Abstract
The fruits of Ziziphus jujuba, known as jujube or Chinese date, are being consumed all around the world because of their health benefits, as both food and herbal medicine. Traditionally, one of the main functions of jujube, as described in herbal medicine, is to benefit our brain by calming down the mind and improving quality of sleep. Here, the activities of jujubes on nervous system are summarized and discussed. Jujube possesses neuroprotective activities, including protecting neuronal cells against neurotoxin stress, stimulating neuronal differentiation, increasing expression of neurotrophic factors, and promoting memory and learning. Flavonoid, cAMP, and jujuboside could be the potential bioactive ingredients to account for the aforesaid biological activities. These findings imply that jujube is a potential candidate for development of health supplements for prevention and/or treatment of neurological diseases.
1. Introduction
Jujubae Fructus, the fruit of Ziziphus jujuba Mill. (Rhamnaceae), also known as jujube, or Chinese date, or red date, has been widely used as food and Chinese herbal medicine for over 3,000 years. Jujube is indigenous to Chinese culture (Figure 1). The description of jujube was first recorded in Classic of Poetry (1046-771 BC). Today, there is a wide distribution of jujube-related products in the world. The consideration of jujube as a vital food and/or medicine has a long history of record in China. In ancient Chinese book on herbal medicine Huangdi Neijing (475-221 BC), jujube was described as one of the five most valuable fruits in China. In Shennong Bencao Jing (300 BC-200 AD), an earlier book recoding medicinal herbs, jujube was considered as one of the superior herbal medicines that prolonged our life-span by nourishing blood, improving quality of sleep, and regulating digestive system.
Figure 1.
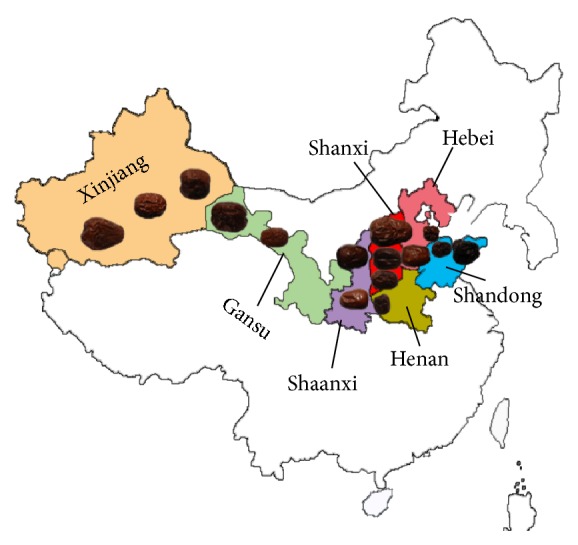
The cultivation areas and major production areas of jujube in China. The cultivation areas of jujube in China were highlighted and shown. The insert shows the location of major production areas of China, including Xinjiang, Gansu, Shaanxi, Shanxi, Hebei, Henan, and Shandong provinces. Shaanxi and Shanxi are considered as the original cultivation areas of jujube.
Jujube has been consumed for thousands of years, which is still gaining influence on our daily life. Recent phytochemical and pharmacological results have revealed that flavonoid, polysaccharide, and triterpenic acid are the main active ingredients within jujube. Based on the literatures, both flavonoid and polysaccharide could account for antioxidative effect of jujube [1–3]. Moreover, jujube polysaccharides were also proposed to be main active ingredients contributing to its immune-modulating and hematopoietic functions [4, 5]. Triterpenic acids were considered as active ingredients for the effect on anti-inflammatory and anticancer activities [6, 7]. In addition, betulinic acid and jujuboside B could be the active components showing beneficial effects on cardiovascular system [8, 9].
The study on biological activities has supported the health benefits of jujube as both food and medicinal herb. According to Chinese medicinal theory, jujube is considered as a medicinal herb that calms the mind and relieves mental tension. Clinically, jujube is commonly prescribed, either as single herb or in tranquillizing formulae combined with other herbal medicines, for the treatment of insomnia and forgetfulness. Previous reviews have summarized the fruit composition and its health benefits [10, 11]. However, the studies focused on neuroprotective activities are rather limited. Therefore, the neurobeneficial roles of jujube will be introduced in this review. In addition, the potential bioactive compounds of jujube related to brain benefits are further discussed.
2. Jujube on Neuron Differentiation
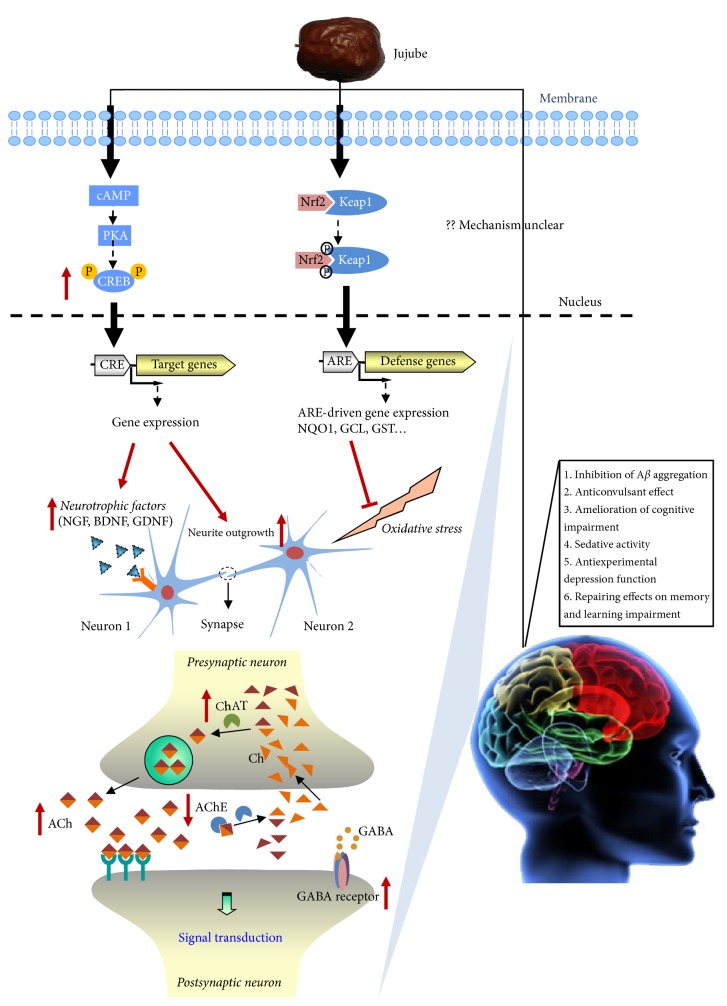
According to historical usage in China, one of the main functions of jujube was considered to benefit our brain by calming down the mind and improving quality of sleep. In modern science, benefiting our brain is usually related to neurobeneficial effects, for example, neuroprotection effect and neurotrophic action. In neurological disorders, for example, neurodegenerative diseases, insomnia, and depression, several common pathological conditions among them are found, that is, neurogenesis impairment, neurotrophic factor deficiency, and oxidative stress. Hence, the traditional function of jujube in benefiting the brain may be closely related to its neurobeneficial effects (Figure 2). Here, the neurobeneficial effects of jujube are summarized (Table 1).
Figure 2.
The neuroprotection effects of jujube. Jujube induces neurite outgrowth and neurothrophic factor expression via cAMP-dependent PKA signaling. Jujube possessed neuroprotection against oxidative stress via enhancing cellular Nrf2-dependent ARE-driven gene expressions. Jujube improves the choline acetyltransferase (ChAT) activity and shows inhibitory activity against acetylcholinesterase (AChE). Jujube increases the level of acetylcholine (ACh) in the brain. Jujube stimulates the transcriptional expression of GABA receptor subunits in rat hippocampal neurons.
Table 1.
Neuronal beneficial properties of jujube.
| Findings | Model | Treatment | Reference |
|---|---|---|---|
| Jujube induced neurite outgrowth and neurofilament expression via cAMP-PKA-CREB signaling | Cultured PC12 cells; neuronal differentiation | Jujube extract compared with forskolin and control | [13] |
| The mature jujube possessed better effect in inducing neurofilament expression than that of the immature one | Cultured PC12 cells; neurofilament expression | Mature jujube extract compared with immature jujube extract | [19] |
| Jujube stimulated the expressions of neurotrophic factors and antioxidant enzymes | Cultured astrocytes; mRNA expression | Jujube at various concentrations (0–3 mg/ml) | [16] |
| Jujube protected neuronal cells against oxidation injury via activation of transcriptional activity of ARE | Cultured PC12 cells; tBHP induced oxidative stress | Jujube extract compared with Vit.C, tBHQb, and negative control | [20] |
| Jujube protected neurons from ischemic damage | Ischemic damage in gerbil hippocampus | Oral administration of jujube extract for 10 days | [21] |
| Jujube increased pentobarbital-induced sleep time and reduce free movement on mice | Kunming mice, behavior and sleeping studies | Jujube at 8 g/kg was administered orally | [22] |
| Jujube promoted learning and memory in ovariectomized rat model | SD rats, Morris water maze test | Ovariectomized rats in 6 groups: jujube groups, positive, model, and sham surgery groups | [23] |
| Hydroalcoholic extract of jujube ameliorates seizures, oxidative stress, and cognitive impairment in epilepsy rat model | Rat, experimental seizure models | Hydroalcoholic extract of jujube (100, 250, 500, and 1000 mg/kg) was administered orally | [24] |
| Jujube had repairing effects on memory and behavioral disorders produced by NBM lesion in rats | Wistar male rats; Morris water maze test | Rats in 7 groups: normal, AD, AD/normal + jujubes at two doses, and sham | [25] |
| Methanolic jujube extract activated ChAT. Oleamide from jujube reversed the memory and/or cognitive impairment in mice model | Cultured MC-IXC cells, mice | Oleamide at 0.4–2.4 mM on ChATa activity; mice were treated with oleamide for 4 weeks | [26] |
| Jujube increased the cAMP content in plasma and hippocampus of animals | ICR male mice, cAMP level in hippocampus and serum | i.g. administration of jujube at 0.35 g/kg | [27] |
| A polypeptide Snakin-Z from jujube possessed cholinesterase inhibitory activity | Cholinesterase inhibitory activity | Snakin-Z at 1.5 mg/mL has 80% inhibitory activity | [28] |
aChAT, choline acetyltransferase. btBHQ, tert-butyl hydroquinone.
Neurogenesis is a well-orchestrated process consisting of neuronal differentiation, synapse formation, and cell proliferation. Some studies suggested that, in pathological condition of neurodegenerative diseases, various types of neurogenesis could be observed, indicating that neurogenesis might be a compensatory mechanism in neurodegenerative processes [12]. Thus, the promotion of neuronal differentiation could be a means to prevent these diseases. Cultured pheochromocytoma PC12 cells are a pertinent model system for the study of neuronal differentiation [13]. The characters of PC12 cells are very similar to sympathetic neuron system, that is, the characters of neurofilament (NF) expression and neurite outgrowth in responding to challenge of nerve growth factor (NGF). The findings on jujube indicated that application of jujube water extract on cultured PC12 cells for 72 hours could induce cell differentiation: the induced differentiated cell accounted for ~25% of the total cells in cultures. The results also showed that application of jujube water extract in cultured PC12 cells for 72 hours could dose-dependently stimulate the expressions of NF68, NF160, and NF200 [13].
Astrocytes are the most abundant cell in the nervous system. One of its main functions is to synthesize and release neurotrophic factors, that is, NGF, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), neurotrophin 3 (NT3), and NT4/5 [14]. These factors are vital for neuronal survival, growth, and differentiation, and the deficiency of which can cause neurological impairments [15]. Thus, the upregulation of neurotrophic factors can play a positive role in treating neurological diseases. The effect of jujube on neurotrophic factor expression was investigated in cultured astrocytes [16]. The treatment with jujube water extract stimulated the expression of neurotrophic factors in a dose-dependent manner, having the highest induction of ~100% for NGF, 100% for BDNF, 100% for GDNF, and 50% for NT3. For NT4 and NT5, no obvious morphological change was observed in jujube-treated astrocytes.
The signaling of cAMP-PKA-CREB is well known to play a role in neuronal differentiation of PC12 cells [17]. Hence, the involvement of cAMP pathway in jujube-induced neurite outgrowth and neurofilament expression was revealed (Figure 2). The findings showed that jujube-induced neurite outgrowth and neurofilament expressions were attenuated by application of H89 (a cyclic AMP-dependent PKA inhibitor) [13]. CREB, the nuclear transcription factor, has been known to play a role in neuronal differentiation [18]. It was reported that jujube was able to stimulate the phosphorylation of CREB, and its inductive effect could be fully blocked by H89 [13]. Besides, the application of jujube water extract could stimulate the transcriptional activity of CRE. Further studies also showed that pretreatment with H89 significantly blocked jujube-induced neurotrophic factors including NGF, BDNF, and GDNF, indicating the possible involvement of PKA signaling in jujube-induced neurotrophic factor expression [16].
3. Neuroprotection against Oxidation Insult
In the process of neurodegeneration, the functions of neuron are markedly decreased. In Parkinson and Alzheimer diseases, oxidative stress, as the main considerable factor, is believed to cause neuronal damage in progress of diseases [29]. In cultured cells, jujube water extract was reported to protect neuronal cells against tert-butyl hydroperoxide- (tBHP-) induced oxidative injury. In addition, it was found that jujube water extract could inhibit tBHP-induced ROS (reactive oxygen species) formation in cultured PC12 cells [20]. The Nrf2 (nuclear factor (erythroid-derived 2-) like 2-) dependent ARE (antioxidant response element-) driven genes, including glutamate-cysteine ligase (GCL), glutathione S-transferase (GST), and NAD(P)H quinone oxidoreductase (NQO1), have been demonstrated to play an important role in protecting cells against oxidative stress [30, 31]. Jujube water extracts stimulated the ARE-mediated transcriptional activity, indicating the activation of Nrf2 pathway (Figure 3). Besides, the application of jujube induced the amounts of NQO1, GCLC (catalytic subunit of GCL), GCLM (modifier subunit of GCL), and GST mRNA levels in cultured astrocytes [16]. Jujube was also revealed to protect ischemic damage in gerbil hippocampus via its antioxidant effect, that is, the upregulation of superoxide dismutase (SOD) 1 and reduction of lipid peroxidation [21].
Figure 3.
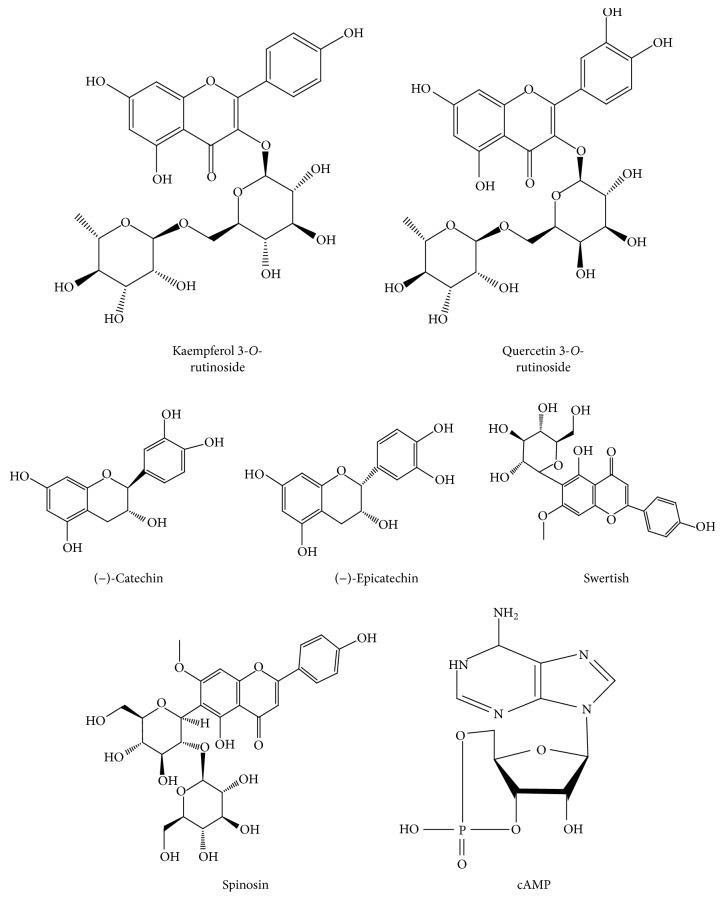
Structures for chemical compounds in jujube having potential neuroprotection effect. Seven chemical markers found in jujube including kaempferol 3-O-rutinoside, quercetin 3-O-rutinoside, (−)-catechin, (−)-epicatechin, swertish, spinosin, and cAMP were reported to possess effect on neuroprotection.
4. Jujube on Insomnia, Learning, and Memory
In Chinese herbal medicine, jujube was prepared as a tea that was used against insomnia [32, 33]. Indeed, jujube was found to increase pentobarbital-induced sleep time and to reduce free movement on mice [22]. In support of this, Peng et al. (2000) reported that the seed of Z. jujuba prolonged the hexobarbital-induced sleeping time in mice and decreased the locomotor activity in rats. Moreover, jujube was shown to possess anxiolytic effect by increasing the first time entry, as well as the total change and time spent in the white chamber of black and white test [34]. Besides, flavonoids and saponins from seed of Z. jujuba showed sedative and hypnotic effects, which caused a significant reduction of walking time and coordinated movement ability of mouse, significantly prolonging its sleeping time [35]. Jujuboside A, one of saponins from seed of Z. jujuba, stimulated the expression of GABA receptor subunits in rat hippocampal neurons [36] and ameliorated behavioral disorders of the dementia mouse model induced by Aβ1–42 [37]. Methanolic extract and oleamide from jujube showed activation effect on choline acetyltransferase (ChAT). Meanwhile, oleamide was found to attenuate the scopolamine-induced amnesia in mice, a useful in vivo model for Alzheimer disease [26]. Jujube was reported to improve learning and memory in ovariectomized rat model, and the effect of which might be due to an increase of estrogen in the blood, as well as the levels of nitric oxide and acetylcholine in the brain [23]. Moreover, hydroalcoholic extract of jujube was also demonstrated to possess anticonvulsant effect and amelioration of cognitive impairment induced by seizures in rats [24]. The seed extract of Z. jujuba showed a promising effect in ameliorating memory in mice with alcohol-induced memory retrieval disorders and might help to improve learning capacity to some extent [38]. In line with the aforementioned reports, jujube was reported to possess repairing effects on memory and learning impairment induced by bilateral electric lesion of the nucleus basalis of Meynert in rats [25]. In addition, jujube extract having 1% of cAMP was found to have antidepression function in animal model [39].
5. Two Developmental Stages of Jujube in Neuroprotection
Two types of jujubes are commonly found in China market: fresh immature jujubes were consumed as fruits, and dried mature jujubes were used as Chinese medicinal herb. Chemically, a sharp decrease of flavonoidic compounds was revealed during maturity [20, 40], while the amounts of selected compounds were significantly lower in immature jujubes than that of mature ones, including xanthine, hypoxanthine, adenine, uridine, guanosine, cGMP, and cAMP [20]. Moreover, the metabolite variations between mature and immature jujubes were also studied. Levels of isoleucine, threonine, acetate, creatine, and glucose were lower in immature jujube than those of mature one, while levels of alanine, sucrose, and formate were lower in mature jujube than those of immature one [19]. For biological assessments, the antioxidative functions of jujube extracts were first compared [20]. The antioxidative effects were varied amongst different maturity jujubes. The fresh immature jujube had better effect on antioxidation activity than that of mature one. However, mature jujube showed better effect in inhibition of ROS formation and activation of Nrf2-antioxidant response element signaling [20]. Furthermore, in terms of neuronal differentiation, mature jujube possessed better effect, about 60% higher than that of immature one [19]. These findings suggested the specific usage of different maturity of jujubes.
6. Jujube-Containing Herbal Decoctions on Neuroprotection
In Chinese herbal medicine, jujube is commonly included in herbal mixture, called Fu Fang, that is having specific combination of different herbs. These specific requirements of herbal preparation have not been changed. Among thousands of different herbal decoctions, Ganmai Dazao Tang and Chaihu Guizhi Tang are commonly used today for the treatment of depressive disorders [41]: both of them contain jujube as a key herb within the formulation. Taking Ganmai Dazao Tang as an example, this prescription having licorice, wheat, and jujube was recorded in a book Jingui Yaolue (Jingui Collection of Prescriptions), written by a well-known Chinese medicine scholar Zhang Zhongjing (150 BC to 219 AD) in Han Dynasty of China. This herbal decoction was traditionally prescribed for women suffering from anxiety. Ganmai Dazao Tang was reported to be one of top 10 Chinese herbal formulae prescribed for insomnia in Taiwan [42]. Although the molecular mechanisms for these herbal formulae are unclear, these antidepressive formulae may possess effect on neurotrophic factor expression, as it is well believed that reduction of BDNF expression is observed in depression patients [43, 44]. Indeed, Ganmai Dazao Tang was reported to induce BDNF expression in depressive animal model [45]. Besides, the jujube-containing herbal decoctions, including Guizhi Tang, Neibu Dangguijianzhong Tang, and ZaoTang, induced neuronal differentiation and expressions of antioxidant enzymes in cultured PC12 cells [46], for example, GCLC, GCLM, GST, and NQO1 via the activation of ARE. Together with the result of jujube as single herb alone, these findings could illustrate the functions of jujube within a multiherbal decoction.
7. Potential Bioactive Ingredients on Neuroprotection
Phenolic compounds are widely found in plants, which comprise a large group of bioactive ingredients. Flavonoids are commonly found in fruits, and some of which have been reported to possess neuroprotective effect (Figure 3) [47, 48]. Among different types of flavonoids in jujube, Zhu et al. (2007) reported that kaempferol 3-O-rutinoside possessed neuroprotective activity against oxidation insult and prevention of Aβ aggregation [47]. Quercetin 3-O-rutinoside was shown to have anti-Aβ aggregation effect. Both (−)-catechin and (−)-epicatechin showed inhibition of Aβ aggregation and Aβ-induced toxicity [47]. In addition, kaempferol 3-O-rutinoside showed protective effect on permanent focal cerebral ischemia and on neuronal cultures [49], as well as in reducing memory dysfunction, energy metabolism failure, and oxidative stress in multi-infarct dementia model rats [50]. Spinosin possessed potentiating effect on pentobarbital-induced sleep, and the effect of which was related to the postsynaptic 5-HT receptor [51, 52]. Indeed, spinosin and swertish, isolated from jujube seeds, were reported to possess significant sedative activity [53]. Yet, the flavonoids within jujube, including (−)-catechin, (−)-epicatechin, kaempferol 3-O-rutinoside, and quercetin 3-O-rutinoside, were tested for their neurotrophic action, yet no significant effect was found among them [48].
Cyclic nucleotides and their derivatives participate in the regulation and modulation of lots of physiological processes in body. These chemicals exhibit multiple bioactivities, that is, neuroprotective effects [54, 55]. Among them, cAMP was reported to be a relative large amount in jujube, and interestingly its content was much higher than other horticultural fruits (Figure 3) [56–58]. The amount of cAMP, at approximately the amount containing within 2 mg/mL of jujube water extract, showed the activity on neuronal differentiation in cultured PC12 cells. This firmly indicated that jujube cAMP exerted a role in neuronal differentiation, as reported for jujube extract [13]. Besides, a report showed that jujube was able to increase the cAMP content in plasma and hippocampus of animal model [27], and the authors hypothesized that the exogenous cAMP in jujube water extract might induce the aforesaid results. In parallel, cAMP is well believed to be involved in formation of depression. Indeed, cAMP isolated from jujube was found to possess antimelancholic property in animal model of depression [39]. In addition, Jujubosides, found in Semen Ziziphi Spinosae and jujube [59], were reported to possess hypnotic effects [35, 60]. To support this, jujuboside A could adjust GABA receptor subunit mRNAs expression in hippocampal neurons [61]. Oleamide from jujube reversed the scopolamine-induced memory and/or cognitive impairment in mice model [26]. In contrast, a polypeptide snakin-Z, isolated from jujube, possessed cholinesterase inhibitory effect [28].
8. Development and Perspective
Having over 3,000 years of history, jujube is still a popular fruit in our daily life for its health benefits [62]. Based on the aforementioned cellular and animal findings, jujube has a great potential in developing further as food and medicinal supplements for brain health. Jujube water extract is the most common usage form. It can be prepared into decoction and drunk daily, or it can be combined with other foods for the preparation of a delicious soup. Clinical studies showed that no adverse and drug interactions have been reported for the consumption of jujube [63–66]. In addition, the recent studies also revealed that jujube water extract contained higher amount of ingredients as compared to that of ethanol extract [20]. Hence, jujube water extract could be a good choice as an alternative to be prescribed for neurological disorder. Moreover, the quality of herbal medicine, as jujube here, could be varied from each other due to numerous factors, for example, harvest season, geographic region, and postharvest treatment. Thus, it is essential to establish different parameters in receiving chemical standardized jujube water extract. In order to chemically standardize jujube water extract in terms of its HPLC profile and chemical contents, the HPLC fingerprint and quantification methods were employed to reveal its HPLC profile and quantify the main ingredients [20]. The standardization parameters can be employed to ensure the consistent quality of jujube water extract and to further make sure of its repeatability of experimental results.
The active ingredients of jujube include nucleotide and flavonoid, which may show benefit effects in brain functions. In consideration of the benefit of cAMP in jujube, numerous studies are targeting on the processes in extracting and separating cAMP from jujube [39, 67, 68]. Hence, the enrichment of jujube nucleotide may be developed into a food or medicinal product for the treatment of diseases, such as depression or neurodegeneration. During the production of beverages and other kinds of food with jujube, such as cake, the peel is usually discarded. Jujube peel extract was found to contain higher amount of phenolic compounds than that of pulp [69]. These findings might imply that jujube peel could serve as an inexpensive source of natural antioxidant to protect neuronal cells against oxidation insult. The seed of jujube is also discarded in either food products or TCM practices. The seed from Z. jujuba var. spinosa, the sour jujube, possessed hypnotic effect [35, 60], indicating that jujube seed might also be a selection for insomnia as either raw material or source for active ingredient.
The usage of jujube in China is not restricted as a single fruit; it is commonly prescribed in multiherbal decoctions for various purposes. Jujube was commonly employed as the main ingredient within all the herbal formulae written by Zhang Zhongjing (150 BC to 219 AD). According to Zhang's theory of Chinese medicine, jujube was included in herbal decoction as to adjust the taste of other herbs, for example, to harmonize the spleen and stomach, to harmonize the nutrient and defense, and to calm the mind. Having jujube as the active ingredient, the major function of jujube-containing decoctions for the treatment of brain related diseases should be expected.
Acknowledgments
This research was supported by Hong Kong Research Grants Council Theme-Based Research Scheme (T13-607/12R), GRF (663012, 662713, M-HKUST604/13), TUYF12SC03, TUYF15SC01, The Hong Kong Jockey Club Charities Trust (HKJCCT12SC01), and Foundation of The Awareness of Nature (TAON12SC01) to Karl Tsim. This research was also funded by Shenzhen Science and Technology Plan Project (JSGG20141017103353178 and ZDSYS201606081515458), Ministry of Human Resources and Social Security of the People's Republic of China, Natural Science Foundation of Guangdong Province (2015A030310247), Traditional Chinese Medicine Bureau of Guangdong Province (20151079), and Health and Family Planning Commission of Shenzhen Municipality (201505015).
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Chang S. C., Hsu B. Y., Chen B. H. Structural characterization of polysaccharides from Zizyphus jujuba and evaluation of antioxidant activity. International Journal of Biological Macromolecules. 2010;47(4):445–453. doi: 10.1016/j.ijbiomac.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Choi S.-H., Ahn J.-B., Kozukue N., Levin C. E., Friedman M. Distribution of free amino acids, flavonoids, total phenolics, and antioxidative activities of jujube (Ziziphus jujuba) fruits and seeds harvested from plants grown in Korea. Journal of Agricultural and Food Chemistry. 2011;59(12):6594–6604. doi: 10.1021/jf200371r. [DOI] [PubMed] [Google Scholar]
- 3.Li J., Shan L., Liu Y., Fan L., Ai L. Screening of a functional polysaccharide from Zizyphus jujuba cv. jinsixiaozao and its property. International Journal of Biological Macromolecules. 2011;49(3):255–259. doi: 10.1016/j.ijbiomac.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Xu Y.-L., Miao M.-S., Sun Y.-H., Miao Y.-Y. Effect of Fructus Jujubae polysaccharide on the hematopoietic function in mice model of both qi and blood deficiencies. Chinese Journal of Clinical Rehabilitation. 2004;8(24):5050–5051. [Google Scholar]
- 5.Zhao Z., Liu M., Tu P. Characterization of water soluble polysaccharides from organs of Chinese Jujube (Ziziphus jujuba Mill. cv. dongzao) European Food Research and Technology. 2008;226(5):985–989. doi: 10.1007/s00217-007-0620-1. [DOI] [Google Scholar]
- 6.Yu L., Jiang B. P., Luo D., et al. Bioactive components in the fruits of Ziziphus jujuba Mill. against the inflammatory irritant action of Euphorbia plants. Phytomedicine. 2012;19(3-4):239–244. doi: 10.1016/j.phymed.2011.09.071. [DOI] [PubMed] [Google Scholar]
- 7.Tahergorabi Z., Abedini M. R., Mitra M., Fard M. H., Beydokhti H. ‘Ziziphus jujuba’: a red fruit with promising anticancer activities. Pharmacognosy Reviews. 2015;9(18):99–106. doi: 10.4103/0973-7847.162108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinkamp-Fenske K., Bollinger L., Xu H., et al. Reciprocal regulation of endothelial nitric-oxide synthase and NADPH oxidase by betulinic acid in human endothelial cells. Journal of Pharmacology and Experimental Therapeutics. 2007;322(2):836–842. doi: 10.1124/jpet.107.123356. [DOI] [PubMed] [Google Scholar]
- 9.Seo E. J., Lee S. Y., Kang S. S., Jung Y.-S. Zizyphus jujuba and its active component jujuboside B inhibit platelet aggregation. Phytotherapy Research. 2013;27(6):829–834. doi: 10.1002/ptr.4809. [DOI] [PubMed] [Google Scholar]
- 10.Mahajan R. T., Chopda M. Z. Phyto-pharmacology of Ziziphus jujuba Mill.—a plant review. Pharmacognosy Reviews. 2009;3(6):320–329. [Google Scholar]
- 11.Gao Q.-H., Wu C.-S., Wang M. The jujube (Ziziphus jujuba Mill.) fruit: a review of current knowledge of fruit composition and health benefits. Journal of Agricultural and Food Chemistry. 2013;61(14):3351–3363. doi: 10.1021/jf4007032. [DOI] [PubMed] [Google Scholar]
- 12.Jin K., Peel A. L., Mao X. O., et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen J. P., Maiwulanjiang M., Lam K. Y. C., et al. A standardized extract of the fruit of Ziziphus jujuba (Jujube) induces neuronal differentiation of cultured PC12 cells: a signaling mediated by protein kinase A. Journal of Agricultural and Food Chemistry. 2014;62(8):1890–1897. doi: 10.1021/jf405093f. [DOI] [PubMed] [Google Scholar]
- 14.Ridet J. L., Malhotra S. K., Privat A., Gage F. H. Reactive astrocytes: cellular and molecular cues to biological function. Trends in Neurosciences. 1997;20(12):570–577. doi: 10.1016/S0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 15.Reuss B., Dono R., Unsicker K. Functions of fibroblast growth factor (FGF)-2 and FGF-5 in astroglial differentiation and blood-brain barrier permeability: evidence from mouse mutants. Journal of Neuroscience. 2003;23(16):6404–6412. doi: 10.1523/JNEUROSCI.23-16-06404.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J. P., Yan A. L., Lam K. Y., et al. A chemically standardized extract of Ziziphus jujuba fruit (jujube) stimulates expressions of neurotrophic factors and anti-oxidant enzymes in cultured astrocytes. Phytotherapy Research. 2014;28(11):1727–1730. doi: 10.1002/ptr.5202. [DOI] [PubMed] [Google Scholar]
- 17.Ravni A., Vaudry D., Gerdin M. J., et al. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Molecular Pharmacology. 2008;73(6):1688–1708. doi: 10.1124/mol.107.044792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ginty D. D., Bonni A., Greenberg M. E. Nerve growth factor activates a Ras-dependent protein kinase that stimulates c-fos transcription via phosphorylation of CREB. Cell. 1994;77(5):713–725. doi: 10.1016/0092-8674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 19.Chen J. P., Chan P. H., Lam C. T. W., et al. Fruit of Ziziphus jujuba (Jujube) at two stages of maturity: distinction by metabolic profiling and biological assessment. Journal of Agricultural and Food Chemistry. 2015;63(2):739–744. doi: 10.1021/jf5041564. [DOI] [PubMed] [Google Scholar]
- 20.Chen J. P., Li Z., Maiwulanjiang M., et al. Dong T. T. X., Tsim K. W. K. Chemical and biological assessment of Ziziphus jujuba fruits from china: different geographical sources and developmental stages. Journal of Agricultural and Food Chemistry. 2013;61(30):7315–7324. doi: 10.1021/jf402379u. [DOI] [PubMed] [Google Scholar]
- 21.Yoo K.-Y., Li H., Hwang I. K., et al. Zizyphus attenuates ischemic damage in the gerbil hippocampus via its antioxidant effect. Journal of Medicinal Food. 2010;13(3):557–563. doi: 10.1089/jmf.2009.1254. [DOI] [PubMed] [Google Scholar]
- 22.Li M., Wang Y. Phamarcological comparison of two Ziziphus jujuba cultivars. Journal of Chinese Medicinal Materials. 1993;16(6):35–37. [Google Scholar]
- 23.Li B., Wang L., Liu Y., et al. Jujube promotes learning and memory in a rat model by increasing estrogen levels in the blood and nitric oxide and acetylcholine levels in the brain. Experimental and Therapeutic Medicine. 2013;5(6):1755–1759. doi: 10.3892/etm.2013.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pahuja M., Mehla J., Reeta K. H., Joshi S., Gupta Y. K. Hydroalcoholic extract of Zizyphus jujuba ameliorates seizures, oxidative stress, and cognitive impairment in experimental models of epilepsy in rats. Epilepsy & Behavior. 2011;21(4):356–363. doi: 10.1016/j.yebeh.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Rabiei Z., Rafieian-Kopaei M., Heidarian E., Saghaei E., Mokhtari S. Effects of Zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus basalis of meynert in rat. Neurochemical Research. 2014;39(2):353–360. doi: 10.1007/s11064-013-1232-8. [DOI] [PubMed] [Google Scholar]
- 26.Heo H.-J., Park Y.-J., Suh Y.-M., et al. Effects of oleamide on choline acetyltransferase and cognitive activities. Bioscience, Biotechnology and Biochemistry. 2003;67(6):1284–1291. doi: 10.1271/bbb.67.1284. [DOI] [PubMed] [Google Scholar]
- 27.Tian J. S., Gao S., Cui Y. L., Wang Q. S., Liu L. P., Zhang Z. G. The cyclic AMP content with time variation after oral administration of the extract of Fructus Jujubae in mice. Chinese Journal of Experimental Traditional Medical Formulae. 2010;16(7):102–104. [Google Scholar]
- 28.Zare-Zardini H., Tolueinia B., Hashemi A., Ebrahimi L., Fesahat F. Antioxidant and cholinesterase inhibitory activity of a new peptide from Ziziphus jujuba fruits. American Journal of Alzheimer's Disease and Other Dementias. 2013;28(7):702–709. doi: 10.1177/1533317513500839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin M. T., Beal M. F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 30.Murphy T. H., De Long M. J., Coyle J. T. Enhanced NAD(P)H:Quinone Reductase Activity Prevents Glutamate Toxicity Produced by Oxidative Stress. Journal of Neurochemistry. 1991;56(3):990–995. doi: 10.1111/j.1471-4159.1991.tb02019.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee J. M., Calkins M. J., Chan K., Kan Y. W., Johnson J. A. Identification of the NF-E2-related factor-2-dependent genes conferring protection against oxidative stress in primary cortical astrocytes using oligonucleotide microarray analysis. The Journal of Biological Chemistry. 2003;278(14):12029–12038. doi: 10.1074/jbc.m211558200. [DOI] [PubMed] [Google Scholar]
- 32.Yeung W.-F., Chung K.-F., Poon M. M.-K., et al. Prescription of Chinese herbal medicine and selection of acupoints in pattern-based traditional Chinese medicine treatment for insomnia: a systematic review. Evidence-based Complementary and Alternative Medicine. 2012;2012:16. doi: 10.1155/2012/902578.902578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dey A., Dey A. Phytotherapy against insomnia: extravagant claims or an alternative medicine? Pakistan Journal of Biological Sciences. 2013;16(3):148–150. doi: 10.3923/pjbs.2013.148.150. [DOI] [PubMed] [Google Scholar]
- 34.Peng W.-H., Hsieh M.-T., Lee Y.-S., Lin Y.-C., Liao J. Anxiolytic effect of seed of Ziziphus jujuba in mouse models of anxiety. Journal of Ethnopharmacology. 2000;72(3):435–441. doi: 10.1016/s0378-8741(00)00255-5. [DOI] [PubMed] [Google Scholar]
- 35.Jiang J.-G., Huang X.-J., Chen J., et al. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus Jujube. Natural Product Research. 2007;21(4):310–320. doi: 10.1080/14786410701192827. [DOI] [PubMed] [Google Scholar]
- 36.You Z.-L., Xia Q., Liang F.-R., et al. Effects on the expression of GABAA receptor subunits by jujuboside A treatment in rat hippocampal neurons. Journal of Ethnopharmacology. 2010;128(2):419–423. doi: 10.1016/j.jep.2010.01.034. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z., Zhao X., Liu B., et al. Jujuboside A, a neuroprotective agent from Semen Ziziphi Spinosae ameliorates behavioral disorders of the dementia mouse model induced by Aβ1−42. European Journal of Pharmacology. 2014;738:206–213. doi: 10.1016/j.ejphar.2014.05.041. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Qiao L., Song M., Wang L., Xie J., Feng H. Hplc-ESI-MS/MS analysis of the water-soluble extract from Ziziphi Spinosae Semen and its ameliorating effect of learning and memory performance in mice. Pharmacognosy Magazine. 2014;10(40):509–516. doi: 10.4103/0973-1296.141777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chi Y. F., Zhang Z. Antimelancholic medicine prepared from jujube cAMP materials. European: CA 2707192; 2009. [Google Scholar]
- 40.Choi S.-H., Ahn J.-B., Kim H.-J., et al. Changes in free amino acid, protein, and flavonoid content in Jujube (Ziziphus jujube) fruit during eight stages of growth and antioxidative and cancer cell inhibitory effects by extracts. Journal of Agricultural and Food Chemistry. 2012;60(41):10245–10255. doi: 10.1021/jf302848u. [DOI] [PubMed] [Google Scholar]
- 41.Pang T. H. H., Ip F. C. F., Ip N. Y. Recent development in the search for effective antidepressants using traditional Chinese medicine. Central Nervous System Agents in Medicinal Chemistry. 2008;8(1):64–71. doi: 10.2174/187152408783790659. [DOI] [Google Scholar]
- 42.Chen F. P., Jong M. S., Chen Y. C., et al. Prescriptions of Chinese herbal medicines for insomnia in Taiwan during 2002. Evidence-Based Complementary and Alternative Medicine. 2011;2011:9. doi: 10.1093/ecam/nep018.236341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogóz Z., Legutko B. Combined treatment with imipramine and metyrapone induces hippocampal and cortical brain-derived neurotrophic factor gene expression in rats. Pharmacological Reports. 2005;57(6):840–844. [PubMed] [Google Scholar]
- 44.Schmidt H. D., Duman R. S. The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behavioural Pharmacology. 2007;18(5-6):391–418. doi: 10.1097/FBP.0b013e3282ee2aa8. [DOI] [PubMed] [Google Scholar]
- 45.Yan Y. M. The effect of Jiawei Gaimai Dazao Tang on BDNF expression in despressive hippocampus rats. Zhejiang Journal Traditioanl Chinese Medicine. 2010;45(8):604–605. [Google Scholar]
- 46.Lam C. T., Gong A. G., Lam K. Y., et al. Jujube-containing herbal decoctions induce neuronal differentiation and the expression of anti-oxidant enzymes in cultured PC12 cells. Journal of Ethnopharmacology. 2016;188:275–283. doi: 10.1016/j.jep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Zhu J. T., Choi R. C., Chu G. K., et al. Flavonoids possess neuroprotective effects on cultured pheochromocytoma PC12 cells: a comparison of different flavonoids in activating estrogenic effect and in preventing β-amyloid-induced cell death. Journal of Agricultural and Food Chemistry. 2007;55(6):2438–2445. doi: 10.1021/jf063299z. [DOI] [PubMed] [Google Scholar]
- 48.Xu S. L., Bi C. W., Choi R. C., et al. Flavonoids induce the synthesis and secretion of neurotrophic factors in cultured rat astrocytes: A signaling response mediated by estrogen receptor. Evidence-based Complementary and Alternative Medicine. 2013;2013:10. doi: 10.1155/2013/127075.127075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li R., Guo M., Zhang G., Xu X., Li Q. Neuroprotection of nicotiflorin in permanent focal cerebral ischemia and in neuronal cultures. Biological and Pharmaceutical Bulletin. 2006;29(9):1868–1872. doi: 10.1248/bpb.29.1868. [DOI] [PubMed] [Google Scholar]
- 50.Huang J.-L., Fu S.-T., Jiang Y.-Y., et al. Protective effects of Nicotiflorin on reducing memory dysfunction, energy metabolism failure and oxidative stress in multi-infarct dementia model rats. Pharmacology Biochemistry and Behavior. 2007;86(4):741–748. doi: 10.1016/j.pbb.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Wang L.-E., Bai Y.-J., Shi X.-R., et al. Spinosin, a C-glycoside flavonoid from Semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacology Biochemistry and Behavior. 2008;90(3):399–403. doi: 10.1016/j.pbb.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Wang L.-E., Cui X.-Y., Cui S.-Y., et al. Potentiating effect of spinosin, a C-glycoside flavonoid of Semen Ziziphi Spinosae, on pentobarbital-induced sleep may be related to postsynaptic 5-HT1A receptors. Phytomedicine. 2010;17(6):404–409. doi: 10.1016/j.phymed.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Cheng G., Bai Y., Zhao Y., et al. Flavonoids from Ziziphus jujuba Mill. var. spinosa. Tetrahedron. 2000;56(45):8915–8920. doi: 10.1016/s0040-4020(00)00842-5. [DOI] [Google Scholar]
- 54.Schmidt A. P., Lara D. R., De Faria Maraschin J., Da Silveira Perla A., Souza D. O. Guanosine and GMP prevent seizures induced by quinolinic acid in mice. Brain Research. 2000;864(1):40–43. doi: 10.1016/S0006-8993(00)02106-5. [DOI] [PubMed] [Google Scholar]
- 55.Urushitani M., Inoue R., Nakamizo T., Sawada H., Shibasaki H., Shimohama S. Neuroprotective effect of cyclic GMP against radical-induced toxicity in cultured spinal motor neurons. Journal of Neuroscience Research. 2000;61(4):443–448. doi: 10.1002/1097-4547(20000815)61:4<443::AID-JNR11>3.0.CO;2-W. doi: 10.1002/1097-4547(20000815)61:4<443::AID-JNR11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 56.Cyong J.-C., Hanabusa K. Cyclic adenosine monophosphate in fruits of Zizyphus jujuba. Phytochemistry. 1980;19(12):2747–2748. doi: 10.1016/S0031-9422(00)83955-2. [DOI] [Google Scholar]
- 57.Hanabusa K., Cyong J., Takahashi M. High-level of cyclic AMP in the jujube plum. Planta Medica. 1981;42(08):380–384. doi: 10.1055/s-2007-971659. [DOI] [PubMed] [Google Scholar]
- 58.Liu M., Wang Y. cAMP contents of Zizyphus jujuba Mill., Zizyphus spinosus Hu. and other twelve horticultural plants. Journal of Agricultural University of Hebei. 1991;14(4):20–23. [Google Scholar]
- 59.Chen X., Wang Y. X., Zheng L. Y., He X. H., Han N. Y. Simultaneous determination of rutin and jujuboside B in Ziziphus jujuba by HPLC. China Pharmacy. 2010;21(3):247–248. [Google Scholar]
- 60.Cao J.-X., Zhang Q.-Y., Cui S.-Y., et al. Hypnotic effect of jujubosides from Semen Ziziphi Spinosae. Journal of Ethnopharmacology. 2010;130(4):163–166. doi: 10.1016/j.jep.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 61.Wang X., Ma G., Xie J., Pang G. Influence of JuA in evoking communication changes between the small intestines and brain tissues of rats and the GABAA and GABAB receptor transcription levels of hippocampal neurons. Journal of Ethnopharmacology. 2015;159:215–223. doi: 10.1016/j.jep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 62.Chen J. P., Lam C. T. W., Li Z. G., et al. Chinese Dates: A Traditional Functional Food. Abingdon: CRC Press; 2016. Chemical and biological assessment of Ziziphus jujuba (jujubes) fruit from china of different geographical sources and developmental stages: chemical composition and possible targets in developing health food products; pp. 83–97. [Google Scholar]
- 63.Chevallier A. An excellent guide to over 500 of the more well known medicinal herbs from around the world. London, UK: Dorling Kindersley; 1996. The encyclopedia of medicinal plants. [Google Scholar]
- 64.Ebrahimi S., Ashkani-Esfahani S., Poormahmudi A. Investigating the efficacy of Ziziphus jujuba on neonatal jaundice. Iranian Journal of Pediatrics. 2011;21(3):320–324. [PMC free article] [PubMed] [Google Scholar]
- 65.Naftali T., Feingelernt H., Lesin Y., Rauchwarger A., Konikoff F. M. Ziziphus jujuba extract for the treatment of chronic idiopathic constipation: a controlled clinical trial. Digestion. 2009;78(4):224–228. doi: 10.1159/000190975. [DOI] [PubMed] [Google Scholar]
- 66.Sabzghabaee A. M., Khayam I., Kelishadi R., et al. Effect of Ziziphus jujuba fruits on dyslipidemia in obese adolescents: a triple-masked randomized controlled clinical trial. Medical Archives. 2013;67(3):p. 156. doi: 10.5455/medarh.2013.67.156-159. [DOI] [PubMed] [Google Scholar]
- 67.Mi D., Wang Y., Li M. R. Research on extraction of cAMP from fruits of Ziziphus. Journal of Shanghai Normal University (Nature Science) 2007;36(3):77–79. [Google Scholar]
- 68.Pan J., You F. H., Wu F. R., Yang Y. Performance of absorption and separation of the macroporeticular resin for cAMP from Ziziphus jujuba. Journal of Anhui University Nature Science Edition. 2007;31(5):87–90. [Google Scholar]
- 69.Xue Z. P., Feng W. H., Cao J. K., Cao D. D., Jiang W. B. Antioxidant activity and total phenolic contents in peel and pulp of Chinese jujube (Ziziphus jujuba Mill.) fruits. Journal of Food Biochemistry. 2009;33(5):613–629. doi: 10.1111/j.1745-4514.2009.00241.x. [DOI] [Google Scholar]