Abstract
The hypernociceptive effects of cytokines [TNF-α, keratinocyte-derived chemokine (KC), and IL-1β] and their participation in carrageenan (Cg)-induced inflammatory hypernociception in mice were investigated. Nociceptor sensitization (hypernociception) was quantified with an electronic version of the von Frey filament test in WT and TNF receptor type 1 knockout mice (TNF-R1–/–). TNF-α-induced hypernociception was abolished in TNF-R1–/– mice, partially inhibited by pretreatment with IL-1 receptor antagonist (IL-1ra) or indomethacin and unaffected by Ab against KC (AbKC) or guanethidine. IL-1ra and indomethacin pretreatment strongly inhibited the hypernociception induced by IL-1β, which was not altered by AbKC or guanethidine or by knocking out TNF-R1. KC-induced hypernociception was abolished by AbKC, inhibited by pretreatment with indomethacin plus guanethidine, and partially inhibited by IL-1ra, indomethacin, or guanethidine. In contrast, KC-induced hypernociception was not altered by knocking out TNF-R1. Cg-induced hypernociception was abolished by administration of indomethacin plus guanethidine, diminished in TNF-R1–/– mice, and partially inhibited in WT mice pretreated with AbKC, IL-1ra, indomethacin, or guanethidine. TNF-α, KC, and IL-1β concentrations were elevated in the skin of Cg-injected paws. The TNF-α and KC concentrations rose concomitantly and peaked before that of IL-1β. In mice, the cytokine cascade begins with the release of TNF-α (acting on TNF-R1 receptor) and KC, which stimulate the release of IL-1β. As in rats, the final mediators of this cascade were prostaglandins released by IL-1β and sympathetic amines released by KC. These results extend to mice the concept that the release of primary mediators responsible for hypernociception is preceded by a cascade of cytokines.
Keywords: inflammation, prostaglandins, TNF, hyperalgesia, pain
Cytokines are produced by various cells types in response to a variety of stimuli and constitute a link between cellular injury or recognition of nonself and the development of local and systemic signs and symptoms of inflammation (1–5). Acute inflammatory pain is characterized by hypernociception due to the sensitization of primary sensory nociceptive neurons, also referred to as hyperalgesia or allodynia (6). After tissue injury, specific primary mediators are released that act on membrane neuronal metabotropic receptors to trigger the activation of second messenger pathways. Eicosanoids and sympathetic amines are the most important primary mediators ultimately responsible for mechanical hypernociception in rats (7–9). These mediators activate second messenger pathways (cAMP, protein kinase A, and PKC) responsible for lowering the nociceptor threshold and increasing neuronal membrane excitability. In this state, nociceptor activation and impulse transmission by the primary nociceptive neurons are facilitated (7, 10–17).
In the last decade, it has been shown that inflammatory stimuli do not directly stimulate the release of primary hypernociceptive mediators but that their release is preceded by a cascade of cytokines (18). In this context, our group developed the concept that, in rats, inflammatory stimuli cause mechanical hypernociception by a well defined sequential release of cytokines. It was shown in rats that carrageenan (Cg) induces mechanical hypernociception through a cascade of cytokines released by resident or migrating cells initiated by production of bradykinin (19). The first cytokine released is TNF-α, which triggers the release of IL-6/IL-1β and cytokine-induced neutrophil chemoattractant-1 (CINC-1) responsible for stimulation of the synthesis of prostaglandins and release of sympathetic amines, respectively (20–22). The suggestion of a cascade of cytokines was further supported by data from rat paws and tail models. In mechanical (von Frey filaments) and thermal (hot plate) hypernociception induced by complete Freund's adjuvant, there was a sequential release of TNF-α→IL-1β→nerve growth factor (23, 24). Also, systemic administration of LPS (i.p.) sensitized rat tail flick responses by means of TNF through releases of IL-1β (25, 26).
These experiments strongly suggest that in rats there is a cascade of release of cytokines that constitutes a link between the injuries and the release of primary hypernociceptive mediators. This concept allows us to understand why the inhibition of one (IL-1β or TNF-α) or several (glucocorticoids) cytokines causes analgesia (27–30). The clinical success of anti-TNF-α in rheumatoid arthritis also exemplifies this concept (31), which opposes to the idea that inflammatory hyperalgesia results from a “soup of mediators.” The relevance of this concept, however, depends on its confirmation in species other than rats. In mice, writhing responses to zymosan and acetic acid were mediated by TNF-α, IL-1β, and IL-8, but these cytokines appeared not to be released sequentially but, rather, were acting concomitantly and synergistically (32). This discrepancy might be a result of the different tests applied or be a species difference or both.
Recently, we adapted an electronic anesthesiometer to detect mechanical inflammatory hypernociception in mice. This technique detects inflammatory hypernociception and allows the quantification of peripheral analgesic drugs with different mechanisms of action (33). In the present study, this mechanical method was used to investigate the hypernociceptive effects of TNF-α, IL-1β, and keratinocyte-derived chemokine (KC) (rat CINC-1 and human IL-8-related chemokine) (34–36) and their role in Cg-induced inflammatory mechanical hypernociception in mice. In an attempt to further clarify the role of the cytokines in hypernociception, knockout mice for TNF receptor type 1 (TNF-R1–/–) were tested because this receptor has been shown to participate in neurophatic pain after nerve injury (37).
Methods
Nociceptive Mechanical Test. We use the term hypernociception rather than hyperalgesia or allodynia to define nociceptor sensitization (38). Mechanical hypernociception was tested in mice as reported in ref. 33. In a quiet room, mice were placed in acrylic cages (12 × 10 × 17 cm) with wire grid floors 15–30 min before the start of testing. The test consisted of evoking a hindpaw flexion reflex with a hand-held force transducer (electronic anesthesiometer, IITC Life Science, Woodland Hills, CA) adapted with a 0.5-mm2 polypropylene tip. The investigator was trained to apply the tip perpendicularly to the central area of the hindpaw with a gradual increase in pressure. The end point was characterized by the removal of the paw followed by clear flinching movements. After the paw withdrawal, the intensity of the pressure was automatically recorded. The value for the response was obtained by averaging three measurements. The animals were tested before and after treatments. The results are expressed by the Δ withdrawal threshold (in grams) calculated by subtracting the zero-time mean measurements from the time interval mean measurements. Withdrawal threshold was 9.8 ± 0.5 g (mean ± SEM; n = 30) before injection of the hypernociceptive agents.
Cytokine Measurements. At indicated times after the inflammatory stimuli injection, animals were killed, the skin tissues were removed from the injected and control paws (saline and naive). The samples were homogenized in 500 μl of the appropriate buffer containing protease inhibitors, and TNF-α, IL-1β, and KC levels were determined by ELISA as described in ref. 23. The results are expressed as picograms of each cytokine per paw. As a control, the concentrations of these cytokines were determined in naive mice and animals injected with saline.
Experimental Protocol. Mechanical hypernociception was measured in mice before and after the intraplantar (i.pl.) injection of one of the following substances: Cg (30–300 μg per paw), TNF-α (1–1,000 pg per paw), KC (1–10 ng per paw), and IL-1β (10–1,000 pg per paw). The antihypernociceptive effects of indomethacin (5 mg/kg delivered i.p. 30 min before stimuli injection), a standard cyclooxygenase inhibitor; guanethidine (30 mg/kg delivered s.c. 60 min before stimuli injection), a sympathomimetic neuron-blocking agent (39); Ab against KC (AbKC; 500 ng in 15 μl delivered i.pl. 5 min before stimuli injection); and IL-1 receptor antagonist (IL-1ra; 500 ng in 15 μl delivered i.pl. 5 min before stimuli injection) on the hypernociceptive effects of Cg (100 μg), TNF-α (100 pg), KC (10 pg), and IL-1 β (1,000 pg) were investigated. The hypernociception was determined 3 h after the i.pl. injections of the stimuli. The hypernociceptive effects of Cg (100 μg), TNF-α (100 pg), KC (10 ng), and IL-1β (1,000 pg) injected into the hindpaw of TNF-R1–/– mice were also evaluated 3 h after the injections.
The concentrations of the TNF-α, KC, and IL-1β on hindpaw were determined 0.5, 1.0, 3.0, and 5.0 h after i.pl. injection of Cg (100 μg) in WT mice. The concentrations of these cytokines into the hindpaws of TNF-R1–/– mice was also measured 3 h after Cg (100 μg) or TNF-α (100 pg) injection.
Drugs. The following materials were obtained from the indicated sources. Murine recombinant TNF-α, IL1-β, and human recombinant IL-1ra were provided by the National Institute for Biological Standards and Control (South Mimms, Hertfordshire, U.K.). Recombinant murine KC and AbKC were purchased from PeproTech (Rocky Hill, NJ). Guanethidine was purchased from Sigma. Cg was obtained from FMC (Philadelphia), and indomethacin was obtained from Prodome Química e Farmacêutica (São Paulo, Brazil).
Animals. The experiments were performed on 25- to 30-g C57BL/6 WT and TNF-R1–/– mice (University of São Paulo, Ribeirão Preto, Brazil) housed in the animal care facility of the School of Medicine of Ribeirão Preto and taken to the testing room at least 1 h before experiments. Food and water were available ad libitum. The mice were used only once. Animal care and handling procedures were in accordance with the International Association for Study of Pain guidelines on the use of animals in pain research, and they were approved by Animal Ethnical Comity of Faculty of Medicine of Ribeirão Preto (University of São Paulo).
Statistical Analysis. Results are presented as means ± SEM for groups of five animals (for in vivo experiments) or four animals (for in vitro experiments), and they are representative of two independent experiments. The differences between the experimental groups were compared by ANOVA and, in the case of statistical significance, individual comparisons were subsequently made with Tukey's post hoc test. The level of significance was set at P < 0.05.
Results and Discussion
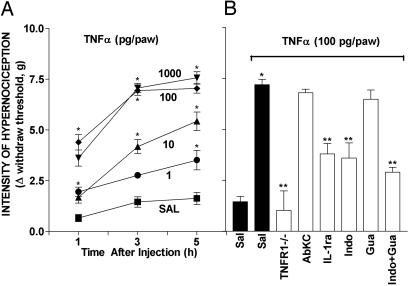
IL-1β-Induced Mechanical Hypernociception. In an earlier study in which the electronic pressure meter described above was applied to the paws of mice, prostaglandin E2 produced a dose–hypernociceptive response curve (33). In the present study, IL-1β evoked mechanical hypernociception in a dose- and time-dependent (10–1,000 pg; 1–5 h) manner (Fig. 1A). The maximum hypernociception was observed with the dose of 1,000 pg of IL-1β. Larger doses did not give larger responses (data not shown). Fig. 1 A shows that the hypernociception was already significant 1 h after IL-1β (100 pg) injection, reached a plateau between 3 and 5 h after injection, and had returned to control levels by 24 h after injection (data not shown). There was no difference between responses at 3 and 5 h. Pretreatment with IL-1ra or indomethacin caused a marked inhibition of the IL-1β-induced hypernociception (–94% and –75%, respectively). These results indicate that, in mice, IL-1β causes hypernociception by means of induction of prostanoids, which is in agreement with results described for rats (40, 41). IL-1β-induced hypernociception (1,000 pg) was not affected by pretreatment with AbKC or guanethidine (Fig. 1B), nor was it altered in TNF-R1–/– mice. Guanethidine coadministrated with indomethacin did not increase the inhibitory effect of indomethacin on IL-1β-induced hypernociception. Also, neither TNF-α nor KC was produced in paw skin in response to IL-1β injection (data not shown). Thus, prostanoids but not TNF-α, KC, or sympathomimetic amines are involved in IL-1β-induced hypernociception.
Fig. 1.
Mechanical hypernociception induced by i.pl. injection of IL-1β: the role of TNF-α, KC, prostanoides, and sympathomimetic amines. (A) Dose– and time–response curves of the hypernociception induced by i.pl. injection of IL-1β (10, 100, and 1,000 pg per paw) or saline (SAL; control). The hypernociceptive effects were determined at 1, 3, and 5 h after the stimuli injection. (B) Nociceptive response induced by IL-1β (1,000 pg per paw) in WT mice pretreated with 15 μl of saline (Sal) or TNF-R1–/– mice and pretreatment of WT mice with 500 ng of AbKC, 500 ng of IL-1ra, 5 mg/kg indomethacin (Indo) delivered i.p., 30 mg/kg guanethidine (Gua) delivered s.c., and indomethacin plus guanethidine. The hypernociceptive responses were taken 3 h after IL-1β injection. The results are expressed by the mean ± SEM of five animals per group. *, Statistically significant different time points (P < 0.05); **, statistically significant difference compared with the control group (P < 0.05).
KC-Induced Mechanical Hypernociception. I.pl. injection of KC evoked mechanical hypernociception in a dose- and time-dependent (1–10 ng; 1–5 h) manner (Fig. 2A). The maximum hypernociception was observed with the dose of 10 ng of KC and the response reached a plateau within 3–5 h and returned to preinjection levels within 24 h (data not shown). Larger doses did not evoke larger responses (data not shown). There was no difference between responses at 3 and 5 h. The KC-induced (10 ng) hypernociception was not altered in TNF-R1–/– mice but was partially inhibited by i.pl. pretreatment with IL-1ra (–56%), indomethacin (–64%) or guanethidine (–60%) and was more effectively inhibited by the coadministration of indomethacin and guanethidine (85%) (Fig. 2B). These results suggest that prostanoids, sympathomimetic amines, and IL-1β, but not TNF-α, are involved in KC-induced mechanical hypernociception in mice. Consistent with this suggestion, KC induced IL-1β production in WT mice paw skin at the same levels as in TNF-RI–/– mice (see Fig. 6B). Thus, KC causes hypernociception by means of an IL-1β/prostaglandins pathway and by stimulating the release of sympathetic amines. The participation of prostanoids and sympathomimetic amines as primary mediators of inflammatory hypernociception has already been shown in mechanical tests in rats (8, 9, 42, 43).
Fig. 2.
Mechanical hypernociception induced by i.pl. injection of KC: the role of TNF-α, IL-1β, prostaglandins, and sympathomimetic amines. (A) Dose– and time–response curves of the hypernociception induced by i.pl. injection of KC (1, 3, and 10 pg per paw) or saline (SAL; control). The hypernociceptive effects were determined at 1, 3, and 5 h after the stimuli injection. (B) Nociceptive response induced by KC (10 ng per paw) in WT mice pretreated with 15 μlof saline (Sal) or TNF-R1–/– mice and pretreatment of WT mice with 500 ng of AbKC delivered i.pl., 500 ng of IL-1ra delivered i.pl, 5 mg/kg indomethacin (Indo) delivered i.p., 30 mg/kg guanethidine (Gua) delivered s.c., and indomethacin plus guanethidine. The hypernociceptive responses were taken 3 h after KC injection. The results are expressed as the mean ± SEM of five animals per group. *, Statistically significant different time points (P < 0.05); **, statistically significant difference compared with the control group (P < 0.05).
Fig. 6.
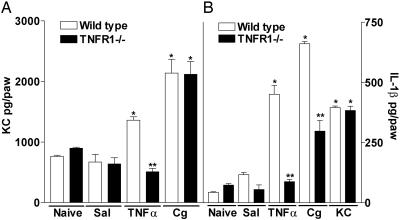
Production of cytokines in TNF-R1–/– mice. Concentration of KC (A) and IL-1β (B) in WT and TNF-R1–/– mice paws injected with 100 μg of Cg, 100 pg of TNF-α, 10 ng of KC, or 25 μl of saline (Sal) or in naïve paws. At 3 h after injection, mice were killed, and paw skin samples were extracted for cytokines, which were measured by ELISA. The results are expressed by the mean ± SEM of four animals per group. *, Statistically significant differences compared with the saline group (P < 0.05); **, statistically significant differences compared with the WT mice group (P < 0.05).
TNF-α-Induced Mechanical Hypernociception. I.pl. injection of TNF-α induced hypernociception in a dose- and time-dependent (1–1,000 pg; 1–5 h) manner (Fig. 3A). The maximum hypernociceptive response was observed with the dose of 100 pg of TNF-α. The hypernociception was already significant 1 h after TNF-α injection, reached a plateau between 3 and 5 h after injection, and returned to control levels by 24 h (data not shown). There was no difference in responses at 3 and 5 h. TNF-α-induced hypernociception was absent in TNF-R1–/– mice, and, in WT mice, it was partially inhibited by pretreatment of paws with IL-1ra (–60%) or indomethacin (–62%) (Fig. 3B). These results suggest that the hypernociceptive effect of TNF-α depends on TNF-R1 receptor activation, which mainly triggers the secretion of IL-1β, which is responsible for prostaglandins production. Consistent with this suggestion, the injection of TNF-α (100 pg delivered i.pl.) stimulated the secretion of IL-1β in paw skin that was absent in TNF-R1–/– mice (see Fig. 6B). It is noteworthy that TNF-α hypernociception was not fully blocked by combined pretreatment with guanethidine and indomethacin. This result suggests that TNF-α either has a direct hypernociceptive effect or releases another nociceptor-sensitizing mediator. Although KC appeared not to be involved in the hypernociceptive effects of TNF-α, because AbKC antibody did not inhibit the TNF-α-induced hypernociception (Fig. 3B), the i.pl. administration of TNF-α appeared to stimulate a TNF-RI-dependent release of KC (see Fig. 6A). A possible explanation of this apparently contradictory result could be that the amounts of KC released by TNF-α-injection were insufficient to cause detectable hypernociception.
Fig. 3.
Mechanical hypernociception induced by i.pl. injection of TNF-α: the role of TNF-R1 receptor, KC, IL-1β, prostaglandins, and sympathomimetic amines. (A) Dose– and time–response curves of the hypernociception induced by i.pl. injection of TNF-α (1, 10, 100, and 1,000 pg per paw) or saline (SAL; control). The hypernociceptive effects were determined at 1, 3, and 5 h after the stimuli injection. (B) Nociceptive response induced by TNF-α (100 pg per paw) in WT mice treated with 20 μl of saline (Sal) or TNF-R1–/– mice and pretreatment of WT mice with 500 ng of AbKC delivered i.pl. 500 ng of IL-1ra delivered i.pl. 5 mg/kg indomethacin (Indo) delivered i.p., 30 mg/kg guanethidine (Gua) delivered s.c., and indomethacin plus guanethidine. The hypernociceptive responses were taken 3 h after TNF-α injection. The results are expressed as the mean ± SEM of five animals per group. *, Statistically significant different time points (P < 0.05); **, statistically significant difference compared with the control group (P < 0.05).
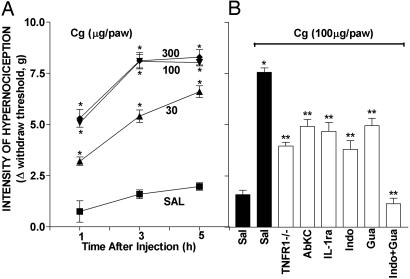
Cg-Induced Hypernociception. I.pl. injection of Cg in WT mice evoked mechanical hypernociception in a dose- and time-dependent (30–300 μg; 1–5 h) manner (Fig. 4A). The maximum hypernociceptive response was observed with the dose of 100 μg of Cg. Significant hypernociception was present 1 h after Cg injection, reached a plateau between 3 and 5 h after injection, and had returned to control levels within 24 h (data not shown). The Cg-induced hypernociception was partially reduced in TNF-R1–/– mice (–60%) (Fig. 4B). Pretreatment with AbKC and IL-1ra that abolished the hypernociception induced by KC (Fig. 2B) and IL-1β (Fig. 1B), respectively, only partially reduced the effect of Cg (AbKC, –41%; IL-1ra, –48%) (Fig. 4B). Partial inhibition of the Cg effect was also seen in WT mice pretreated with indomethacin (–63%) or guanethidine (–43%) and was abolished by pretreatment with both indomethacin and guanethidine (–100%) (Fig. 4B).
Fig. 4.
Mechanical hypernociception induced by i.pl. injection of Cg in WT and TNF-R1–/– mice: effects of IL-1ra, AbKC, indomethacin, guanethidine, and association of indomethacin and guanethidine. (A) Dose– and time–response curves of the hypernociception induced by i.pl. injection of Cg (30, 100, and 300 μg per paw) or saline (SAL; control). The hypernociceptive effects were determined at 1, 3, and 5 h after the stimuli injection. (B) Nociceptive response induced by Cg (100 μg per paw) in WT mice pretreated with 15 μl of saline (Sal) or TNF-R1–/– mice and pretreatment of WT mice with 500 ng of AbKC delivered i.pl., 500 ng of IL-1ra delivered i.pl., 5 mg/kg indomethacin (Indo) delivered i.p., 30 mg/kg guanethidine (Gua) delivered s.c., and indomethacin plus guanethidine. The hypernociceptive responses were made 3 h after Cg injection. The results are expressed by the mean ± SEM of five animals per group. *, Statistically significant different time points (P < 0.05); **, statistically significant difference compared with the control group (P < 0.05).
These results can be interpreted as Cg stimulating the release of TNF-α and KC and with TNF-α acting on TNF-R1 to stimulate the release of IL-1β, which stimulates the release of prostanoids. Cg also appears to directly stimulate release of KC, which, besides releasing sympathomimetic amines, is itself partially responsible for the hypernociception by also stimulating the release of IL-1β/prostanoids. This suggestion is further supported by measurements of the concentrations of TNF-α, KC, and IL-1β in paw skin injected with Cg.
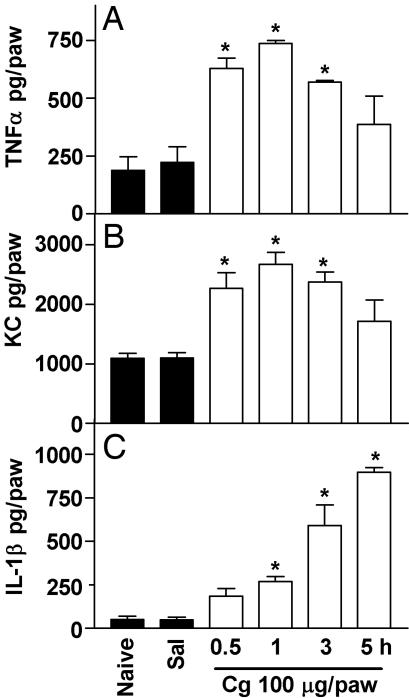
Cg-Induced Paw Inflammation: Detection of TNF-α, KC, and IL-1β in Skin. To confirm the involvement of TNF-α, KC, and IL-1β in Cg-induced hypernociception in mice, the levels of these cytokines were measured in inflamed paw skin. The administration of Cg (100 μg delivered i.pl.) stimulated the production of TNF-α, KC, and IL-1β in WT mice (Fig. 5). The Cg-evoked increase in TNF-α (Fig. 5A) and KC (Fig. 5B) in paw skin was detected 30 min after Cg injection, peaked at 1 h, and diminished thereafter (3–5 h). However, the Cg-evoked increased in IL-1β (Fig. 5C) was significant 1 h after Cg injection and increased progressively thereafter, reaching its maximum value at 5 h. The i.pl. administration of Cg (100 μg) in TNF-R1–/– mice did not alter the production of KC in paw skin (Fig. 6A) but halved IL-1β production (Fig. 6B). TNF-α (100 pg delivered i.pl.) stimulated the release of KC and IL-1β in WT but not TNF-R1–/– mice (Fig. 6 A and B, respectively). Furthermore, the i.pl. administration of KC (10 ng) stimulated the production of IL-1β (Fig. 6B) but not TNF-α (data not shown) in WT mice, and TNF-α and KC were not detected in paw skin in response to IL-1β injection (data not shown).
Fig. 5.
I.pl. injection of Cg-stimulated TNF-α, KC, and IL-1β production in paw skin. Concentrations of TNF-α (A), KC (B), and IL-1β (C) in mice paws injected with 100 μg of Cg or saline (Sal) or in naive paws. At 0.5, 1, 3, and 5 h after injection, mice were killed and paw skin samples were extracted for cytokines, which were measured by ELISA. The results are expressed by the mean ± SEM of four animals per group. *, Statistically significant differences compared with the control group (P < 0.05).
Role of Cytokines on Mechanical Inflammatory Hypernociception: Similarities and Differences Between Mice and Rats. There are qualitative similarities and differences between mice and rats in terms of the cytokines released by inflammatory stimuli and the hypernociceptive mechanisms of action of those cytokines. TNF-α, IL-1β, and CINC/KC (human IL-8 counterparts produced by rats and mice, respectively) are released in rats and mice challenged with nociceptive stimuli, and these cytokines trigger two basic mechanical hypernociceptive inflammatory pathways: the prostanoid and the sympathetic (18). In both animal species, prostanoid release is stimulated by IL-1β, because indomethacin blocks the hypernociceptive effect of IL-1β (40). However, TNF-α, which stimulates both pathways in rats and only the prostanoid pathway in mice (21). Furthermore, whereas CINC stimulates only the sympathetic component in rats, KC stimulates both of the hypernociceptive pathways in mice (22). Another species difference is the hypernociceptive role of IL-6. Although IL-6 induces the production of prostanoids in both species, in rats, but not in mice, IL-6 is an intermediate in TNF-α-induced IL-1β release, i.e., TNF-α→IL-6→IL-1β→prostanoids (21). In contrast, i.pl. injection of IL-6 in mice caused hypernociception, which was inhibited by indomethacin but not by IL-1ra (data not shown), at a dose similar to that which inhibited IL-6-induced hypernociception in rats.
The fact that indomethacin and IL1ra, at doses that abolished IL-1β hypernociception, only partially inhibited Cg-induced hypernociception in mice and rats suggested that, besides the prostanoid component, Cg-induced paw inflammatory hypernociception is mediated by another pathway: the sympathetic component (8). In fact, the sympathetic amine blocker, guanethidine, at a dose that inhibited KC/CINC-induced hypernociception, also partially inhibited Cg-induced hypernociception. Also, the association of guanethidine and indomethacin abolished Cg hypernociception in both animal species (18). However, in contrast to responses in rats, activation of the sympathetic pathway in mice was not inhibited by a β-adrenoreceptor blocker (atenolol), but it was inhibited by the nonselective α-antagonist-phentolamine (results not shown). These observations confirm that mechanical inflammatory hypernociception in both species has a sympathetic component but that in mice sympathetic hypernociception is transduced by another class of adrenoreceptor. Actually, in man, α-adrenoreceptor agonists are associated with the triggering of hyperalgesia (44, 45) and β-adrenoreceptor antagonists are ineffective in sympathetic pain syndromes; although guanethidine, injected retrogradely into the injured tissue, produces a long-lasting analgesia (46–48)
Another difference between rats and mice is the participation of the kinin system as the trigger of the mechanical hypernociceptive cytokine cascade. In Cg-induced hypernociception in rats, an early event is the activation of the plasma kinin system with consequent production of bradykinin that induces TNF-α production by means of the activation of B1 and B2 receptors (18). In contrast, in mice, bradykinin-mediated inflammatory hypernociception does not depend on cytokines; instead, bradykinin directly activates the release of prostanoids and sympathetic amines (data not shown). However, such direct activation is not unique to mice, because, in rats, there is no requirement for activation of the kinin system if the hypernociceptive stimulus is of sufficient magnitude, e.g., a relatively large dose of LPS (49).
Sequential release of cytokines has been also described in complete Freund's adjuvant-induced inflammatory hypernociception in rats. In this model, in both mechanical and thermal hypernociception, TNF-α was the first cytokine released, and it induced IL-1β secretion, and both acted to stimulate nerve growth factor production. Nerve growth factor was believed to act directly without the participation of prostanoids (23, 24). However, it seems that its hypernociception is at least in part mediated by leukotrienes and sympathetic amines (50, 51). Furthermore, hypernociception in response to i.p. administration of LPS in rats is thought to be mediated by TNF-α-dependent IL-1β release, which in turn activates subdiaphragmatic vagal afferents (26). In this model, it appears that cytokine-mediated hypernociception is independent of prostanoids, suggesting a direct effect of IL-1β, although other primary mediators may participate (e.g., sympathetic amines, leukotrienes, and/or endothelins). Thus, the results discussed above reinforce the idea of a sequential–functional role of mediators in inflammatory hypernociception, instead of a “soup of mediators” (52).
There are also discrepancies in the literature concerning the sequential participation of the cytokines in inflammatory hypernociception in mice. The use of different experimental models may at least in part explain these differences. Although in a model of overt nociception (writhing induced by acetic acid or zymosan) the cytokines acted concomitant and synergistically (32), in mechanical inflammatory hypernociceptive models, cytokines appear to act sequentially, as discussed above. The paw mechanical pressure tests are based on the triggering of a behavioral end point by the application of a mechanical stimulus to a paw previously sensitized by administration of an inflammatory stimulus. However, in writhing or formalin tests, the number of successive characteristic behavioral responses (contortions/stretches or licking behaviors) is triggered by i.p. or i.pl. injections of strong injurious nociceptive stimuli without previous sensitization (53), and these can promote the concomitant release of many different mediators by the injured cells. Therefore, in these tests there is no time interval between the sensitization and the stimulus triggering the behavior; i.e., the sensitizing and activating nociceptor mediators may be release together and act at the same time, making it difficult to analyze the role of sensitizing mediators.
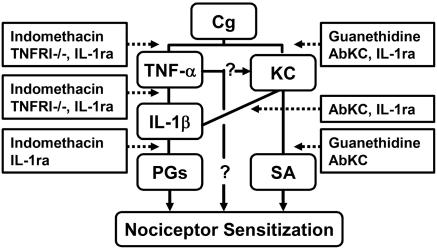
Fig. 7 summarizes the essential conclusions of this study: We report that Cg-induced mechanical hypernociception in mice depends on two key cytokines, TNF-α and KC. Both act through IL-1β release and the release of prostanoids by the IL-1β. In addition, KC acts additionally by means of sympathomimetic amines. Finally, the TNF-α-related events largely depend on the TNF-R1 receptor.
Fig. 7.
Representative diagram of Cg-induced inflammatory hypernociceptive cytokine cascade in mice. Shown is the sequential release (solid line, stimulation) of inflammatory mediators initiated by Cg and intermediated by cytokines and the final induction by prostanoids (PGs) and sympathetic amines (SA). The cascade pharmacological and genetic approach susceptibility is also shown (dotted line, inhibition) along with the other possibilities for TNF-α action (question marks).
Conclusion
Although there is a functional difference in the sequence of release of cytokines in rats and mice, a group of cytokines common to both species have roles in the injury and the induction of hypernociception. These cytokines act in a distinct sequence, but the final effect is indirect and mediated through the release of prostanoids and sympathomimetic amines. Thus, the present study reinforces the importance of cytokines in inflammatory hypernociception. Therefore, if similar mechanisms occur in man, the inhibition of cytokine production or action appears to constitute a real target for a new therapeutic approach to control the inflammatory pain.
Acknowledgments
We thank Ieda Regina dos Santos Schivo, Sérgio Roberto Rosa, and Giuliana Bertozi Francisco for excellent technical support. This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo and Conselho Nacional de Desenvolvimento Científico e Technológico. T.M.C. is a recipient of a Master's Studentship from Fundação de Amparo à Pesquisa do Estado de São Paulo.
Abbreviations: Cg, carrageenan; TNF-R1–/–, TNF receptor type 1 knockout; KC, keratinocyte-derived chemokine; AbKC, Ab against KC; i.pl., intraplantar; CINC-1, cytokine-induced neutrophil chemoattractant-1; IL-1ra, IL-1 receptor antagonist.
References
- 1.Blackwell, T. S. & Christman, J. W. (1996) Br. J. Anaesth. 77, 110–117. [DOI] [PubMed] [Google Scholar]
- 2.Dinarello, C. A. (2000) Chest 118, 503–508. [DOI] [PubMed] [Google Scholar]
- 3.Hopkins, S. J. (2003) Leg. Med. (Tokyo) 5, S45–S57. [DOI] [PubMed] [Google Scholar]
- 4.Cunha, F. Q. & Ferreira, S. H. (2003) Adv. Exp. Med. Biol. 521, 22–39. [PubMed] [Google Scholar]
- 5.Conti, B., Tabarean, I., Andrei, C. & Bartfai, T. (2004) Front. Biosci. 9, 1433–1449. [DOI] [PubMed] [Google Scholar]
- 6.Millan, M. J. (1999) Prog. Neurobiol. 57, 1–164. [DOI] [PubMed] [Google Scholar]
- 7.Ferreira, S. H. & Nakamura, M. (1979) Prostaglandins 18, 179–190. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura, M. & Ferreira, S. H. (1987) Eur. J. Pharmacol. 135, 145–153. [DOI] [PubMed] [Google Scholar]
- 9.Khasar, S. G., McCarter, G. & Levine, J. D. (1999) J. Neurophysiol. 81, 1104–1112. [DOI] [PubMed] [Google Scholar]
- 10.Taiwo, Y. O., Bjerknes, L. K., Goetzl, E. J. & Levine, J. D. (1989) Neuroscience 32, 577–580. [DOI] [PubMed] [Google Scholar]
- 11.Coderre, T. J. (1992) Neurosci. Lett. 140, 181–184. [DOI] [PubMed] [Google Scholar]
- 12.Hingtgen, C. M., Waite, K. J. & Vasko, M. R. (1995) J. Neurosci. 15, 5411–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold, M. S., Reichling, D. B., Shuster, M. J. & Levine, J. D. (1996) Proc. Natl. Acad. Sci. USA 93, 1108–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malmberg, A. B., Chen, C., Tonegawa, S. & Basbaum, I. A. (1997) Science 278, 279–283. [DOI] [PubMed] [Google Scholar]
- 15.Lynn, B. & O'shea, N. R. (1998) Brain Res. 780, 320–362. [DOI] [PubMed] [Google Scholar]
- 16.Cunha, F. Q., Teixeira, M. M. & Ferreira, S. H. (1999) Br. J. Pharmacol. 127, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza, A. L., Moreira, F. A., Almeida, K. R., Bertollo, C. M., Costa, K. A. & Coelho, M. M. (2002) Br. J. Pharmacol. 135, 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poole, S., Cunha, F. Q. & Ferreira, S. H. (1999) in Cytokines and Pain, eds. Watkins, L. R. & Maier, S. F. (Springer, Berlin), pp. 59–87.
- 19.Ferreira, S. H., Lorenzetti, B. B. & Poole, S. (1993) Br. J. Pharmacol. 110, 1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cunha, F. Q., Lorenzetti, B. B., Poole, S. & Ferreira, S. H. (1991) Br. J. Pharmacol. 104, 765–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cunha, F. Q., Poole, S., Lorenzetti, B. B. & Ferreira, S. H. (1992) Br. J. Pharmacol. 107, 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lorenzetti, B. B., Veiga, F. H., Canetti, C. A., Poole, S., Cunha, F. Q. & Ferreira, S. H. (2002) Eur. Cytokine Network 13, 456–461. [PubMed] [Google Scholar]
- 23.Safieh-Garabedian, B., Poole, S., Allchorne, A., Winter, J. & Woolf, C. J. (1995) Br. J. Pharmacol. 115, 1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolf, C. J., Allchorne, A., Safieh-Garabedian, B. & Poole, S. (1997) Br. J. Pharmacol. 121, 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watkins, L. R., Wiertelak, E. P., Goehler, L. E., Smith, K. P., Martin, D. & Maier, S. F. (1994) Brain Res. 654, 15–26. [DOI] [PubMed] [Google Scholar]
- 26.Watkins, L. R., Goehler, L. E., Relton, J., Brewer, M. T. & Maier, S. F. (1995) Brain Res. 692, 244–250. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira, S. H., Cunha, F. Q., Lorenzetti, B. B., Michelin, M. A., Perretti, M., Flower, R. J. & Poole, S. (1997) Br. J. Pharmacol. 121, 883–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanaan, S. A., Safieh-Garabedian, B., Haddad, J. J., Atweh, S. F., Abdelnoor, A. M., Jabbur, S. J. & Saade, N. E. (1997) Pharmacology 54, 285–297. [DOI] [PubMed] [Google Scholar]
- 29.Ribeiro, R. A., Vale, M. L., Ferreira, S. H. & Cunha, F. Q. (2000) Eur. J. Pharmacol. 391, 97–103. [DOI] [PubMed] [Google Scholar]
- 30.Sommer, C. & Kress, M. (2004) Neurosci. Lett. 361, 184–187. [DOI] [PubMed] [Google Scholar]
- 31.Rankin, E. C., Choy, E. H., Kassimos, D., Kingsley, G. H., Sopwith, A. M., Insenberg, D. A. & Panayi, G. S. (1995) Br. J. Rheumatol. 34, 334–342. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro, R. A., Vale, M. L., Thomazzi, S. M., Paschoalato, A. B., Poole, S., Ferreira, S. H. & Cunha, F. Q. (2000) Eur. J. Pharmacol. 387, 111–118. [DOI] [PubMed] [Google Scholar]
- 33.Cunha, T. M., Verri, W. A., Jr., Vivancos, G. G., Moreira, I. F., Reis, S., Parada, C. A., Cunha, F. Q. & Ferreira, S. H. (2004) Brazil J. Med. Biol. Res. 37, 401–407. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe, K., Konishi, K., Fujioka, M., Kinoshita, S. & Nakagawa, H. (1989) J. Biol. Chem. 264, 19559–19563. [PubMed] [Google Scholar]
- 35.Baggiolini, M., Dewald, B. & Moser, B. (1994) Adv. Immunol. 55, 97–179. [PubMed] [Google Scholar]
- 36.Bozic, C. R., Gerard, N. P., von Uexkull-Guldenband, C., Kolakowski, L. F., Jr., Conklyn, M. J., Breslow, R., Showell, H. J. & Gerard, C. (1994) J. Biol. Chem. 269, 29355–29358. [PubMed] [Google Scholar]
- 37.Sommer, C., Schmidt, C. & George, A. (1998) Exp. Neurol. 151, 138–142. [DOI] [PubMed] [Google Scholar]
- 38.Parada, C. A., Vivancos, G. G., Tambeli, C. H., deq Ueiroz Cunha, F. & Ferreira, S. H. (2003) Proc. Natl. Acad. Sci. USA 100, 2923–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coderre, T. J., Abbott, F. V. & Melzack, R. (1984) Pain 18, 13–23. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira, S. H., Lorenzetti, B. B., Bristow, A. F. & Poole, S. (1988) Nature 334, 689–700. [DOI] [PubMed] [Google Scholar]
- 41.Schweizer, A., Feige, U., Fontana, A., Muller, K. & Dinarello, C. A. (1988) Agents Actions 25, 246–251. [DOI] [PubMed] [Google Scholar]
- 42.Ferreira, S. H., Nakamura, M. & de Abreu Castro, M. S. (1978) Prostaglandins 16, 31–37. [DOI] [PubMed] [Google Scholar]
- 43.Safieh-Garabedian, B., Poole, S., Haddad, J. J., Massaad, C. A., Jabbur, S. J. & Saade, N. E. (2002) Neuropharmacology 42, 864–872. [DOI] [PubMed] [Google Scholar]
- 44.Drummond, P. D., Skipworth, S. & Finch, P. M. (1996) Clin. Sci. (London) 91, 73–77. [DOI] [PubMed] [Google Scholar]
- 45.Ali, Z., Raja, S. N., Wesselmann, U., Fuchs, P. N., Meyer, R. A. & Campbell, J. N. (2000) Pain 88, 161–168. [DOI] [PubMed] [Google Scholar]
- 46.Hannington-Kiff, J. G. (1979) Br. Med. J. 2, 367–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonezzi, C., Miotti, D., Bettaglio, R. & Stephen, R. (1994) J. Pain Symptom Manage. 9, 39–43. [DOI] [PubMed] [Google Scholar]
- 48.Wahren, L. K., Gordh, T., Jr., & Torebjork, E. (1995) Pain 62, 379–385. [DOI] [PubMed] [Google Scholar]
- 49.Poole, S., Lorenzetti, B. B., Cunha, J. M., Cunha, F. Q. & Ferreira, S. H. (1999) Br. J. Pharmacol. 126, 649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andreev, N. Y., Dimitrieva, N., Koltzenburg, M. & McMahon, S. B. (1995) Pain 63, 109–115. [DOI] [PubMed] [Google Scholar]
- 51.Bennett, G., al-Rashed, S., Hoult, J. R. & Brain, S. D. (1998) Pain 77, 315–322. [DOI] [PubMed] [Google Scholar]
- 52.Julius, D. & Basbaum, A. I. (2001) Nature 413, 203–210. [DOI] [PubMed] [Google Scholar]
- 53.Le Bars, D., Gozariu, M. & Cadden, S. W. (2001) Pharmacol. Rev. 53, 597–652. [PubMed] [Google Scholar]