Summary
Objective
Obesity is an established risk factor for cardiovascular disease. The mechanisms by which obesity affects cardiovascular risk have not been fully elucidated. This paper reports a comprehensive systematic review and meta‐analysis on obesity and two key aspects of vascular health using gold‐standard non‐invasive measures – arterial endothelial function (brachial flow‐mediated dilatation) and subclinical atherosclerosis (carotid intima‐media thickness).
Methods
Electronic searches for ‘Obesity and flow‐mediated dilatation’ and ‘Obesity and intima‐media thickness’ were performed using Ovid Medline and Embase databases. A meta‐analysis was undertaken for brachial flow‐mediated dilatation and carotid intima‐media thickness to obtain pooled estimates for adults with obesity and those with healthy weight.
Results
Of the 5,810 articles retrieved, 19 studies on flow‐mediated dilatation and 19 studies on intima‐media thickness were included. Meta‐analysis demonstrated that obesity was associated with lower flow‐mediated dilatation (−1.92 % [95% CI −2.92, −0.92], P = 0.0002) and greater carotid intima‐media thickness (0.07 mm [95% CI 0.05, 0.08], P < 0.0001).
Conclusions
Obesity is associated with poorer arterial endothelial function and increased subclinical atherosclerosis, consistent with these aspects of vascular health at least partially contributing to the increased risk of cardiovascular events in adults with obesity. These estimated effect sizes will enable vascular health benefits in response to weight loss treatment to be put in greater perspective, both in the research setting and potentially also clinical practice.
Keywords: Atherosclerosis, endothelial function, meta‐analysis, obesity
Introduction
Cardiovascular events such as myocardial infarction and stroke are a major cause of morbidity and mortality 1. Atherosclerosis is the disease process that underlies the majority of cardiovascular events and is characterized by endothelial dysfunction and arterial wall thickening. The non‐invasive methodologies most commonly used to assess these aspects of vascular health in research studies are brachial artery flow‐mediated dilatation (FMD) and carotid intima‐media thickness (IMT). Both have been used extensively to study the effects of putative risk factors on the vasculature and predict future risk of cardiovascular events 2, 3.
Obesity is an important cardiovascular risk factor. A number of studies have determined the associations of obesity with markers of vascular health, albeit with mixed findings. Studies of obesity and brachial FMD have shown either preserved or impaired endothelial function 4, 5, 6, 7. In contrast, studies of carotid IMT have usually shown increased IMT in people with obesity, although with markedly different effect size estimates. Clarifying these associations has implications for the mechanisms linking obesity with cardiovascular events and will have implications for treatment strategies, particularly those that specifically seek to lower cardiovascular risk in obesity. For instance, a clear indication of the effect size of the increased carotid IMT in obesity will enable a more accurate estimation of the proportion of cardiovascular risk in obesity that is related to atherosclerotic vascular disease, and as such assist in identifying the mechanisms to target for the likely most efficacious approach to reducing risk of heart disease in people with obesity. Similarly if obesity is accompanied by lower brachial FMD, the use of strategies that improve endothelial function, independent of weight loss, may become viable to lower risk of cardiovascular events. Weight loss itself has been demonstrated to be associated with increases in brachial FMD and reductions in carotid IMT 8, 9.
Nonetheless, unlike weight loss, such strategies aimed at improving vascular function per se would not infer benefits for other obesity‐related comorbidities but may be of benefit as an addendum to weight loss or for people who are resistant to weight loss.
Accordingly, a comprehensive systematic review of the available literature on obesity and vascular health (as assessed by brachial FMD, and carotid IMT) was undertaken, with subsequent meta‐analysis to determine whether these markers of vascular health differ between adults with obesity and those with healthy weight, and the estimated effect size of any such difference. Studies were restricted to those that defined obesity using body mass index (BMI), which despite being unable to differentiate between different types of obesity, is the most commonly used measure in clinical practice.
Methods
Search strategy and selection criteria
The MOOSE checklist was used as a guide to prepare this systematic review and meta‐analysis. Electronic searches were performed on 29 January 2017 using Ovid Medline and Embase databases, to identify papers published through end‐2016. Searches were run in each database, relating to ‘Obesity and FMD’ and ‘Obesity and IMT’. Relevant search terms under each search category ‘Obesity’ (Obesity, morbid obesity, overweight, obes*, BMI, body mass index), ‘Flow‐mediated dilatation’ (flow mediated dilat* or vasodilat*, FMD) and ‘Intima media thickness’ (intima media thickness, IMT) were identified and used.
To maximize sensitivity of the search, relevant terms were combined as keywords or subject heading (MeSH) terms within each category. After the initial screening of titles and abstracts, the full text of potentially relevant articles were obtained and evaluated. To further identify relevant studies, a secondary search was also carried out by reviewing the reference lists of all shortlisted articles.
Inclusion criteria
To ensure consistency, only studies that used BMI to define obesity and studies which measured brachial FMD and common carotid IMT as endpoints were included. Studies were included if participants were categorized on the basis of study‐specific BMI cut‐points, into those with some degree of obesity and those with healthy weight. A sensitivity analysis was undertaken in studies using World Health Organization criteria (Controls <25 kg m−2, Obesity ≥30 kg m−2). The limits ‘humans’ and ‘adults ≥18 years old’ were added to make the searches more specific. Date limits were applied according to when the FMD and IMT techniques were first described (1992 and 1984, respectively). No language limits were applied.
Exclusion criteria
The following types of studies were excluded: Studies in which the participants with obesity all had a specific co‐morbidity, studies without a healthy weight comparator, studies which did not assess FMD and IMT as endpoints and studies from which the mean, standard deviations or standard error were unable to be extracted or converted. For studies in which the group with obesity was stratified into two groups (with and without a specific co‐morbidity), the group with the specific co‐morbidity was excluded. As obesity is associated with an increased risk of metabolic co‐morbidities, studies that included participants with metabolic risk factors, but did not specifically select based on these risk factors, were not excluded. Abstracts, case reports, conference presentations, editorials and expert opinions were excluded.
Screening of articles, data extraction and critical appraisal
All 5,810 titles, relevant abstracts and relevant full‐text articles from the primary and secondary search were screened by the main reviewer (J.Y.A.N). All titles and relevant abstracts from the primary search were also independently screened by a second reviewer (T.Y.C). Discrepancies were resolved by discussion and consensus. Relevant data were extracted from the articles, tables and figures by the main reviewer.
Statistical analysis
The sample size, mean and SD of relevant data (age, BMI, FMD and IMT) from the group with obesity and the control group were extracted from each study. For studies with measures of both mean and maximum carotid IMT, or a composite measure derived from multiple areas of the carotid tree, the measure of mean common carotid IMT was used preferentially in analysis. To determine the net effect of obesity on vascular health, a meta‐analysis was performed by combining the mean difference of FMD or IMT between the adults with obesity and those with healthy weight from the different studies to obtain pooled estimates. Weighted mean and pooled variance were used when the group with obesity or the control group were stratified into two groups according to age, type of obesity or amount of inflammatory markers (e.g. high sensitivity C‐reactive protein). The random effects model was used to account for the variation in baseline characteristics of different populations as well as the methodological variation among the different studies. An I2 value of greater than 50% indicated substantial heterogeneity. Possible causes of substantial heterogeneity, specifically age, sex, BMI cut‐points for healthy weight and obesity, measurement methodology (for IMT only) and site of occlusion (for FMD only), were explored by conducting sensitivity analyses stratified by subgroup. Statistical significance was inferred at P < 0.05. Statistical analysis was undertaken with Review Manager Version 5.3 (The Nordic Cochrane Centre, Copenhagen, Denmark).
Results
Search results – brachial flow‐mediated dilatation (FMD)
A total of 1,611 references were identified through the two electronic database searches in the primary search. A secondary search of references from shortlisted articles from the primary search yielded an additional 944 articles. After removal of duplicates, 1,994 references remained for screening, of which 1,811 were excluded because they did not meet the inclusion criteria. The remaining 183 full‐text articles were assessed for eligibility. After screening these articles based on the selection criteria, 19 studies remained for analysis.
Nineteen of these FMD studies are cross‐sectional studies of adults with obesity and controls. In these studies, a total of 2,733 participants were compared, including 1,680 controls and 1,053 adults with obesity. A summary of characteristics of the study populations is presented in Table 1.
Table 1.
Summary of studies included in meta‐analysis of obesity and brachial FMD
| Mean age (year) | Gender (% males) | BMI cut‐off (kg m−2) | Mean BMI (kg m−2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Study design | Controls | Obesity | Controls | Obesity | Controls | Obesity | Controls | Obesity |
| Hashimoto 16 | 1998 | Cross‐sectional | 37.3 ± 1.9 |
S:37.9 ± 4.1*
V:37.5 ± 1.5* |
100 | 100 | <26 | ≥26 | 22.5 ± 0.4* |
S:30.4 ± 1.1*, V:29.7 ± 0.5* |
| Oflaz 7 | 2003 | Cross‐sectional | 30.6 ± 7.2 | 31.4 ± 7.5 | 0 | 0 | <24 | >30 | 20.4 ± 1.6 | 34.2 ± 3.8 |
| Yu 26 | 2003 | Cross‐sectional | 33.4 ± 12.3 | 34.1 ± 12.2 | 100 | 100 | <24 | >28 | 21.9 ± 1.9 | 32.8 ± 4.1 |
| Pulerwitz 27 | 2006 | Cross‐sectional | 65.2 ± 8.0‡ | 41.7 | <25 | ≥30 | 28.0 ± 4.6‡ | |||
| Olson 28 | 2006 | Cross‐sectional | 34.0 ± 2.0* | 37.0 ± 1.0* | 0 | 0 | 18.0–24.9 | 30.0–34.9 | 22.0 ± 0.5* | 31.8 ± 0.4* |
| Zhu 14 | 2007 | Cross‐sectional | 45.3 ± 5.3 | 44.2 ± 6.4 | 62.8 | 63.7 | <25 | ≥30 | 22.8 ± 1.7 | 28.3 ± 2.0 |
| Mizia‐Stec 29 | 2008 | Cross‐sectional |
Control A <35: 28.0 ± 7.6, Control B >45: 51.9 ± 4.4 |
29.7 ± 6.2 | 0 | 0 | Control A: 20.8–27.6; Control B: 21.9–28.3 | >30 | Control A: 24.0 ± 3.8; Control B: 25.8 ± 2.9 | 33.6 ± 2.9 |
| Patel 30 | 2009 | Cross‐sectional | 51 ± 1‡ | 0 | 0 | <25 | ≥30 | NIL | ||
| Skilton 31 | 2008 | Cross‐sectional | 42.8 ± 2.5* | 44.4 ± 1.3* | 27.3 | 20.5 | <24 | >30 | 21.9 ± 0.4* | 44.6 ± 1.6* |
| Lind 32 | 2009 | Cross‐sectional | 40.0 ± 19.0 | 41.0 ± 11.0 | 26.0 | 26.0 | NIL | NIL | 23.1 ± 2.0 | 43.8 ± 3.1 |
| Fahs 33 | 2009 | Cross‐sectional | 25.7 ± 1.2* | 33.8 ± 1.3* | 100 | 100 | <25.9 | ≥29.5 | 23.6 ± 0.3* | 32.8 ± 0.5* |
| Ayer 34 | 2010 | Cross‐sectional | 31.6 ± 5.3 | 32.1 ± 6.3 | 63.6 | 63.6 | 18–25 | >30 | 22.6 (21.7–24.2) † | 46.4 (35.4–53.6) † |
| Biasucci 4 | 2010 | Cross‐sectional | 48.4 ± 5.6 | 51.0 ± 6.2 | 38.5 | 57.1 | NIL | NIL | 23.2 ± 1.6 | 32.6 ± 2.5 |
| Zhu 35 | 2010 | Cross‐sectional | 22.4 ± 1.5 | 22.5 ± 0.9 | 100 | 100 | NIL | NIL | 20.1 ± 2.5 | 32.2 ± 1.7 |
| Ayer 36 | 2011 | Cross‐sectional | 31.0 ± 5.3 | 32.7 ± 5.3 | 52.6 | 52.6 | 18–25 | >30 | 22.4 ± 1.6 | 44.1 ± 9.0 |
| Mah 37 | 2011 | Cross‐sectional | 22.0 ± 1.3* | 21.0 ± 1.0* | 100 | 100 | 18–25 | 27–40 | 21.9 ± 0.65* | 35.9 ± 1.9* |
| Doupis 38 | 2011 | Cross‐sectional | 54.0 ± 13.0 | 51.0 ± 10.0 | 50.0 | 51.0 | <30 | >30 | 24.9 ± 3.4 | 38.1 ± 7.1 |
| Vinet 39 | 2011 | Cross‐sectional | 48.2 ± 1.6* | 51.0 ± 2.5* | 100 | 100 | <25 | ≥30 | 24.3 ± 0.3* | 33.9 ± 1.1* |
| Martin 40 | 2013 | Cross‐sectional | 48.3 ± 10.4 | 52.2 ± 8.0 | 100 | 100 | <30 | ≥30 | 26.7 ± 2.0 | 32.9 ± 2.7 |
Data presented as mean ± SD except:
Mean ± SE.
Median, IQR.
Value for controls and obesity combined.
S, subcutaneous obesity.
V, visceral obesity.
Search results – carotid intima‐media thickness (IMT)
A total of 4,081 references were identified through the two electronic database searches in the primary search. A secondary search of references from shortlisted articles from the primary search yielded an additional 1,064 articles. After removal of duplicates, 3,901 references remained for screening, of which 3,760 were excluded because they did not meet the inclusion criteria. The remaining 141 full‐text articles were assessed for eligibility. After screening these articles based on the selection criteria, 19 studies remained for analysis.
Of the 19 studies included in the meta‐analysis, 14 of them are cross‐sectional studies, and 5 of them are longitudinal studies. In these studies, a total of 9,821 participants were compared, including 4,999 controls and 4,822 adults with obesity. A summary of characteristics of the study populations is presented in Table 2.
Table 2.
Summary of studies included in meta‐analysis of obesity and carotid IMT
| Mean age (year) | Gender (% males) | BMI cut‐off (kg m−2) | Mean BMI (kg m−2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Study design | Controls | Obesity | Controls | Obesity | Controls | Obesity | Controls | Obesity |
| Karason 41 | 1999 | Baseline data from intervention study | 49 ± 8 | 49 ± 5 | 77 | 79 | 20–28 | 30–40 | 24 ± 2 | 37 ± 4 |
| Ciccone 42 | 1999 | Cross‐sectional | 28.9 ± 6.3 | 29.2 ± 8.4 | 38.3 | 41.7 | <27 | >27 | 22.7 ± 2.6 | 35.6 ± 6.8 |
| Mavri 43 | 2001 | Baseline data from intervention study (weight loss) | 41 | 40 | 0 | 0 | NIL | NIL | NIL | 30.6 ± 5.0 |
| De Michele 44 | 2002 | Baseline data from prospective cohort | 54.0 ± 0.9* | 55.0 ± 0.8* | 0 | 0 | <25 | ≥30 | 23.0 ± 0.1* | 33.8 ± 0.3* |
| Yu 17 | 2003 | Cross‐sectional | 33.4 ± 12.3 | 34.1 ± 12.2 | 100 | 100 | <24 | >28 | 21.9 ± 1.9 | 32.8 ± 4.1 |
| Oflaz 7 | 2003 | Cross‐sectional | 30.6 ± 7.2 | 31.4 ± 7.5 | 0 | 0 | <24 | >30 | 20.4 ± 1.6 | 34.2 ± 3.8 |
| Marini 13 | 2007 | Cross‐sectional | 34 ± 9 | 35 ± 8 | 0 | 0 | <27 | >30 | 23.8 ± 2.8 | 37.7 ± 9.9 |
| Skilton 31 | 2008 | Cross‐sectional | 42.8 ± 2.5* | 44.4 ± 1.3* | 27.3 | 20.5 | <24 | >30 | 21.9 ± 0.4* | 44.6 ± 1.6* |
| Mizia‐Stec 29 | 2008 | Cross‐sectional |
Control A <35: 28.0 ± 7.6 Control B >45: 51.9 ± 4.4 |
29.7 ± 6.2 | 0 | 0 | Control A: 20.8–27.6, Control B: 21.9–28.3 | >30 |
Control A: 24.0 ± 3.8, Control B: 25.8 ± 2.9 |
33.60 ± 2.90 |
| Yu 17 | 2008 | Cross‐sectional | 56.4 ± 3.3† | 0 | 0 | <25 | ≥25 | 23.6 ± 3.7† | ||
| Stefan 45 | 2008 | Cross‐sectional | 44.8 ± 1.6* | 46.5 ± 1.9* | 16.7 | 38.6 | <25 | ≥30 | 22.0 | 34.0 |
| Fahs 33 | 2009 | Cross‐sectional | 25.7 ± 1.2* | 33.8 ± 1.3* | 100 | 100 | <25.9 | ≥29.5 | 23.6 ± 0.3* | 32.8 ± 0.5* |
| Biasucci 4 | 2010 | Cross‐sectional | 48.4 ± 5.6 | 51.0 ± 6.2 | 38.5 | 57.1 | NIL | NIL | 23.2 ± 1.6 | 32.6 ± 2.5 |
| Blaha 46 | 2011 | Baseline data from population‐based prospective cohort |
hsCRP < 2: 61.7 ± 11 hsCRP ≥ 2: 63.0 ± 10 |
hsCRP < 2: 62.5 ± 10 hsCRP ≥ 2: 62.0 ± 10 |
hsCRP < 2: 61 hsCRP ≥ 2: 53 |
hsCRP < 2: 48 hsCRP≥ 2: 31 |
<30 | ≥30 |
hsCRP < 2: 24.2 ± 3 hsCRP ≥ 2: 24.8 ± 3 |
hsCRP < 2: 30.6 ± 4 hsCRP ≥ 2: 32.9 ± 5 |
| Ayer 36 | 2011 | Cross‐sectional | 31.0 ± 5.3 | 32.7 ± 5.3 | 52.6 | 52.6 | 18–25 | >30 | 22.4 ± 1.6 | 44.1 ± 9.0 |
| Park 47 | 2011 | Cross‐sectional | 52.0 ± 5.5 | 52.2 ± 6.1 | 45.0 | 52.0 | <23 | ≥25 | 21.3 ± 1.1 | 26.3 ± 1.0 |
| Csongradi 48 | 2011 | Cross‐sectional, case‐control | 39.7 ± 10.0 | 40.4 ± 11.8 | 33.9 | NIL | NIL | NIL | 22.1 ± 2.0 | 37.7 ± 8.1 |
| Ecemis 49 | 2012 | Cross‐sectional | 41.8 ± 11.2 | 44.7 ± 12.5 | 0 | 0 | 18.5–24.9 | 30.0–39.9 | 22.3 ± 1.9 | 34.9 ± 2.5 |
| Aydin 12 | 2013 | Baseline data from prospective cohort | 45 ± 18 | 50 ± 13 | 48 | 20 | <25 | 30–39.9 | NIL | NIL |
Data presented as mean ± SD except:
Mean ± SE.
Value for controls and obesity combined.
hsCRP, high‐sensitivity C‐reactive protein.
Meta‐analysis results – brachial FMD and carotid IMT
The meta‐analysis showed potentially meaningful and statistically significant differences in brachial FMD and carotid IMT between the adults with obesity and those who are healthy weight, with the adults with obesity having lower FMD (−1.92 % [95% CI: −2.92, −0.92], P = 0.0002, n = 1,053; Figure 1) and higher carotid IMT (0.07 mm [95% CI: 0.05, 0.08], P < 0.0001, n = 4,822; Figure 2) than the healthy weight adults (n = 1,680 [FMD], n = 4,999 [IMT]). However, there was significant heterogeneity, based on I2 test (I2> 50%). The association of obesity with lower brachial FMD and greater carotid IMT did not differ by age or sex (Figures 3, 4, 7 and 8), with the estimated mean differences being profoundly similar between all subgroups. Similar results were found in studies that used standard World Health Organization criteria for obesity and healthy weight, and studies that used alternative criteria (Figures 5 and 9). Heterogeneity between studies remained high in these stratified analyses, with the exception of studies of brachial FMD in participants aged >50 years, in which findings were relatively consistent.
Figure 1.
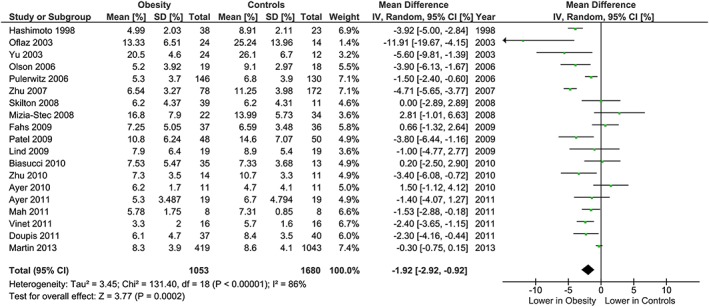
Forest plot of mean difference in brachial FMD between adults with obesity and healthy weight adults.
Figure 2.
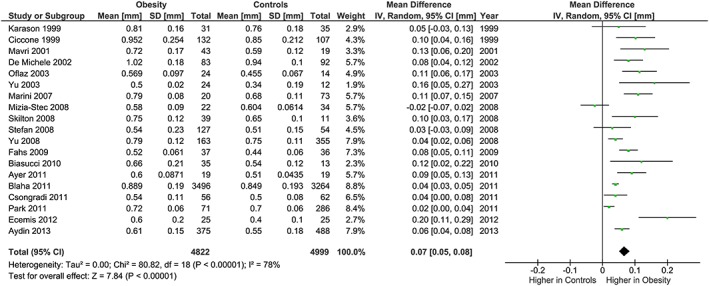
Forest plot of mean difference in carotid IMT between adults with obesity and healthy weight adults.
Figure 3.
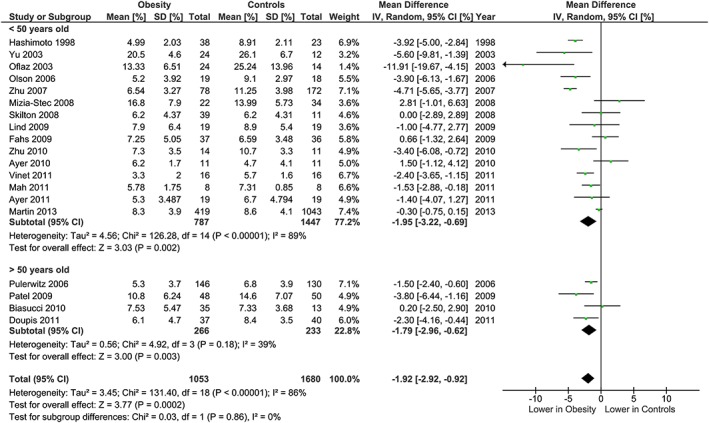
Brachial FMD and obesity: analysis stratified by age. Forest plot of mean difference in brachial FMD between adults with obesity and healthy weight adults stratified by age above and below 50 years.
Figure 4.
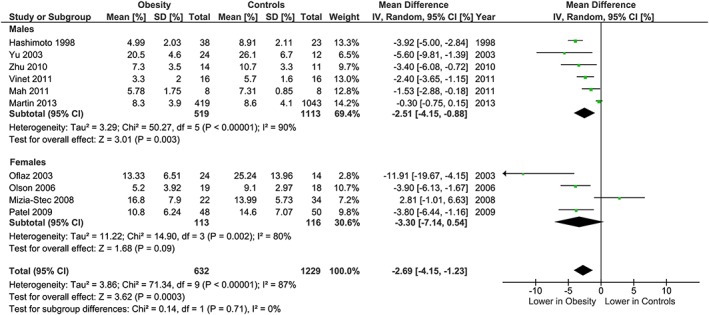
Brachial FMD and obesity: analysis stratified by gender. Forest plot of mean difference in brachial FMD between adults with obesity and healthy weight adults stratified by gender.
Figure 5.
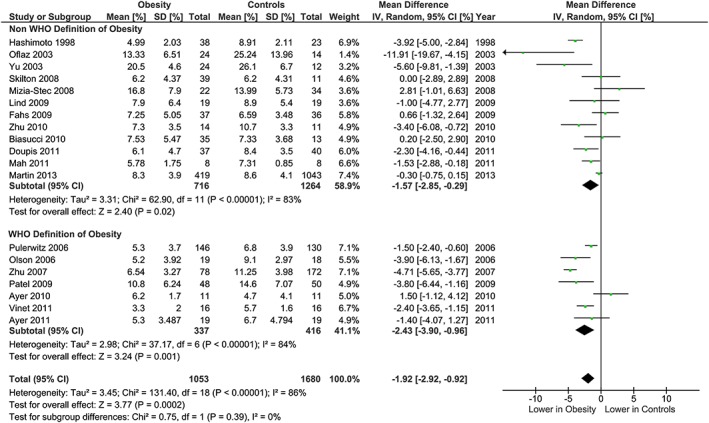
Brachial FMD and obesity: analysis stratified by obesity and healthy weight criteria. Forest plot of mean difference in brachial FMD between adults with obesity and healthy weight adults stratified by use of World Health Organization criteria (Controls <25 kg m−2, Obesity ≥30 kg m−2), or alternative BMI cut‐points.
Figure 7.
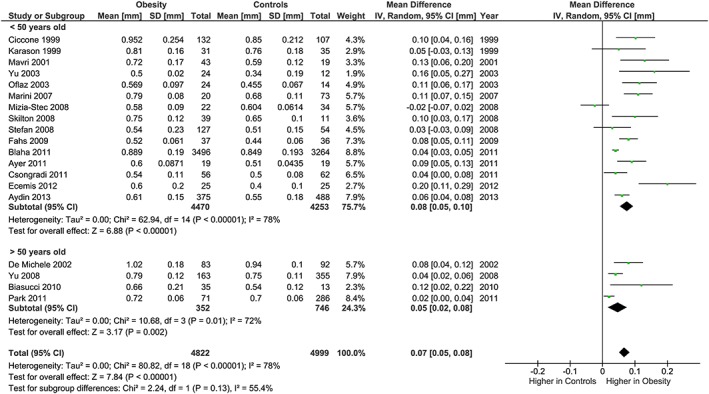
Carotid IMT and obesity: analysis stratified by age. Forest plot of mean difference in carotid IMT between adults with obesity and healthy weight people stratified by age above and below 50 years.
Figure 8.
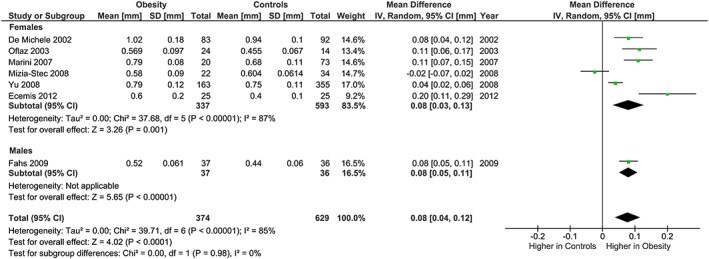
Carotid IMT and obesity: analysis stratified by gender. Forest plot of mean difference in carotid IMT between adults with obesity and healthy weight people stratified by gender.
Figure 9.
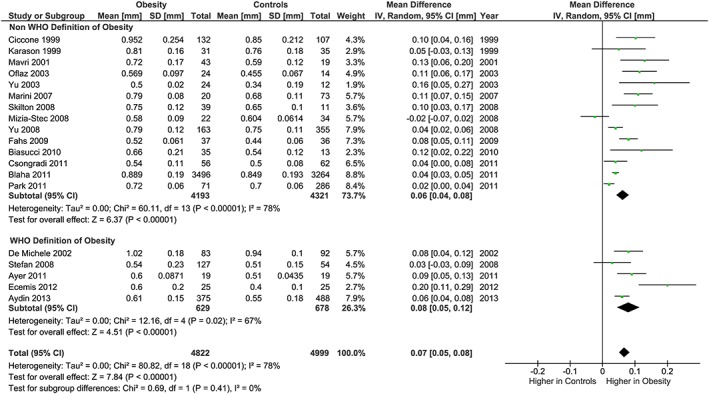
Carotid IMT and obesity: analysis stratified by obesity and healthy weight criteria. Forest plot of mean difference in carotid IMT between adults with obesity and healthy weight adults stratified by use of World Health Organization criteria (Controls <25 kg m−2, Obesity ≥30 kg m−2), or alternative BMI cut‐points.
Regarding methodological differences, analysis stratified by carotid IMT measurement methodology found no differences between subgroups (Figure 10). For the brachial FMD methodology, subgroups were analysed based on site of occlusion, which has important implications for the proportion of the response due to endothelial nitric oxide. The subgroup analysis showed some evidence that the association of obesity with lower FMD was more marked in studies which used distal (forearm) occlusion, than in studies which used proximal (upper arm) occlusion (P heterogeneity = 0.10; Figure 6).
Figure 10.
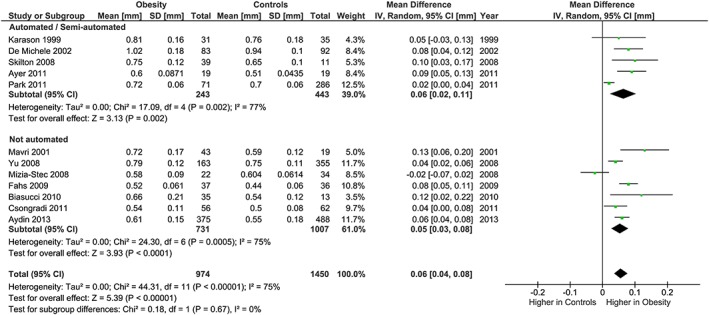
Carotid IMT and obesity: analysis stratified by IMT measurement methodology. Forest plot of mean difference in carotid IMT between adults with obesity and healthy weight people stratified by IMT measurement methodology (manual or semi‐automated/automated).
Figure 6.
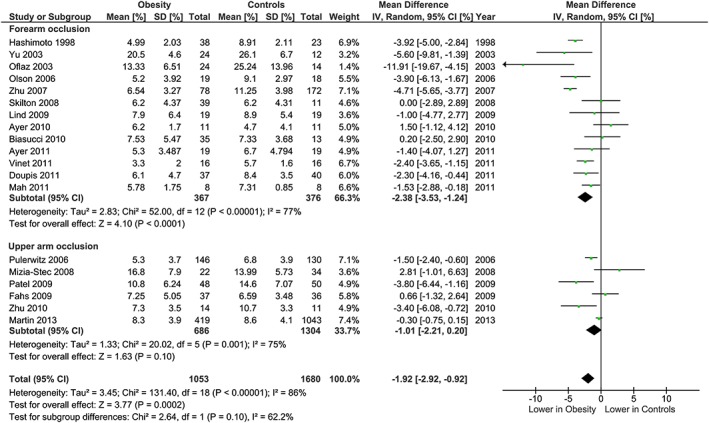
Brachial FMD and obesity: analysis stratified by site of occlusion. Forest plot of mean difference in brachial FMD between adults with obesity and healthy weight adults stratified by site of occlusion: forearm (distal to site of scan) and upper arm occlusion (proximal to site of scan).
Assessment of publication bias
Funnel plots for both brachial FMD and carotid IMT demonstrate a lack of smaller studies (Figures 11 and 12). The funnel plot for brachial FMD shows a symmetrical distribution of studies about the mean effect size. The funnel plot for carotid IMT is slightly skewed to the right, indicating that smaller studies with negative results or reverse associations might not have been published.
Figure 11.
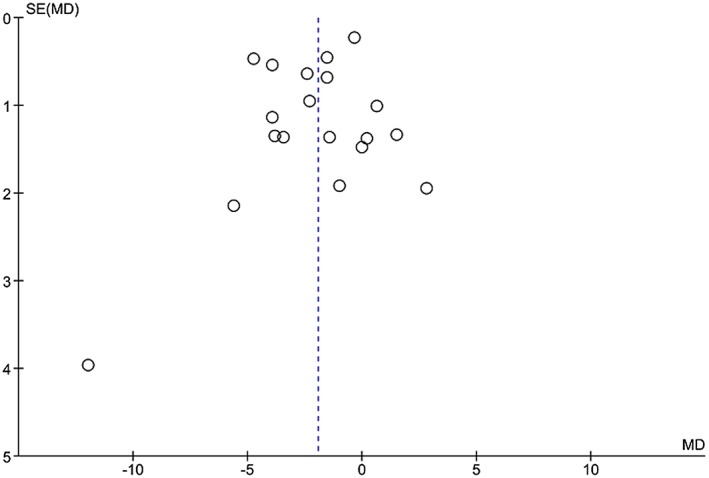
Funnel plot for obesity and brachial FMD.
Figure 12.
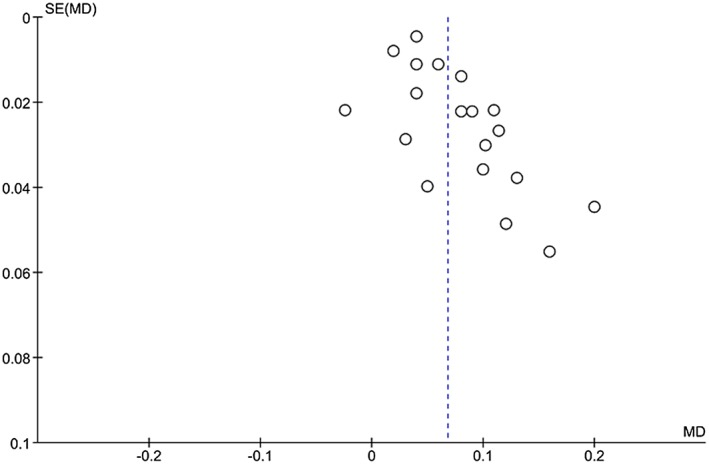
Funnel plot for obesity and carotid IMT.
Discussion
The results of this systematic review and meta‐analysis indicate that obesity is associated with functional and structural changes to the arterial vasculature, specifically lower brachial FMD and greater carotid IMT. This evidence of worsened arterial endothelial function and increased subclinical atherosclerosis is consistent with theoretically meaningful differences in the risk of cardiovascular events. Specifically, the impairment of brachial FMD is consistent with a 12 to 33% higher risk of cardiovascular events 10, and the differences in carotid IMT are associated with a 7 to 12% higher risk of myocardial infarction and a 9 to 14% higher risk of stroke 11.
Cardiometabolic risk profile in individuals with obesity may contribute to the poor vascular health observed in this condition. Indeed, brachial FMD is impaired, and carotid IMT is increased in people with the metabolic syndrome 12, 13, 14. Approximately 64% of the risk of coronary artery disease events attributable to obesity are due to altered levels of the total cholesterol to HDL cholesterol ratio, systolic blood pressure and diabetes mellitus 15. Studies that included only adults with obesity and a specific comorbidity were specifically excluded, in order to at least partially limit the impact of these comorbidities. However, the rates of non‐selected comorbidities remained high overall and may have contributed to the magnitude of the vascular health disturbances observed. There has been considerable discussion of metabolically healthy obesity, and as noted, the lack of consistent findings formed the basis for this current meta‐analysis. Identification of a phenotype of people with obesity and optimal vascular health is beyond the scope of the current study, although if such a phenotype exists, it may relate to adipose tissue distribution 16, 17, adipokine profile or genetic predisposition.
Subgroup analysis based on the site of occlusion for arterial endothelial function testing found some evidence for a greater difference between adults with obesity and those with healthy weight in studies utilizing the forearm occlusion method than in those with an upper arm occlusion. This may be due to the different underlying mechanisms of vasodilation in response to occlusion from these two sites, with the forearm occlusion eliciting vasodilatation through predominantly nitric oxide mediated pathways, and upper arm occlusion via both endothelium‐derived nitric oxide and ischemic metabolites 18, 19. Accordingly, these findings are consistent with obesity being associated with worsened endothelial function, and in particular, nitric oxide mediated vasodilation, putatively via enhanced oxidative stress, increased production of inflammatory cytokines and a direct effect of some adipokines and obesity‐related peptides 20.
Interestingly, the difference in FMD observed is less than that described in a recent meta‐analysis of weight loss and FMD 8, in which the net effect of weight loss on FMD was an improvement of 3.29% (absolute units). It is not clear why the beneficial effect of weight loss on endothelial function would be greater than the detrimental effect of obesity, although this may potentially relate to energy balance at the time of assessment or an independent effect of concurrent improvements in nutrition and physical activity.
This systematic review and meta‐analysis has a number of strengths. First, there has been no previous systematic review or meta‐analysis on obesity and these two measures of vascular health (brachial FMD and carotid IMT), both of which are established methodologies that have well‐documented associations with cardiovascular outcomes. A second reviewer independently screened all titles and abstracts identified in the primary search in order to minimize bias. Furthermore, a comprehensive search strategy was utilized that involved the use of both keyword and subject heading terms, US (Medline) and European (Embase) databases, and without application of language limits, thus searching for and reviewing both English and non‐English articles. The sample size of research subjects (n = 12,554) is large, and sensitivity analyses were relatively consistent, suggesting that the findings are likely robust. Weaknesses and limitations of this work include the significant heterogeneity of results between studies. This may possibly relate to methodological differences in assessment of vascular health, for which there remains little standardization between laboratories. The different BMI cut‐points and differences in prevalence of cardiometabolic risk factors may also have contributed to the heterogeneity. Furthermore, the majority of included studies are cross‐sectional, and as such no causal relationship can be inferred. Only studies with data on obesity as defined by BMI, as widely used in clinical practice, were included. Although this facilitated comparison between studies, BMI lacks discriminatory power to differentiate between body fat and lean mass 21, as well as between subcutaneous and visceral obesity 22. Other measures of obesity, particularly those relating to regional distribution of adiposity, such as waist–hip ratio and waist circumference, in addition to direct measures of percentage body fat, may potentially be more closely linked to vascular health disturbances 23. Studies were excluded that treated BMI as a continuous variable. The findings are not able to inform on the progression to endothelial dysfunction, changes in intima‐media thickening over time or whether the effects of obesity on brachial FMD and carotid IMT are reversible. Not all studies presented data specifically for the different subgroups (age, gender, IMT measurement methodology), and as such the reported results are derived from a subset of studies, which may have reduced statistical power.
Whether the assessment of these markers of vascular health may have a role in the clinical management of obesity is unclear. The base‐level equipment required is commonly available in the hospital setting, most notably B mode ultrasound with linear array transducer. Both techniques are routinely assessed in research, although they require a significant level of training to ensure reproducibility 24. Neither currently has a clearly defined role in clinical practice, although the use of these techniques to monitoring individual vascular responses to weight loss therapy as a potential means by which to identify strategies with the greatest likely cardiovascular benefit is an attractive concept that warrants further investigation 25.
In conclusion, these findings indicate that brachial FMD is lower and carotid IMT is greater in adults with obesity. Accordingly, changes in brachial FMD and carotid IMT may be important surrogate markers suitable for use in clinical trials of weight loss interventions. Furthermore, these findings provide a robust estimation of the effect sizes likely due to obesity, which will enable the effect sizes observed with weight loss interventions to be put into appropriate context.
Funding
J.Y.A.N was supported by a Sydney Medical School MBBS‐MPhil scholarship. M.R.S is supported by a Future Leader fellowship from the National Heart Foundation of Australia (#100419).
Conflict of Interest Statement
M.R.S. received consultancy fees from AstraZeneca for his contribution to the development of educational resources relating to clinical management of type 2 diabetes. I.D.C. is a board member of the EXSCEL Trial Operations Committee. For this, he has received an honorarium. He has performed and still performs clinical trials of obesity treatment and prevention, some of which have been funded by the government and others by the pharmaceutical industry. Current trials are funded by the NHMRC (3), NovoNordisk, Pfizer, BMS and SFI. He has given talks for NovoNordisk, Servier Laboratories, Ache and Pfizer in the last 3 years. The Boden Institute of Obesity, Nutrition, Exercise and Eating Disorders (M.R.S., J.Y.A.N., I.D.C., C.M.Y.L.) currently receives or has grants pending, from Pfizer, NovoNordisk, Bristol‐Myers Squibb, Egg Board and Soho Floridis International.
Author Contributions
J.Y.A.N. carried out the literature search, data collection, statistical analysis and drafted the manuscript; T.Y.C. independently screened articles; D.S.C. contributed to study conception; I.D.C. contributed to study conception; T.G. contributed to study conception; C.M.Y.L. assisted with statistical design and interpretation; M.R.S. contributed to study conception and interpretation of data. All authors were involved in writing the paper and had final approval of the submitted and published versions.
Ne, J. Y. A. , Cai, T. Y. , Celermajer, D. S. , Caterson, I. D. , Gill, T. , Lee, C. M. Y. , and Skilton, M. R. (2017) Obesity, arterial function and arterial structure – a systematic review and meta‐analysis. Obesity Science & Practice, 3: 171–184. doi: 10.1002/osp4.108.
References
- 1. Tunstall‐Pedoe H, Kuulasmaa K, Amouyel P, et al. Myocardial infarction and coronary deaths in the world health organization monica project. Registration procedures, event rates, and case‐fatality rates in 38 populations from 21 countries in four continents. Circulation 1994; 90: 583–612. [DOI] [PubMed] [Google Scholar]
- 2. O'Leary DH, Polak JF, Kronmal RA, et al. Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular health study collaborative research group. N Engl J Med 1999; 340: 14–22. [DOI] [PubMed] [Google Scholar]
- 3. Fathi R, Haluska B, Isbel N, Short L, Marwick TH. The relative importance of vascular structure and function in predicting cardiovascular events. J Am Coll Cardiol 2004; 43: 616–623. [DOI] [PubMed] [Google Scholar]
- 4. Biasucci LM, Graziani F, Rizzello V, et al. Paradoxical preservation of vascular function in severe obesity. Am J Med 2010; 123: 727–734. [DOI] [PubMed] [Google Scholar]
- 5. Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: risk factor, paradox, and impact of weight loss. J Am Coll Cardiol 2009; 53: 1925–1932. [DOI] [PubMed] [Google Scholar]
- 6. Arcaro G, Zamboni M, Rossi L, et al. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord 1999; 23: 936–942. [DOI] [PubMed] [Google Scholar]
- 7. Oflaz H, Ozbey N, Mantar F, et al. Determination of endothelial function and early atherosclerotic changes in healthy obese women. Diabetes Nutr Metabol ‐ Clin Exp 2003; 16: 176–181. [PubMed] [Google Scholar]
- 8. Joris PJ, Zeegers MP, Mensink RP. Weight loss improves fasting flow‐mediated vasodilation in adults: a meta‐analysis of intervention studies. Atherosclerosis 2015; 239: 21–30. [DOI] [PubMed] [Google Scholar]
- 9. Skilton MR, Yeo SQ, Ne JYA, et al. Weight loss and carotid intima‐media thickness – a meta‐analysis. Obesity 2017; 25: 357–362. [DOI] [PubMed] [Google Scholar]
- 10. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging 2010; 26: 631–640. [DOI] [PubMed] [Google Scholar]
- 11. Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima‐media thickness: a systematic review and meta‐analysis. Circulation 2007; 115: 459–467. [DOI] [PubMed] [Google Scholar]
- 12. Aydin M, Bulur S, Alemdar R, et al. The impact of metabolic syndrome on carotid intima media thickness. Eur Rev Med Pharmacol Sci 2013; 17: 2295–2301. [PubMed] [Google Scholar]
- 13. Marini MA, Succurro E, Frontoni S, et al. Metabolically healthy but obese women have an intermediate cardiovascular risk profile between healthy nonobese women and obese insulin‐resistant women. Diabetes Care 2007; 30: 2145–2147. [DOI] [PubMed] [Google Scholar]
- 14. Zhu LY, Hu LY, Wang GY, Wang XH. Comparison of vascular endothelial function in simple obesity and metabolic syndrome patients. [Chinese]. J Clin Rehabil Tissue Eng Res 2007; 11: 2240–2242. [Google Scholar]
- 15. Wilson PW, Bozeman SR, Burton TM, et al. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation 2008; 118: 124–130. [DOI] [PubMed] [Google Scholar]
- 16. Hashimoto M, Akishita M, Eto M, et al. The impairment of flow‐mediated vasodilatation in obese men with visceral fat accumulation. Int J Obes Relat Metab Disor: J Int Assoc Stud Obes 1998; 22: 477–484. [DOI] [PubMed] [Google Scholar]
- 17. Yu RH, Ho SC, Ho SS, Woo JL, Ahuja AT. Association of general and abdominal obesities and metabolic syndrome with subclinical atherosclerosis in asymptomatic chinese postmenopausal women. Menopause 2008; 15: 185–192. [DOI] [PubMed] [Google Scholar]
- 18. Green DJ, Jones H, Thijssen D, Cable N, Atkinson G. Flow‐mediated dilation and cardiovascular event prediction does nitric oxide matter? Hypertension 2011; 57: 363–369. [DOI] [PubMed] [Google Scholar]
- 19. Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial‐dependent flow‐mediated vasodilation of the brachial artery: A report of the international brachial artery reactivity task force. J Am Coll Cardiol 2002; 39: 257–265. [DOI] [PubMed] [Google Scholar]
- 20. Dolores Mauricio M, Aldasoro M, Ortega J, Vila JM. Endothelial dysfunction in morbid obesity. Curr Pharm Des 2013; 19: 5718–5729. [DOI] [PubMed] [Google Scholar]
- 21. Prentice AM, Jebb SA. Beyond body mass index. Obes Rev 2001; 2: 141–147. [DOI] [PubMed] [Google Scholar]
- 22. Janssen I, Heymsfield SB, Allison DB, Kotler DP, Ross R. Body mass index and waist circumference independently contribute to the prediction of nonabdominal, abdominal subcutaneous, and visceral fat. Am J Clin Nutr 2002; 75: 683–688. [DOI] [PubMed] [Google Scholar]
- 23. Ho SC, Chen YM, Woo JL, Leung SS, Lam TH, Janus ED. Association between simple anthropometric indices and cardiovascular risk factors. Int J Obes Relat Metab Disor: J Int Assoc Stud Obes 2001; 25: 1689–1697. [DOI] [PubMed] [Google Scholar]
- 24. Touboul PJ, Hennerici MG, Meairs S, et al. Mannheim carotid intima‐media thickness consensus (2004–2006). Cerebrovasc Dis 2007; 23: 75–80. [DOI] [PubMed] [Google Scholar]
- 25. Flammer AJ, Anderson T, Celermajer DS, et al. The assessment of endothelial function: From research into clinical practice. Circulation 2012; 126: 753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yu Y, Li H, Yu H, Wang C, Pu S. The relationship between insulin resistance and endothelium‐dependent vasodilatation in obese subjects. Zhonghua Yi Xue Za Zhi 2003; 83: 1467–1470. [PubMed] [Google Scholar]
- 27. Pulerwitz T, Grahame‐Clarke C, Rodriguez CJ, et al. Association of increased body mass index and impaired endothelial function among hispanic women. Am J Cardiol 2006; 97: 68–70. [DOI] [PubMed] [Google Scholar]
- 28. Olson TP, Schmitz KH, Leon AS, Dengel DR. Vascular structure and function in women: relationship with body mass index. Am J Prev Med 2006; 30: 487–492. [DOI] [PubMed] [Google Scholar]
- 29. Mizia‐Stec K, Gasior Z, Zahorska‐Markiewicz B, et al. The indexes of arterial structure and function in women with simple obesity: a preliminary study. Heart Vessels 2008; 23: 224–229. [DOI] [PubMed] [Google Scholar]
- 30. Patel AR, Hui H, Kuvin JT, Pandian NG, Karas RH. Modestly overweight women have vascular endothelial dysfunction. Clin Cardiol 2009; 32: 269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Skilton MR, Sieveking DP, Harmer JA, et al. The effects of obesity and non‐pharmacological weight loss on vascular and ventricular function and structure. Diabetes Obes Metab 2008; 10: 874–884. [DOI] [PubMed] [Google Scholar]
- 32. Lind L, Zethelius B, Sundbom M, Eden Engstrom B, Karlsson FA. Vasoreactivity is rapidly improved in obese subjects after gastric bypass surgery. Int J Obes (Lond) 2009; 33: 1390–1395. [DOI] [PubMed] [Google Scholar]
- 33. Fahs CA, Smith DL, Horn GP, et al. Impact of excess body weight on arterial structure, function, and blood pressure in firefighters. Am J Cardiol 2009; 104: 1441–1445. [DOI] [PubMed] [Google Scholar]
- 34. Ayer JG, Harmer JA, Steinbeck K, Celermajer DS. Postprandial vascular reactivity in obese and normal weight young adults. Obesity 2010; 18: 945–951. [DOI] [PubMed] [Google Scholar]
- 35. Zhu W, Zeng J, Yin J, et al. Both flow‐mediated vasodilation procedures and acute exercise improve endothelial function in obese young men. Eur J Appl Physiol 2010; 108: 727–732. [DOI] [PubMed] [Google Scholar]
- 36. Ayer JG, Harmer JA, David C, et al. Severe obesity is associated with impaired arterial smooth muscle function in young adults. Obesity 2011; 19: 54–60. [DOI] [PubMed] [Google Scholar]
- 37. Mah E, Matos MD, Kawiecki D, et al. Vitamin c status is related to proinflammatory responses and impaired vascular endothelial function in healthy, college‐aged lean and obese men. J Am Diet Assoc 2011; 111: 737–743. [DOI] [PubMed] [Google Scholar]
- 38. Doupis J, Rahangdale S, Gnardellis C, et al. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity 2011; 19: 729–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vinet A, Karpoff L, Walther G, et al. Vascular reactivity at rest and during exercise in middle‐aged obese men: effects of short‐term, low‐intensity, exercise training. Int J Obes (Lond) 2011; 35: 820–828. [DOI] [PubMed] [Google Scholar]
- 40. Martin BJ, Verma S, Charbonneau F, et al. The relationship between anthropometric indexes of adiposity and vascular function in the fate cohort. Obesity 2013; 21: 266–273. [DOI] [PubMed] [Google Scholar]
- 41. Karason K, Wikstrand J, Sjostrom L, Wendelhag I. Weight loss and progression of early atherosclerosis in the carotid artery: a four‐year controlled study of obese subjects. Int J Obes Relat Metab Disord 1999; 23: 948–956. [DOI] [PubMed] [Google Scholar]
- 42. Ciccone M, Maiorano A, De Pergola G, et al. Microcirculatory damage of common carotid artery wall in obese and non obese subjects. Clin Hemorheol Microcirc 1998; 21: 365–374. [PubMed] [Google Scholar]
- 43. Mavri A, Stegnar M, Sentočnik JT, Videčnik V. Impact of weight reduction on early carotid atherosclerosis in obese premenopausal women. Obes Res 2001; 9: 511–516. [DOI] [PubMed] [Google Scholar]
- 44. De Michele M, Panico S, Iannuzzi A, et al. Association of obesity and central fat distribution with carotid artery wall thickening in middle‐aged women. Stroke 2002; 33: 2923–2928. [DOI] [PubMed] [Google Scholar]
- 45. Stefan N, Kantartzis K, Machann J, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 46. Blaha MJ, Rivera JJ, Budoff MJ, et al. Association between obesity, high‐sensitivity c‐reactive protein ≥2 mg/l, and subclinical atherosclerosis: implications of jupiter from the multi‐ethnic study of atherosclerosis. Arterioscler Thromb Vasc Biol 2011; 31: 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Park J, Kim SH, Cho GY, et al. Obesity phenotype and cardiovascular changes. J Hypertens 2011; 29: 1765–1772. [DOI] [PubMed] [Google Scholar]
- 48. Csongradi E, Nagy B Jr, Fulop T, et al. Increased levels of platelet activation markers are positively associated with carotid wall thickness and other atherosclerotic risk factors in obese patients. Thromb Haemost 2011; 106: 683–692. [DOI] [PubMed] [Google Scholar]
- 49. Ecemis GC, Kahraman H, Nural MS, Aslan HS, Atmaca A. The relationship between insulin resistance and carotid artery intima‐media thickness in obese and morbidly obese women. Turk J Med Sci 2012; 42: 1121–1128. [Google Scholar]


