Abstract
To investigate the role of Angptl4, an inhibitor of lipoprotein lipase that is induced by >3-fold in the heart after rosiglitazone treatment, we generated transgenic mice that overexpress Angptl4 in the heart (MHC-Angptl4). We show that MHC-Angptl4 mice exhibit cardiac-restricted expression of the transgene and inhibition of cardiac lipoprotein lipase (LPL) activity. However, LPL activities in other tissues or that released into plasma by heparin are not affected. In addition, MHC-Angptl4 mice also exhibit hypertriglyceridemia after 6 h of fasting. We use echocardiography to show that MHC-Angptl4 mice develop left-ventricular dysfunction. Comparison of the metabolic profiles of isolated working hearts demonstrates that cardiac impairment in MHC-Angptl4 mice is positively associated with decreased triglyceride (TG) utilization. When bred to transgenic mice that overexpress acyl-CoA synthetase in the heart, a strain that exhibits elevated cardiac TG accumulation, cardiac TG content in double transgenic mice is reversed to that of wild-type mice. Taken together, our data support the hypothesis that induction of Angptl4 in the heart inhibits lipoprotein-derived fatty acid delivery. This mouse model will be useful to elucidate the role of reduced fatty acid supply in the pathogenesis of heart failure and related disorders.
Keywords: lipase, nuclear receptor, cardiomyopathy
Under normal physiologic conditions, cardiac myocytes in the postnatal mammalian heart rely on β-oxidation of long-chain fatty acids to generate ATP. Up to 70% of the energy requirements of the heart may be derived from fatty acids, originating from three sources: circulating free fatty acids (FFAs) bound to albumin, circulating chylomicrons and very low-density lipoprotein, which requires the action of lipoprotein lipase (LPL) before utilization, and hydrolysis of triglycerides (TG) present in the cardiomyocyte (1–3). Because FFA concentrations are in the low nanomolar range in serum, cardiac myocytes must rely on the action of LPL to hydrolyze circulating lipoproteins as the main source of fatty acids for uptake (4). In support of the critical role of LPL in cardiac fuel metabolism, cardiac muscle has a higher level of LPL expression than other forms of muscle tissue (5). However, the role of posttranslational regulation of LPL activity in the control of cardiac metabolism is still not fully understood.
Two members in the family of angiopoietin-like proteins, Angptl3 and -4, have been shown to be potent inhibitors of LPL activity (6–8). In contrast to Angptl3, whose expression is restricted to the liver and is not affected by nutrient availability (9), the relevance of Angptl4 in fuel metabolism is further supported by the observation that its expression is induced by fasting (10, 11). Because fasting causes a change in the metabolic patterns of many tissues, induction of Angptl4 during fasting might play a role in the switch of the metabolic patterns of tissues to ensure adequate energy partitioning that is essential for survival. A role of Angptl4 in metabolic regulation is also suggested by the observation that Angptl4 expression is modulated by all members of the peroxisome proliferator-activated receptor (PPAR) family of transcription factors (10, 12–14), demonstrated by genetic as well as pharmacological studies (15).
Of clinical relevance, agonists of PPARγ, such as rosiglitazone and pioglitazone, are now widely prescribed in the treatment of type 2 diabetes. Because expression of Angptl4 is increased by PPARγ activation both in vitro and in vivo, a role of Angptl4 in mediating the biological activity of thiazolidinediones (TZDs) has been suggested (13). Notably, treatment with TZDs has been shown to be associated with increased incidence of cardiac hypertrophy and diminished cardiac fatty acid catabolism in preclinical species (16–18). However, the molecular events leading to this observation remain elusive.
To test the hypothesis that Angptl4, by inhibiting the action of LPL in heart, which relies on the availability of fatty acids as the main source of fuel (4), serves a gatekeeping role in cardiac metabolism and is a key regulator of cardiac function, we generated transgenic mouse lines with cardiac-restricted overexpression of Angptl4. These mice exhibit marked inhibition of cardiac LPL activity, fasting hypertriglyceridemia, and develop left-ventricular dysfunction. This study thus provides insight into the role of perturbations of myocardial lipid metabolism in the pathogenesis of cardiomyopathy.
Materials and Methods
Reagents. [1,3-13C2]Acetoacetate, [3-13C1]lactate, and [3-13C1]pyruvate were purchased from Cambridge Isotopes (Andover, MA). Uniformly labeled (99%) long-chain fatty acids were obtained from Isotec (Miamisburg, OH). Intralipid (20% i.v. fat emulsion) was obtained from Baxter Healthcare (Deer-field, IL). Heparin, LPL standard, unlabelled triolein, lecithin, PGO enzyme preparation, and Serum Triglyceride Determination kit were obtained from Sigma. [9,10-3H(N)]triolein was from PerkinElmer Life Sciences.
Animal Treatment Procedure and Microarray Studies. All procedures were performed following the guidelines of Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center and Merck Research Laboratories. C57BL/6 mice (Charles River Laboratories), ≈9–11 weeks old, were group housed (four mice per cage) and fed standard rodent chow for at least 1 week before study initiation. Each animal group (n = 12) was dosed orally with PPARα agonist Wyeth14643 or PPARγ agonist rosiglitazone, once daily in the morning for 6 h, 1, 3, or 7 days; dose volume was 10 ml/kg. The vehicle group was dosed with 0.25% methylcellulose. Animals were killed by CO2 asphyxiation. Tissues were harvested for microarray analysis as described (19).
Generation of α-MHC-Angptl4 Transgenic Mice and Angptl4/ACS Double Transgenic Mice. The MHC-Angptl4 transgene consists of a 5.5-kb segment of the α-MHC (myosin heavy chain) gene promoter (kindly provided by Deepak Srivastava, University of Texas Southwestern Medical Center), driving transcription of the 1.2-kb rat Angptl4 cDNA, downstream of which lies a human growth hormone (hGH) gene polyadenylation signal. Injection of the transgene was performed by the transgenic core facility at University of Texas Southwestern Medical Center. Three founders yielded true breeding lines and were used for subsequent analyses. Angptl4/ACS double transgenic mice were generated by breeding MHC-Angptl4 mice with MHC-ACS mice (20). Mice were genotyped by PCR on tail-biopsied samples.
Northern and Western Blotting Analysis of Transgene Expression in MHC-Angptl4 Transgenic Mice. Total RNA was isolated from the hearts and other tissues of MHC-Angptl4 transgenic mice or control littermates, run on denaturing agarose gel, and transferred onto nylon membrane. Hybridization was performed according to standard protocols (21). Transgenic expression of Angptl4 protein was detected with an antibody that recognizes the c-myc epitope.
Plasma Preparation and Analysis. Blood was collected from 6-h-fasted 2-month-old mice into Eppendorf tubes coated with EDTA. Plasma was prepared by low-speed centrifugation (5,000 × g for 5 min). MHC-Angptl4 (n = 6) and wild-type (n = 5) mice were gavaged with corn oil (5 ml/kg). Blood samples were collected from tail vein at 0, 2, 4, 6, 8, and 10 h after gavage, and plasma TG measured (22).
TG Content of Heart. Animals were killed under sodium pentobarbital anesthesia. Ventricular structures were rapidly isolated and snap frozen. Total lipids were extracted from ≈50–100 mg of tissue by the method of Folch et al. (23), and dried under N2 gas. TG content of tissue was measured by the method of Danno et al. (24).
Determination of LPL Activity in Tissues and Postheparin Plasma. Fresh tissue or frozen tissue powder were homogenized at 4°C as described (25). Insoluble material was removed by centrifugation (26). Phospholipid-stabilized emulsion in glycerol was prepared as described (27). Postheparin plasma (PHP) was obtained from age-matched wild-type or transgenic mice. Heparin (1 unit/g body weight) was injected into the jugular vein, and blood was collected 5 min later. LPL activity in PHP was determined as above.
Incubation of tissue lysate or postheparin plasma with [9,10-3H(N)]triolein emulsions was carried out for 30–60 min at 25°C in a shaking water bath. After incubation, released fatty acid was extracted as described (28). Labeled triolein was also incubated with buffer alone to obtain the background reading that is subtracted from all samples. Lipoprotein lipase standard was used to determine the linear range of the assay. Readings from all experiments are within this range.
Echocardiography. Echocardiography was performed on wild-type and MHC-Angptl4 mice fed ad libitum by using a Vivid 7 GE echo machine. Two-dimensional and M-mode images were obtained from parasternal short axis position in nonsedated mice. Percentage fractional shortening was calculated from M-mode images. Measurements were performed on mice at 2 and 7 months of age, with similar results.
Heart Perfusions. Hearts were perfused by using conventional Langendorff methods (retrograde) as described (29). Two different substrate conditions were tested: (i) [U-13C]FFA (0.63 mM algal mix bound to albumin), [1,3-13C2]Acetoacetate (0.28 mM), [3-13C1]lactate (4.9 mM), [3-13C1]pyruvate (0.49 mM), glucose (8.2 mM), and glycerol (0.13 mM); and (ii) [U-13C]Acetoacetate (0.28 mM), [3-13C1]lactate (4.9 mM), [3-13C1]pyruvate (0.49 mM), and 10 ml of 20% Intralipid per liter of perfusate (200 mg/dl triglycerides).
Mouse hearts were rapidly excised from MHC-Angptl4 or control mice weighing ≈35 g and either fed ad libitum or fasted for 24 h. Heart rate and developed pressure was monitored. The pO2 difference and measure of coronary flow were used to evaluate MVO2 (30). Hearts were then freeze-clamped, ground to a fine powder, extracted, and finally dissolved in D2O for 13C and 1H analysis (31, 32). Total substrate oxidation or citric acid cycle flux was determined by using the reported relationship (33, 34), Ct = Qt/(FC0R0 + FC1R1 + FC2R2 + FC3R3), where Ct is citric acid cycle flux, Qt is oxygen consumption, Fc0 is the fraction of acetyl-CoA derived from unlabeled substrates, FC1 is the fraction of [1-13C]acetyl-CoA ([1,3-13C2]acetoacetate under condition i), FC2 is the fraction of [2-13C]acetyl-CoA ([3-13C2]lactate/pyruvate), and FC3 is the fraction of [U-13C2]acetyl-CoA ([U-13C]FFA under condition i or [U-13C4]acetoacetate under condition ii). R0-R3 is the proportionality factor between citric acid cycle flux and oxygen consumption as reported and was taken as follows. R0 = 3.0 (endogenous substrates), R1 = 2.0 (acetoacetate), R2 = 2.95 (lactate/pyruvate), and R3 = 2.71 (FFA mix/triglycerides). Absolute substrate oxidation was calculated as: endogenous substrate oxidation = Fc0 × Ct; acetoacetate = Fc1 × Ct; lactate/pyruvate = Fc2 × Ct; FFA = Fc3 × Ct.
Statistical Analysis. All data were analyzed by using prism (Graph-Pad, San Diego) software, expressed as means ± SEM. Differences between groups are calculated by performing an unpaired t test and considered statistically significant when P values are <0.05.
Results
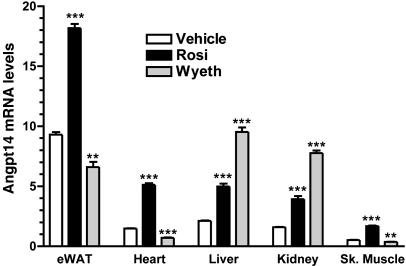
Angptl4 Expression Is Robustly Induced in the Heart After Treatment with Rosiglitazone. To identify target genes of PPARα and PPARγ in vivo, microarray experiments were performed on several tissues treated with Wyeth14643, a PPARα agonist, or rosiglitazone, a PPARγ agonist. Among the genes whose expression levels were changed by these treatments, Angptl4 was one of a number of genes that is robustly regulated by both Wyeth14643 and rosiglitazone (Fig. 1). Furthermore, Angptl4 expression was differentially regulated by these PPARα and PPARγ agonists. Wyeth14643 selectively and robustly up-regulated Angptl4 expression in liver and kidney by >3-fold, but down-regulated it in epididymal white adipose tissue, muscle, and heart by ≈2-fold. In contrast, rosiglitazone up-regulated Angptl4 expression across all five tissues that were examined. Notably, Angptl4 transcript was up-regulated by almost 3-fold in the heart. The tissue-specific induction patterns of Angptl4 suggest that it may act locally in an autocrine/paracrine fashion. This hypothesis is also supported by the observation that processing of Angptl4, which we reported recently (35), is regulated by its site of expression (14).
Fig. 1.
Angptl4 gene expression in five tissues from C57BL/6 mice treated with a PPARα agonist, Wyeth14643 (Wyeth), or a PPARγ agonist, rosiglitazone (Rosi), for 7 days. Tissues were harvested 6 h after the last dose, RNA was isolated, and global gene transcription profiling was performed as described in Materials and Methods. Pharmacological activation of PPARα causes robust up-regulation of Angptl4 transcript (3- to 4-fold) in liver and kidney but modest down-regulation (>2-fold) in epididymal white adipose tissue, muscle, and heart. PPARγ activation resulted in up-regulation (>2-fold) of Angptl4 transcript across all tissues. **, P < 0.01; ***, P < 0.0001 vs. vehicle.
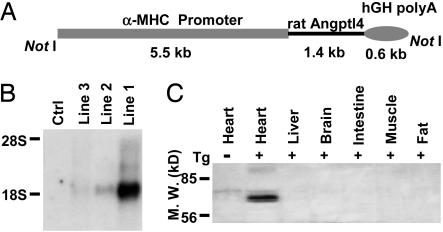
Cardiac Overexpression of Angptl4 Causes Decreased LPL Activity and TG Content in the Heart. Because cardiac tissue relies on LPL-derived fatty acid as a fuel source and Angptl4 is a potent inhibitor of LPL, we investigated whether overexpression of Angptl4 in the heart of transgenic mice causes a disturbance of fuel utilization and functional impairment of the heart. We overexpressed Angptl4 in the postnatal cardiac ventricles of mice by using the myosin heavy-chain gene promoter (Fig. 2A). At the C terminus of Angptl4, we inserted a c-myc and histidine epitope tag that does not interfere with the expression or hyperlipidemic effect of Angptl4 (35). Founder mice were screened by PCR and Southern blot analysis (data not shown). We characterized three independent MHC-Angptl4 lines and analyzed their progenies. The average litter size was 10 pups, and there was no evidence that transgene expression resulted in any changes in development. Expression of the transgene was evaluated by Northern blotting of total RNA from heart as well as Western blotting of lysates from various tissues of control or transgenic mice (Fig. 2 B and C and data not shown). MHC-Angptl4 mice exhibited high expression levels of the transgene in the heart, with no detectable expression in several other tissues examined, including brain, liver, skeletal muscle, intestine, and fat (Fig. 2C).
Fig. 2.
Generation and characterization of transgenic mice overexpressing Angptl4 in cardiomyocytes. (A) Diagram of construct used to generate MHC-Angptl4 mice. The lengths of each segment in the transgene are indicated under the construct. NotI denotes the restriction enzyme used to liberate the transgene before injection into fertilized eggs. (B) Determination of levels of transgenic Angptl4 expression in different lines of MHC-Angptl4 mice by Northern blotting analysis. Ctrl, nontransgenic littermates. (C) Detection of transgenic expression of Angptl4 protein in the heart by Western blotting for c-myc tag. Minus and plus signs denote the origin of tissues from nontransgenic (minus sign) or transgenic (plus sign) mouse. Tg, transgenic.
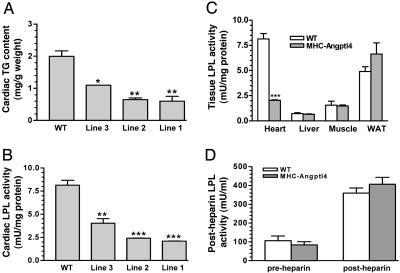
Because heart uses lipoprotein-derived FFA as the main source of fuel (4), biochemical analyses were carried out to quantitatively assess the effects of Angptl4 overexpression on cardiac LPL activity and myocardial lipid content. Treatment of tissue lysates from hearts of each line of MHC-Angptl4 mice with [3H]triolein emulsion revealed a transgene expression-dependent inhibition of cardiac LPL activity (Fig. 3A) in parallel to decreased cardiac TG content (Fig. 3B). The inhibition of cardiac LPL activity in lines 1 and 2 are similar (Fig. 3B), yet the mRNA level of Angptl4 is much higher in line 1 (Fig. 2B), suggesting that once the amount of Angptl4 protein reaches a threshold level, further LPL inhibition does not occur.
Fig. 3.
Dosage-dependent inhibition of cardiac TG content (A) and LPL activity (B) by transgenic expression of Angptl4, tissue-associated LPL activity (C) and heparin-releasable LPL activity (D) in control and MHC-Angptl4 mice. Hearts from 9-month-old MHC-Angptl4 mice or control littermates were rapidly removed, and ventricles were dissected. Cardiac TG accumulation was assayed as described in Materials and Methods. LPL activity in heart, liver, skeletal muscle, and adipose tissue (WAT) of MHC-Angptl4 mice or control littermates were measured by using [3H]triolein as substrate. LPL activity was significantly decreased only in the hearts of MHC-Angptl4 mice (C). (D) Heparin-releasable LPL activity was determined in 2-month-old MHC-Angptl4 mice or control littermates. Plasma was collected 5 min after heparin injection, and LPL activity was determined as in C. Heparin-releasable LPL activity was similar between MHC-Angptl4 and control mice before (pre-heparin) and after (post-heparin) heparin injection. n = 4 – 6 mice per group. *, P < 0.05; **, P < 0.01; ***, P < 0.0001.
Because Angptl4 protein is processed and secreted into media or plasma when expressed in 293 cells or the liver of mice (35), we asked whether cardiac expression of Angptl4 serves to inhibit LPL locally in an autocrine/paracrine fashion or whether it also affects the activity of LPL in other tissues. We determined LPL activity of heart, liver, skeletal muscle, and white adipose tissue of both MHC-Angptl4 mice and their nontransgenic littermates. We chose line 1 for this analysis because this line expresses Angptl4 at the highest level as well as inhibiting cardiac LPL activity most completely (Fig. 3). The presence of the Angptl4 transgene in the heart caused a reduction in cardiac LPL activity by ≈80%, whereas the normally low levels of hepatic LPL activity were unchanged (Fig. 3C). LPL activities in skeletal muscle and WAT also remained essentially unchanged (Fig. 3C). Correspondingly, the TG content in hearts, but not livers, of MHC-Angptl4 mice was also significantly decreased compared with control littermates (data not shown). Interestingly, heparin-releasable LPL activity was comparable between MHC-Angptl4 mice and control littermates (Fig. 3D). These data suggest that cardiac overexpression of Angptl4 results in heart-specific inhibition of LPL activity, thus directly controlling lipoprotein partitioning.
Increased Fasting and Postprandial TG Levels in the Plasma of MHC-Angptl4 Mice. Given that cardiac specific LPL knockout mice develop hypertriglyceridemia after 6 h of fasting (22), we investigated whether Angptl4-mediated LPL inhibition in the heart also recapitulates the findings. In agreement with earlier studies, 6-h-fasted 2-month-old male MHC-Angptl4 mice showed a >2-fold increase in plasma TG levels (58.4 ± 2.8 mg/dl in WT vs. 133.1 ± 5.8 mg/dl in MHC-Angptl4, P < 0.0001). However, plasma glucose levels at 6 h of fasting are similar (87.6 ± 3.1 mg/dl in WT vs. 87.5 ± 5.8 mg/dl in MHC-Angptl4), even after mice are fasted for up to 24 h (data not shown). However, after corn oil gavage, postprandial TG levels are increased similarly in WT and MHC-Angptl4 mice, which peaked at 2 h (190.4 ± 15.3 mg/dl for MHC-Angptl4 mice and 112.0 ± 27.4 mg/dl in control mice, P < 0.05). Similar differences were seen at additional points for up to 10 h after oil gavage (data not shown). Cardiac glycogen levels are similar in WT and MHC-Angptl4 mice (data not shown). Because transgene expression is limited to heart tissue, these data indicate that a significant portion of plasma triglyceride turnover can be attributed to cardiac utilization. This finding is in agreement with earlier studies demonstrating that the heart is a dominant site of lipoprotein utilization in vivo (4).
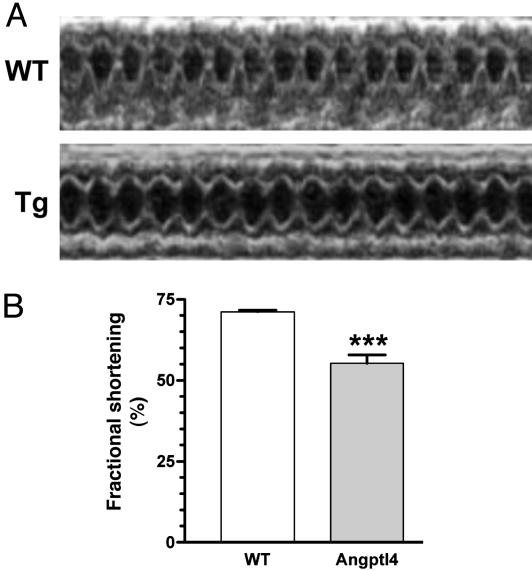
Cardiac Overexpression of Angptl4 Causes Impairment of Heart Function. Because inhibition of cardiac LPL activity by Angptl4 would limit utilization of lipoprotein-derived fatty acids, we asked whether such inhibition of cardiac LPL activity by Angptl4 would affect cardiac function. We performed echocardiography measurements on MHC-Angptl4 mice and control littermates at 5 months of age. Previously, it has been reported that increased cardiac lipid uptake by overexpression of LPL or ACS in the heart causes lipotoxic cardiomyopathy (20, 36). The 2D and M-mode images from the parasternal short axis position in nonsedated mice (Fig. 4A) and calculated percentage fractional shortening from M-mode images (Fig. 4B) show significant impairment of heart function in MHC-Angptl4 mice. These data suggest that similar to models of cardiac dysfunction caused by increased lipid uptake (20), inhibition of lipoprotein-derived FFA also impairs cardiac function, further highlighting the importance of balanced FFA demand and availability on the maintenance of normal cardiac function.
Fig. 4.
Left ventricular dysfunction in MHC-Angptl4 transgenic mice. (A) Echocardiogram of 5-month-old wild-type littermates (WT) and MHC-Angptl4 (Tg) mice. (B) Quantification of fractional shortening for age- and sex-matched wild-type and transgenic mice. n = 7 mice per group. ***, P < 0.0001.
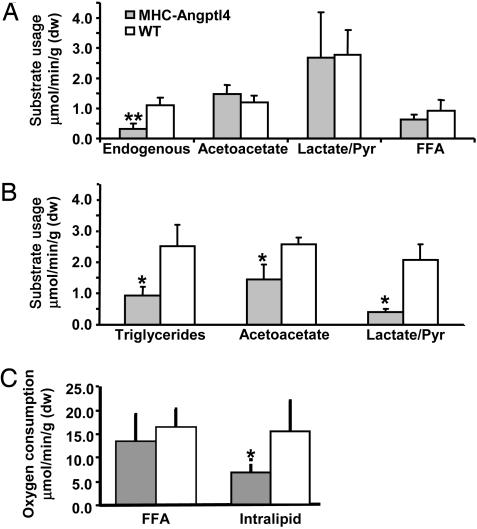
Although overexpression of Angptl4 clearly correlates with decreased LPL activity and TG accumulation as well as impaired heart function, the relationship between these abnormalities and cardiac oxidative metabolism is less clear. Thus, cardiac metabolism was assessed by using well established NMR isotopomer methods, which report the oxidation rates of selected substrates. Isolated hearts from MHC-Angptl4 and control mice were perfused in the Langendorff mode with a mixture of 13C-labeled substrates. There was no difference in the utilization of [1,3-13C2]acetoacetate, [3-13C1]lactate, [3-13C1]pyruvate, and [U-13C]FFA in hearts from ad libitum fed control and MHC-Angpotl4 mice. However, in parallel with decreased cardiac TG content, there was a significant decrease in the utilization of endogenous (unlabeled) substrates in hearts from MHC-Angptl4 mice (Fig. 5A). Additionally, heart function measured by the rate pressure product (RPP) was not different between hearts from MHC-Angptl4 and control mice. To examine TG oxidation, hearts from fasted mice were again perfused with [U-13C2]acetoacetate and [3-13C1]lactate/[3-13C1]pyruvate, but a TG emulsion (Intralipid) was used instead of FFA. In parallel with the reduced LPL activity seen in the hearts of MHC-Angptl4 mice, intralipid utilization was reduced by >60% (Fig. 5B). Acetoacetate and lactate/pyruvate utilization were also reduced in the hearts of MHC-Angptl4 mice, owing to a >50% decrease in oxygen consumption and significant decrease in RPP (Fig. 5C and data not shown).
Fig. 5.
Substrate oxidation is impaired and oxygen consumption reduced in isolated heart of MHC-Angptl4 mice. (A) MHC-Angptl4 mice or control littermates were fed ad libitum before being killed. Langendorff heart preparations were made and provided labeled substrates ([1,3-13C2]acetoacetate, [3-13C1]lactate, [3-13C1]pyruvate, and [U-13C]FFA) as well as unlabelled glucose. The absolute amount of usage of each labeled substrate was calculated as described in Materials and Methods. (B) MHC-Angptl4 mice or control littermates were fasted for 24 h before being killed. Langendorff heart preparations were made and provided labeled substrates ([1,3-13C2]acetoacetate, [3-13C1]lactate, [3-13C1]pyruvate) as well as unlabelled Intralipid emulsion. The absolute amount of usage of each labeled substrate was calculated as in A. (C) Oxygen consumption of hearts under conditions as in A and B. A decrease of oxygen consumption by >50% was observed in hearts of MHC-Angptl4 mice when Intralipid was used in the perfusate as a source of FFA. *, P < 0.05; **, P < 0.01. n = 3–4 mice per group.
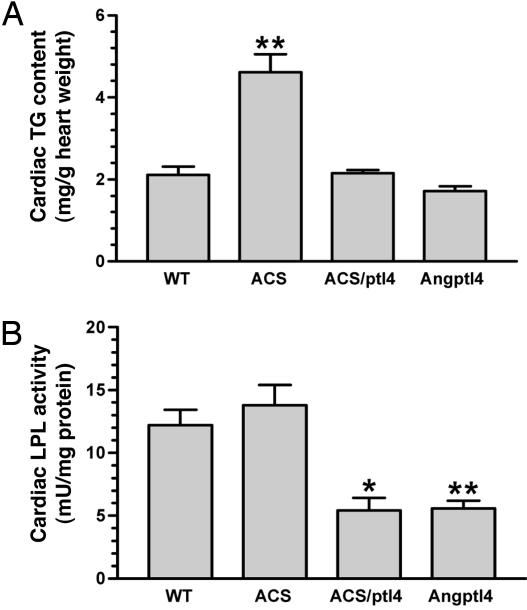
Angptl4 Reverses Lipid Overaccumulation in Hearts of a Model of Lipotoxic Cardiomyopathy. To further establish the gatekeeping role of Angptl4 in cardiac fuel utilization, we crossed MHC-Angptl4 mice with a model of lipotoxic cardiomyopathy, MHC-ACS mice (20). The latter overexpresses long-chain acyl-CoA synthetase, a protein that plays an important role in the vectorial transport of fatty acid across the plasma membrane and their subsequent metabolism (37). Hearts of MHC-ACS mice show marked triglyceride accumulation and exhibit hypertrophy and left ventricular dysfunction. Bigenic mice were obtained by crossing MHC-Angptl4 mice and MHC-ACS mice and were obtained at predicted frequency, suggesting that this manipulation does not cause embryonic lethality. We measured cardiac LPL activity and TG content of the monogenic and bigenic mice at 2 months of age. We found that the cardiac TG content in MHC-Angptl4 tends to be lower than in wild-type mice, although this difference did not reach statistical significance (Fig. 6A). In contrast, as has been reported (20), overexpression of ACS in heart caused a significant increase in cardiac TG content over controls (Fig. 6A). Notably, coexpression of Angptl4 with ACS reduced cardiac TG content to levels similar to that found in wild-type mice, suggesting that fatty acid delivery to heart is restricted by Angptl4 overexpression. In addition, whereas the activity of LPL in MHC-ACS mice is not increased, coexpression of ACS with Angptl4 lowered cardiac LPL activity of bigenic mice to a level similar to that seen in MHC-Angptl4 mice (Fig. 6B). These data provide additional evidence that, even in the presence of irreversible fatty acid import by the action of acyl-CoA synthetase, Angptl4 plays a more dominant role in restricting the availability of lipoprotein-derived fatty acids. When accessed by echocardiography, cardiac function in bigenic mice also showed a trend toward normalization, although still abnormal (data not shown), raising the possibility that the compensatory increase of glucose uptake and oxidation might be insufficient to restore cardiac function in bigenic mice.
Fig. 6.
Reversal of lipotoxicity of MHC-ACS mice by cardiac overexpression of Angptl4. Bigenic mice (6 weeks old) were created by crossing MHC-Angptl4 mice with MHC-ACS mice, which exhibit increased cardiac TG content due to overexpression of Acyl-CoA synthetase. (A) The introduction of Angptl4 reversed cardiac TG content to wild-type levels. **, P < 0.01. n = 5 mice per group. ACS/ptl4, bigenic mice for ACS/Angptl4. (B) Cardiac LPL activity in bigenic mice was reduced to similar levels as that in MHC-Angptl4 mice, suggesting that Angptl4 overexpression limits the availability of lipoprotein-derived FFA delivery, even though ACS activity is high within cardiomyocytes. *, P < 0.05; **, P < 0.01.
Discussion
Our results provide several lines of evidence for a critical role of Angptl4 as a gatekeeper of fuel partitioning: (i) cardiac overexpression of Angptl4 selectively inhibits LPL activity only in heart, highlighting an autocrine/paracrine role for Angptl4 expression in that tissue; (ii) the extent of cardiac LPL inhibition is positively correlated with the level of transgene expression; (iii) inhibition of cardiac LPL causes fasting plasma hypertriglyceridemia, similar to that of cardiac LPL knockout mice (22); (iv) overexpression of Angptl4 in the heart alters substrate oxidation and impairs cardiac function; and (v) cardiac-specific coexpression of Angptl4 rescues excessive lipid accumulation in the hearts of MHC-ACS transgenic mice.
Earlier, we reported that when Angptl4 is expressed in 293 cells, a variable-sized oligomer is synthesized that undergoes regulated processing (35). We and others also reported that both i.v. injection of bacterially produced recombinant Angptl4 and adenoviral overexpression of the protein in the liver potently raise plasma TG levels (7, 8). Angptl4 has also been reported to be differentially processed in human liver and adipose tissue (14). These findings raise the intriguing possibility that, depending on the site of Angptl4 expression, it may be processed differently, and therefore function differently. For example, when immediately processed upon secretion, as in the liver (14), its coiled-coil domain may circulate systematically and act in an endocrine manner to inhibit LPL activities in various tissues. In contrast, when it is not processed at the site of its production, such as in the heart, it may act in an autocrine/paracrine manner to only inhibit local LPL activity. The correlation between the ability of Angptl4 to be processed and the level of expression of LPL at the same site therefore warrants additional studies.
The activity of LPL in the capillary bed of tissues is a key mechanism of lipoprotein-derived FFA delivery. In vivo, fatty acids provide >70% of the heart's energy supply, most of which is derived from lipoproteins (4). Numerous studies have demonstrated that regulation of LPL activity by nutritional status occurs by posttranslational mechanism (38). However, the mechanisms involved are still poorly understood (39, 40). As a recently discovered LPL inhibitor, the role of Angptl4 in the regulation of LPL activity during fasting or pharmacological treatment has not been well defined, in large part because of its variable expression in multiple tissues. Our current study demonstrates that, when Angptl4 is overexpressed in the heart, it specifically inhibits cardiac LPL activity. The residual LPL activity, at a level similar to that found in cardiac LPL knockout mice (22), might be due to LPL expression in noncardiomyocytes (41). We suggest that, during the secretion of Angptl4 in LPL-rich tissues, such as heart, it might be “tethered” to LPL, thus delaying its proteolytic processing. This hypothesis is supported by the observation that, in humans, processing of Angptl4 does not occur in adipose tissue, where LPL expression is high, but does occur in liver, where LPL expression is low (14). When Angptl4 is not proteolytically processed in tissues where it is induced, local inhibition of LPL activity will divert fatty acids contained in lipoproteins away to other tissues for utilization. In particular, TZD inhibition of fatty acid acquisition through the induction of Angptl4 in tissues, such as skeletal muscle and heart, may result in a net decrease in tissue triglyceride levels and an apparent redistribution of lipids to adipose tissue, a phenomenon that has been observed in the clinic during the treatment of diabetic patients with TZDs.
Certain important questions regarding Angptl4 function still remain. An apparent, and as yet inexplicable, discrepancy exists between the observed hyperlipidemic effect of Angptl4 and the lack of such an effect by TZDs. LPL activity in adipose tissue of rosi-treated mice is similar to that in controls (data not shown). This finding suggests that other PPARγ target genes are likely involved to counteract the effects of Angptl4's hyperlipidemic action. For example, it has been shown that PPARγ agonists increase LPL expression and activity in white adipose tissue (42). The physiological role of the Angptl4 carboxyl fibrinogen-like domain, which is present in the circulation at detectable levels and is found at elevated levels in the plasma of db/db mice (data not shown), also remains to be clarified. Additionally, the level of angptl4 expression from the transgene is likely to be much higher than that induced by TZD treatment. The transgene also becomes active very early on, before birth, which may also modify the phenotypes observed. These factors in aggregate may contribute to the differences caused by Angptl4 overexpression and TZD action.
Based on our in vivo evidence, we propose that Angptl4 is likely to have a variety of biological consequences, both positive and negative, as a result of its control of fuel utilization in different tissues. In skeletal muscle, elevation of Angptl4 will inhibit LPL activity, thereby causing a net decrease in intramyo-cellular TG content and indirectly potentiating insulin sensitivity. In macrophages, where increased LPL activity is associated with elevated levels of atherosclerosis, inhibition of this enzyme by induction of Angptl4 should provide protection from the induction of atherosclerosis by excessive dietary intake of lipids. However, in heart, where lipoprotein-derived fatty acid is the major energy source, we have shown that induction of Angptl4 inhibits LPL activity, resulting in cardiomyopathy.
PPARγ is the primary target of TZD antidiabetic agents; the receptor is abundantly expressed in adipocytes, which are thought to mediate the major insulin-sensitizing activities of such ligands (43). However, adipose tissue-specific deletion of PPARγ has revealed the presence of PPARγ actions that may be generated through other tissues (43). In support of this assertion, skeletal muscle-selective deletion of PPARγ in mice results in glucose intolerance and progressive insulin resistance (44). Interestingly, the microarray experiments we present here demonstrate that Angptl4 is significantly induced by rosiglitazone in multiple tissues, suggesting that it might play a role in the pleiotropic biological effects of PPARγ agonists. In particular, on the basis of our characterization of mice overexpressing cardiac Angptl4, it is interesting to speculate that the shift in cardiac fuel utilization from lipids to carbohydrates and the concomitant induction in cardiac hypertrophy observed in preclinical species treated with TZDs (15–17) could be a consequence, at least in part, of PPARγ-mediated augmentation of Angptl4 expression. Further studies using cardiac-specific Angptl4 null mice will be necessary to demonstrate that this conjecture is correct.
In summary, cardiac-restricted overexpression of Angptl4 in our mouse model leads to marked inhibition of cardiac LPL activity and a parallel decrease in cardiomyocyte lipid accumulation and catabolism. This perturbation in lipid homeostasis results in the impairment of left ventricular function. Therefore, MHC-Angptl4 mice may prove useful as a model of altered cardiac lipid metabolism.
Acknowledgments
We thank Drs. Roger Unger, Steve Kliewer, and Joyce Repa for many discussions and a critical reading of the manuscript, Dr. Jean Schaffer (Washington University, St. Louis) for providing the MHC-ACS mice, and Charles Storey and Angela Milde for excellent technical assistance. We also acknowledge the efforts of the Merck/Rosetta Diabetes Microarray Group. This work was supported by National Institutes of Health Grants RR02584, U24-DK59632, HL-34557, and DK-60137.
Abbreviations: FFA, free fatty acid; LPL, lipoprotein lipase; TG, triglycerides; PPAR, peroxisome proliferator-activated receptor; TZD, thiazolidinediones.
References
- 1.Merkel, M., Eckel, R. H. & Goldberg, I. J. (2002) J. Lipid Res. 43, 1997–2006. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg, I. J. & Merkel, M. (2001) Front. Biosci. 6, D388–D405. [DOI] [PubMed] [Google Scholar]
- 3.Kim, J. K., Fillmore, J. J., Chen, Y., Yu, C., Moore, I. K., Pypaert, M., Lutz, E. P., Kako, Y., Velez-Carrasco, W., Goldberg, I. J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 7522–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustus, A. S., Kako, Y., Yagyu, H. & Goldberg, I. J. (2003) Am. J. Physiol. 284, E331–E339. [DOI] [PubMed] [Google Scholar]
- 5.Enerback, S. & Gimble, J. M. (1993) Biochim. Biophys. Acta 1169, 107–125. [DOI] [PubMed] [Google Scholar]
- 6.Shimizugawa, T., Ono, M., Shimamura, M., Yoshida, K., Ando, Y., Koishi, R., Ueda, K., Inaba, T., Minekura, H., Kohama, T. & Furukawa, H. (2002) J. Biol. Chem. 277, 33742–33748. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida, K., Shimizugawa, T., Ono, M. & Furukawa, H. (2002) J. Lipid Res. 43, 1770–1772. [DOI] [PubMed] [Google Scholar]
- 8.Ge, H., Yang, G., Yu, X., Pourbahrami, T. & Li, C. (2004) J. Lipid Res. 45, 2071–2079. [DOI] [PubMed] [Google Scholar]
- 9.Inukai, K., Nakashima, Y., Watanabe, M., Kurihara, S., Awata, T., Katagiri, H., Oka, Y. & Katayama, S. (2004) Biochem. Biophys. Res. Commun. 317, 1075–1079. [DOI] [PubMed] [Google Scholar]
- 10.Kersten, S., Mandard, S., Tan, N. S., Escher, P., Metzger, D., Chambon, P., Gonzalez, F. J., Desvergne, B. & Wahli, W. (2000) J. Biol. Chem. 275, 28488–28493. [DOI] [PubMed] [Google Scholar]
- 11.Wiesner, G., Morash, B. A., Ur, E. & Wilkinson, M. (2004) J. Endocrinol. 180, R1–R6. [DOI] [PubMed] [Google Scholar]
- 12.Akiyama, T. E., Sakai, S., Lambert, G., Nicol, C. J., Matsusue, K., Pimprale, S., Lee, Y. H., Ricote, M., Glass, C. K., Brewer, H. B., Jr., & Gonzalez, F. J. (2002) Mol. Cell. Biol. 22, 2607–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon, J. C., Chickering, T. W., Rosen, E. D., Dussault, B., Qin, Y., Soukas, A., Friedman, J. M., Holmes, W. E. & Spiegelman, B. M. (2000) Mol. Cell. Biol. 20, 5343–5349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandard, S., Zandbergen, F., Tan, N. S., Escher, P., Patsouris, D., Koenig, W., Kleemann, R., Bakker, A., Veenman, F., Wahli, W., et al. (2004) J. Biol. Chem. 279, 34411–34420. [DOI] [PubMed] [Google Scholar]
- 15.Schmuth, M., Haqq, C. M., Cairns, W. J., Holder, J. C., Dorsam, S., Chang, S., Lau, P., Fowler, A. J., Chuang, G., Moser, A. H., et al. (2004) J. Invest. Dermatol. 122, 971–983. [DOI] [PubMed] [Google Scholar]
- 16.Moller, D. E. & Greene, D. A. (2001) Adv. Protein Chem. 56, 181–212. [DOI] [PubMed] [Google Scholar]
- 17.Berger, J. P., Petro, A. E., Macnaul, K. L., Kelly, L. J., Zhang, B. B., Richards, K., Elbrecht, A., Johnson, B. A., Zhou, G., Doebber, T. W., et al. (2003) Mol. Endocrinol. 17, 662–676. [DOI] [PubMed] [Google Scholar]
- 18.Carley, A. N., Semeniuk, L. M., Shimoni, Y., Aasum, E., Larsen, T. S., Berger, J. P. & Severson, D. L. (2004) Am. J. Physiol. 286, E449–E455. [DOI] [PubMed] [Google Scholar]
- 19.Hughes, T. R., Mao, M., Jones, A. R., Burchard, J., Marton, M. J., Shannon, K. W., Lefkowitz, S. M., Ziman, M., Schelter, J. M., Meyer, M. R., et al. (2001) Nat. Biotechnol. 19, 342–347. [DOI] [PubMed] [Google Scholar]
- 20.Chiu, H. C., Kovacs, A., Ford, D. A., Hsu, F. F., Garcia, R., Herrero, P., Saffitz, J. E. & Schaffer, J. E. (2001) J. Clin. Invest. 107, 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, L., Wang, Z. & Li, C. (2001) J. Biol. Chem. 276, 6343–6349. [DOI] [PubMed] [Google Scholar]
- 22.Augustus, A., Yagyu, H., Haemmerle, G., Bensadoun, A., Vikramadithyan, R. K., Park, S. Y., Kim, J. K., Zechner, R. & Goldberg, I. J. (2004) J. Biol. Chem. 279, 25050–25057. [DOI] [PubMed] [Google Scholar]
- 23.Folch, J., Lees, M. & Sloane Stanley, G. H. (1957) J. Biol. Chem. 226, 497–509. [PubMed] [Google Scholar]
- 24.Danno, H., Jincho, Y., Budiyanto, S., Furukawa, Y. & Kimura, S. (1992) J. Nutr. Sci. Vitaminol. (Tokyo) 38, 517–521. [DOI] [PubMed] [Google Scholar]
- 25.Hocquette, J. F., Graulet, B. & Olivecrona, T. (1998) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 121, 201–212. [DOI] [PubMed] [Google Scholar]
- 26.Ruge, T., Bergo, M., Hultin, M., Olivecrona, G. & Olivecrona, T. (2000) Am. J. Physiol. 278, E211–E218. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson-Ehle, P. & Schotz, M. C. (1976) J. Lipid Res. 17, 536–541. [PubMed] [Google Scholar]
- 28.Belfrage, P. & Vaughan, M. (1969) J. Lipid Res. 10, 341–344. [PubMed] [Google Scholar]
- 29.Burgess, S. C., Babcock, E. E., Jeffrey, F. M., Sherry, A. D. & Malloy, C. R. (2001) FEBS Lett. 505, 163–167. [DOI] [PubMed] [Google Scholar]
- 30.Neely, J. R., Liebermeister, H., Battersby, E. J. & Morgan, H. E. (1967) Am. J. Physiol. 212, 804–814. [DOI] [PubMed] [Google Scholar]
- 31.Jeffrey, F. M., Diczku, V., Sherry, A. D. & Malloy, C. R. (1995) Basic Res. Cardiol. 90, 388–396. [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey, F. M., Rajagopal, A., Malloy, C. R. & Sherry, A. D. (1991) Trends Biochem. Sci. 16, 5–10. [DOI] [PubMed] [Google Scholar]
- 33.Malloy, C. R., Jones, J. G., Jeffrey, F. M., Jessen, M. E. & Sherry, A. D. (1996) Magma 4, 35–46. [DOI] [PubMed] [Google Scholar]
- 34.Boucher, A., Lu, D., Burgess, S. C., Telemaque-Potts, S., Jensen, M. V., Mulder, H., Wang, M. Y., Unger, R. H., Sherry, A. D. & Newgard, C. B. (2004) J. Biol. Chem. 279, 27263–27271. [DOI] [PubMed] [Google Scholar]
- 35.Ge, H., Yang, G., Huang, L., Motola, D. L., Pourbahrami, T. & Li, C. (2004) J. Biol. Chem. 279, 2038–2045. [DOI] [PubMed] [Google Scholar]
- 36.Yagyu, H., Chen, G., Yokoyama, M., Hirata, K., Augustus, A., Kako, Y., Seo, T., Hu, Y., Lutz, E. P., Merkel, M., et al. (2003) J. Clin. Invest. 111, 419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gargiulo, C. E., Stuhlsatz-Krouper, S. M. & Schaffer, J. E. (1999) J. Lipid Res. 40, 881–892. [PubMed] [Google Scholar]
- 38.Preiss-Landl, K., Zimmermann, R., Hammerle, G. & Zechner, R. (2002) Curr. Opin. Lipidol. 13, 471–481. [DOI] [PubMed] [Google Scholar]
- 39.Bergo, M., Wu, G., Ruge, T. & Olivecrona, T. (2002) J. Biol. Chem. 277, 11927–11932. [DOI] [PubMed] [Google Scholar]
- 40.Wu, G., Olivecrona, G. & Olivecrona, T. (2003) J. Biol. Chem. 278, 11925–11930. [DOI] [PubMed] [Google Scholar]
- 41.Babaev, V. R., Fazio, S., Gleaves, L. A., Carter, K. J., Semenkovich, C. F. & Linton, M. F. (1999) J. Clin. Invest. 103, 1697–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laplante, M., Sell, H., MacNaul, K. L., Richard, D., Berger, J. P. & Deshaies, Y. (2003) Diabetes 52, 291–299. [DOI] [PubMed] [Google Scholar]
- 43.He, W., Barak, Y., Hevener, A., Olson, P., Liao, D., Le, J., Nelson, M., Ong, E., Olefsky, J. M. & Evans, R. M. (2003) Proc. Natl. Acad. Sci. USA 100, 15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hevener, A. L., He, W., Barak, Y., Le, J., Bandyopadhyay, G., Olson, P., Wilkes, J., Evans, R. M. & Olefsky, J. (2003) Nat. Med. 9, 1491–1497. [DOI] [PubMed] [Google Scholar]