Abstract
Objective(s):
Neurodegenerative diseases have been associated with glutamatergic dysfunction. Berberine, an isoquinoline alkaloid broadly present in different medicinal herbs, has been reported to have neuroprotective effect. In the present study, the effects of berberine against glutamate-induced oxidative damage and apoptosis were investigated.
Materials and Methods:
The cultured PC12 and N2a cells were pretreated (2 hr) with varying concentrations of berberine (50-1000 µM), followed by exposure to glutamate (10 mM) for 24 hr. The cells viability, intracellular reactive oxygen species (ROS), lipid peroxidation, glutathione (GSH) content, superoxide dismutase (SOD) activity, DNA fragmentation and the expressions of pro-apoptotic (cleaved caspase-3 and bax) and anti-apoptotic (bcl-2) proteins were then measured.
Results:
In both cell lines, pretreatment with berberine (especially at low concentrations) significantly decreased ROS generation, lipid peroxidation, and DNA fragmentation, while improving glutathione content and SOD activity in glutamate-injured cells. Moreover, berberine showed anti-apoptotic effects by reducing the glutamate-evoked caspase-3 and bax/bcl-2 overexpression.
Conclusion:
The results of present study suggest that berberine protects against glutamate-induced PC12 and N2a cells injury by decreasing oxidative stress and subsequently inhibiting apoptosis. This is relevant to berberine treatment in neurodegenerative disorders, such as dementia (Alzheimer’s disease), seizures, and stroke.
Keywords: Apoptosis, Berberine, Glutamate cytotoxicity, Neuroprotection, Oxidative injury
Introduction
Glutamate is the main excitatory neurotrans-mitter in the brain (1). It has a critical role in acute and chronic neurodegenerative disorders such as epileptic seizures, ischemia, traumatic brain injury, multiple sclerosis and Alzheimer’s disease (1). Two pathways for glutamate toxicity have been proposed: The first pathway, called excitotoxicity, is mediated by over-stimulation of glutamate receptors resulting in large amounts of extracellular Ca2+ influx (2). The second one is oxidative glutamate toxicity, which occurs through competitive inhibition of cystine/glutamate antiporter system leading to decreased cystine uptake, depleted intracellular glutathione content, increased reactive oxygen species (ROS) production and also NADPH oxidase-dependent extracellular hydrogen peroxide accumulation (3, 4). These processes can lead to neuronal apoptosis (5).
Berberine is an isoquinoline alkaloid, broadly present in different medicinal herbs, particularly in the genus Berberis (6). Berberine is known to have a wide range of biological activities (7-9) such as anticonvulsant (10), neuroprotective in ischemic brain damage (11), antifungal (12), antiviral (13), anti-inflammatory (14), anti-tumor (15) and antidiabetic (16). Berberine has also been shown to modulate mitochondrial and caspase pathways, N-methyl-D-aspartate (NMDA) receptors and potassium currents (17).
Nowadays, more attention has been focused on medicinal plants with advantages of antioxidative and anti-apoptotic effects and low toxicity, as neuro-protective agents (18). Considering the aforementioned beneficial effects of berberine and since N2a and PC12 cells have been widely applied as a neuronal model system for glutamate-induced cytotoxicity (19-21), the present study was conducted to investigate the effects of berberine against glutamate-induced oxidative stress and apoptosis in PC12 and N2a cells.
Materials and Methods
Cell lines and reagents
PC12 and N2a cell lines were purchased from Pasteur Institute (Tehran, Iran). High glucose Dulbecco’s Modified Eagles Medium (DMEM) and fetal calf serum (FCS) were purchased from Gibco (Carlsbad, CA). The 3-(4,5-dimethylthiazol-2-yl)- 2,5-diphenyl tetrazolium (MTT), 2-thiobarbi-turicacid (TBA), trichloroacetic acid (TCA), berberine, monocholorobimane (MCB), 2’,7’-dichlorodihydro-fluorescein diacetate (H2DCF-DA), pyrogallol and glutamate were obtained from Sigma (St. Louis, MO, USA). Low melting point (LMP) agarose and normal melting point (NMP) agarose were purchased from Fermentas (Glen Burnie, MD). Anti-β-actin (4967), caspase-3 (9665), bax (2772), bcl-2 (2870), and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (7074) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Other chemicals mainly ethylene diamine tetraacetic acid disodium salt (Na2EDTA), Tris (hydroxymethyl) aminomethane (Trizma base), toctylphenoxy polyethoxyethanol (Triton X-100), dimethyl sulfoxide (DMSO), sodium lauroylsarcosinate (sarkosyl, SLS), sodium dodecyl-sulfate (SDS) and ethidium bromide were obtained from Merck (Darmstadt, Germany).
Cell proliferation (MTT) assay
N2a and PC12 cells were cultured in high glucose DMEM (4.5 g/l) supplemented with 10% FCS and 100 units/ml of penicillin/streptomycin. All cells were maintained in a humidified atmosphere (90%) containing 5% CO2 at 37 °C. The cell viability was determined using MTT assay as previously described (22). Briefly, N2a and PC12 cells (5000/well) were seeded out in 96 well tissue culture plates, and after 24 hr, the cells were incubated with increasing concentrations of glutamate (0-10 mM) or berberine (0-4000 µM) for 24 hr to calculate the fifty percent inhibitory concentrations (IC50). Stock solutions of glutamate and berberine were prepared in sterile water and DMSO, respectively. The final working concentration of DMSO (plus compound) in working assays was less than 1%, except for 4000 µM berberine which it was 2%. In another set of experiments, the cells were pretreated with berberine (0-2000 µM) for 2 hr and then incubated simultaneously for another 24 hr in complete culture medium which contained 8 mM glutamate. After that, aliquots of 10 μl of MTT solution (5 mg/ml) were added to culture medium and the reaction mixture incubated for 2 hr. Then, the mixture was removed and the resulting formazan dissolved by adding 100 μl DMSO to each well of plate. The optical density of formazan dye was read at 570 and 620 nm (background) using a Stat FAX303 plate reader. The concentrations and times were chosen based on earlier experiments.
Measurement of intracellular reactive oxygen species
The determination of intracellular reactive oxygen species (ROS) levels was accomplished as described previously with minor modifications (23). In brief, PC12 and N2a cells (104) were incubated with 5 µM H2DCF-DA at 37 °C for 30 min in the dark. The H2DCF-DA diffuses through the cell membrane and is hydrolyzed by intracellular esterases to H2DCF, which is then oxidized to highly fluorescent 2’,7’-dichlorofluorescein in the presence of ROS. The cells were pretreated with three different concentrations of berberine (50, 250 and 1000 µM) for 2 hr, then subjected to glutamate (8 mM) toxicity for 1 hr. At the end of treatment, the fluorescence intensity was detected with excitation/emission of 485/530 nm using VICTOR X multilabel plate reader (PerkinElmer, USA). The temperature was maintain-ed at 37°C throughout the experiment.
Lipid peroxidation assay
The lipid peroxidation level was determined by measuring the concentration of malondialdehyde (MDA), which is the end product of lipid peroxida-tion and reacts with TBA to form a fluorescence adduct. In brief, PC12 and N2a cells (104) were incubated for 2 hr with different concentrations of berberine (50, 250, 1000 and 2000 µM) then subjected to glutamate (8 mM) for 24 hr, at the end of treatment, the cells were scraped into TCA (2.5%, 1 ml) and centrifuged at 13000 g at 4 °C for 2 min. The lysate supernatant (500 μl) was removed and added to TCA (15%, 400 μl) and TBA 0.67%/butylated hydroxytoluene 0.01% (800 μl). This mixture was boiled for 20 min and then the reaction was stopped by cooling in an ice water bath. After centrifugation at 2500 rpm for 10 min at 4°C, the fluorescence intensity of supernatant was read in excitation/emission of 530/550 nm. The MDA amounts were expressed as nmol/mg protein. Protein content was determined using BCA kit (24).
Glutathione (GSH) determination
Relative change of intracellular glutathione (GSH) in cells after exposure to glutamate was assayed using monochlorobimane (MCB). The assay is based on the ability of the non-fluorescent substrate MCB to form a fluorescent adduct with GSH. The reaction is catalyzed by the enzyme glutathione-S-transferase (25). The fluorescent probe was dissolved in dimethyl sulfoxide and working solutions in PBS at 20 times the required final concentration (50 µM) was prepared immediately before use and added directly to the cultures in 96-well plates (5 d 103), treated as above. After incubation at 37°C for 30 min in the dark, the fluorescence intensity was monitored at excitation/emission of 360/460 nm. Results were obtained by subtracting blank values and presented as a percentage of control cells.
Determination of superoxide dismutase (SOD) activity
At the end of the treatment period, the cells (105) were harvested from the plates and washed with ice-cold PBS. The cells were then resuspended and incubated with lysis buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1 mM EDTA, 0.2% SDS for 30 min. Cell lysates were prepared by centrifugation at 10,000 rpm for 20 min at 4 °C and assayed for soluble protein content using BCA kit. SOD activity in cell lysates was evaluated using the procedure previously described based on the ability of the enzyme to inhibit the autoxidation of pyrogallol (26). Briefly, 120 µl of cell lysate was added to the reaction mixture containing 0.2 mM pyrogallol in Tris-HCl buffer (pH 8.2, 50 mM) and EDTA (1 mM) to initiate the reaction and absorbance decrease of pyrogallol was spectrophotometrically recorded at 420 nm. The amount of the SOD inhibiting the rate of pyrogallol auto-oxidation by 50% was defined as one enzyme unit and was expressed as U per mg protein (U/mg protein) (27).
Single cell gel electrophoresis (SCGE, comet) assay
The alkaline SCGE assay was conducted based on the method described previously (22). Briefly, PC12 and N2a cells (3 × 105) were incubated for 2 hr with three different concentrations of berberine (50, 250 and 1000 µM (then subjected to glutamate (8 mM) toxicity for another 24 hr. After removing the medium, the cells were washed three times with cold PBS, harvested and centrifuged at 3000 rpm for 5 min at 4°C. The pellets were then resuspended in PBS at a cell density of 1 × 105. For the comet assay, 100 µl NMP agarose was quickly layered on conventional slides, covered with a cover slip, and then the slides were placed on ice to allow agarose to gel. 10 µl of the cell suspension, prepared as above, was mixed with 100 µl LMP agarose, and the mixture was quickly layered over the NMP agarose layer after removal of the cover slip. Finally, another layer of LMP agarose was added on top. The slides were immersed immediately in a chilled lysing solution (pH = 10) made up of 2.5 M NaCl, 100 mM Na2EDTA, 10 mM Trizma, 1% sarkosyl, 10% DMSO, and 1% Triton X-100 and kept at 0°C in the dark overnight. Then, the slides were placed on a horizontal gel electrophoresis platform and covered with a prechilled alkaline solution made up of 300 mM NaOH and 1 mM Na2EDTA (pH > 13). They were left in the solution in the dark at 0°C for 40 min and then electrophoresed at 0°C in the dark for 30 min at 25V and approximately 300 mA. The slides were rinsed gently three times with 400 mM Trizma solution (adjusted to pH 7.5 by HCl) to neutralize the excess alkali, stained with 50 µl of 20 µg/ml ethidium bromide and covered with a cover slip. For comet analysis, 150 nuclei were randomly selected from three replicated slides (50 nuclei on one slide), examined and photographed through a fluorescence microscope (Nikon, Japan), at 400x magnification equipped with an excitation filter of 520-550 nm and a barrier filter of 580 nm. Undamaged cells resemble an intact nucleus without a tail, and damaged cells have the appearance of a comet.
The percent of DNA in the comet tail (% tail DNA), which is an estimation of DNA damage, was analyzed using the computerized image analysis software (CASP software). The experiments were done in triplicate.
Western blot analysis
Western blot analysis was performed as described previously (28). In brief, at the end of treatments, the PC12 cells were washed with ice-cold phosphate buffer saline and lysed with lysis buffer containing 50 mM Tris-HCl, 150 mM NaCl, 2 mM EDTA, 5 mM sodium fluoride, 1 mM sodium orthovanadate, 1% Nonidet P-40 and a protease inhibitor. The lysates were centrifuged at 13000 g for 20 min at 4 °C. The protein concentration of the supernatants was measured using BCA kit. Then, equal amounts of protein from each sample were mixed with loading buffer and boiled for 5 min. Samples were then separated by SDS-polyacrylamide gels electrophoresis and transferred onto polyvinyli-dene fluoride membranes. The membrane was incubated in blocking buffer (50 mM Tris/HCl, 150 mM NaCl, 0.1% Tween 20 and 5% skimmed milk) and the blots were then probed with anti-caspase-3 primary antibody at 4 °C for overnight. The bound antibody was made visible using horse radish peroxidase-conjugated goat anti-rabbit secondary antibody and an enhanced chemiluminescence system. Bands were analyzed by densitometry using Image J software and normalized with respect to the corresponding β-actin band and expressed as fold of control.
Statistical analysis
All results were presented as mean ± SEM. Statistical differences between groups were analyzed by one-way analysis of variance with subsequent Tukey’s tests. P<0.05 was considered to indicate statistical significance.
Results
Berberine increased viability of PC12 and N2a cells after glutamate-induced cytotoxicity
Assessment of glutamate cytotoxicity through the MTT assay exhibited the dose-dependent toxic effects on the viability of PC12 and N2a cells in a concentration range of 0-10 mM. Exposure of N2a and PC12 cells to glutamate (10 mM) condition for 24 hr leads to 92 and 90% reduction in cells viability, respectively. The fifty percent inhibitory concentration (IC50) after 24 hr treatment with glutamate were 1.4 mM and 2.1 mM in N2a and PC12 cells, respectively (Figure 1A).
Figure 1.
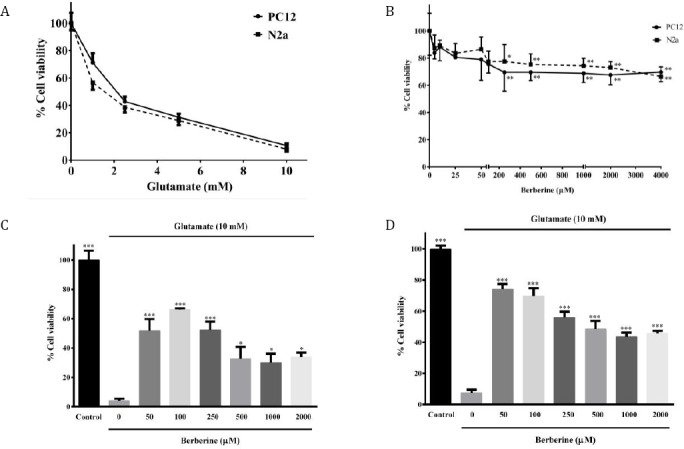
A: Effect of glutamate on viability of N2a and PC12 cells following 24 hr treatment. B: Effect of berberine on viability of N2a and PC12 cells following 24 hr treatment. *P<0.05, ** P<0.01 as compared to untreated control cells. C and D: Effect of berberine on glutamate-induced N2a (C) and PC12 (D) cell death. The cells were pre-treated (2 hr) with various concentrations of berberine and then exposed to glutamate (10 mM) for 24 hr, in which the same treatments were applied. * P<0.05, ** P<0.01, *** P<0.001 as compared to untreated cells cultured in glutamate condition. The data were expressed as percentage viability of control cells and presented as means ± SEM from three independent experiments
As illustrated in Figure 1B, treatment with berbe-rine alone (0-4000 µM) for 24 hr, concentration-dependently decreased cells viability at concentra-tions above 200 μM with a maximum reduction of cells viability of 30% and 34% upon treatment of PC12 and N2a cells with 4000 µM berberine, respectively. However, no significant toxicity was observed following incubation of PC12 and N2a cells with berberine (0-4000 µM) for 2 hr (data not shown).
On the other hand, berberine significantly decreas-ed glutamate-induced N2a and PC12 cells death. Figure 1C shows that pretreatment with berberine)50-2000 μM) for 2 hr significantly increased the viability of N2a cells to 51.7±8.0 (50 μM, P< 0.001); 66.2±1.0 (100 μM, P< 0.001); 52.4±5.5 (250 μM, P<0.001); 32.7±8.0 (500 μM, P<0.05); 30.1 ± 6.0 (1000 μM, P<0.05) and 33.9±3.0 (2000 μM, P<0.05), as compared with the untreated cells in 10 mM glutamate condition (4.0± 1.4%). It is obvious that the protective effect of berberine was more significant at lower concentrations and decreased with increasing concentration. Similarly, when PC12 cells were pretreated with 50, 100, 250, 500, 1000 and 2000 μM berberine for 2 hr and then exposed to glutamate cytotoxicity, the viability of the cells wasincreased from 10.0±1.0 % (untreated cells in 10 mM glutamate condition) to 74.0±3.4, 69.8±4.9, 56.0 ±3.7, 48.4±5.3, 43.5±2.8 and 45.7±1.6, respectively (Figure 1D, P<0.001 for all). Again, more significant neuroprotective effects were seen at lower concentrations.
Berberine attenuated glutamate-induced genera-tion of ROS
ROS generation was significantly elevated in PC12 (approximately 2 fold) and N2a (approximately 3 fold) cells after exposure to 10 mM glutamate for 2 hr, compared to the control group (Figure 2A-B P<0.001). Therefore, glutamate-induced cytotoxicity in PC12 cells was indeed associated with increased ROS. As shown in Figure 2A, pre-treatment of N2a cells with berberine (for 2 hr) at different concentrations (50, 100, 250, 500 and 1000 μM) significantly reduced glutamate-induced ROS production to 96.7±1.8 %, 152.8 ± 24.8 %, 117.7± 3.8 %, 104.5±2.4 % and 108.6±3.5 %, respectively (P<0.001 for all). Again, PC12 cultures pretreated with berberine at different concentrations (50, 100, 250, 500 and 1000 μM) showed significantly (P <0.001 for all) reduced intensity of DCF labeled cells (86.1±9.4, 132.1±11.9, 125.3±8.8, 100.1±2.4 and 112.7±3.0 %, respectively) when compared to glutamate-treated cultures (202.7±7.8) (Figure 2B).
Figure 2.
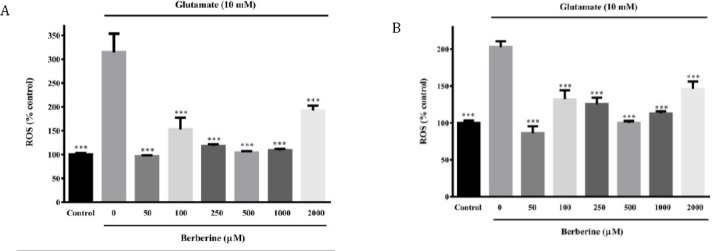
Effect of berberine on intracellular reactive oxygen species (ROS) content of glutamate-injured N2a (A) and PC12 (B) cells. The cells were pre-treated (2 hr) with various concentrations of berberine and then exposed to glutamate (10 mM) for 24 hr, in which the same treatments were applied. The data were expressed as means ± SEM from three independent experiments. ***P<0.001 as compared to untreated cells cultured in glutamate condition
Berberine decreased glutamate-induced lipid peroxidation increase
When N2a and PC12 cells were exposed to glutamate (10 mM) for 24 hr, an increase in lipid peroxidation level, as indicated by the excessive formation of MDA, was observed to approximately 2.1 and 1.7 folds of control values (Figure 3A-B).
Figure 3.
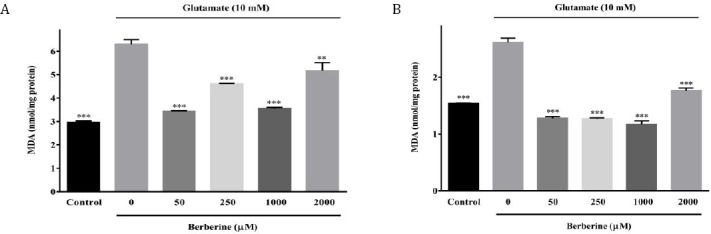
Effect of berberine on intracellular malondialdehyde (MDA) content of glutamate-injured N2a (A) and PC12 (B) cells. The cells were pre-treated (2 hr) with various concentrations of berberine and then exposed to glutamate (10 mM) for 24 hr, in which the same treatments were applied. The data were expressed as means ± SEM from three independent experiments. **P<0.01, ***P<0.001 as compared to untreated cells cultured in glutamate condition
As shown in Figure 3A, pre-treatment of the N2a cells with 50, 250 and 1000 μM berberine signifi-cantly alleviated glutamate-induced lipid peroxida-tion from 6.30±0.20 nmol/mg protein (untreated cells in 10 mM glutamate condition) to 3.44±0.02, 4.62±0.02 and 3.56±0.05 nmol/mg protein, respectively (P<0.001 for all). In the same manner, when berberine (50, 250 and 1000 μM)-pretreated PC12 cells were exposed to glutamate injury, MDA formation was significantly (P<0.001 for all) decreased to 1.28±0.03, 1.27±0.02 and 1.17±0.06 nmol/mg protein, as compared to glutamate-injured cells (2.62 ± 0.08 nmol/mg protein) (Figure 3B).
Berberine enhanced GSH level upon oxidative glutamate toxicity
Exposure of PC12 and N2a cells to glutamate (10 mM for 24 hr) resulted in a significant decrease of GSH percentage (33.2%±5.3 and 21.6%±3.7 for PC12 and N2a cells, respectively) compared to control cells. Significant increases in GSH content were observed with pretreatments (2 hr) with all berberine concentrations (50-1000 µM), compared to glutamate- injured cells (Figure 4).
Figure 4.
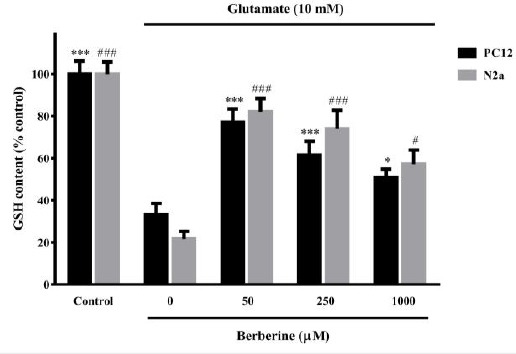
Effect of berberine on intracellular glutathione (GSH) content of glutamate-injured N2a and PC12 cells. The cells were pre-treated (2 hr) with various concentrations of berberine and then exposed to glutamate (10 mM) for 24 hr, in which the same treatments were applied. The data were expressed as means±SEM from three independent experiments. #P<0.05, ###P<0.001 as compared to untreated N2a cells cultured in glutamate condition. *P<0.05, ***P<0.001 as compared to untreated PC12 cells cultured in glutamate condition
Berberine improved SOD activity in the glutamate-injured cells
Glutamate exposure caused a significant decrease of SOD activity in PC12 (P<0.001) and N2a (P<0.01) cells from 2.8 and 2.2 IU/min/mg protein to 1.3 and 1.0 IU/min/mg protein, respectively (Figure 5). In PC12 cells, berberine (50, 250 and 1000 µM) significantly improved SOD activity to 2.9, 3.2 and 2.6 IU/min/mg protein, respectively (P<0.001 for all). In the same way, treatment with 50, 250 or 1000 µM berberine-induced a marked increase in SOD activity in N2a cells to 2.5 (P<0.001), 2.2 (P<0.001) and 1.9 (P<0.05) IU/min/mg protein, respectively (Figure 5).
Figure 5.
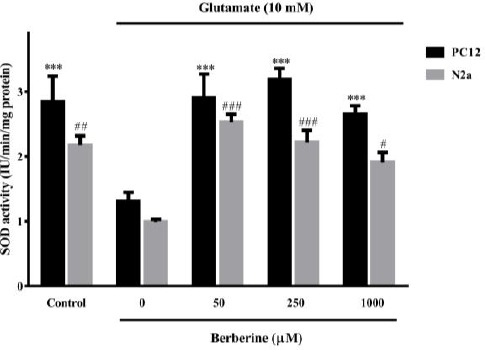
Effect of berberine on superoxide dismutase (SOD) activity in glutamate-injured N2a and PC12 cells. The cells were pre-treated (2 hr) with various concentrations of berberine and then exposed to glutamate (10 mM) for 24 hr, in which the same treatments were applied. The data were expressed as means±SEM from three independent experiments. #P<0.05, ##P<0.01, ###P<0.001 as compared to untreated N2a cells cultured in glutamate condition. *** P<0.001 as compared to untreated PC12 cells cultured in glutamate condition
Berberine significantly diminished glutamate-induced DNA fragmentation
In this study, % tail DNA was measured as an indicator of DNA damage. The results showed that exposure of PC12 and N2a cells to glutamate (10 mM for 24 hr) significantly increased DNA fragmentation to 28.8%±4.2 and 31.8%±5.0, respectively (P<0.001 for both cell lines) (Fig 6A-B).
Figure 6.
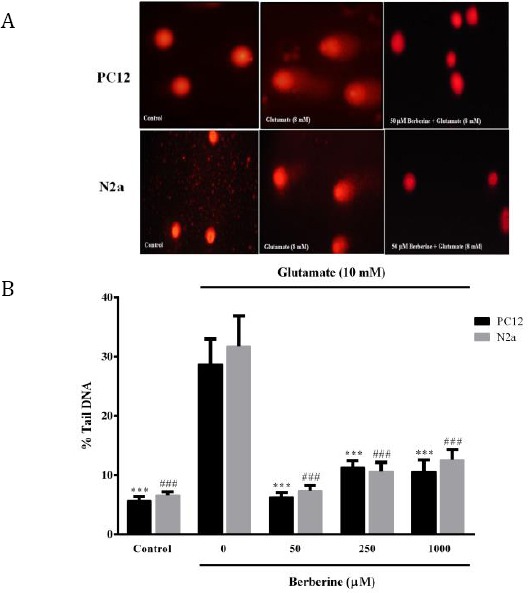
Effect of berberine on glutamate-induced DNA damage in N2a and PC12 cells. A: Representative comet images of N2a and PC12 cells from different treatment groups. B: Bar graphs representing the percent of DNA in the comet tail (% tail DNA) of N2a and PC12 cells, as an index for the extent of DNA damage. The data were expressed as means±SEM from three independent experiments. ###P<0.001 as compared to untreated N2a cells cultured in glutamate condition. ***P<0.001 as compared to untreated PC12 cells cultured in glutamate condition
All three concentrations of berberine (50, 250 and 1000 µM) markedly decreased glutamate-induced DNA damage approximately 4.5-, 2.5- and 2.7-fold in PC12 cells and 4.3-, 3.0- and 2.5-fold in N2a cells, respectively (Figure 6A-B).
Berberine significantly decreased cleaved caspase-3 and bax/bcl-2 expressions in the glutamate-injured cells
As illustrated in Figure 7A-C, glutamate oxidative injury led to a substantial increase in the expression of cleaved caspase-3 (5.1 fold) and bax/bcl-2 ratio (3.6 fold), as compared to the control cells (P<0.001). Treatment with berberine (50 µM) markedlydecreased cleaved caspase-3 and bax/bcl-2 expre-ssion to 2.2 (P<0.001) and 2.0 (P<0.05) fold of control value, respectively (Figure 7A-C).
Figure 7.
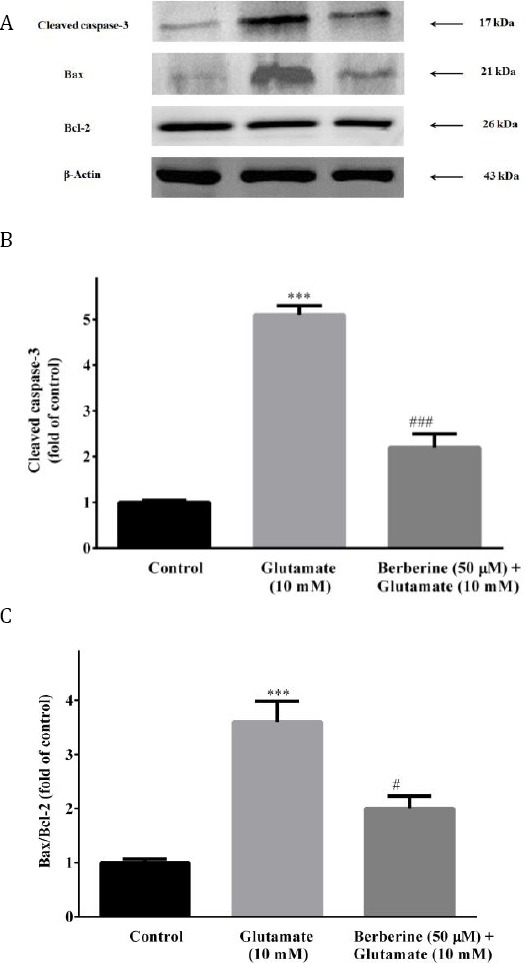
Effects of berberine on expressions of pro-apoptotic (cleaved caspase-3 and bax) and anti-apoptotic (bcl-2) proteins in glutamate-injured PC12 cells. The blots (A) are representative of six different experiments with similar results and the bar graphs showing relative protein band expressions of cleaved caspase-3 (B) and bax/bcl-2 ratio (C) with β-actin as a protein loading control. The data were expressed as means as mean ± SEM (n=5). ***P<0.01 as compared to untreated control cells. #P<0.05, ###P< 0.001 as compared to glutamate-injured cells
Discussion
The present study showed that treatment of PC12 and N2a cells with low concentrations of berberine up to 200 µM for 24 hr or concentrations as high as 4000 µM for 2 hr did not significantly affect cellular viability. Moreover, the current study demonstrated that berberine exerts neuroprotective effects against glutamate-induced N2a and PC12 cytotoxicity via antioxidant and anti-apoptotic mechanisms. Berberine, especially at concentrations as low as 50 µM, significantly decreased ROS generation and lipid peroxidation, while improving glutathione content and SOD activity in glutamate-injured cells. In addition, berberine significantly attenuated glutamate-evoked DNA fragmentation, caspase-3, and bax/bcl-2 overexpressions. Besides, the protective effect of berberine was more significant at lower concentrations and decreased with increasing concentration. The present findings are in line with other researches; Peng et al reported that 20 mg/kg berberine increased norepinephrine and serotonin levels in the rat’s hippocampus and frontal cortex but at higher doses (100 and 500 mg/kg) decreased the concentrations of these neurotransmitters in brain stem (29, 30). Vaziri et al also showed that 10 and 20 mg/kg berberine attenuated harmaline-induced tremor in rats, however at 50 mg/kg not only failed to recover but also showed an adverse effect on tremors (31). It has also been shown that berberine exhibits multiphasic action in melanoma cells. Berberine at low concentrations mainly causes mitochondrial dysfunction while at high concentrations directly interacts with DNA, triggering apoptosis (32). As DNA binding molecule, high doses of berberine is required to translocate to the nucleus and induce mitotic arrest by interfering with DNA and block G2 cells from entering the M phase (32, 33). Berberine has also been shown to have neurotoxic actions against dopaminergic neurons, in vitro or in vivo, at high doses (34, 35).
In this study, about 90% cells loss was seen under glutamate toxicity for 24 hr. Consistent with our results; Froissard and Duval revealed that treatment of PC12 with 10 mM glutamate for 24 hr led to 70% cell lysis, as estimated by lactate dehydrogenase release (36). In another study, Calderon et al reported that the viability of N2a and PC12 cells exposed to 20 mM glutamate for 4 hr decreased about 60 % and 96 %, respectively (37). Another study also showed that exposure to 5, 50 and 30 mM glutamate for 24 hr induced approximately 80% cell death in HT22, SH-SY5Y and PC12, respectively (4). Ma et al also showed that the viability of PC12 cells exposed to 10 mM glutamate for 12 hr was about 51 % (19).
The improved cellular redox status and the decreased apoptosis by berberine described here are in agreement with other findings. Zhang et al showed that berberine protects hypoxia-treated mesenchymal stem cells by suppressing ROS-dependent and JNK-driven cell apoptosis via PI3K/Akt signaling pathway (38). In the same way, it was found that berberine at nanomolar concentrations attenuates hydrogen peroxide-induced oxidative stress and apoptosis in motor neuronal NSC34 cells through PI3K/Akt-dependent mechanisms. They found that berberine increases endogenous antioxidants (GSH level and SOD activity), oxidant-sensitive proteins (heme oxygenase 1 and nuclear factor-like 2) and the antiapoptotic protein bcl-2 while decreases the expressions of proapoptotic proteins (cytochrome c, bax and cleaved caspase-3 and -9) in hydrogen peroxide-treated NSC34 cells (39). Zhou et al also described the neuroprotective effects of berberine against ischemic brain injury, in vitro and in vivo, by inhibiting generation of ROS and releases of cytochrome c and apoptosis-inducing factors (AIFs) (40). It was reported that berberine is able to restore the glutamate-evoked changes in oxidative status and tissue transglutaminase, a calcium-dependent enzyme of transglutaminase family involved in several neurodegenerative disorders including Alzheimer’s, and Parkinson’s diseases, in astroglial cells cultures system (41). A recent study also showed that 25 µg/ml berberine markedly improved viability, axonal outgrowth and transport in calyculin A-injured N2a cells. The effects were shown to be mediated by modulating the activity of protein phosphatase 2A, MDA level, and SOD activity and also by inhibiting the hyperphosphorylation of tau and neurofilaments (42).
It is well documented that glutamate induces neurotoxicity through over-activation of glutamate (including NMDA) receptors and competitive inhibition of cysteine/glutamate antiporter, leading to excitotoxicity and oxidative damages resulting apoptotic cell death (43). Several studies suggested that berberine might have antagonistic effects on ionotropic glutamate receptors. Berberine has been shown to inhibit kainic acid-induced clonic convulsions and NMDA-induced turning behavior (44). Consistently, Cui et al revealed that berberine protects ischemia and NMDA-induced neuronal damage in mice hippocampal slice cultures which is comparable with the NMDA antagonist MK-801 and is mediated, at least in part, via the suppression of Bcl-2 phosphorylation (45). In another study, berberine promoted survival of hydrogen peroxide-induced neuronal cells death and MK801-induced brain degeneration in developing neonatal rats (46). A recent study also confirmed that a standardized berberine extract of barberry protects against ischemic damage via the reduction of NMDA receptor type 1 (NR1) immunoreactivity in the gerbil hippocampal CA1 region (47). It has also been shown that berberine reduces dopamine D1 and NMDA receptors bindings in mouse cortex and inhibits NMDA receptor channel current in Xenopus oocytes with voltage-independent manner (48). In contrast, a recent study revealed that berberine at micromolar concentrations decreased the viability of primary neurons in a caspase-independent but mitochondria- and NMDA-dependent manner. In addition, pretreat-ment with berberine at nanomolar concentrations sensitized neurons to both glutamate excitotoxicity and rotenone injury (17).
Previous studies proposed that voltage-activated potassium currents, especially delayed rectified potassium current (IK) and transient outward current (IA), in addition to NMDA receptor-mediated K+ efflux, contribute to glutamate-induced apoptosis and therefore inhibition of outward potassium currents (especially IK) might have therapeutic effects against some neurodegenerative diseases (49, 50). Indeed, it is believed that physiologic intracellular potassium concentration (∼140 mM) inhibits caspase-3 activation and apoptotic DNA fragmentation (51). Wang et al reported that berberine blocks IA and IK currents in CA1 pyramidal neurons of rat hippocampus (52). In the same way, Wu et al showed that tetrahydroberberine inhibits receptor-mediated outward currents in acutely dissociated CA1 neurons from rat hippocampus via direct blockade of membrane K+ channels (53). Therefore, blockade of K+ channels by berberine might be suggested as one of the protective mechanisms for the suppression of apoptosis and the increased rate of cell survival, described in the current study. It is noteworthy to mention that the inhibition of NMDA receptors may compensate by blockade of K+ channels at high doses of berberine, resulting in decreased protective effects (54).
Also, The role of calcium in the cytotoxic action of glutamate has been well documented and calcium channel blockers have been shown to oppose glutamate-induced apoptosis or necrosis (55). The blocking effects on both L- and T-type calcium channels have been described for berberine (56). Nadjafi et al demonstrated that berberine protects OLN-93 oligodendrocytes against ischemic-induced cell death by attenuating the intracellular Ca2+ overload similar to the NMDA or the AMPA/kainate receptors antagonists (57).
Conclusion
The results of present study suggest that berberine protects against glutamate-induced PC12 and N2a cells injury by decreasing oxidative stress and subsequently inhibiting apoptosis.
Acknowledgment
The present study was extracted from the Pharm D thesis of Monireh Kolangikhah and financially supported by a grant (No. 900593) from the Office of the Vice Chancellor for Research and Technology of Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflügers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- 2.Mehta A, Prabhakar M, Kumar P, Deshmukh R, Sharma PL. Excitotoxicity:bridge to various triggers in neurodegenerative disorders. Eur J Pharmacol. 2013;698:6–18. doi: 10.1016/j.ejphar.2012.10.032. [DOI] [PubMed] [Google Scholar]
- 3.Bridges RJ, Natale NR, Patel SA. System xc(-) cystine/glutamate antiporter:an update on molecular pharmacology and roles within the CNS. Br J Pharmacol. 2012;165:20–34. doi: 10.1111/j.1476-5381.2011.01480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ha JS, Lim HM, Park SS. Extracellular hydrogen peroxide contributes to oxidative glutamate toxicity. Brain Research. 2010;1359:291–297. doi: 10.1016/j.brainres.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 5.Froissard P, Monrocq H, Duval D. Role of glutathione metabolism in the glutamate-induced programmed cell death of neuronal-like PC12 cells. Eur J Pharmacol. 1997;326:93–99. doi: 10.1016/s0014-2999(97)00155-6. [DOI] [PubMed] [Google Scholar]
- 6.Ahmed T, Abdollahi M, Daglia M, Nabavi SF, Nabavi SM. Berberine and neurodegeneration:A review of literature. Pharmacological Reports. 2015;67:970–979. doi: 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Imenshahidi M, Hosseinzadeh H. Berberis vulgaris and berberine:an update review. Phytother Res. 2016;30:1745–1764. doi: 10.1002/ptr.5693. [DOI] [PubMed] [Google Scholar]
- 8.Imanshahidi M, Hosseinzadeh H. Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 9.Tabeshpour J, Imenshahidi M, Hosseinzadeh H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome Iran J Basic Med Sci. 2017;20:557–568. doi: 10.22038/IJBMS.2017.8682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhutada P, Mundhada Y, Bansod K, Dixit P, Umathe S, Mundhada D. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav. 2010;18:207–210. doi: 10.1016/j.yebeh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang F, Zhao G, Cheng L, Zhou H-Y, Fu L-Y, Yao W-X. Effects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Brain Res. 2004;999:91–97. doi: 10.1016/j.brainres.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 12.Sarma B, Pandey V, Mishra G, Singh U. Antifungal activity of berberine iodide, a constituent of Fumaria indica. Folia Microbiol. 1999;44:164–166. [Google Scholar]
- 13.Hayashi K, Minoda K, Nagaoka Y, Hayashi T, Uesato S. Antiviral activity of berberine and related compounds against human cytomegalovirus. Bioorg Med Chem Lett. 2007;17:1562–1564. doi: 10.1016/j.bmcl.2006.12.085. [DOI] [PubMed] [Google Scholar]
- 14.Kuo C-L, Chi C-W, Liu T-Y. The anti-inflammatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Katiyar SK, Meeran SM, Katiyar N, Akhtar S. p53 cooperates berberine-induced growth inhibition and apoptosis of non-small cell human lung cancer cells in vitro and tumor xenograft growth in vivo. Mol Carcinog. 2009;48:24–37. doi: 10.1002/mc.20453. [DOI] [PubMed] [Google Scholar]
- 16.Punitha ISR, Shirwaikar A, Shirwaikar A. Antidiabetic activity of benzyl tetra isoquinoline alkaloid berberine in streptozotocin-nicotinamide induced type 2 diabetic rats. Diabetol Croat. 2005;34:117–128. [Google Scholar]
- 17.Kysenius K, Brunello CA, Huttunen HJ. Mitochondria and NMDA receptor-dependent toxicity of berberine sensitizes neurons to glutamate and rotenone injury. PloS one. 2014;9:e107129. doi: 10.1371/journal.pone.0107129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Snafi AE. Therapeutic properties of medicinal plants:a review of medicinal plants with central nervous effects (part 1) Int J Pharmacol Toxicol. 2015;5:177–192. [Google Scholar]
- 19.Ma S, Liu H, Jiao H, Wang L, Chen L, Liang J, et al. Neuroprotective effect of ginkgolide K on glutamate-induced cytotoxicity in PC 12 cells via inhibition of ROS generation and Ca 2+influx. Neurotoxicology. 2012;33:59–69. doi: 10.1016/j.neuro.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y, Wang W, Hao C, Mao X, Zhang L. Astaxanthin protects PC12 cells from glutamate-induced neurotoxicity through multiple signaling pathways. J Func Foods. 2015;16:137–151. [Google Scholar]
- 21.Lee SM, Yang EJ, Choi S-M, Kim SH, Baek MG, Jiang JH. Effects of bee venom on glutamate-induced toxicity in neuronal and glial cells. Evid Based Complement Alternat Med. 2011;2012:368196. doi: 10.1155/2012/368196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forouzanfar F, Afkhami Goli A, Asadpour E, Ghorbani A, Sadeghnia HR. Protective effect of Punica granatum L. against serum/glucose deprivation-induced PC12 cells injury. Evid Based Complement Alternat Med. 2013;2013:3716730. doi: 10.1155/2013/716730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Rad Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 24.Ghorbani A, Sadeghnia HR, Asadpour E. Mechanism of protective effect of lettuce against glucose/serum deprivation-induced neurotoxicity. Nutr Neurosci. 2015;18:103–109. doi: 10.1179/1476830513Y.0000000107. [DOI] [PubMed] [Google Scholar]
- 25.Sebastia J, Cristofol R, Martin M, Rodriguez-Farre E, Sanfeliu C. Evaluation of fluorescent dyes for measuring intracellular glutathione content in primary cultures of human neurons and neuroblastoma SH-SY5Y. Cytometry A. 2003;51:16–25. doi: 10.1002/cyto.a.10003. [DOI] [PubMed] [Google Scholar]
- 26.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 27.Percival SS. Cu/Zn superoxide dismutase activity does not parallel copper levels in copper supplemented HL-60 cells. Biol Trace Elem Res. 1993;38:63–72. doi: 10.1007/BF02783983. [DOI] [PubMed] [Google Scholar]
- 28.Ghorbani A, Sadeghnia HR, Asadpour E. Mechanism of protective effect of lettuce against glucose/serum deprivation-induced neurotoxicity. Nutr Neurosci. 2015;18:103–109. doi: 10.1179/1476830513Y.0000000107. [DOI] [PubMed] [Google Scholar]
- 29.Peng WH, Wu CR, Chen CS, Chen CF, Leu ZC, Hsieh MT. Anxiolytic effect of berberine on exploratory activity of the mouse in two experimental anxiety models:interaction with drugs acting at 5-HT receptors. Life Sci. 2004;75:2451–2462. doi: 10.1016/j.lfs.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 30.Peng WH, Lo KL, Lee YH, Hung TH, Lin YC. Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 2007;81:933–938. doi: 10.1016/j.lfs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Vaziri Z, Abbassian H, Sheibani V, Haghani M, Nazeri M, Aghaei I, et al. The therapeutic potential of Berberine chloride hydrate against harmaline-induced motor impairments in a rat model of tremor. Neurosci Lett. 2015;590:84–90. doi: 10.1016/j.neulet.2015.01.078. [DOI] [PubMed] [Google Scholar]
- 32.Serafim TL, Oliveira PJ, Sardao VA, Perkins E, Parke D, Holy J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemother Pharmacol. 2008;61:1007–1018. doi: 10.1007/s00280-007-0558-9. [DOI] [PubMed] [Google Scholar]
- 33.Tsang CM, Lau EPW, Di K, Cheung PY, Hau PM, Ching YP, et al. Berberine inhibits Rho GTPases and cell migration at low doses but induces G2 arrest and apoptosis at high doses in human cancer cells. Int J Mol Med. 2009;24:131. doi: 10.3892/ijmm_00000216. [DOI] [PubMed] [Google Scholar]
- 34.Shin KS, Choi HS, Zhao TT, Suh KH, Kwon IH, Choi SO, et al. Neurotoxic effects of berberine on long-term L-DOPA administration in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Arch Pharm Res. 2013;36:759–767. doi: 10.1007/s12272-013-0051-4. [DOI] [PubMed] [Google Scholar]
- 35.Kwon IH, Choi HS, Shin KS, Lee BK, Lee CK, Hwang BY, et al. Effects of berberine on 6-hydroxydopamine-induced neurotoxicity in PC12 cells and a rat model of Parkinson’s disease. Neurosci Lett. 2010;486:29–33. doi: 10.1016/j.neulet.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 36.Froissard P, Duval D. Cytotoxic effects of glutamic acid on PC12 cells. Neurochem Int. 1994;24:485–493. doi: 10.1016/0197-0186(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 37.Calderon FH, Bonnefont A, Muñoz FJ, Fernández V, Videla LA, Inestrosa NC. PC12 and neuro 2a cells have different susceptibilities to acetylcholinesterase–amyloid complexes, amyloid25–35 fragment, glutamate, and hydrogen peroxide. J Neurosci Res. 1999;56:620–631. doi: 10.1002/(SICI)1097-4547(19990615)56:6<620::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W, Su X, Gao Y, Sun B, Yu Y, Wang X, et al. Berberine protects mesenchymal stem cells against hypoxia-induced apoptosis in vitro. Biol Pharmac Bull. 2009;32:1335–1342. doi: 10.1248/bpb.32.1335. [DOI] [PubMed] [Google Scholar]
- 39.Hsu Y-Y, Chen C-S, Wu S-N, Jong Y-J, Lo Y-C. Berberine activates Nrf2 nuclear translocation and protects against oxidative damage via a phosphatidylinositol 3-kinase/Akt-dependent mecha-nism in NSC34 motor neuron-like cells. Eur J Pharmac Sci. 2012;46:415–425. doi: 10.1016/j.ejps.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Zhou X-Q, Zeng X-N, Kong H, Sun X-L. Neuroprotective effects of berberine on stroke models in vitro and in vivo. Neurosci Lett. 2008;447:31–36. doi: 10.1016/j.neulet.2008.09.064. [DOI] [PubMed] [Google Scholar]
- 41.Campisi A, Acquaviva R, Mastrojeni S, Raciti G, Vanella A, De Pasquale R, et al. Effect of berberine and Berberis aetnensis C. Presl. alkaloid extract on glutamate-evoked tissue transglutaminase up-regula-tion in astroglial cell cultures. Phytother Res. 2011;25:816–820. doi: 10.1002/ptr.3340. [DOI] [PubMed] [Google Scholar]
- 42.Liu X, Zhou J, Abid MDN, Yan H, Huang H, Wan L, et al. Berberine attenuates axonal transport impairment and axonopathy induced by calyculin a in N2a cells. PloS one. 2014;9 doi: 10.1371/journal.pone.0093974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kritis AA, Stamoula EG, Paniskaki KA, Vavilis TD. Researching glutamate-induced cytotoxicity in different cell lines:a comparative/collective analysis/study. Front Cell Neurosci. 2015;9:91. doi: 10.3389/fncel.2015.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bhutada P, Mundhada Y, Bansod K, Dixit P, Umathe S, Mundhada D. Anticonvulsant activity of berberine, an isoquinoline alkaloid in mice. Epilepsy Behav. 2010;18:207–210. doi: 10.1016/j.yebeh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Cui HS, Matsumoto K, Murakami Y, Hori H, Zhao Q, Obi R. Berberine exerts neuroprotective actions against in vitro ischemia-induced neuronal cell damage in organotypic hippocampal slice cultures:involvement of B-cell lymphoma 2 phosphorylation suppression. Biol Pharmac Bull. 2009;32:79–85. doi: 10.1248/bpb.32.79. [DOI] [PubMed] [Google Scholar]
- 46.Lee T, Heo H, Kim Kwon Y. Effect of berberine on cell survival in the developing rat brain damaged by MK-801. Exp Neurobiol. 2010;19:140–145. doi: 10.5607/en.2010.19.3.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoo KY, Hwang IK, Lim BO, Kang TC, Kim DW, Kim SM, et al. Berberry extract reduces neuronal damage and N-Methyl-D-aspartate receptor 1 immunoreactivity in the gerbil hippocampus after transient forebrain ischemia. Biol Pharmac Bull. 2006;29:623–628. doi: 10.1248/bpb.29.623. [DOI] [PubMed] [Google Scholar]
- 48.Yoo JH, Yang EM, Cho JH, Lee JH, Jeong SM, Nah SY, et al. Inhibitory effects of berberine against morphine-induced locomotor sensitization and analgesic tolerance in mice. Neuroscience. 2006;142:953–961. doi: 10.1016/j.neuroscience.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Zhao YM, Sun LN, Zhou HY, Wang XL. Voltage-dependent potassium channels are involved in glutamate-induced apoptosis of rat hippocampal neurons. Neurosci Lett. 2006;398:22–27. doi: 10.1016/j.neulet.2005.12.073. [DOI] [PubMed] [Google Scholar]
- 50.Yu SP, Yeh C, Strasser U, Tian M, Choi DW. NMDA receptor-mediated K+efflux and neuronal apoptosis. Science. 1999;284:336–339. doi: 10.1126/science.284.5412.336. [DOI] [PubMed] [Google Scholar]
- 51.Yu SP. Regulation and critical role of potassium homeostasis in apoptosis. Prog Neurobiol. 2003;70:363–386. doi: 10.1016/s0301-0082(03)00090-x. [DOI] [PubMed] [Google Scholar]
- 52.Wang F, Zhao G, Cheng L, Zhou HY, Fu LY, Yao WX. Effects of berberine on potassium currents in acutely isolated CA1 pyramidal neurons of rat hippocampus. Brain Res. 2004;999:91–97. doi: 10.1016/j.brainres.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Jin GZ. Tetrahydroberberine blocks membrane K+channels underlying its inhibition of intracellular message-mediated outward currents in acutely dissociated CA1 neurons from rat hippocampus. Brain Res. 1997;775:214–218. doi: 10.1016/s0006-8993(97)00960-8. [DOI] [PubMed] [Google Scholar]
- 54.Vaziri Z, Abbassian H, Sheibani V, Haghani M, Nazeri M, Aghaei I, et al. The therapeutic potential of Berberine chloride hydrate against harmaline-induced motor impairments in a rat model of tremor. Neurosci Lett. 2015;590:84–90. doi: 10.1016/j.neulet.2015.01.078. [DOI] [PubMed] [Google Scholar]
- 55.Sendrowski K, Rusak M, Sobaniec P, Ilendo E, Dabrowska M, Bockowski L, et al. Study of the protective effect of calcium channel blockers against neuronal damage induced by glutamate in cultured hippocampal neurons. Pharmacol Rep. 2013;65:730–736. doi: 10.1016/s1734-1140(13)71052-1. [DOI] [PubMed] [Google Scholar]
- 56.Xu S, Zhang Y, Ren J, Zhou Z. Effects of berberine of L-and T-type calcium channels in guinea pig ventricular myocytes. Zhongguo Yao Li Xue Bao. 1997;18:515–518. [PubMed] [Google Scholar]
- 57.Nadjafi S, Ebrahimi S-A, Rahbar-Roshandel N. Protective effects of berberine on oxygen-glucose deprivation/reperfusion on oligodendrocyte cell line (OLN-93) Int J Prevent Med. 2014;5:1153–1160. [PMC free article] [PubMed] [Google Scholar]


