbiol. 1969;15:1067–76. doi: 10.1139/m69-190. [DOI] [PubMed] [Google Scholar]
- 31.Ni W-J, Ding H-H, Tang L-Q. Berberine as a promising anti-diabetic nephropathy drug:An analysis of its effects and mechanisms. Eur J Pharmacol. 2015;760:103–12. doi: 10.1016/j.ejphar.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Yin J, Xing H, Ye J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metab Clin Exp. 2008;57:712–7. doi: 10.1016/j.metabol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cui G, Qin X, Zhang Y, Gong Z, Ge B, Zang YQ. Berberine differentially modulates the activities of ERK, p38 MAPK, and JNK to suppress Th17 and Th1 T cell differentiation in type 1 diabetic mice. J Biol Chem. 2009;284:28420–9. doi: 10.1074/jbc.M109.012674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freile ML, Giannini F, Pucci G, Sturniolo A, Rodero L, Pucci O, et al. Antimicrobial activity of aqueous extracts and of berberine isolated from Berberis heterophylla. Fitoterapia. 2003;74:702–5. doi: 10.1016/s0367-326x(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 35.Wang N, Feng Y, Zhu M, Tsang C-M, Man K, Tong Y, et al. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells:The cellular mechanism. J Cell Biochem. 2010;111:1426–36. doi: 10.1002/jcb.22869. [DOI] [PubMed] [Google Scholar]
- 36.Goff DC, Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, et al. Dyslipidemia prevalence, treatment, and control in the Multi-Ethnic Study of Atherosclerosis (MESA):gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–56. doi: 10.1161/CIRCULATIONAHA.105.552737. [DOI] [PubMed] [Google Scholar]
- 37.Gulati OP. Pycnogenol®in metabolic syndrome and related disorders. Phytother Res. 2015;29:949–68. doi: 10.1002/ptr.5341. [DOI] [PubMed] [Google Scholar]
- 38.Dong S-F, Hong Y, Liu M, Hao Y-Z, Yu H-S, Liu Y, et al. Berberine attenuates cardiac dysfunction in hyperglycemic and hypercholesterolemic rats. Eur J Pharmacol. 2011;660:368–74. doi: 10.1016/j.ejphar.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 39.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 40.Kong W-J, Wei J, Zuo Z-Y, Wang Y-M, Song D-Q, You X-F, et al. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism. 2008;57:1029–37. doi: 10.1016/j.metabol.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 41.Taheri S, Zarei A, Changizi Ashtiyani S, Rezaei A, Zaheiri S. Evaluation of the effects of hydroalcoholic extract of Berberis vulgaris root on the activity of liver enzymes in male hypercholesterolemic rats. Avicenna J Phytomed. 2012;2:153–61. [PMC free article] [PubMed] [Google Scholar]
- 42.Wang Y, Yi X, Ghanam K, Zhang S, Zhao T, Zhu X. Berberine decreases cholesterol levels in rats through multiple mechanisms, including inhibition of cholesterol absorption. Metabolism. 2014;63:1167–77. doi: 10.1016/j.metabol.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Chang X, Yan H, Fei J, Jiang M, Zhu H, Lu D, et al. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010;51:2504–15. doi: 10.1194/jlr.M001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y, Cao S, Wang Y, Xu P, Yan J, Bin W, et al. Berberine metabolites could induce low density lipoprotein receptor up-regulation to exert lipid-lowering effects in human hepatoma cells. Fitoterapia. 2014;92:230–7. doi: 10.1016/j.fitote.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Zhao W, Xue R, Zhou Z-X, Kong W-J, Jiang J-D. Reduction of blood lipid by berberine in hyperlipidemic patients with chronic hepatitis or liver cirrhosis. Biomed Pharmacother. 2008;62:730–1. doi: 10.1016/j.biopha.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Mazza A, Lenti S, Schiavon L, Zuin M, D’Avino M, Ramazzina E, et al. Nutraceuticals for serum lipid and blood pressure control in hypertensive and hypercholesterolemic subjects at low cardiovascular risk. Adv Ther. 2015;32:680–90. doi: 10.1007/s12325-015-0229-x. [DOI] [PubMed] [Google Scholar]
- 47.Zhu L, Li H. Therapeutic efficacy of combined Berberine and Glipizide on type 2 diabete. J Clin Res. 2007;1:20. [Google Scholar]
- 48.Shanobin J, Lirong T, Yi S. Interventioal effect of berberine liposome on impaired glucose tolerance accompanied with hyperlipemia. J Pract Tradit Chin Med. 2007;8:008. [Google Scholar]
- 49.Brusq J-M, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, et al. Inhibition of lipid synthesis through activation of AMP kinase:an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Grover SA, Kaouache M, Rempel P, Joseph L, Dawes M, Lau DCW, et al. Years of life lost and healthy life-years lost from diabetes and cardiovascular disease in overweight and obese people:a modelling study. Lancet Diabetes Endocrinol. 2015;3:114–22. doi: 10.1016/S2213-8587(14)70229-3. [DOI] [PubMed] [Google Scholar]
- 51.El-Sayed MM, Ghareeb DA, Talat HA, Sarhan EM. High fat diet induced insulin resistance and elevated retinol binding protein 4 in female rats;treatment and protection with Berberis vulgaris extract and vitamin A. Pak J Pharm Sci. 2013;26:1189–95. [PubMed] [Google Scholar]
- 52.Hu Y, Davies GE. Berberine inhibits adipogenesis in high-fat diet-induced obesity mice. Fitoterapia. 2010;81:358–66. doi: 10.1016/j.fitote.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 53.Iloon Kashkooli R, Najafi SS, Sharif F, Hamedi A, Hoseini Asl MK, Najafi Kalyani M, et al. The effect of Berberis vulgaris extract on transaminase activities in non-alcoholic fatty liver disease. Hepat Mon. 2015;15:e25067. doi: 10.5812/hepatmon.25067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Prevention of cardiovascular disease:guideline for assessment and management of cardiovascular risk. 2007 [Google Scholar]
- 55.Psaty BM, Smith NL, Siscovick DS, et al. Health outcomes associated with antihypertensive therapies used as first-line agents:A systematic review and meta-analysis. JAMA. 1997;277:739–45. [PubMed] [Google Scholar]
- 56.Guo Z, Sun H, Zhang H, Zhang Y. Anti-hypertensive and renoprotective effects of berberine in spontaneously hypertensive rats. Clin Exp Hypertens. 2015;37:332–9. doi: 10.3109/10641963.2014.972560. [DOI] [PubMed] [Google Scholar]
- 57.Fatehi-Hassanabad Z, Jafarzadeh M, Tarhini A, Fatehi M. The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother Res. 2005;19:222–5. doi: 10.1002/ptr.1661. [DOI] [PubMed] [Google Scholar]
- 58.Chun YT, Yip TT, Lau KL, Kong YC, Sankawa U. A biochemical study on the hypotensive effect of berberine in rats. General Pharmacology:The Vascular System. 1979;10:177–82. doi: 10.1016/0306-3623(79)90085-5. [DOI] [PubMed] [Google Scholar]
- 59.Kang DG, Sohn EJ, Kwon EK, Han JH, Oh H, Lee HS. Effects of berberine on angiotensin-converting enzyme and NO/cGMP system in vessels. Vascul Pharmacol. 2002;39:281–6. doi: 10.1016/s1537-1891(03)00005-3. [DOI] [PubMed] [Google Scholar]
- 60.Huang WM, Yan H, Jin JM, Yu C, Zhang H. Beneficial effects of berberine on hemodynamics during acute ischemic left ventricular failure in dogs. Chin Med J. 1992;105:1014–9. [PubMed] [Google Scholar]
- 61.Marin-Neto JA, Maciel BC, Secches AL, Gallo Junior L. Cardiovascular effects of berberine in patients with severe congestive heart failure. Clin Cardiol. 1988;11:253–60. doi: 10.1002/clc.4960110411. [DOI] [PubMed] [Google Scholar]
- 62.Trimarco V, Cimmino C, Santoro M, Pagnano G, Manzi M, Piglia A, et al. Nutraceuticals for blood pressure control in patients with high-normal or grade 1 hypertension. High Blood Press Cardiovasc Prev. 2012;19:117–22. doi: 10.1007/BF03262460. [DOI] [PubMed] [Google Scholar]
- 63.Pang B, Zhao L-H, Zhou Q, Zhao T-Y, Wang H, Gu C-J, et al. Application of berberine on treating type 2 diabetes mellitus. Int J Endocrinol. 2015;2015:12. doi: 10.1155/2015/905749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gu Y, Zhang Y, Shi X, Li X, Hong J, Chen J, et al. Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta. 2010;81:766–72. doi: 10.1016/j.talanta.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 65.García-Fontana B, Morales-Santana S, Díaz Navarro C, Rozas-Moreno P, Genilloud O, Vicente Pérez F, et al. Metabolomic profile related to cardiovascular disease in patients with type 2 diabetes mellitus:A pilot study. Talanta. 2016;148:135–43. doi: 10.1016/j.talanta.2015.10.070. [DOI] [PubMed] [Google Scholar]
- 66.Abd El-Wahab A, Ghareeb D, Sarhan E, Abu-Serie M, El Demellawy M. In vitro biological assessment of Berberis vulgaris and its active constituent, berberine:antioxidants, anti-acetylcholinesterase, anti-diabetic and anticancer effects. BMC Complement Altern Med. 2013;13:218. doi: 10.1186/1472-6882-13-218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shidfar F, Ebrahimi SS, Hosseini S, Heydari I, Shidfar S, Hajhassani G. The Effects of Berberis vulgaris fruit extract on serum lipoproteins, apoB, apoA-I, homocysteine, glycemic control and total antioxidant capacity in type 2 diabetic patients. Iran J Pharm Res. 2012;11:643–52. [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang H, Wei J, Xue R, Wu J-D, Zhao W, Wang Z-Z, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285–92. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Li X, Zou D, Liu W, Yang J, Zhu N, et al. Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J Clin Endocrinol Metab. 2008;93:2559–65. doi: 10.1210/jc.2007-2404. [DOI] [PubMed] [Google Scholar]
- 70.Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan J, et al. Meta-analysis of the effect and safety of berberine in the treatment of type 2 diabetes mellitus, hyperlipemia and hypertension. J Ethnopharmacol. 2015;161:69–81. doi: 10.1016/j.jep.2014.09.049. [DOI] [PubMed] [Google Scholar]
- 71.Zhang M, Lv X, Li J, Meng Z, Wang Q, Chang W, et al. Sodium caprate augments the hypoglycemic effect of berberine via AMPK in inhibiting hepatic gluconeogenesis. Mol Cell Endocrinol. 2012;363:122–30. doi: 10.1016/j.mce.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhou J, Zhou S, Tang J, Zhang K, Guang L, Huang Y, et al. Protective effect of berberine on beta cells in streptozotocin- and high-carbohydrate/high-fat diet-induced diabetic rats. Eur J Pharmacol. 2009;606:262–8. doi: 10.1016/j.ejphar.2008.12.056. [DOI] [PubMed] [Google Scholar]
- 73.Chatuphonprasert W, Lao-ong T, Jarukamjorn K. Improvement of superoxide dismutase and catalase in streptozotocin–nicotinamide-induced type 2-diabetes in mice by berberine and glibenclamide. Pharm Biol. 2014;52:419–27. doi: 10.3109/13880209.2013.839714. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Q, Xiao X, Feng K, Wang T, Li W, Yuan T, et al. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid Based Complement Alternat Med. 2011;2011:10. doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu S-S, Yu Y-L, Zhu H-J, Liu X-D, Liu L, Liu Y-W, et al. Berberine promotes glucagon-like peptide-1 (7–36) amide secretion in streptozotocin-induced diabetic rats. J Endocrinol. 2009;200:159–65. doi: 10.1677/JOE-08-0419. [DOI] [PubMed] [Google Scholar]
- 76.Cicero AF, Tartagni E. Antidiabetic properties of berberine:from cellular pharmacology to clinical effects. Hosp Pract. 2012;40:56–63. doi: 10.3810/hp.2012.04.970. [DOI] [PubMed] [Google Scholar]
- 77.Chang W, Zhang M, Li J, Meng Z, Wei S, Du H, et al. Berberine improves insulin resistance in cardiomyocytes via activation of 5′-adenosine monophosphate-activated protein kinase. Metabolism. 2013;62:1159–67. doi: 10.1016/j.metabol.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 78.Ko B-S, Choi SB, Park SK, Jang JS, Kim YE, Park S. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–7. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 79.Pan G-Y, Huang Z-J, Wang G-J, Fawcett JP, Liu X-D, Zhao X-C, et al. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69:632–6. doi: 10.1055/s-2003-41121. [DOI] [PubMed] [Google Scholar]
- 80.Lou T, Zhang Z, Xi Z, Liu K, Li L, Liu B, et al. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation. 2011;34:659–67. doi: 10.1007/s10753-010-9276-2. [DOI] [PubMed] [Google Scholar]
- 81.Yin J, Gao Z, Liu D, Liu Z, Ye J. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. 2008;294:E148–E56. doi: 10.1152/ajpendo.00211.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jeong HW, Hsu KC, Lee J-W, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–E64. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 83.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit Basic Res. 2011;17:RA164–RA7. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Changizi Ashtiyani S, Zarei A, Taheri S, Rezaei A, Golshan M, Ghafarzadegan R. A comparative study of hypolipidemic activities of the extracts of Melissa officinalis and Berberis vulgaris in rats. J Med Plant. 2013;12:38–47. [Google Scholar]
- 85.Zheng B, Wei J, Jiang J, GONG H-h, ZHANG R-s. The therapeutic effects of combination of simvastatin with berberine on the patients with hyperlipemia. Acta Univ Med Nanjing (Nat Sci) 2009;29:1493–7. [Google Scholar]
- 86.Ye W. Clinical efficacy of berberine treatment of diabetes. Mod Hosp. 2010;10:9–10. [Google Scholar]
- 87.JANG C-S. The action of berberine on mammalian hearts. J Pharmacol Exp Ther. 1941;71:178–86. [Google Scholar]
- 88.Kong W-J, Zhang H, Song D-Q, Xue R, Zhao W, Wei J, et al. Berberine reduces insulin resistance through protein kinase C–dependent up-regulation of insulin receptor expression. Metabolism. 2009;58:109–19. doi: 10.1016/j.metabol.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 90.Liu C, Wang Z, Song Y, Wu D, Zheng X, Li P, et al. Effects of berberine on amelioration of hyperglycemia and oxidative stress in high glucose and high fat diet-induced diabetic hamsters in vivo. Biomed Res Int. 2015;2015:9. doi: 10.1155/2015/313808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Di Pierro F, Bellone I, Rapacioli G, Putignano P. Clinical role of a fixed combination of standardized Berberis aristata and Silybum marianum extracts in diabetic and hypercholesterolemic patients intolerant to statins. Diabetes Metab Syndr Obes. 2015;8:89–96. doi: 10.2147/DMSO.S78877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen G, Lu F, Xu L, Dong H, Yi P, Wang F, et al. The anti-diabetic effects and pharmacokinetic profiles of berberine in mice treated with Jiao-Tai-Wan and its compatibility. Phytomedicine. 2013;20:780–6. doi: 10.1016/j.phymed.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 93.Dai P, Wang J, Lin L, Zhang Y, Wang Z. Renoprotective effects of berberine as adjuvant therapy for hypertensive patients with type 2 diabetes mellitus:Evaluation via biochemical markers and color Doppler ultrasonography. Exp Ther Med. 2015;10:869–76. doi: 10.3892/etm.2015.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Huang Y, Lam KSL, Li Y, Wong WT, Ye H, et al. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc Res. 2009;82:484–92. doi: 10.1093/cvr/cvp078. [DOI] [PubMed] [Google Scholar]


