Abstract
Apoptotic stimuli augment intracellular calcium concentration through inositol 1,4,5-trisphosphate receptors (IP3R) on endoplasmic reticulum calcium stores. We previously discovered an apoptotic cascade wherein cytochrome c binds to IP3R early in apoptosis, resulting in dysregulated calcium release. Here we show that cytochrome c binding to IP3R depends on a cluster of glutamic acid residues within the C terminus of the channel. A cell permeant peptide derived from this sequence displaces cytochrome c from IP3R and abrogates cell death induced by staurosporine treatment of HeLa cells and Fas ligand stimulation of Jurkat cells. Small-molecule inhibitors of cytochrome c/IP3R interactions may prove useful in treating disorders associated with inappropriate intrinsic and extrinsic apoptotic signaling.
Keywords: apoptosis, calcium, Fas, caspase, T lymphoma
Calcium signaling plays a major role in cell death pathways, because many apoptotic stimuli augment cytosolic and mitochondrial calcium (1, 2). Calcium overload in mitochondria leads to opening of the permeability transition pore, releasing cytochrome c, which binds to apoptotic protease-activating factor 1, recruiting and activating caspase-9 to form the apoptosome (3). Caspase-9 then activates executioner caspases such as caspase-3, which, in turn, cleave many intracellular targets (4). The release of calcium from endoplasmic reticulum stores by inositol 1,4,5-trisphosphate receptors (IP3R) has been implicated in multiple models of apoptosis as being directly responsible for mitochondrial calcium overload (5-9), due in part to the privileged communication of the IP3R with closely adjacent mitochondria (10-12). Indeed, it is becoming increasingly appreciated that endoplasmic reticulum-mitochondrial calcium signaling is crucial in several models of apoptosis (2, 13-16). The requirement of IP3R for calcium-dependent cell death is exemplified by the resistance to apoptosis of cells with antisense knockdown or genetic deletion of IP3R gene (17-19).
Recently we demonstrated a cell death signaling cascade involving IP3R and cytochrome c (20, 21). During apoptosis, cytochrome c exits mitochondria and binds directly and selectively to IP3R in the closely adjacent endoplasmic reticulum, restricting most of the cytosolic access of cytochrome c early in apoptosis. Cytochrome c binding to IP3R abolishes the calcium-mediated inhibition of IP3-associated calcium release (20). By blocking the feedback regulation of IP3R, cytochrome c greatly augments the release of calcium, which then enters mitochondria to provoke further cytochrome c release. This feed-forward amplification is manifested by sustained increases in cytosolic calcium in early apoptosis, which elicit massive release of cytochrome c from all mitochondria (20, 22).
In our initial study, we focused on a very restricted set of stimuli that activate the intrinsic apoptotic pathway (20). The intrinsic pathway is characterized by mitochondrial membrane permeabilization, cytochrome c release, and apoptosome formation in response to cytotoxic stimuli (3). In contrast, extrinsic apoptotic pathways are activated in response to ligand binding to death receptors of the TNF-α superfamily (23). Receptor activation can result in cytochrome c release through caspase-dependent cleavage and translocation of the proapoptotic protein Bid to mitochondria (24-26). Antisense knockout of the type I IP3R suppresses cell death in response to extrinsic apoptotic stimuli, suggesting that intracellular calcium release is a critical mediator of this cell death pathway (19). The mechanistic basis linking IP3R to the extrinsic pathway has not been determined.
We established functional relevance for cytochrome c/IP3R binding by showing that a transfected 20-kDa fragment of IP3R encoding the cytochrome c binding domain suppresses the increase in cytosolic calcium in response to apoptotic stimuli, presumably by competing for cytochrome c binding to IP3R (20). However, we did not directly establish an influence on subsequent cell death. In the present study we map the binding sequence in IP3R to a 16-aa sequence (amino acids 2621-2636) in the rat type I IP3R. A peptide derived from this sequence potently blocks IP3R/cytochrome c interactions. We describe a cell permeant derivative of this peptide that attenuates apoptotic cell death via both intrinsic and extrinsic pathways and which may help elucidate calcium-dependent apoptotic mechanisms.
Materials and Methods
Materials. Staurosporine (STS) was purchased from Biomol (Plymouth Meeting, PA). Membrane-bound Fas ligand (FasL) was from Upstate USA (Charlottesville, VA). Complete protease inhibitor mixture without EDTA was from Roche Applied Science (Indianapolis). Cytochrome c-agarose, protein A-Sepharose, and all other chemicals were from Sigma-Aldrich. Peptide synthesis and purification were performed by the Synthesis and Sequencing Facility of The Johns Hopkins University. Coupling of the peptide to BODIPY 577/618 maleimide was performed as suggested by the manufacturer (Molecular Probes).
Antibodies. Production of rabbit polyclonal antiserum specific to the type I IP3R has been reported previously (20). Rabbit anti-heme oxygenase-2 antisera has been described previously (27). Mouse anti-cytochrome c was purchased from Zymed. Mouse anti-cytochrome c oxidase subunit IV was from Molecular Probes. Goat anti-lactate dehydrogenase was purchased from Chemicon. Anti-hemagglutinin (HA) conjugated to horseradish peroxidase was from Roche Applied Science.
Expression Constructs. A GST fusion protein encoding amino acids 2589-2749 of the rat type I IP3R (GST-FL) was cloned by PCR into the EcoR1/NotI sites of pGEX-4T-1 (Amersham Pharmacia Biosciences, Piscataway, NJ). C-terminal IP3R mammalian expression constructs encompassing amino acids 2589-2749 (FL), 2589-2669 (CT1), 2669-2749 (CT2), 2629-2709 (CT3), 2605-2749 (2605), 2621-2749 (2621), 2637-2749 (2637), and 2653-2749 (2653) of the rat type I IP3R were cloned by PCR into the EcoR1/NotI sites of pCMV-HA (Clontech). Mutations E2628/2629Q and E2633/2634Q were introduced by the QuikChange site-directed mutagenesis kit (Stratagene) into the pCMV-HA-FL construct according to the manufacturer's instructions. The sequences of the PCR primers are available upon request.
Cytochrome c Mapping. C-terminal constructs of the IP3R in pCMV-HA were transfected and expressed in HEK-293 cells for 48 h. Lysates were prepared in buffer A (150 mM NaCl/50 mM Tris, pH 7.8/1% Triton X-100/1 mM EDTA), and expression levels were normalized by running 50 μg of lysate on an SDS/PAGE gel and blotting with anti-HA. Expression corrected lysates (300 μg) were incubated with 20 μl of cytochrome c agarose (5 mg/ml) in 500 μl of buffer A overnight with rotation at 4°C protected from light. The agarose beads were washed vigorously four times with 0.5 ml of buffer A, separated on SDS/PAGE, and blotted with anti-HA. In some experiments, BSA-agarose was used as a negative control (data not shown).
GST-FL Expression and Purification. GST-FL in BL21 bacteria were grown to an OD600 of ≈0.7. Cultures were induced for 18 h at room temperature with 0.1 mM isopropyl-β-d-thiogalactopyranoside. Cells were pelleted and disrupted by sonication in PBS containing 100 mM EDTA, 1 mM PMSF, 5 mM DTT, and Complete protease inhibitor mixture. The lysate was cleared by centrifugation, and the supernatant was incubated with GST-Sepharose (Amersham Pharmacia Biosciences, Piscataway, NJ) for 30 min at 4°C with rotation. The Sepharose beads were transferred to a minicolumn and washed five times with ice-cold PBS, then eluted with 50 mM Tris, pH 8.0/10 mM reduced glutathione/0.1% Triton X-100/0.5 mM DTT at room temperature. Excess glutathione was removed by extensive dialysis against PBS.
In Vitro GST Binding Assays. GST-FL (100 nM) was incubated with 80 nM cytochrome c in 500 μl of buffer A for 18 h with rotation at 4°C protected from light. Where indicated, IP3R inhibitory peptide (which we refer to as IP3RCYT) was added at various concentrations. Fifty microliters of 50% GST-Sepharose was added, and the incubation was continued for 30 min at room temperature. The beads were washed four times with 1.0 ml of binding buffer and electrophoresed on an SDS/PAGE gel. After transfer to nitrocellulose, the blot was Ponceau-stained to confirm equal loading of GST-FL and blotted for cytochrome c.
Cell Viability. Cell viability was assayed by trypan blue exclusion as described elsewhere (28). Caspase-3 like activity was measured fluorometrically as described previously (20) by using Z-DEVD-AMC as a substrate.
Calcium Imaging. Calcium measurements were as previously described (20, 29) using the Intracellular Imaging Calcium Imaging System and incyt2 imaging software (Intracellular Imaging, Cincinnati). Fura 2-acetoxymethyl ester loading (Molecular Probes) was conducted for 25 min at room temperature. All measurements shown are an average of 50 cells unless otherwise stated and are representative of a minimum of three independent experiments.
Results
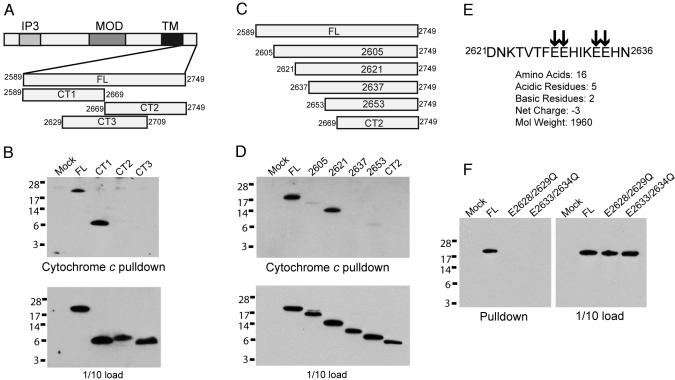
Mapping the Region in IP3R That Mediates Cytochrome c Binding. The rat type I IP3R is a 2,749-aa protein. In our earlier study, we mapped the region responsible for binding cytochrome c to the C-terminal 160 aa that follow the last transmembrane domain of the channel pore. We now provide more detailed mapping (Fig. 1). Three overlapping 80-aa fragments of the C-terminal portion of IP3R localize the binding site to amino acids 2589-2669 (Fig. 1B). Successive deletions of ≈20 aa within this sequence localize the critical binding domain to a 16-aa fragment encompassing amino acids 2621-2636, an acidic sequence containing two pairs of glutamic acid residues (Fig. 1 D and E). Substitution of either of these pairs of glutamic acid residues with glutamine abolishes binding (Fig. 1F). Because cytochrome c is highly basic and binds to other proteins such as apoptotic protease-activating factor 1 through electrostatic interactions (30, 31), we presume that a similar mechanism underlies IP3R/cytochrome c interactions. Interestingly, the cytochrome c binding region overlaps a small, highly conserved region of the protein that participates in channel assembly and oligomerization (32).
Fig. 1.
Cytochrome c binding determinants of IP3R. (A) The IP3R domain structure includes an N-terminal IP3 binding domain (IP3), the modulatory domain (MOD), and the C-terminal transmembrane domain (TM). Previously we demonstrated that cytochrome c binds IP3R within amino acids 2589-2749 encompassing the full-length C terminus (FL) (20). Three 80-aa fragments of FL (CT1, CT2, and CT3) were generated to facilitate cytochrome c mapping. Rat type I IP3R amino acid boundaries are indicated. (B) HEK 293 lysates expressing FL, CT1, CT2, and CT3 were subjected to in vitro pull-down with cytochrome c agarose. Only CT1 (amino acids 2589-2669) bound cytochrome c agarose. Blot is anti-HA. (C) Schematic diagram of ≈20-aa deletion constructs of FL within amino acids 2589-2669. (D) Binding of deletion constructs to cytochrome c agarose. IP3R fragment 2621 binds, whereas 2637, 2653, and CT2 do not. Construct 2605 had minimal binding but expressed at very low levels and was susceptible to degradation (data not shown), suggesting improper folding. Experiments were performed exactly as in B. (E) Sequence and physicochemical properties of amino acids 2621-2636. Arrows indicate a cluster of glutamic acid residues. (F) Effect on cytochrome c binding of mutations replacing glutamic acids residues 2628 and 2629 to glutamine (E2628/2629Q) or glutamic acid residues 2633 and 2634 to glutamine (E2633/2634Q). Both mutations eliminate binding to cytochrome c agarose.
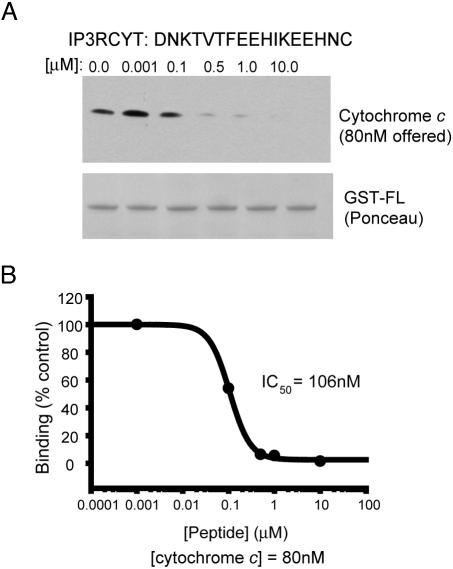
We synthesized a 17-aa peptide encompassing the cytochrome c binding domain with the addition of a C-terminal cysteine (referred to as IP3RCYT) and examined its influence on the binding of the C terminus of IP3R to cytochrome c (Fig. 2). The peptide inhibits binding with an IC50 of ≈100 nM. Because cytochrome c is used in these experiments at an 80 nM concentration, IP3RCYT is presumably stoichiometrically sequestering cytochrome c under these experimental conditions, suggesting that the peptide's binding constant is substantially lower than 100 nM. Because of technical limitations, we have not been able to ascertain IC50 values at lower concentrations of cytochrome c to estimate a Kd value.
Fig. 2.
A 17-aa IP3R peptide displaces cytochrome c from IP3R. (A) A peptide encompassing the minimal cytochrome c binding domain (amino acids 2621-2636) with a C-terminal cysteine (IP3RCYT) was tested for the ability to disrupt cytochrome c/IP3R binding. Cytochrome c (80 nM) was incubated with 100 nM GST-FL and varying concentrations of IP3RCYT. Complexes were immobilized on GST-Sepharose and analyzed for cytochrome c binding by Western blotting. A Ponceau stain of the nitrocellulose blot is shown to illustrate equal loading of GST-FL. (B) Densitometric analysis of cytochrome c pull-down and subsequent graphing of percent binding (vs. no peptide) demonstrates an IC50 value of 106 nM for IP3RCYT. Data were fit to the monophasic Hill equation y = min + (max-min)/1 + (x/IC50)Hillslope. The experiment was repeated three times with essentially identical results.
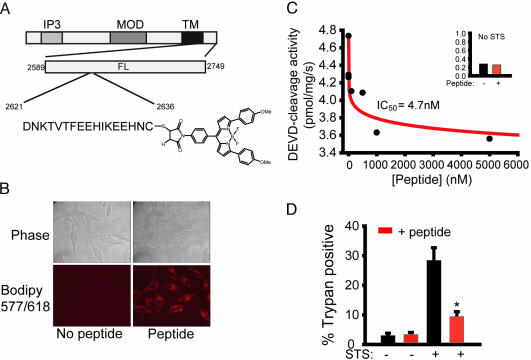
A Cell-Permeant IP3R Peptide Potently Blocks STS-Induced Cell Death. To ascertain influences of the IP3RCYT on apoptosis of intact cells, we coupled the C-terminal cysteine to the hydrophobic fluorescent molecule BODIPY 577/618 (Fig. 3). This modification rendered the peptide cell permeant, as confocal microscopy demonstrates that BODIPY-IP3RCYT accumulates in the cytosol of living HeLa cells (Fig. 3B).
Fig. 3.
A cell permeant fluorescent derivative of IP3RCYT blocks caspase activation and cell death in HeLa cells. (A) Schematic diagram of IP3RCYT peptide conjugated to BODIPY 577/618 via maleimide linkage to the C-terminal cysteine. (B) Phase and confocal fluorescent images of HeLa cells incubated with or without 400 nM BODIPY-IP3RCYT. Image capture times were equal. Note the diffuse cytoplasmic localization. (C) HeLa cells pretreated for 30 min with varying concentrations of BODIPY-IP3RCYT were stimulated with 1 μM STS for 6 h. Caspase activity was measured fluorometrically by using Z-DEVD-AMC as a substrate. Data were fit by using the Hill equation. Maximal inhibition was ≈30% of basal caspase activity (Inset). (D) HeLa cells pretreated with 400 nM BODIPY-IP3RCYT were stimulated with 1 μM STS for 6 h. Viability was assessed by trypan blue staining of nuclei. Cells were counted blind to the treatment, and at least 5,000 cells were scored over three separate experiments. *, P < 0.01.
We evaluated the influence of BODIPY-IP3RCYT on STS-induced apoptosis in HeLa cells by monitoring both caspase-3-like activity and trypan blue staining (Fig. 3 C and D). Preincubation with increasing concentrations of peptide causes a potent (IC50 = 5 nM) decrease in caspase activity when measured 6 h after STS treatment (Fig. 3C). Maximal inhibition is ≈30% compared to basal caspase activity (Fig. 3C Inset). When cell survival is examined by trypan blue exclusion 6 h after STS treatment, preincubation with 400 nM peptide results in a 65% reduction in cell death (28.3 ± 4.3 vs. 9.4 ± 1.6 trypan positive; Fig. 3D). The inhibition of STS-induced cell death in HeLa cells by 400 nM BODIPY-IP3RCYT is comparable to the antiapoptotic effects of preincubation with 200 μM Bax inhibitory peptide or caspase inhibitor z-VAD-fmk (33).
The Extrinsic Pathway of Apoptosis Involves Cytochrome c/IP3R Interactions and Mobilization of Internal Calcium Stores. Apoptosis can proceed via two distinct pathways. In the intrinsic pathway, noxious stimuli elicit cytochrome c release from mitochondria, which triggers a cascade of caspase activation. By contrast, the extrinsic pathway mediates physiologic cell death during embryogenesis and tissue homeostasis, such as developmental loss neurons in the brain or of lymphocytes during immune tolerance (4). The extrinsic pathway is mediated by members of the TNF-α superfamily of cell-surface receptors. Binding of ligand to these receptors causes multimerization, resulting in recruitment and activation of caspase-8 via adapter proteins to form the death-inducing signaling complex (23). The extrinsic and intrinsic pathways can converge, as in some instances the extrinsic pathway elicits mitochondrial permeabilization and cytochrome c release [the so-called type II pathway (34, 35)].
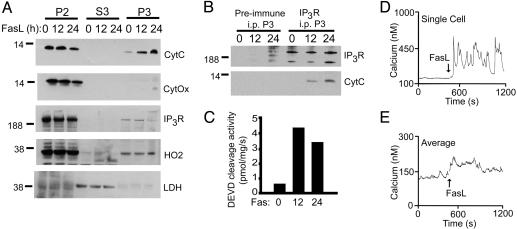
Because our previous study examined only the intrinsic pathway (20), we investigated the potential role of cytochrome c/IP3R interactions during FasL-mediated cell death of Jurkat T lymphoma cells (Fig. 4). We chose this model system because (i) it is a prototypical type II extrinsic cell death pathway (34) and (ii) antisense knockout of the type I IP3R blocks cell death in this experimental paradigm (19). After treatment with FasL, we observe a translocation of cytochrome c from the mitochondrial-enriched 10,000 × g pellet (P2) into the 100,000 × g light membrane pellet (P3; Fig. 4A). Cytochrome c oxidase distribution was used to control for the presence of mitochondrial contaminants in the P3 fraction. Heme oxygenase-2 was used as a fractionation control for microsomal constituents (36), and lactate dehydrogenase was used as a fractionation control for cytoplasm. Detergent solubilization and immunoprecipitation of IP3R from the P3 fraction demonstrate a FasL-dependent coimmunoprecipitation of cytochrome c and IP3R (Fig. 4B). At both 12 and 24 h we observe a marked increase in caspase-3-like activity, ensuring that apoptosis takes place under our treatment protocol.
Fig. 4.
Extrinsic apoptotic signaling via FasL is associated with cytochrome c binding to IP3R and calcium mobilization. (A) Jurkat cells were treated with 1 ng/ml FasL vesicles for 0, 12, or 24 h, and the subcellular distribution of cytochrome c (CytC), cytochrome c oxidase (CytOx), IP3R, heme oxygenase-2 (HO2), and lactate dehydrogenase (LDH) was monitored in the mitochondrial-enriched 10,000 × g pellet (P2), the 100,000 × g cytosol (S3), or the light membrane-enriched 100,000 × g pellet (P3). (B) Immunoprecipitation of IP3R from P3 fraction solubilized in buffer A with anti-IP3R or preimmune serum. Samples were immunoblotted for IP3R and cytochrome c. (C) DEVD cleavage activity of the S3 fraction. (D and E) Calcium mobilization in response to FasL examined in individual fura 2-acetoxymethyl ester-loaded Jurkat cells. A representative single cell (D) and 50-cell average (E) from one experiment are shown. Approximately 30% of cells responded with an immediate and oscillatory rise in cytoplasmic calcium in response to FasL. All data are from the same experiment and are representative of at least three separate determinations.
In our previous study, we demonstrated that STS treatment resulted in rapid, oscillatory increases in cytoplasmic calcium that were due at least in part to cytochrome c binding to IP3R (20). Other groups have shown that a crosslinking antibody that stimulates Fas does not mobilize internal calcium and, in fact, may decrease cytosolic calcium (37-39). This finding is in contrast to other reports showing a crucial role for the IP3R in FasL-mediated cell death (19). We measured cytosolic calcium in fura 2-acetoxymethyl ester-loaded Jurkat cells in response to FasL vesicles (Fig. 4 D and E). In ≈30% of cells, we observed an immediate oscillatory increase in cytoplasmic calcium in response to FasL, consistent with the hypothesis that calcium mobilization is linked directly to Fas signaling.
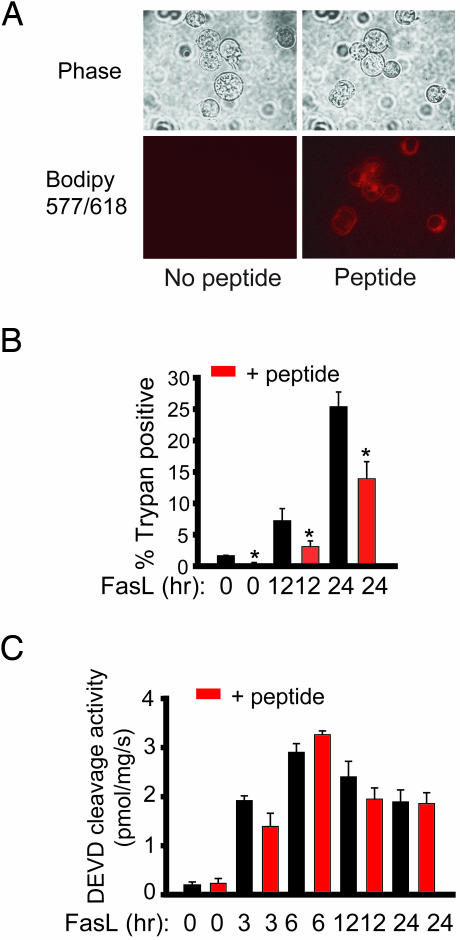
FasL-Induced Death Is Attenuated by BODIPY-IP3RCYT. We evaluated the effect of the BODIPY-IP3RCYT peptide on Fas-mediated cell death in Jurkat cells (Fig. 5). As in HeLa cells, the peptide readily penetrates into Jurkat cells as determined by confocal microscopy (Fig. 5A). At 12 and 24 h after FasL treatment, the peptide significantly reduces cell death monitored by trypan blue exclusion (Fig. 5B). Cell death is reduced less for the Fas extrinsic pathway than for the STS-elicited intrinsic pathway, which fits with the known mechanisms of the two pathways. Thus, the intrinsic pathway depends almost exclusively on cytochrome c release, whose interaction with IP3R is the target of BODIPY-IP3RCYT. By contrast, the extrinsic pathway can activate multiple caspases independent of mitochondrial permeabilization and cytochrome c release. Consistent with this notion, the BODIPY-IP3RCYT does not influence overall caspase activity of Jurkat cells monitored by DEVD cleavage (Fig. 5C).
Fig. 5.
Fas-mediated cell death is attenuated by BODIPY-IP3RCYT. (A) Phase and confocal fluorescent images of Jurkat cells incubated with or without 400 nM BODIPY-IP3RCYT. Image capture times were equal. (B and C) Jurkat cells pretreated with 400 nM BODIPY-IP3RCYT or vehicle for 30 min and 1 ng/ml FasL vesicles were added for the indicated amount of time. Trypan blue staining (B) and DEVD-cleavage activity (C) were assayed as in Fig. 3. *, P < 0.05.
Discussion
In the present study we have mapped the region of IP3R that binds cytochrome c and is responsible for an apoptotic signaling cascade. Despite the large size of IP3R, we identified a 16-aa sequence that is responsible for cytochrome c binding and found two pairs of glutamate residues whose substitution to glutamine abolishes binding, implicating an electrostatic interaction between this acidic region and the positively charged cytochrome c. These observations may facilitate detailed elucidation of IP3R/cytochrome c interactions at the structural level.
We have established that the extrinsic apoptotic pathway also involves IP3R/cytochrome c interactions. To our knowledge, this is the first demonstration of an endogenous physiologic apoptotic stimulus resulting in cytochrome c translocation to the endoplasmic reticulum and provides a possible mechanism for the involvement of the IP3R in death receptor-mediated apoptosis. Furthermore, we have shown that treatment with FasL vesicles results in rapid calcium mobilization in Jurkat cells. This finding is in contrast with previous observations suggesting no role of calcium in Fas-mediated signaling (37-39). This discrepancy might simply be explained by the different stimuli used, because previous studies used a crosslinking antibody to stimulate Fas instead of FasL vesicles. Interestingly, Scoltock et al. (40) showed that ≈30% of Jurkat cells had elevated cytosolic calcium 1 h after treatment with anti-Fas antibody as determined by flow cytometry, consistent with our findings. Further experiments will be necessary to elucidate in more detail the role of calcium in Fas signaling. Our results clarify previous observations that lymphocyte apoptosis via the extrinsic pathway involves augmented numbers of type III IP3R (17) and that deletion of IP3R diminishes such apoptosis (17, 19).
Apoptotic stimuli are well known to augment intracellular calcium (2, 41). We previously discovered that cytochrome c binding to IP3R causes calcium oscillations leading to massive, cell-wide, coordinated cytochrome c release (20). Consistent with these observations, transfecting the 20-kDa C-terminal cytochrome c binding domain of the IP3R into HeLa cells prevents these oscillations. It would be expected that BODIPY-IP3RCYT would have a similar effect, resulting in the suppression of calcium oscillations. Paradoxically, we found that BODIPY-IP3RCYT had precisely the opposite effect, resulting in an enhancement of STS-induced calcium mobilization in HeLa cells and Fas-mediated signaling in Jurkat cells (data not shown). The reason for this discrepancy is unknown, but it may reflect a direct effect of the peptide on channel function because of the importance of this region in gating and assembly (32, 42). We have ruled out a nonspecific effect of the BODIPY moiety, because transduction of the peptide without the BODIPY molecule produced the same effect. So how does BODIPY-IP3RCYT attenuate cell death? It may function as a cytoplasmic sponge for released cytochrome c, thereby blocking apoptotic protease-activating factor 1 activation. Alternatively, it may affect cell death by altering IP3R function autonomously of the apoptotic stimulus, thereby disrupting the “normal” regulation of IP3R by cytochrome c.
A major finding of our study is that a cell permeant peptide fragment of the IP3R calcium channel can block cell death when administered at low nM concentrations. The peptide is antiapoptotic for both intrinsic and extrinsic pathways. Related agents that block IP3R/cytochrome c binding may offer therapeutic potential for the many disease states associated with apoptotic cell death, including inflammatory disturbances, neurodegenerative diseases, and disorders elicited by xenobiotics.
Acknowledgments
This work was supported by U.S. Public Health Service Grants MH-18501 and DA-000266 and Research Scientist Award DA-00074 (to S.H.S.) and National Research Service Awards NS-043850 (to D.B.) and NH65090 (to R.L.P.).
Abbreviations: STS, staurosporine; IP3R, inositol 1,4,5-trisphosphate receptor; FasL, Fas ligand; HA, hemagglutinin.
References
- 1.Berridge, M. J., Lipp, P. & Bootman, M. D. (2000) Nat. Rev. Mol. Cell Biol. 1, 11-21. [DOI] [PubMed] [Google Scholar]
- 2.Orrenius, S., Zhivotovsky, B. & Nicotera, P. (2003) Nat. Rev. Mol. Cell Biol. 4, 552-565. [DOI] [PubMed] [Google Scholar]
- 3.Jiang, X. & Wang, X. (2004) Annu. Rev. Biochem. 73, 87-106. [DOI] [PubMed] [Google Scholar]
- 4.Strasser, A., O'Connor, L. & Dixit, V. M. (2000) Annu. Rev. Biochem. 69, 217-245. [DOI] [PubMed] [Google Scholar]
- 5.Szalai, G., Krishnamurthy, R. & Hajnoczky, G. (1999) EMBO J. 18, 6349-6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajnoczky, G., Csordas, G., Madesh, M. & Pacher, P. (2000) Cell Calcium 28, 349-363. [DOI] [PubMed] [Google Scholar]
- 7.Hajnoczky, G., Csordas, G., Krishnamurthy, R. & Szalai, G. (2000) J. Bioenerg. Biomembr. 32, 15-25. [DOI] [PubMed] [Google Scholar]
- 8.Pinton, P., Ferrari, D., Rapizzi, E., Di Virgilio, F., Pozzan, T. & Rizzuto, R. (2001) EMBO J. 20, 2690-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Csordas, G., Madesh, M., Antonsson, B. & Hajnoczky, G. (2002) EMBO J. 21, 2198-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzuto, R., Pinton, P., Carrington, W., Fay, F. S., Fogarty, K. E., Lifshitz, L. M., Tuft, R. A. & Pozzan, T. (1998) Science 280, 1763-1766. [DOI] [PubMed] [Google Scholar]
- 11.Csordas, G., Thomas, A. P. & Hajnoczky, G. (1999) EMBO J. 18, 96-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajnoczky, G., Csordas, G., Madesh, M. & Pacher, P. (2000) J. Physiol. 529, 69-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rizzuto, R., Pinton, P., Ferrari, D., Chami, M., Szabadkai, G., Magalhaes, P. J., Virgilio, F. D. & Pozzan, T. (2003) Oncogene 22, 8619-8627. [DOI] [PubMed] [Google Scholar]
- 14.Scorrano, L., Oakes, S. A., Opferman, J. T., Cheng, E. H., Sorcinelli, M. D., Pozzan, T. & Korsmeyer, S. J. (2003) Science 300, 135-139. [DOI] [PubMed] [Google Scholar]
- 15.Demaurex, N. & Distelhorst, C. (2003) Science 300, 65-67. [DOI] [PubMed] [Google Scholar]
- 16.Wang, S. & El-Deiry, W. S. (2004) Cancer Biol. Ther. 3, 44-46. [DOI] [PubMed] [Google Scholar]
- 17.Khan, A. A., Soloski, M. J., Sharp, A. H., Schilling, G., Sabatini, D. M., Li, S. H., Ross, C. A. & Snyder, S. H. (1996) Science 273, 503-507. [DOI] [PubMed] [Google Scholar]
- 18.Sugawara, H., Kurosaki, M., Takata, M. & Kurosaki, T. (1997) EMBO J. 16, 3078-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayaraman, T. & Marks, A. R. (1997) Mol. Cell. Biol. 17, 3005-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boehning, D., Patterson, R. L., Sedaghat, L., Glebova, N. O., Kurosaki, T. & Snyder, S. H. (2003) Nat. Cell Biol. 5, 1051-1061. [DOI] [PubMed] [Google Scholar]
- 21.Mattson, M. P. & Chan, S. L. (2003) Nat. Cell Biol. 5, 1041-1043. [DOI] [PubMed] [Google Scholar]
- 22.Goldstein, J. C., Waterhouse, N. J., Juin, P., Evan, G. I. & Green, D. R. (2000) Nat. Cell Biol. 2, 156-162. [DOI] [PubMed] [Google Scholar]
- 23.Wallach, D., Varfolomeev, E. E., Malinin, N. L., Goltsev, Y. V., Kovalenko, A. V. & Boldin, M. P. (1999) Annu. Rev. Immunol. 17, 331-367. [DOI] [PubMed] [Google Scholar]
- 24.Wang, K., Yin, X. M., Chao, D. T., Milliman, C. L. & Korsmeyer, S. J. (1996) Genes Dev. 10, 2859-2869. [DOI] [PubMed] [Google Scholar]
- 25.Luo, X., Budihardjo, I., Zou, H., Slaughter, C. & Wang, X. (1998) Cell 94, 481-490. [DOI] [PubMed] [Google Scholar]
- 26.Gross, A., Yin, X. M., Wang, K., Wei, M. C., Jockel, J., Milliman, C., Erdjument-Bromage, H., Tempst, P. & Korsmeyer, S. J. (1999) J. Biol. Chem. 274, 1156-1163. [DOI] [PubMed] [Google Scholar]
- 27.Baranano, D. E., Wolosker, H., Bae, B. I., Barrow, R. K., Snyder, S. H. & Ferris, C. D. (2000) J. Biol. Chem. 275, 15166-15173. [DOI] [PubMed] [Google Scholar]
- 28.Dawson, V. L., Dawson, T. M., London, E. D., Bredt, D. S. & Snyder, S. H. (1991) Proc. Natl. Acad. Sci. USA 88, 6368-6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatachalam, K., Ma, H. T., Ford, D. L. & Gill, D. L. (2001) J. Biol. Chem. 276, 33980-33985. [DOI] [PubMed] [Google Scholar]
- 30.Yu, T., Wang, X., Purring-Koch, C., Wei, Y. & McLendon, G. L. (2001) J. Biol. Chem. 276, 13034-13038. [DOI] [PubMed] [Google Scholar]
- 31.Purring-Koch, C. & McLendon, G. (2000) Proc. Natl. Acad. Sci. USA 97, 11928-11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galvan, D. L. & Mignery, G. A. (2002) J. Biol. Chem. 277, 48248-48260. [DOI] [PubMed] [Google Scholar]
- 33.Sawada, M., Hayes, P. & Matsuyama, S. (2003) Nat. Cell Biol. 5, 352-357. [DOI] [PubMed] [Google Scholar]
- 34.Scaffidi, C., Fulda, S., Srinivasan, A., Friesen, C., Li, F., Tomaselli, K. J., Debatin, K. M., Krammer, P. H. & Peter, M. E. (1998) EMBO J. 17, 1675-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barnhart, B. C., Alappat, E. C. & Peter, M. E. (2003) Semin. Immunol. 15, 185-193. [DOI] [PubMed] [Google Scholar]
- 36.Tenhunen, R., Marver, H. S. & Schmid, R. (1968) Proc. Natl. Acad. Sci. USA 61, 748-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahn, E. Y., Pan, G., Oh, J. H., Tytler, E. M. & McDonald, J. M. (2003) Am. J. Pathol. 163, 2053-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sen, C. K., Sashwati, R. & Packer, L. (1999) Cell Death Differ. 6, 481-491. [DOI] [PubMed] [Google Scholar]
- 39.Ayub, K., Laffafian, I., Dewitt, S. & Hallett, M. B. (2004) Immunology 112, 454-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scoltock, A. B., Bortner, C. D., St. J. Bird, G., Putney, J. W., Jr., & Cidlowski, J. A. (2000) J. Biol. Chem. 275, 30586-30596. [DOI] [PubMed] [Google Scholar]
- 41.Hanson, C. J., Bootman, M. D. & Roderick, H. L. (2004) Curr. Biol. 14, R933-R935. [DOI] [PubMed] [Google Scholar]
- 42.Patterson, R. L., Boehning, D. & Snyder, S. H. (2004) Annu. Rev. Biochem. 73, 437-465. [DOI] [PubMed] [Google Scholar]