Abstract
Synthetic cannabinoids (SCs) represent an emerging class of abused drugs associated with psychiatric complications and other substantial health risks. These ligands are largely sold over the internet for human consumption, presumably because of their high cannabinoid 1 receptor (CB1R) affinity and their potency in eliciting pharmacological effects similar to Δ9-tetrahydrocannabinol (THC), as well as circumventing laws illegalizing this plant. Factors potentially contributing to the increased prevalence of SC abuse and related hospitalizations, such as increased CB1R efficacy and non-CB1R targets, highlight the need for quantitative pharmacological analyses to determine receptor mediation of the pharmacological effects of cannabinoids. Accordingly, the present study used pA2 and pKB analyses for quantitative determination of CB1R mediation in which we utilized the CB1R-selective inverse agonist/antagonist rimonabant to elicit rightward shifts in the dose-response curves of five SCs (i.e., A-834,735D; WIN55,212-2; CP55,950; JWH-073; and CP47,497) and THC in producing common cannabimimetic effects (i.e., catalepsy, antinociception, and hypothermia). The results revealed overall similarity of pA2 and pKB values for these compounds and suggest that CB1Rs, and not other pharmacological targets, largely mediated the central pharmacological effects of SCs. More generally, affinity estimation offers a powerful pharmacological approach to assess potential receptor heterogeneity subserving in vivo pharmacological effects of SCs.
Introduction
Δ9-Tetrahydrocannabinol (THC), the primary psychoactive constituent of marijuana, exerts the bulk of its pharmacological effects, including the subjective high in humans (Huestis et al., 2001), discriminative stimulus effects (Järbe et al., 2001, 2014), and other common pharmacological effects (e.g., hypomotility, antinociception, catalepsy, and hypothermia) in rodents (Compton and Martin, 1996; Ledent et al., 1999), through the activation of cannabinoid receptor 1 (CB1R). Originally developed as research tools and potential medications, synthetic cannabinoids (SCs) bind and activate CB1Rs to produce similar pharmacological effects as THC in preclinical assays (Devane et al., 1988; Compton et al., 1992; Järbe et al., 2011). Since the emergence of SCs as drugs of abuse (Presley et al., 2013), their consumption has been associated with untoward effects more severe than cannabis, including reports of life-threatening medical complications and death (Thornton et al., 2013; Gerostamoulos et al., 2015; Trecki et al., 2015). Although CB1(−/−) mice represent useful tools to determine CB1R mediation of cannabinoids (Ledent et al., 1999; Zimmer et al., 1999; Grim et al., 2016), limitations of this approach include compensatory changes across ontogeny, hitchhiking genes, epistasis effects, and other potential confounds related to constitutive knockout mouse models (Lariviere et al., 2001). Conversely, rigorous pharmacological approaches utilizing CB1R-selective antagonists lack these confounds and enable crossspecies comparisons to provide complementary and converging evidence to determine CB1R mediation.
Approaches employing pA2 and pKB analyses with competitive, reversible pharmacological antagonists provide the quantitative basis for assessing receptor heterogeneity in modulating the in vivo effects of drug classes (Tallarida et al., 1979). In pA2 analysis, agonist dose-effect curves are determined after pretreatment with a range of doses of a competitive, reversible antagonist, thus relating antagonist dose to the magnitude of the rightward shift in the agonist dose-effect curve. This antagonist dose-effect curve is typically expressed as a Schild plot (see Data Analysis), and if the Schild plot yields a slope of –1 (predicted for an antagonist competing with an agonist at a single population of receptors), then the antagonist dose sufficient to produce a 2-fold rightward shift in the agonist dose-effect curve is interpreted as an estimate of antagonist affinity for the receptor that mediates agonist effects. This antagonist dose is commonly expressed as a “pA2” value (i.e., the –log antagonist dose in units of mol/kg sufficient to produce a 2-fold rightward shift in the agonist dose-effect curve). Similar pA2 values for an antagonist to block effects of two different agonists, or to block two different effects of a given agonist, provide evidence of a common receptor type with a common affinity for the antagonist. Conversely, different pA2 values for an antagonist to block effects of two different agonists, or to block two different effects of a given agonist, implicate the involvement of different receptor populations. This approach may be applied to in vitro- or in vivo-dependent measures, and has been employed to compare receptor types that mediate subjective effects of THC and SCs (i.e., CP55,940; WIN55,212-2; and JWH-018) in nonhuman primates trained to discriminate THC or the anandamide analog arachidonylcycloproplamide (McMahon, 2006; Rodriguez and McMahon, 2014; Ginsburg et al., 2012). This analysis has also been used in the mouse radiant-heat tail-flick assay (Reche et al., 1996).
A primary goal of the present study was to provide a straightforward pharmacological approach using pA2 analysis to determine the extent to which CB1Rs mediate common in vivo pharmacological effects of SCs. Specifically, we administered to mice vehicle or various doses of the CB1R-selective antagonist rimonabant and then evaluated the cataleptic, antinociceptive, and hypothermic effects produced by THC and five SCs (i.e., CP47,497; JWH-073; CP55,940; WIN55,212-2; and A-834,735D), which varied in CB1R affinity, selectivity, and efficacy. Of note, A-834,735D was recently detected in an herbal sample containing synthetic cannabinoids (Byrska et al., 2016) but remains largely uncharacterized. We determined pKB values for compounds whose solubility limitations constrained testing a full dose range in the presence of more than two rimonabant doses. A previous study employing CB1 (+/+), (+/−), and (−/−) mice revealed that CB1Rs play a necessary role in the antinociceptive, hypothermic, and cataleptic effects of all six agonists (Grim et al., 2016). Curiously, THC retained weak, but significant, hypothermic and antinociceptive effects in CB1(−/−) mice, suggesting contribution of a non-CB1R site of action. In view of these findings, we predicted that rimonabant would dose-dependently antagonize all three effects of each SC with Schild-plot slopes of –1 and similar pA2 and pKB values, whereas THC-induced antinociception and hypothermia would display different sensitivity to rimonabant antagonism owing to non-CB1R contribution.
Methods
Animal Subjects.
A total of 72 male and female CB1(+/+) mice backcrossed for at least 15 generations on a C57BL/6J background were used for these studies. Mice were kept on a 12-hour light/dark cycle with ad libitum access to food and water and were at least 10 weeks of age at the beginning of testing. All procedures and protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Drugs.
THC, CP55,940, and rimonabant were generously provided by the National Institute on Drug Abuse Drug Supply Program (Rockville, MD). JWH-073; CP47,497; and WIN55,212-2 were purchased from Cayman Chemicals (Ann Arbor, MI), and A-834,735D was a gift from the same company. All drugs, except for WIN55,212-2 and rimonabant, were initially dissolved in ethanol or methanol at concentrations insufficient for testing; thus, the solvent was evaporated under a stream of nitrogen, and drugs were redissolved in ethanol at the appropriate stock concentrations. WIN55,212-2 was delivered in powder crystal form and dissolved in ethanol at the appropriate stock concentrations. Rimonabant was supplied in powder form and was prepared at the start of each experiment. Concentrated drug stocks were then diluted with emulphor and 0.9% saline to yield a final vehicle containing 1 part ethanol, 1 part emulphor, and 18 parts saline.
Behavioral Testing.
Six groups of mice, consisting of six males and six females per group, were used to determine dose-response relationships of the six selected agonists, both alone and in the presence of varying doses of rimonabant (0.3–10 mg/kg). As previously described (Grim et al., 2016; Falenski et al., 2010), the bar test was used to assess catalepsy, the warm-water tail-withdrawal test was employed to assess antinociception, and rectal temperature was taken to evaluate hypothermia. Prior to each dose-response determination, baseline measurements of all dependent measures were recorded. Catalepsy was defined as a rigid, immobile posture, except for involuntary movements such as breathing, and was measured by placing the mouse’s forepaws on a bar elevated 4.5 cm above the bench top. If the mouse removed its paws, it was replaced up to four times, or until 60 seconds was reached, whichever occurred first. For antinociception, the distal 1 cm of the tail was inserted in 52°C water, and latency to remove the tail from the water was recorded, with a 10-second cut-off to prevent tissue damage. Hypothermia was measured by inserting a thermocouple probe 2 cm into the rectum. Mice received an intraperitoneal injection of vehicle or the indicated rimonabant dose 30 minutes before administration of the first dose of the cannabinoid receptor agonist. Each subject was assessed for catalepsy, antinociception, and hypothermia 30 minutes after each subsequent injection of agonist. Assessment of these three measures for each cohort of six mice required 10 minutes, after which mice were injected with the next dose, so that 40 minutes elapsed between injections throughout testing. Cumulative doses were administered until maximum effects were observed for catalepsy (60 seconds), antinociception (10 seconds), and hypothermia (–8°C from baseline). The solubility of each drug limited the number of possible determinations of dose-response curves in the presence of rimonabant, so that only one was determined for THC and JWH-073, and two were determined for WIN55,212-2 and CP47,497. A-834,735D and CP55,940 afforded three or more redeterminations necessary for pA2 analysis utilizing linear regression. Most injections were administered in a volume of 10 μl/g, though to achieve maximal doses for some dose-effect curves, the injection volumes for the final doses were: 14.4 μl/g for CP55,940, 23.3 μl/g for JWH-073, 18.5 μl/g for CP47,497, and 30 μl/g for THC. At least 1 week separated determination of agonist dose-response curves, and each group of mice only received one agonist (i.e., one group of 12 mice for A-834,735D; WIN55,212-2; CP55,940; JWH-073; CP47,497; and THC) and rimonabant.
Data Analysis.
To facilitate calculation of ED50 values, all data were transformed to percent maximum possible effect (%MPE) utilizing the following formula:
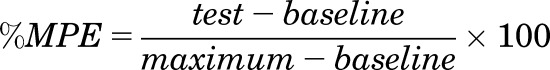 |
(1) |
After transformation, ED50 values and 95% confidence limits were estimated via linear regression of log dose versus %MPE, and ED50 values were considered to differ significantly if the 95% confidence limits did not overlap. For hypothermia, a loss of 8°C was used as the maximum. Dose ratios (DR) were calculated by dividing the ED50 value of the agonist with rimonabant pretreatment by the ED50 of the agonist with vehicle pretreatment.
Two methods were used to calculate the potency of rimonabant to antagonize each agonist under these conditions: pA2 (Tallarida et al., 1979) and pKB (Negus et al., 1993). For pA2 analysis, antagonist effects quantified as dose ratios were determined for at least three antagonist doses, and these data were plotted on a Schild plot, which shows antagonist dose expressed as –log antagonist dose in units of mol/kg on the x-axis, and dose ratio expressed as log (dose ratio –1) on the y-axis. In all cases reported here, Schild-plot slopes did not differ from the value of –1 theoretically expected when an antagonist competes with an agonist for a single population of receptors. Accordingly, linear regression with slope constrained to –1 was used to determine the pA2 value (i.e., the antagonist dose required to produce a dose ratio of 2, indicating a 2-fold rightward shift in the agonist dose-effect curve). Also determined were 95% confidence limits of the pA2 value, and pA2 values were considered to be different if 95% confidence limits did not overlap. Full pA2 analysis requires evaluation of antagonist effects produced by at least three antagonist doses, and the highest of these doses can produce large rightward shifts in agonist dose-effect curves. In the present study, only A-834,735D and CP55,940, the two most potent agonists tested, permitted assessment of these large rightward shifts and the use of full pA2 analysis. For the remaining compounds, solubility limitations constrained the range of doses that could be tested, and antagonism could only be assessed using one or two rimonabant doses. In these cases, a value related to the pA2, known as the pKB value, was determined for each antagonist dose using the equation pKB = –log [B/(DR-1)], where B equals the antagonist dose in mol/kg, and DR equals the dose ratio produced by that antagonist dose. pKB analysis essentially plots the effect of a single antagonist dose on a Schild plot and infers the antagonist dose required to produce a dose ratio of 2 by assuming a Schild-plot slope of –1 through the empirically determined point. Accordingly, in cases where the Schild-plot slope equals –1, pA2 and pKB values should be identical. pKB values were also determined for each antagonist dose in combination with A-834,735D and CP55,940 to permit direct comparison of pA2 and pKB values. Unlike pA2 analysis, pKB estimations are single points and do not afford calculation of error. However, where possible, mean pKB values and 95% confidence limits were determined across rimonabant doses for antagonism of effects produced by a given agonist on a given endpoint. Additionally, if pKB estimations for rimonabant antagonism of a test drug fell outside the confidence limits generated via pA2 analysis for rimonabant antagonism of A-834,735D and CP55,940, they were considered significantly different.
Results
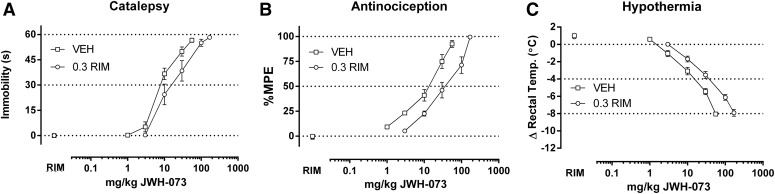
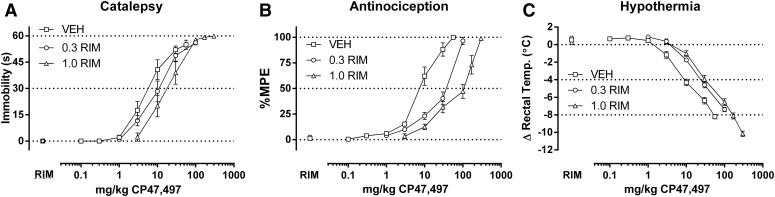
Rimonabant produced dose-dependent rightward shifts in the catalepsy, antinociception, and hypothermia dose-effect curves for A-834,735D; CP55,940; WIN55,212-2; CP47,497; JWH-073; and THC (Figs. 1–6, respectively). The ED50 (95% confidence limits) values of all cannabinoids for each of the three dependent measures in the absence and presence of rimonabant are shown in Table 1. Dose ratios for antagonism of each cannabinoid on each dependent measure by each rimonabant dose are shown in Table 2. Because the highest THC dose (900 mg/kg) produced a maximal antinociceptive effect of less than 50% MPE (46.8 ± 8.4% MPE) in mice pretreated with 0.3 mg/kg rimonabant, its ED50 value for this measure was calculated by extrapolation.
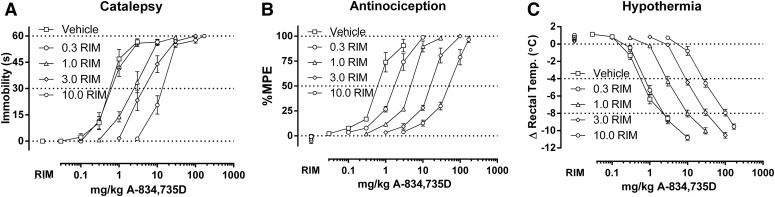
Fig. 1.
Rimonabant (0.3-10 mg/kg) dose-dependently elicits rightward shifts in the dose-response relationships of A-834,735D (0.03–170 mg/kg) in producing catalepsy (A), antinociception (B), and hypothermia (C) in CB1(+/+) mice. ED50 values (Table 1) and dose ratios (Table 2) were determined by linear regression. Four redeterminations of the dose-effect relationship in the presence of rimonabant enabled calculation of pA2 values (Table 3, Figs. 7 and 8).
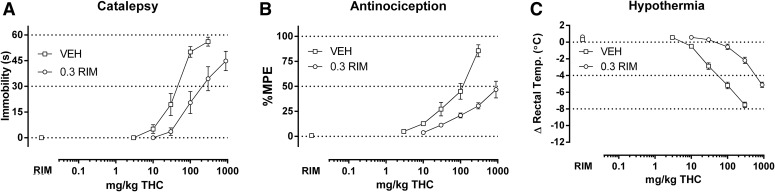
Fig. 6.
Rimonabant (0.3 mg/kg) produced a rightward shift in the dose-response relationship of THC (3–900 mg/kg) in producing catalepsy (A), antinociception (B), and hypothermia (C) in CB1(+/+) mice. ED50 values (Table 1) and dose ratios (Table 2) were determined by linear regression, though solubility of THC limited the number of redeterminations with rimonabant to one. Thus, only pKB values were calculated (Table 3, Fig. 8).
TABLE 1.
ED50 values (95% CL, expressed in mg/kg) for all treatments
Male and female (CB1 (+/+) mice (n = 10–12) were cumulatively dosed with increasing doses of the indicated agonist in absence or presence of rimonabant. For the antinociception measure, the ED50 for THC preceded by treatment with 0.3 mg/kg rimonabant was calculated by extrapolation, as the maximum effect determined was below 50%.
| Drug | Vehicle (mg/kg) | Rimonabant (mg/kg) |
|||
|---|---|---|---|---|---|
| 0.3 | 1 | 3 | 10 | ||
| Catalepsy | |||||
| A-834,735D | 0.50 (0.39–0.64) | 0.71 (0.47–1.08) | 2.21 (1.77–2.76)* | 5.01 (3.96–6.34)* | 14.04 (11.46–17.19)* |
| WIN55,212-2 | 5.43 (4.11–7.17) | 14.12 (10.31–19.37)* | 17.44 (10.21–29.78)* | ||
| CP55,940 | 0.65 (0.52–0.82) | 1.46 (1.08–1.99)* | 4.80 (3.51–6.56)* | 10.89 (8.87–13.39)* | |
| JWH-073 | 8.87 (7.58–10.41) | 18.86 (14.66–24.25)* | |||
| CP47,497 | 6.20 (4.77–8.07) | 9.41 (7.13–12.41) | 17.52 (13.52–22.69)* | ||
| THC | 46.47 (35.86–60.16) | 237.38 (150.20–375.15)* | |||
| Antinociception | |||||
| A-834,735D | 0.61 (0.47–0.77) | 1.60 (1.34–1.91)* | 4.34 (3.70–5.10)* | 14.62 (12.31–17.36)* | 40.93 (11.32–147.18)* |
| WIN55,212-2 | 7.42 (5.83–9.00) | 16.14 (13.36–19.51)* | 51.88 (41.28–65.21)* | ||
| CP55,940 | 0.47 (0.40–0.57) | 1.46 (1.15–1.83)* | 4.34 (3.51–5.39)* | 10.00 (7.77–12.86)* | |
| JWH-073 | 9.49 (7.77–11.60) | 28.37 (22.74–35.41)* | |||
| CP47,497 | 9.03 (7.92–10.29) | 22.34 (19.76–25.26)* | 53.41 (41.21–69.22)* | ||
| THC | 71.69 (51.32–130.04) | 1523 (891–2606)* | |||
| Hypothermia | |||||
| A-834,735D | 0.63 (0.52–0.78) | 0.63 (0.51–0.78) | 3.08 (2.69–3.53)* | 9.63 (8.79–10.54)* | 27.75 (23.69–32.51)* |
| WIN55,212-2 | 7.65 (6.43–9.09) | 16.09 (14.32–18.08)* | 31.46 (27.90–35.48)* | ||
| CP55,940 | 0.59 (0.49–0.71) | 2.05 (1.76–2.38)* | 4.47 (3.79–5.26)* | 16.45 (14.16–19.1)* | |
| JWH-073 | 11.25 (9.62–13.16) | 30.33 (25.88–35.54)* | |||
| CP47,497 | 7.21 (5.69–9.14) | 23.54 (18.91–29.31)* | 34.09 (29.90–38.58)* | ||
| THC | 55.38 (47.33–64.81) | 581.01 (474.77–711.04)* | |||
Confidence limits for a given drug in the presence of the dose of rimonabant in the header did not overlap with the confidence limits of the respective agonist tested in the absence of rimonabant.
TABLE 2.
Dose ratios of rimonabant (ED50 value in the presence of the indicated dose of rimonabant/ED50 value in the absence of the antagonist) for all treatments used for pA2 and pKB calculations
| Drug | Dose Ratio |
|||
|---|---|---|---|---|
| 0.3 | 1 | 3 | 10 | |
| Catalepsy | ||||
| A-834,735D | 1.42 | 4.41 | 9.99 | 27.99 |
| WIN55,212-2 | 2.60 | 3.21 | ||
| CP55,940 | 2.23 | 7.29 | 16.55 | |
| JWH-073 | 2.12 | |||
| CP47,497 | 1.47 | 2.83 | ||
| THC | 5.11 | |||
| Antinociception | ||||
| A-834,735D | 2.64 | 7.16 | 24.10 | 67.47 |
| WIN55,212-2 | 2.23 | 7.16 | ||
| CP55,940 | 3.06 | 9.13 | 21.02 | |
| JWH-073 | 2.99 | |||
| CP47,497 | 2.47 | 5.91 | ||
| THC | 21.2 | |||
| Hypothermia | ||||
| A-834,735D | 0 | 3.08 | 9.63 | 27.75 |
| WIN55,212-2 | 2.10 | 4.11 | ||
| CP55,940 | 3.47 | 7.57 | 27.85 | |
| JWH-073 | 2.70 | |||
| CP47,497 | 3.26 | 4.73 | ||
| THC | 10.49 | |||
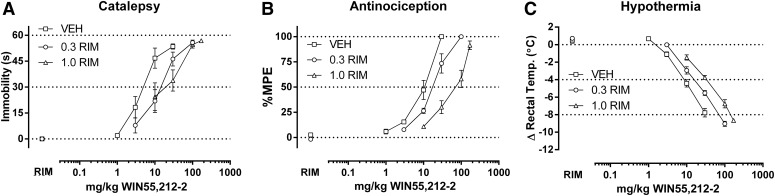
Fig. 2.
Rimonabant (0.3–1.0 mg/kg) elicits rightward shifts in the dose-response relationships of WIN55,212-2 (1.0–170 mg/kg) in producing catalepsy (A), antinociception (B), and hypothermia (C) in CB1(+/+) mice. ED50 values (Table 1) and dose ratios (Table 2) were determined by linear regression, though solubility of WIN55,212-2 limited the number of redeterminations possible with rimonabant to two. Thus, only pKB values were calculated (Table 3, Fig. 8).
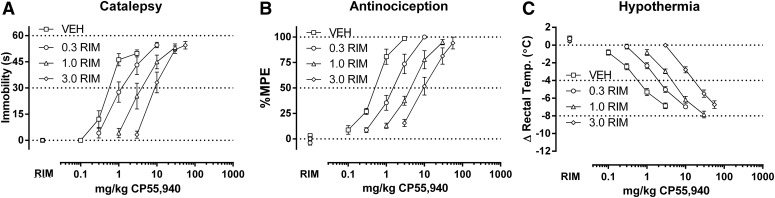
Fig. 3.
Rimonabant (0.3–3 mg/kg) dose dependently elicits rightward shifts in the dose-response relationships of CP55,940 (0.1–56 mg/kg) in producing catalepsy (A), antinociception (B), and hypothermia (C) in CB1(+/+) mice. ED50 values (Table 1) and dose ratios (Table 2) were determined by linear regression. Three redeterminations of the dose-effect relationship in the presence of rimonabant enabled calculation of pA2 values (Table 3, Figs. 7 and 8).
Fig. 4.
Rimonabant (0.3 mg/kg) elicits a rightward shift in the dose-response relationship of JWH-073 (1.0–170 mg/kg) in producing catalepsy (A), antinociception (B), and hypothermia (C) in CB1(+/+) mice. ED50 values (Table 1) and dose ratios (Table 2) were determined by linear regression, though solubility of JWH-073 limited the number of redeterminations with rimonabant to one. Thus, only pKB values were calculated (Table 3, Fig. 8).
Fig. 5.
Rimonabant (0.3–1.0 mg/kg) elicits rightward shifts in the dose-response relationships of CP47,497 (0.1–300 mg/kg) in producing catalepsy (A), antinociception (B), and hypothermia (C) in CB1(+/+) mice. ED50 values (Table 1) and dose ratios (Table 2) were determined by linear regression, though solubility of CP47,497 limited the number of redeterminations with rimonabant to two. Thus, only pKB values were calculated (Table 3, Fig. 8).
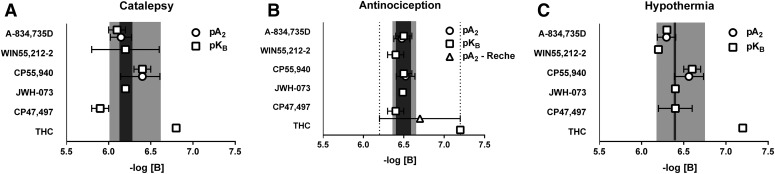
Table 3 shows pA2 and pKB values for the effects of rimonabant antagonism of A-834,735D and CP55,940 on catalepsy, antinociception, and hypothermia. Table 4 shows that the 95% confidence limits of all Schild-plot slopes included the value of –1 expected for competition between an antagonist and agonist at a single population of receptors. Accordingly, pA2 values were determined by linear regression with slopes constrained to –1 (see Fig. 7). In most cases, the 95% confidence limits of these rimonabant pA2 values overlapped across drugs as well as endpoints. The exceptions were as follows: The rimonabant pA2 value in antagonizing the cataleptic effects of A-834,735D were lower than those for antagonizing its antinociceptive effects, as well as those for antagonizing CP55,940-induced antinociception and hypothermia. In general, pKB values calculated for individual rimonabant doses fell within the 95% confidence limits of pA2 values for rimonabant antagonism of a given drug effect, and confidence limits for mean pKB values overlapped with confidence limits for pA2 values. However, a notable exception was that the pKB value for 0.3 mg/kg rimonabant antagonism of A-834,735D-induced catalepsy was below the 95% confidence limits of the pA2 value.
TABLE 3.
Calculated estimations of rimonabant affinity to antagonize the cataleptic, antinociceptive, and hypothermic effects of each agonist via pA2 and pKB analyses
| Drug |
pA2 (95% CL) |
Rimonabant (mg/kg), individual pKB values | Avg. pKB (95% CL) |
|||
|---|---|---|---|---|---|---|
| 0.3 | 1 | 3 | 10 | |||
| Catalepsy | ||||||
| A-834,735D | 6.2 (6.0–6.3) | 5.8*,^ | 6.2 | 6.1 | 6.1 | 6.1 (6.0–6.2) |
| WIN55,212-2 | 6.4* | 6.0^ | 6.2 (5.8–6.6) | |||
| CP55,940 | 6.4 (6.1–6.6) | 6.3 | 6.5* | 6.4* | 6.4 (6.3–6.5) | |
| JWH-073 | 6.2 | 6.2 | ||||
| CP47,497 | 5.9*,^ | 5.9*,^ | 5.9 (5.8–6.0)^ | |||
| THC | 6.8*,^ | 6.8 | ||||
| Antinociception | ||||||
| A-834,735D | 6.5 (6.4–6.6) | 6.4 | 6.5 | 6.6 | 6.5 | 6.5 (6.4–6.6) |
| WIN55,212-2 | 6.3*,^ | 6.5 | 6.4 (6.3–6.5) | |||
| CP55,940 | 6.5 (6.4–6.6) | 6.5 | 6.6* | 6.5 | 6.5 (6.5–6.6) | |
| JWH-073 | 6.5 | 6.5 | ||||
| CP47,497 | 6.4*,^ | 6.4*,^ | 6.4 (6.3–6.4) | |||
| THC | 7.2*,^ | 7.2 | ||||
| Hypothermia | ||||||
| A-834,735D | 6.3 (6.2–6.4) | 6.3^ | 6.3^ | 6.3^ | 6.3 (6.3–6.3) | |
| WIN55,212-2 | 6.2^ | 6.2*,^ | 6.2 (6.2–6.2) | |||
| CP55,940 | 6.6 (6.4–6.7) | 6.6* | 6.5* | 6.6* | 6.6 (6.5–6.6) | |
| JWH-073 | 6.4 | 6.4 | ||||
| CP47,497 | 6.5* | 6.2^ | 6.4 (6.2–6.6) | |||
| THC | 7.2*,^ | 7.2 | ||||
pKB value which fell outside the 95% confidence limits (CL) of the pA2 estimation of rimonabant affinity in the presence of A-834,735D.
pKB value which fell outside of the confidence limits of the pA2 estimation of rimonabant affinity in the presence of CP55,940.
TABLE 4.
Unconstrained Schild slopes (95% confidence limits) calculated for pA2 analysis of rimonabant versus A-834,735D and CP55,940 did not statistically differ from unity for catalepsy, antinociception, and hypothermia
For subsequent pA2 analyses, slopes were constrained to –1.
| Unconstrained Slope | Catalepsy | Antinociception | Hypothermia |
|---|---|---|---|
| A-834,735D | −0.69 (–1.13 to –0.25) | −1.07 (–1.21 to –0.86) | −1.08 (–1.23 to –0.92) |
| CP55,940 | −1.11 (–3.0 to 0.85) | −0.99 (–2.165 to 0.18) | −1.03 (–2.75 to 0.68) |
Fig. 7.
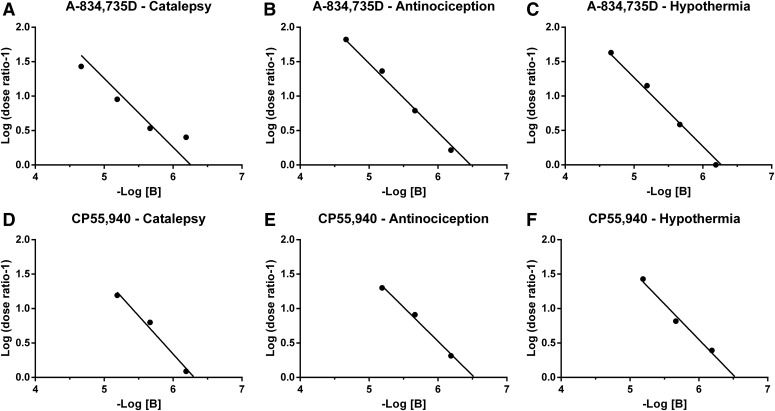
Apparent pA2 estimates of rimonabant in mice treated with A-834,735D and CP55,940. For A-834,735D, there was a 1:1 relationship between the log of the dose of rimonabant (mol/kg) treatment and the resulting log of the dose ratio –1 (log(DR-1)) for catalepsy (A), antinociception (B), and hypothermia (C). This relationship between rimonabant (mol/kg) and the ensuing log(DR-1) also held for CP55,940-induced catalepsy (D), antinociception (E), and hypothermia (F). In all cases, the 95% confidence limits of the unconstrained slope derived from linear regression included –1 (Table 4). Therefore, the slope was constrained to –1 in subsequent analyses to facilitate accurate estimations of the x-intercept (i.e., the dose of the antagonist that elicits a twofold shift in the dose effect curve of the agonist).
Because solubility constraints of WIN55-212-2; JWH-073; CP47,497; and THC precluded determination of their complete dose-response curves in the presence of at least three rimonabant doses, pKB values were determined for each rimonabant dose tested. Table 3 shows pKB values for each rimonabant dose, as well as average pKB values (with 95% confidence limits) across rimonabant doses, for each agonist and endpoint. In most cases, the pKB confidence limits overlapped with each other as well as with the pA2 confidence limits for rimonabant antagonism of A-834,735D and/or CP55,940. The most reliable exception to this general finding was 0.3 mg/kg rimonabant antagonism of THC, which yielded relatively high pKB values. The pKB values for individual rimonabant doses generally fell within the pA2 confidence limits for rimonabant antagonism of A-834,735D and/or CP55,940, though several exceptions of lower or higher pKB values were observed. For catalepsy, the exceptions included WIN55,212-2 at 1.0 mg/kg rimonabant, CP47,497 at both 0.3 and 1.0 mg/kg rimonabant, and THC at 0.3 mg/kg rimonabant. For antinociception, the exceptions included WIN55,212-2 at 0.3 mg/kg rimonabant and CP47,497 at 0.3 and 1.0 mg/kg rimonabant. Because THC (at doses up to 900 mg/kg) in the presence of 0.3 mg/kg rimonabant elicited a maximum of 46.8 ± 8.4% MPE for antinociception, we extrapolated its ED50 value to calculate its dose ratio and pKB value. For hypothermia, outlier pKB values were observed for WIN55,212-2 at 1.0 mg/kg rimonabant and THC at 0.3 mg/kg rimonabant. Figure 8 graphically illustrates pKB values compared with pA2 values for A-834,735D and/or CP55,940. In general, the variance in pKB values was reduced for antinociception compared with catalepsy or hypothermia.
Fig. 8.
Apparent pA2 values of rimonabant for A-834,735D and CP55,940 along with average pKB (with 95% confidence limits) for all doses of rimonabant tested against each agonist are depicted for catalepsy (A), antinociception (B), and hypothermia (C). Overlapping confidence limits of pA2 and pKB values were considered not to differ significantly. In cases in which only one re-determination of the dose-effect relationship in the presence of rimonabant was possible, if the single pKB value fell within the confidence limits of either pA2 or other pKB they were considered to not differ significantly. For each triad endpoint, the light gray background indicates the span of the 95% confidence limits of the pA2 for A-834,735D and CP55,940, while the dark gray indicates the overlap between the two. For antinociception, the dotted lines indicate the 95% confidence limits for the recalculation of the results from Reche et al., 1996, in which the pA2 of rimonabant versus THC was determined utilizing the radiant heat tail flick assay with intravenous drug administration.
Because both male and female mice were used in all experiments, two-way analysis of variance followed by Holm-Sidak post hoc analyses were performed for each agonist alone and in the presence of all doses of rimonabant tested for each agonist. Although sporadic significant sex differences were detected, none of the dependent measures varied in a systematic manner (see Supplemental Table 1).
Discussion
The primary contribution of the present study was the use of pA2/pKB analyses to determine CB1R heterogeneity of THC, the well characterized SCs CP55,940 and WIN55,212-2, the well characterized abused SCs JWH-073 and CP47,497, and the emerging novel abused SC A834,735D (Frost et al., 2008; Grim et al., 2016b; Marshell et al., 2014; Pertwee, 2006) in producing common in vivo cannabimimetic pharmacological effects (i.e., catalepsy, antinociception, and hypothermia) in male and female mice. These findings build on our recent study demonstrating that these drugs do not elicit relevant antinociceptive, cataleptic, and hypothermic effects in CB1(−/−) mice (Grim et al., 2016a). Moreover, the present study offers a quantitative approach to unmask potential subtle dissimilarities of emerging SCs across the endpoints.
The full dose-effect curves established for A-834,735D and CP55,940 in the presence of at least three doses of rimonabant permitted the generation of Schild plots and determination of pA2 values. The overall findings that Schild-plot slopes did not differ from the expected value of –1 and that the 95% confidence limits of the pA2 values overlapped for each endpoint suggest that a common receptor with a shared affinity for rimonabant mediated the effects of both drugs. An exception to this general finding was that the rimonabant pA2 value against A-834,735D-induced catalepsy was lower than those for A-834,735D-induced antinociception and CP55,940-induced antinociception and hypothermia. Although this exception raises the possibility that additional receptors with reduced rimonabant affinity may contribute to cataleptic effects of this largely uncharacterized SC, the fact that the pA2 confidence limits differed by only 0.1 log units provide, at best, weak support for a contribution of non-CB1Rs. The present finding are consistent with the resistance of CB1(−/−) mice to the cataleptic and hypothermic effects of both agonists as well as A-834,735D-induced antinociception (Grim et al., 2016). Although CP55,940 retained weak but significant antinociceptive effects in CB1(−/−) mice, suggesting the possibility of another target, the present results with rimonabant suggest CB1Rs were the sole target. Collectively, these studies suggest a major and nearly exclusive role for CB1Rs in mediating the cataleptic, antinociceptive, and hypothermic effects of A-834,735D and CP55,940 in mice.
Although constraints in THC; WIN55,212-2; JWH-073; and CP47,497 solubility precluded pA2 analysis, their dose-response curves were amenable to pKB analysis. A limitation of pKB analysis is the assumption of a Schild-plot slope of –1, rather than generating an empirically determined slope. However, this approach may be applied to studies with single antagonist doses, which allows antagonist affinity estimates that can be compared with more rigorously determined pA2 values. From this perspective, pKB values for rimonabant antagonism of WIN55,212-2; JWH-073; and CP47,497 were generally consistent with pA2 values for rimonabant antagonism of A-834,735D and CP55,940, suggesting that their effects were mediated by a common population of CB1Rs. This conclusion is again consistent with the previous finding that pharmacological effects of WIN55,212-2; JWH-073; and CP47,497 were absent in CB(−/−) mice. An exception to this general conclusion is that the pKB values for rimonabant antagonism of CP47,497-induced catalepsy fell below the A-834,735D and CP55,940 pA2 confidence limits, suggesting a population of receptors with relatively low affinity for rimonabant may have contributed to the cataleptic effects of CP47,497. As with A-834,735D-induced catalepsy, this difference was small and CB1(−/−) mice did not display CP47,497-induced antinociception (Grim et al., 2016). Thus, the overall results are consistent with a major role of CB1Rs, and a minimal role, if any, for other targets in mediating effects of these SCs.
As solubility constraints precluded pA2 value determination for rimonabant antagonism of THC, the interpretation of results again relied on less definitive pKB analysis. In contrast to results with the SCs, these pKB values for THC were higher across all three endpoints than the upper pA2 confidence limits for rimonabant antagonism of the pharmacological effects of A-834,735D and CP55,940. A conventional interpretation of these results would suggest that the pharmacological effects of THC are mediated by a distinct population of receptors with higher affinity for rimonabant than those mediating the effects of the actions of SCs. This conclusion is also consistent with the previous finding that THC retained weak but significant antinociceptive and hypothermic effects in CB1(−/−) mice, and suggest that these effects of THC might be mediated by non-CB1Rs with high affinity for rimonabant. However, this conclusion should be considered with caution, especially because a non-CB1R with exceptionally high affinity for rimonabant has not been elucidated. Moreover, CB1R deletion eliminated THC-induced catalepsy, but similar pKB values for rimonabant antagonism of THC-induced catalepsy, antinociception, and hypothermia suggest that a factor other than CB1R mediation influenced rimonabant pKB values. A plausible contributing factor could be the poor solubility of THC at the very high concentrations needed to probe right-shifted dose-effect curves in this study, and the resulting potential for incomplete or altered dose delivery and absorption. Specifically, evaluation of right-shifted THC dose-effect curves in this study required delivery of cumulative THC doses up to 900 mg/kg in double-the-normal injection volumes of THC suspended in a high concentration of 30 mg/ml. It is possible that the apparently large rightward shifts in THC dose-effect curves after rimonabant pretreatment resulted at least in part from incomplete delivery/absorption of these large doses rather than from rimonabant antagonism. Finally, Reche et al. (1996) conducted a radiant heat tail-flick mouse study yielding a pA2 value 6.7 (6.2–7.2) for rimonabant antagonism of the antinociceptive effects of intravenously administered THC in mice (recalculated pA2 estimate in Supplemental Fig. 1). Notably, THC was more than 20-fold more potent by intravenous route of administration (Reche et al., 1996) than by the intraperitoneal route used here. This increased potency of THC following intravenous injection permitted determination of right-shifted dose-effect curves in the presence of multiple rimonabant doses. The reduced drug concentrations via the intravenous route of administration may have improved reliability of dose delivery and absorption and more accurate quantification of rimonabant antagonism.
In the present study, rimonabant pA2/pKB values were most consistent across agonists in the warm water tail-withdrawal assay and least consistent in the bar test (i.e., catalepsy), suggesting contribution of non-CB1Rs to the latter measure. Indeed, many other drugs acting at non-CB1R targets elicit catalepsy, including GABAergic allosteric modulators (Mierzejewski et al., 2013), dopaminergic antagonists (Langlois et al., 2012), and antipsychotic drugs (Grim et al., 2016; Wiley and Martin, 2003). Interestingly, a discrepancy in potency between rimonabant and another structurally similar CB1R antagonist, AM251, to antagonize cannabinoid-induced catalepsy, but not cannabinoid-induced hypothermia, may reflect a difference in the underlying mechanisms (McMahon and Koek, 2007).
In conclusion, pA2/pKB analyses with competitive reversible CB1R antagonists provides a valuable complement to the use of CB1R mutant mice to examine suspected non-CB1R effects of novel SCs. CB1(+/−) mice and CB1(−/−) may be used, respectively, to examine drug efficacy at the target receptor and overall reliance of drug effects on the target receptor (Grim et al., 2016). However, compensatory mechanisms and epistatic effects associated with constitutive receptor mutations may confound interpretation (Lariviere et al., 2001). Thus, pharmacological approaches of receptor antagonism avoid these confounds, and studies with competitive, reversible antagonists, such as rimonabant, are not influenced by agonist efficacy (i.e., all other factors being equal, a given dose of a competitive antagonist will produce similar antagonism of high- and low-efficacy agonists). With the advent of CB1R-selective irreversible antagonists (Hua et al., 2016), quantitative determination of in vivo efficacy may now be possible and offer an opportunity to expand our previous and present findings. These approaches quantitatively establish the relative efficacy and receptor mediation of cannabinoids, and by extension the degree to which activation of CB1R differs among novel SCs, as well THC and other naturally occurring cannabinoids.
Abbreviations
- A-834,735 degradant
3,3,4-trimethyl-1-(1-((tetrahydro-2H-pyran-4-yl)methyl)-1H-indol-3-yl)pent-4-en-1-one
- CB1R
cannabinoid receptor 1
- CP47,497
rel-5-(1,1-dimethylheptyl)-2-[(1R,3S)-3-hydroxycyclohexyl]-phenol
- CP55,9440
5-(1,1-dimethylheptyl)- 2-[(1R,2R,5R)-5-hydroxy-2-(3-hydroxypropyl)cyclohexyl]-phenol
- JWH-018
1-pentyl-3-(1-naphthoyl)-indole
- JWH-073
(1-butyl-1H-indol-3-yl)-1-naphthalenyl-methanone or naphthalen-1-yl(1-pentyl-1H-indol-3-yl)methanone
- SC
synthetic cannabinoids
- THC
Δ9-tetrahydrocannabinol
- WIN55,212-2
(R)-(1)-[2,3- dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-napthalenylmethanone
Authorship Contributions
Participated in research design: Grim, Morales, Lichtman, Negus, Thomas, Wiley.
Conducted experiments: Grim, Morales.
Contributed new reagents or analytic tools: Endres, Thomas, Wiley.
Performed data analysis: Grim, Lichtman, Negus.
Wrote or contributed to the writing of the manuscript: Grim, Morales, Lichtman, Negus, Thomas, Wiley, Endres.
Footnotes
This work was funded by the National Institutes of Health (Grants R01DA030404, R01DA03672, R01DA040460, R01DA039942, P30DA033934, T32DA007027; and by Virginia Commonwealth University, School of Pharmacy start-up funds.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Byrska B, Stanaszek R, Zuba D. (2016) Alpha-PVP as an active component of herbal highs in Poland between 2013 and 2015. Drug Test Anal doi:10.1002/dta.2151. [DOI] [PubMed] [Google Scholar]
- Compton DR, Johnson MR, Melvin LS, Martin BR. (1992) Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J Pharmacol Exp Ther 260:201–209. [PubMed] [Google Scholar]
- Compton R, Aceto MD, Lowe J, and Martin BR (1996). In vivo characterization of a specific cannabinoid receptor antagonist (SR141716A): inhibition of delta 9-tetrahydrocannabinol-induced responses and apparent agonist activity. J Pharmacol Exp Ther 277: 586–594. [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. (1988) Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34:605–613. [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ. (2010) FAAH-/- mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology 35:1775–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (2008) Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity. J Med Chem 51:1904–12. [DOI] [PubMed] [Google Scholar]
- Gerostamoulos D, Drummer OH, Woodford NW. (2015) Deaths linked to synthetic cannabinoids. Forensic Sci Med Pathol 11:478. [DOI] [PubMed] [Google Scholar]
- Ginsburg BC, Schulze DR, Hruba L, McMahon LR. (2012) JWH-018 and JWH-073: Δ9-tetrahydrocannabinol-like discriminative stimulus effects in monkeys. J Pharmacol Exp Ther 340:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Gonek MM, Wiley JL, Thomas BF, Endres GW, et al. (2016a) Stratification of cannabinoid 1 receptor (CB1R) agonist efficacy: Manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J Pharmacol Exp Ther doi:10.1124/jpet.116.233163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Samano KL, Ignatowska-Jankowska B, Tao Q, Sim-Selly LJ, Selley DE, et al. (2016b) Pharmacological characterization of repeated administration of the first generation abused synthetic cannabinoid CP47,497. J Basic Clin Physiol Pharmacol 27:217–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grim TW, Morales AJ, Gonek MM, Wiley JL, Thomas BF, Endres GW, Sim-Selley LJ, Selley DE, Negus SS, Lichtman AH. (2016) Stratification of cannabinoid 1 receptor (CB1R) agonist efficacy: Manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J Pharmacol Exp Ther 359:329–339 DOI: 10.1124/jpet.116.233163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua T, Vemuri K, Pu M, Qu L, Han GW, Wu Y, Zhao S, Shui W, Li S, Korde A, et al. (2016) Crystal structure of the human cannabinoid receptor CB1. Cell 167:750–762.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, Frank RA. (2001) Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry 58:322–328. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, Deng H, Vadivel SK, Makriyannis A. (2011) Cannabinergic aminoalkylindoles, including AM678=JWH018 found in ‘Spice’, examined using drug (Δ(9)-tetrahydrocannabinol) discrimination for rats. Behav Pharmacol 22:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järbe TU, Lamb RJ, Lin S, Makriyannis A. (2001) (R)-methanandamide and Delta 9-THC as discriminative stimuli in rats: tests with the cannabinoid antagonist SR-141716 and the endogenous ligand anandamide. Psychopharmacology (Berl) 156:369–380. [DOI] [PubMed] [Google Scholar]
- Järbe TUC, LeMay BJ, Halikhedkar A, Wood J, Vadivel SK, Zvonok A, Makriyannis A. (2014) Differentiation between low- and high-efficacy CB1 receptor agonists using a drug discrimination protocol for rats. Psychopharmacology (Berl) 231:489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois X, Megens A, Lavreysen H, Atack J, Cik M, te Riele P, Peeters L, Wouters R, Vermeire J, Hendrickx H, et al. (2012) Pharmacology of JNJ-37822681, a specific and fast-dissociating D2 antagonist for the treatment of schizophrenia. J Pharmacol Exp Ther 342:91–105. [DOI] [PubMed] [Google Scholar]
- Lariviere WR, Chesler EJ, Mogil JS. (2001) Transgenic studies of pain and analgesia: mutation or background genotype? J Pharmacol Exp Ther 297:467–473. [PubMed] [Google Scholar]
- Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Böhme GA, Imperato A, Pedrazzini T, Roques BP, et al. (1999) Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283:401–404. [DOI] [PubMed] [Google Scholar]
- Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, et al. (2014) In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: inhalation versus intraperitoneal injection. Pharmacol Biochem Behav 124:40–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon LR. (2006) Characterization of cannabinoid agonists and apparent pA2 analysis of cannabinoid antagonists in rhesus monkeys discriminating Delta9-tetrahydrocannabinol. J Pharmacol Exp Ther 319:1211–1218. [DOI] [PubMed] [Google Scholar]
- McMahon LR, Koek W. (2007) Differences in the relative potency of SR 141716A and AM 251 as antagonists of various in vivo effects of cannabinoid agonists in C57BL/6J mice. Eur J Pharmacol 569:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierzejewski P, Kolaczkowski M, Nowak N, Korkosz A, Scinska A, Sienkiewicz-Jarosz H, Samochowiec J, Kostowski W, Bienkowski P. (2013) Pharmacological characteristics of zolpidem-induced catalepsy in the rat. Neurosci Lett 556:99–103. [DOI] [PubMed] [Google Scholar]
- Negus SS, Burke TF, Medzihradsky F, Woods JH. (1993) Effects of opioid agonists selective for mu, kappa and delta opioid receptors on schedule-controlled responding in rhesus monkeys: antagonism by quadazocine. J Pharmacol Exp Ther 267:896–903. [PubMed] [Google Scholar]
- Pertwee RG. (2006) The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes (Lond) 30 Suppl 1:S13–8. [DOI] [PubMed] [Google Scholar]
- Presley BC, Jansen-Varnum SA, Logan BK. (2013) Analysis of synthetic cannabinoids in botanical material: a review of analytical methods and findings. Forensic Sci Rev 25:27–46. [PubMed] [Google Scholar]
- Reche I, Fuentes JA, Ruiz-Gayo M. (1996) A role for central cannabinoid and opioid systems in peripheral delta 9-tetrahydrocannabinol-induced analgesia in mice. Eur J Pharmacol 301:75–81. [DOI] [PubMed] [Google Scholar]
- Rodriguez JS, McMahon LR. (2014) JWH-018 in rhesus monkeys: differential antagonism of discriminative stimulus, rate-decreasing, and hypothermic effects. Eur J Pharmacol 740:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ, Cowan A, Adler MW. (1979) pA2 and receptor differentiation: a statistical analysis of competitive antagonism. Life Sci 25:637–654. [DOI] [PubMed] [Google Scholar]
- Thornton SL, Wood C, Friesen MW, Gerona RR. (2013) Synthetic cannabinoid use associated with acute kidney injury. Clin Toxicol (Phila) 51:189–190. [DOI] [PubMed] [Google Scholar]
- Trecki J, Gerona RR, Schwartz MD. (2015) Synthetic cannabinoid-related illnesses and deaths. N Engl J Med 373:103–107. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR. (2003) Cannabinoid pharmacological properties common to other centrally acting drugs. Eur J Pharmacol 471:185–193. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. (1999) Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA 96:5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]