Abstract
Aldosterone-producing adenoma is a major subtype of primary aldosteronism. The number of cases of these adenomas, which are below the detection limit of computed tomography but diagnosed by adrenal venous sampling, has recently been increasing. However, the pathophysiology of these adenomas, especially those manifesting clinically overt hyperaldosteronism despite their small size, remains unknown. Therefore, we examined the correlation between tumor size and the status of intratumoral steroidogenic enzymes involved in aldosterone biosynthesis using immunohistochemistry. Forty patients with surgically proven aldosterone-producing adenomas were retrospectively studied. Multidetector computed tomography, adrenal venous sampling, and laparoscopic adrenalectomy were performed in all of the patients studied. The tumor area at the maximum diameter of the sections was precisely measured by ImageJ software. The status of the steroidogenic enzymes was immunohistochemically analyzed, and the findings were evaluated according to the H-score system, based on both the number of immunopositive cells and relative immunointensity. Adrenal masses were not detected by computed tomography in 20 patients. Blood pressure, plasma aldosterone concentration, urinary aldosterone excretion, and the number of antihypertensive agents also decreased significantly after the surgery in these patients, as well as in the patients with adenomas detectable by computed tomography. Maximum tumor area obtained in the specimens was significantly correlated with preoperative plasma aldosterone concentration, urinary aldosterone excretion, and the H score of 11β-hydroxylase and was inversely correlated with the H score of aldosterone synthase. These results demonstrated that small adenomas could produce sufficient aldosterone to cause clinically overt primary aldosteronism because of the significantly higher aldosterone synthase expression per tumor area.
Keywords: adrenocortical adenoma, CYP11B1, CYP11B2, hyperaldosteronism, hypertension, immunohistochemistry
Primary aldosteronism (PA) is the most common form of secondary hypertension. The prevalence of PA is reported to be ≈5% to 10% in patients with hypertension and ≈20% in patients with resistant hypertension.1–4 Patients with PA are well known to have higher incidences of cardiovascular and cerebrovascular diseases than those with essential hypertension, even at the same blood pressure.5,6 In addition, we recently reported that PA should be detected early and treated to prevent the prevalence of chronic kidney disease.7 Therefore, it has become important to detect PA early in its clinical course.
More than 90% of PA cases involve bilateral adrenal hyperplasia and aldosterone-producing adenoma (APA); rare cases include unilateral adrenal hyperplasia, aldosterone-producing adrenocortical carcinoma, and familial forms.8 Patients with APA or unilateral adrenal hyperplasia can benefit from adrenalectomy, whereas those with bilateral hyperaldosteronism should be treated with mineralocorticoid receptor antagonists.9 Therefore, classifying the subtypes of PA is critical for developing clinical algorithms for patients. The Endocrine Society guidelines recommend adrenal computed tomographic (CT) scanning and adrenal venous sampling (AVS) before the surgical treatment of PA.10 AVS is currently considered the only reliable method for differentiating unilateral disease from bilateral hyperaldosteronism.11 Young et al12 reported that 110 of 194 patients were diagnosed with a unilateral source of hyperaldosteronism by AVS, whereas 31 (30%) of 102 patients with unilateral APA had a small tumor undetectable by CT; in addition, 8 of these 31 patients had CT-detectable nonfunctioning nodules in the contralateral adrenal gland. Furthermore, smaller APAs undetectable by CT, which can only be diagnosed by AVS, have been reported in other institutions.13–16 The prevalence of CT-undetectable APA among all APA patients is currently estimated to be 13% to 30%. In addition, hypertension was cured or markedly improved after adrenalectomy in almost all reported cases.12–16 Such small APAs undetectable by CT have been histopathologically analyzed, and the reasons why aldosterone hypersecretion from CT-undetectable small adenomas is sufficient to cause clinically overt PA have remained unknown. The main purpose of this study was to explore the reasons why the mean aldosterone secretion capacity of CT-undetectable small APA (microadenomas) could reach as much as that of CT-detectable large APA (macroadenomas) and the reasons why the clinical improvement after surgical treatment in both APA could be similar. Therefore, we evaluated the correlation between tumor size and the status of steroidogenic enzymes including 3 β-hydroxysteroid dehydrogenase (HSD3B), 17 α-hydroxylase (CYP17A1), 11β-hydroxylase (CYP11B1), and aldosterone synthase (CYP11B2), which are all related to aldosterone production, using immunohistochemistry to clarify the status of aldosterone biosynthesis in small APAs.
Methods
Patients
From May 2010 to October 2012, we experienced 100 APA cases that consisted of 20 CT-undetectable cases and 80 CT-detectable cases. We then selected 1 of every 4 cases continually among CT-detectable cases to be able to compare the same number of the cases. Therefore, we could study 40 patients with APAs in this study. The study protocols were approved by the Ethics Committee of Tohoku University School of Medicine, and all patients provided informed consent before participation. All patients were diagnosed with PA on the basis of our previously published protocols.16 Blood pressure was measured with Omron Hem 907 (Omron Healthcare Co. Ltd, Kyoto, Japan) after ≥15-minute rest in a sedentary position, and the average of 3 consecutive measurements was recorded.17 Patients were treated with calcium channel blockers and α1-blockers during the workup of PA. Patients with a plasma aldosterone concentration (PAC)/plasma renin activity ratio (ng·dL−1 per ng·mL−1·h−1) >20 after challenge with captopril 50 mg were diagnosed with PA. No patients examined had concurrent cortisol-producing adenoma, which was confirmed by an overnight dexamethasone 1 mg suppression test.
Measurements
CT scanning was performed with a 64-channel multidetector row CT (Aquilion, Toshiba, Tokyo, Japan), which can analyze adrenal glands in contiguous 1.0-mm-thick slices at 1.0-mm intervals. A nonionic iodinated contrast agent was administered intravenously in a routine manner.18 All the cases were evaluated by 3 radiologists well trained in adrenal imaging, and discordant interpretations were resolved by consensus.
All the cases underwent AVS following the protocol, which is shown in the online-only Data Supplement. All patients underwent laparoscopic unilateral adrenalectomy on the basis of AVS findings and subsequent pathological examination to confirm the existence of APA in the resected adrenal gland.
Quantitative Histopathology and Immunohistochemistry
Forty adrenal gland specimens were retrieved from the surgical pathology files of Tohoku University Hospital. The specimens had been fixed in 10% formalin for 48 hours at room temperature, sectioned at 2-mm intervals, and embedded in paraffin. Adrenocortical adenomas were morphologically defined as well-circumscribed solitary adrenocortical masses of different sizes. Because adrenal tumors are not always rounded and are often elliptical, the maximum diameter and area of each tumor were determined on hematoxylin and eosin–stained tissue slides by ImageJ version 1.47 (National Institutes of Health, Bethesda, MD). The adrenal tumors were photographed by a CCD camera attached to a light microscope.
Immunohistochemical staining was performed with antibodies against CYP11B1 (1:200, rat monoclonal), CYP11B2 (1:750, mouse monoclonal), HSD3B (rabbit polyclonal),19 and CYP17A1 (rabbit polyclonal),20 and the antibodies against CYP11B1 and CYP11B2 were recently developed in the laboratory of Dr Gomez Sanchez (Department of Endocrinology, University of Mississippi Medical Center, Jackson, MS).21 Detailed technical aspects of immunohistochemical staining are summarized in the online-only Data Supplement.
In each case, 500 parenchymal cells were evaluated in each region, and the ratio of positive cells in the tumors was subsequently obtained. Immunoreactivity was assessed semiquantitatively according to the McCarty H score, in which the percentage of stained cells is multiplied by a number from 0 to 3, reflecting the intensity of their immunopositivity.22 The relative immunointensity of specific immunoreactivity was characterized as not present (0), weak but detectable above control (1+), distinct (2+), or very strong (3+). In addition, in all APA cases examined, 3 independent observers (Y.O., Y.N., T.M.) evaluated the H scores, the averages of which were obtained in the blind fashion. The individual clinical data were not informed when they evaluated the H scores. When there was discordance or differences in their evaluation, the immunostained slides were simultaneously re-evaluated using multiheaded light microscopy until the consensus was reached. Therefore, the evaluation using H score of immunoreactivity of the enzymes is considered the best method currently available to allow the estimation of steroidogenic enzyme activity in the objective fashion in clinical materials of the patients.
Statistical Analysis
All data are presented as mean±SEM and 25th to 75th percentile ranges. Differences in measured parameters between groups were evaluated using the Mann–Whitney U test and χ2 test. Univariate correlations were determined by calculating Spearman rank correlation coefficients. The level of significance was set at P<0.05. All analyses were performed using JMP version 10 (SAS Institute Inc, Cary, NC).
Results
Clinicopathological Examination
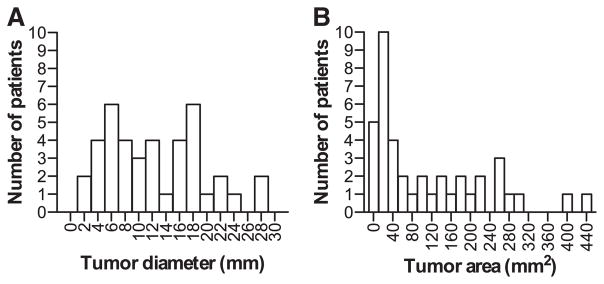
Histological examination indicated the range of the maximum tumor diameter and area as 2 to 28 mm and 3 to 439 mm2 (median, 60.6 mm2), respectively (Figure 1). Because of the noncircular shapes of the tumors, the precise area determined by ImageJ (113.8±18.5 mm2) differed significantly from the circular area calculated on the basis of the maximum diameter (148.4±24.0 mm2; P<0.001). Therefore, we tentatively classified 40 APA cases into the following 2 groups: the smaller and larger groups determined at 60 mm2, which represents the median of the area of APAs that we could obtain using ImageJ. The average diameter and area of adrenal tumors were significantly smaller in the smaller group than in the larger group (P<0.001). There were no significant differences between these 2 groups with respect to the histological features (eg, clear/compact cell type predominance).
Figure 1.
Distributions of the diameters and tumor areas of the aldosterone-producing adenomas (APAs) examined. A, APAs ranged from 2 to 28 mm in diameter. B, APAs ranged from 3 to 439 mm2 in tumor area, with a median of 60.6 mm2.
The preoperative clinical and endocrinologic characteristics of the patients are summarized in the Table. The prevalence of hypokalemia (P<0.001), PAC (P<0.001), and urinary aldosterone excretion (P<0.05) were significantly lower in the smaller group than in the larger group. However, the ratio of male patients, age, hypertension duration, and serum potassium level were significantly greater in the smaller than in the larger group (all P<0.05). The tumor area ratio of the larger/ the smaller group reached to >9×, but the capacity of aldosterone secretion per tumor area of the smaller group was estimated to be much higher than that of the larger group because the plasma aldosterone concentration ratio and an aldosterone urine excretion ratio in the larger group were ≈2.5× and 2× higher than those in the smaller group, respectively (Table).
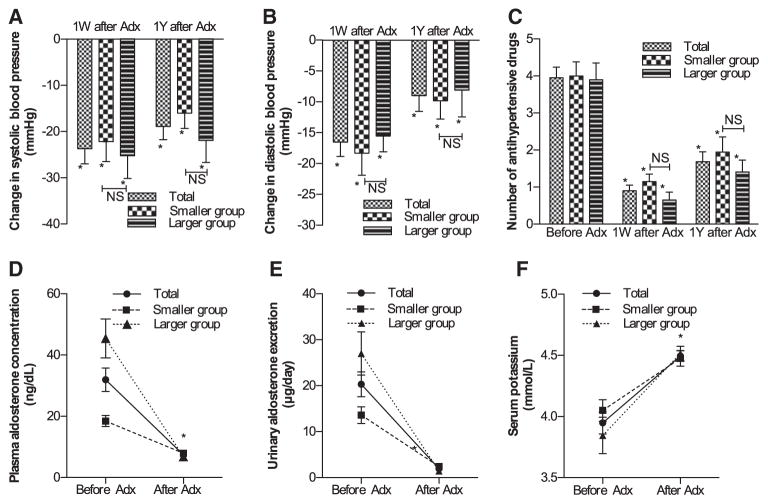
This estimation could account for one of the reasons why the clinical improvements after surgical treatments in both groups were similar (shown in Figure 2). In addition, after unilateral adrenalectomy, the systolic/diastolic blood pressures significantly decreased by 22.2±4.3/18.3±3.6 and 25.3±5.0/15.6±2.5 mm Hg in the smaller and larger groups, respectively (mean±SEM; all P<0.05). Blood pressure reductions remained stable for ≥1 year of postoperative follow-up. Neither systolic blood pressure reductions nor diastolic blood pressure reductions differed significantly between the 2 groups during the 1-year postoperative follow-up period (Figure 2A and 2B). The number of antihypertensive agents, PAC, and urinary aldosterone excretion all decreased significantly in both groups, and serum potassium level was significantly elevated (Figure 2C–2F).
Figure 2.
Summary of the clinico-endocrinologic features of the patients before and after adrenalectomy. Changes in (A) systolic blood pressure, (B) diastolic blood pressure, (C) the number of antihypertensive drugs, (D) plasma aldosterone concentration after adrenalectomy, and (E) urinary aldosterone excretion during 1 year of postoperative follow-up. F, Changes in serum potassium level after adrenalectomy. 1W indicates 1 week; 1Y, 1 year; Adx, adrenalectomy; and NS, not significantly different between the smaller and larger aldosterone-producing adenoma groups. Error bar: SEM. *P<0.05, vs before adrenalectomy.
Immunohistochemistry of Steroidogenic Enzymes
Representative immunohistochemistry results of HSD3B, CYP17A1, CYP11B1, and CYP11B2 in the smaller APA group (case 1) and larger APA group (case 2) are shown in Figure 3. HSD3B immunoreactivity was markedly and diffusely present in all APA cases examined (Figure 3B and 3G). In contrast, CYP17A1-positive tumor cells were heterogeneously distributed, and their relative immunoreactivity was weak (Figure 3C and 3H). The immunoreactivity of CYP11B1 was weaker in the smaller APA group than in the larger APA group (Figure 3D and 3I). In contrast, the immunoreactivity of CYP11B2 was comparatively stronger in the smaller APA group than in the larger APA group (Figure 3E and 3J). The immunoreactivity of both CYP11B1 and CYP17A1 was mainly detected in the same tumor cells.
Figure 3.
Histopathologic findings of the patients with aldosterone-producing adenoma (case 1: A–E; case 2: F–J). Scale bars, 1 mm (A–E) and 10 mm (F–J). A and F, Hematoxylin and eosin (HE) staining. B and G, HSD3B immunostaining. C and H, CYP17A1 immunostaining. D and I, CYP11B1 immunostaining. E and J, CYP11B2 immunostaining.
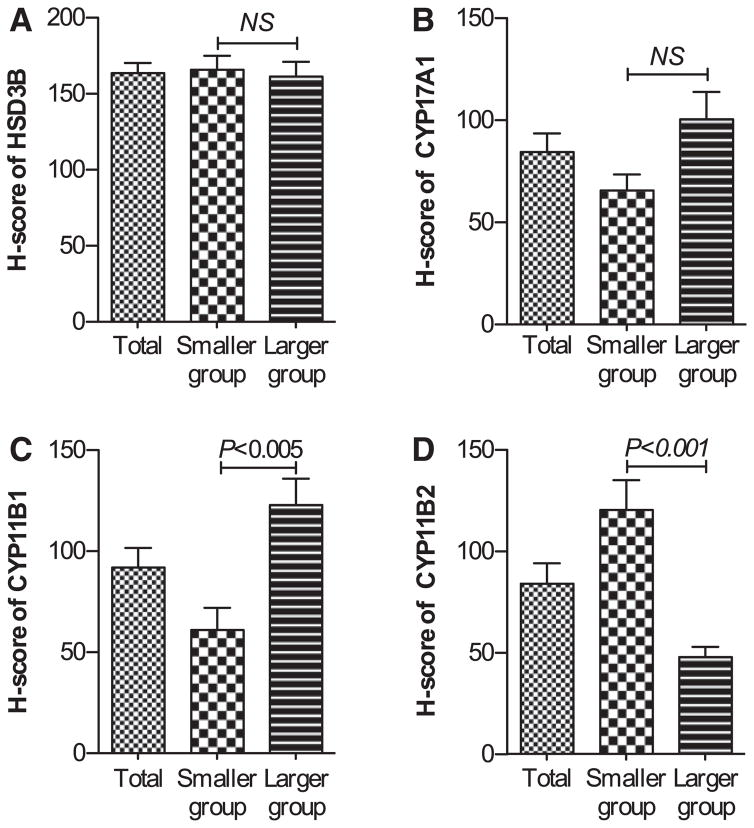
We evaluated these steroidogenic immunoreactivities according to the H-score system (Figure 4). The H score of HSD3B was not significantly different between the 2 groups (Figure 4A). The H score of CYP17A1 immunoreactivity in adenoma cells tended to be higher in the larger APA group, but the difference did not reach statistical significance (Figure 4B). In the smaller APA group, the H score of CYP11B1 was significantly lower (P<0.005) and the H score of CYP11B2 was significantly higher (P<0.001) than the corresponding scores in the larger APA group, respectively (Figure 4C and 4D).
Figure 4.
H scores of HSD3B (A), CYP17A1 (B), CYP11B1 (C), and CYP11B2 (D) in the smaller and larger aldosterone-producing adenoma (APA) groups. There were no significant differences between groups with respect to the H scores of HSD3B or CYP17A1, whereas there were significant differences with respect to the H scores of CYP11B1 and CYP11B2. NS indicates not significantly different between the smaller and larger APA groups. Error bar: SEM.
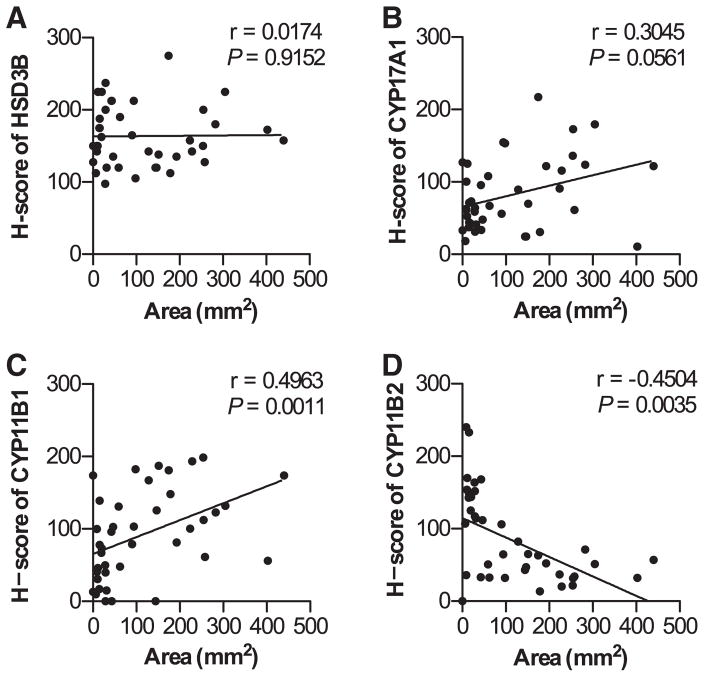
Furthermore, we examined the correlation between the H scores and tumor area (Figure 5). Although the H scores of HSD3B and CYP17A1 were not significantly correlated with tumor area (Figure 5A and 5B), the H score of CYP11B1 was significantly correlated with tumor area (Figure 5C; P<0.005), and the H score of CYP11B2 was inversely significantly correlated with tumor area (Figure 5D; P<0.005). Both preoperative PAC and urinary aldosterone excretion were significantly correlated with the values of H score of CYP11B2 multiplied by tumor area (Figure S1A and S1B in the online-only Data Supplement) in our present study. Therefore, in the larger group, the higher levels of PAC and urinary aldosterone excretion were mainly postulated to be a result of the much larger volume of tumor. In addition, the values of PAC and urinary aldosterone excretion were also significantly correlated with those of H score of CYP11B1 multiplied by tumor area (Figure S1C and S1D). It is therefore reasonably postulated that the higher H score of CYP11B1 could synergistically contribute to the higher aldosterone secretion in the larger group.
Figure 5.
Correlations between tumor area and H scores of HSD3B (A), CYP17A1 (B), CYP11B1 (C), and CYP11B2 (D). There were no significant correlations between tumor area and the H scores of HSD3B or CYP17A1, whereas tumor area was significantly positively and negatively correlated with the H scores of CYP11B1 and CYP11B2, respectively.
Discussion
To the best of our knowledge, this is the first study to evaluate the correlation between APA tumor area and CYP11B1 and CYP11B2 immunoreactivity using specific monoclonal antibodies. Immunoreactivity was analyzed in a quantitative fashion according to the H-score system to evaluate both the percentage and the intensity of positively stained cells, whereas tumor area was precisely measured by ImageJ. In both smaller and larger groups, laparoscopic adrenalectomy based on the results of AVS significantly improved blood pressure, PAC, urinary aldosterone excretion, and the number of anti-hypertensive drugs. These results also support the clinical use and validity of the AVS-based diagnosis and management of APA. In our present study, the H score of CYP11B1 was significantly higher in the larger group but that of CYP11B2 was significantly higher in the smaller group. However, the status of autonomous cortisol production in the tumors diagnosed by the dexamethasone suppression test or urinary free cortisol excretion was not significantly different between these 2 groups. Therefore, the different H-score status of intratumoral CYP11B1 and CYP11B2 in these 2 groups does not necessarily account for the difference in cortisol production but could explain the difference of aldosterone production between these 2 groups of the patients detected in our present study. It is also entirely true that CYP11B1 was partly correlated with the aldosterone production system. In our present study, the larger APA also tended to have higher expression of CYP11B1. In all APA cases examined in this study, CYP11B2 immunoreactivity was not necessarily completely suppressed in hyperplastic zona glomerulosa of adjacent adrenal cortex. These findings did indicate that much larger amounts of precursors of aldosterone such as corticosterone could be produced by CYP11B1 in the larger APA than in the smaller APA group, and such precursors could be converted to aldosterone by CYP11B2 in the hyperplastic zona glomerulosa of adjacent adrenal cortex, but this hypothesis required further studies.
The tumor area of APAs determined by quantitative histological analysis was significantly correlated with preoperative aldosterone levels and urinary aldosterone excretion. The total production of steroids including aldosterone was generally considered higher in the larger APA group than in the smaller APA group. However, tumor area was inversely correlated with the H score of CYP11B2, which is the rate-limiting step of aldosterone biosynthesis, and positively correlated with the H score of CYP11B1. These findings did demonstrate that the smaller tumors had higher CYP11B2 expression per area and cell. It is entirely true that CYP11B2 levels alone do not necessarily represent abundant aldosterone production, because several other factors (eg, the levels of steroidogenic enzymes upstream of CYP11B2) also play pivotal roles in overall aldosterone production.23,24 However, this marked expression of CYP11B2 per area and cell in the tumors may at least explain why small APAs below the detection limit of CT can result in clinically overt hyperaldosteronism. Of particular interest, both PAC and urinary aldosterone excretion were significantly correlated with the values of H scores of CYP11B2 multiplied by tumor area. These findings did indicate that both higher levels of PAC and urinary aldosterone excretion in the larger group could occur mainly because of the volume effects of tumor despite the lower H score of CYP11B2 compared with the smaller group. In addition, the values of PAC and urinary aldosterone excretion were also significantly correlated with those of H score of CYP11B1 multiplied by tumor area. Therefore, both the higher H score of CYP11B1 and volume effect of tumor could synergistically contribute to the higher PAC and urinary aldosterone excretion detected in the larger group of APA patients. However, further investigations are also required to clarify the reasons why larger APA expressed the low H score of CYP11B2, despite the high H scores of HSD3B and CYP11B1.
CT is currently considered one of the best diagnostic tools for detecting adrenal tumors. However, it is also difficult to differentiate unilateral from bilateral hyperaldosteronism solely on the basis of CT findings, because CT and AVS findings are reported to be concordant in only 50% to 70% of cases.11,12,16 In addition, CT has a detection limit on the size of adrenal mass lesions. For example, Omura et al13 reported that CT could not detect adrenal tumors ≤6 mm in diameter. In the smaller APA group in our present study, there were 7 cases in which the tumor diameter evaluated on histological sections exceeded 6 mm (range, 7–10 mm), whereas CT did not detect these tumors. The maximum widths of the 95th percentile measurements of normal adrenal glands were 12.2 and 9.9 mm in the left and right sides, respectively.25 Therefore, if a small adrenocortical tumor is located and embedded in a relatively thick section, CT would be unable to detect it. These clinical difficulties in detecting smaller sized adrenocortical tumors could account for the older age and longer duration of hypertension in the smaller group than in the larger group.
Perspectives
Results of the present study did reveal that hypertension was markedly ameliorated after adrenalectomy in both smaller and larger groups. In addition, the relatively higher CYP11B2 expression per area in the smaller group could clinically cause PA, despite their CT-undetectable tumor size. Therefore, surgical treatment after AVS could be selected if there were sufficient clinical symptoms and desire for surgery in these patients with microadenomas, as well as those with macroadenomas. Given the successful cannulation of the adrenal vein, AVS is the only reliable method for determining the localization of hyper-aldosteronism, especially in patients with microadenomas. Right adrenal veins imaged by contrast-enhanced CT could also be technically helpful for successful AVS.16,18,26 Universal protocols for AVS performance should be established to facilitate the dissemination of this technique, which might prevent cardiovascular and renal complications in patients with APA by means of surgical selection. Finally, somatic mutations of KCNJ5, ATPase, and CACNA1D have been reported mainly in aldosterone-producing macroadenoma.27–29 The somatic mutations, however, may also be responsible for aldosterone over-secretion in microadenoma, but it awaits further investigations to prove this interesting hypothesis.
Supplementary Material
Table.
Clinical Characteristics of the Smaller and Larger Aldosterone-Producing Adenoma Groups
| Characteristics | Total
|
Smaller Group (<60 mm2)
|
Larger Group (≥60 mm2)
|
P Value |
|---|---|---|---|---|
| n=40 | n=20 | n=20 | ||
| Sex (male, female)* | 28, 12 | 17, 3 | 11, 9 | <0.05 |
| Age, y* | 52.2±1.9 (43–61.5) | 57.7±2.8 (51.2–66.0) | 46.7±2.1 (38.3–53.0) | <0.05 |
| Duration of hypertension, y* | 10.6±1.3 (5.0–13.5) | 13.6±2.2 (5.5–20) | 7.6±1.4 (4.0–11) | <0.05 |
| No. of antihypertensive drugs | 3.95±0.29 (3.0–5.0) | 4.00±0.39 (2.3–5.0) | 3.90±0.45 (3.0–4.0) | NS |
| Body mass index, kg/m2 | 25.4±0.5 (23.0–27.4) | 25.9±0.7 (24.1–28.1) | 24.8±0.8 (22.6–26.8) | NS |
| Systolic blood pressure, mm Hg | 149.2±3.0 (134–161) | 144.9±4.0 (130–161) | 153.6±4.6 (139–165) | NS |
| Diastolic blood pressure, mm Hg | 94.1±2.0 (85–102) | 93.5±3.2 (84–102) | 94.6±2.4 (88–102) | NS |
| Serum creatinine, mg/dL | 0.79±0.04 (0.7–0.9) | 0.81±0.04 (0.7–0.9) | 0.77±0.06 (0.6–1.0) | NS |
| Serum potassium, mmol/L* | 3.55±0.08 (3.1–4.0) | 3.75±0.10 (3.5–4.0) | 3.37±0.12 (2.9–3.8) | <0.05 |
| Prevalence of hypokalemia, %† | 42.5 | 20 | 65 | <0.001 |
| Plasma aldosterone concentration, ng/dL† | 31.9±3.8 (16.8–44.9) | 18.5±1.9 (13.4–20.5) | 45.4±6.2 (29.6–52.3) | <0.001 |
| Serum cortisol, μg/dL | 7.55±0.53 (5.0–9.5) | 7.38±0.80 (4.5–8.8) | 7.72±0.73 (5.2–10) | NS |
| Plasma renin activity, ng·mL−1·h−1 | 0.20±0.02 (0.1–0.3) | 0.16±0.10 (0.10–0.28) | 0.24±0.04 (0.1–0.3) | NS |
| Aldosterone/renin activity, ng·dL−1 per ng·mL−1·h−1 | 220.5±29.5 (71.5–283) | 156.4±23.2 (64–199) | 284.5±51.1 (81.4–522) | NS |
| Urinary aldosterone excretion, μg/d* | 20.3±2.7 (10.5–25.0) | 13.6±1.8 (8.5–15.7) | 27.0±4.7 (16.0–30.3) | <0.05 |
| Urinary free cortisol excretion, μg/d | 46.4±5.1 (25.4–54.3) | 48.3±8.2 (24.3–58.6) | 44.4±6.2 (27.2–49.6) | NS |
| Captopril challenge ARR, ng·dL−1 per ng·mL−1·h−1 | 217.9±61.6 (57.2–261) | 93.2±9.7 (64.9–120) | 342.6±117.7 (43.2–356) | NS |
| Cortisol after DST, μg/dL | 1.30±0.11 (0.8–1.8) | 1.27±0.16 (0.8–1.8) | 1.33±0.16 (0.8–1.8) | NS |
| Lateralization index in AVS before cosyntropin loading* | 18.19±4.04 (3.4–26.9) | 11.18±3.30 (3.0–12.1) | 26.00±7.53 (5.6–36.7) | <0.05 |
| Lateralization index in AVS after cosyntropin loading | 9.47±1.05 (4.5–13.1) | 7.59±0.97 (4.0–9.7) | 11.46±1.83 (6.3–15.5) | NS |
| Diameter of adrenal tumor, mm† | 11.9±1.1 (6.3–17.3) | 5.9±0.6 (4.0–7.8) | 17.8±1.0 (15.0–20.5) | <0.001 |
| Area of adrenal tumor, mm2† | 113.8±18.5 (16.6–189.4) | 22.2±3.5 (9.8–31.0) | 205.5±23.0 (132.3–257.0) | <0.001 |
All data are shown in the following order: mean±SEM (25th–75th percentile), except for sex and prevalence of hypokalemia. Hypokalemia is defined as serum potassium concentration <3.5 mmol/L. ARR indicates plasma aldosterone concentration per plasma renin activity; AVS, adrenal venous sampling; DST, 1 mg dexamethasone suppression test; and lateralization index, aldosterone/cortisol ratio.
P<0.05,
P<0.001, both are significantly different between smaller group and larger group.
Novelty and Significance.
What Is New?
The present study is the first to investigate the correlations between aldosterone-producing adenoma tumor area size and CYP11B1 and CYP11B2 immunoreactivity using specific monoclonal antibodies.
What Is Relevant?
Significantly higher CYP11B2 expression in small aldosterone-producing adenomas could explain why small aldosterone-producing adenomas cause aldosteronism, although they are undetectable by computed tomography.
Summary
Small adenomas could produce sufficient aldosterone to cause clinically overt primary aldosteronism because of the significantly higher CYP11B2 expression per tumor area.
Acknowledgments
We thank Kazue Ise for the technical support of immunohistochemical analysis and Akane Sugawara and Yasuko Tsukada for their secretarial assistance.
Footnotes
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.113.02944/-/DC1.
Disclosures
Y. Nakamura was partly supported by the Takeda Science Foundation. The other authors report no conflicts.
References
- 1.Fardella CE, Mosso L, Gómez-Sánchez C, Cortés P, Soto J, Gómez L, Pinto M, Huete A, Oestreicher E, Foradori A, Montero J. Primary hyperaldosteronism in essential hypertensives: prevalence, biochemical profile, and molecular biology. J Clin Endocrinol Metab. 2000;85:1863–1867. doi: 10.1210/jcem.85.5.6596. [DOI] [PubMed] [Google Scholar]
- 2.Stowasser M, Gordon RD. Primary aldosteronism–careful investigation is essential and rewarding. Mol Cell Endocrinol. 2004;217:33–39. doi: 10.1016/j.mce.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society. Guidelines for the diagnosis and treatment of primary aldosteronism—the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- 4.Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension. 2002;40:892–896. doi: 10.1161/01.hyp.0000040261.30455.b6. [DOI] [PubMed] [Google Scholar]
- 5.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Born-Frontsberg E, Reincke M, Rump LC, Hahner S, Diederich S, Lorenz R, Allolio B, Seufert J, Schirpenbach C, Beuschlein F, Bidlingmaier M, Endres S, Quinkler M Participants of the German Conn’s Registry. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn’s Registry. J Clin Endocrinol Metab. 2009;94:1125–1130. doi: 10.1210/jc.2008-2116. [DOI] [PubMed] [Google Scholar]
- 7.Iwakura Y, Morimoto R, Kudo M, Ono Y, Takase K, Seiji K, Arai Y, Nakamura Y, Sasano H, Ito S, Satoh F. Predictors of decreasing glomerular filtration rate and prevalence of chronic kidney disease after treatment of primary aldosteronism: renal outcome of 213 cases. J Clin Endocrinol Metab. 2014;99:1586–1588. doi: 10.1210/jc.2013-2180. [DOI] [PubMed] [Google Scholar]
- 8.Mulatero P, Monticone S, Rainey WE, Veglio F, Williams TA. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat Rev Endocrinol. 2013;9:104–112. doi: 10.1038/nrendo.2012.230. [DOI] [PubMed] [Google Scholar]
- 9.Lim PO, Young WF, MacDonald TM. A review of the medical treatment of primary aldosteronism. J Hypertens. 2001;19:353–361. doi: 10.1097/00004872-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM Endocrine Society. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 11.Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 12.Young WF, Stanson AW, Thompson GB, Grant CS, Farley DR, van Heerden JA. Role for adrenal venous sampling in primary aldosteronism. Surgery. 2004;136:1227–1235. doi: 10.1016/j.surg.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 13.Omura M, Sasano H, Saito J, Yamaguchi K, Kakuta Y, Nishikawa T. Clinical characteristics of aldosterone-producing microadenoma, macroadenoma, and idiopathic hyperaldosteronism in 93 patients with primary aldosteronism. Hypertens Res. 2006;29:883–889. doi: 10.1291/hypres.29.883. [DOI] [PubMed] [Google Scholar]
- 14.Karashima S, Takeda Y, Cheng Y, Yoneda T, Demura M, Kometani M, Ohe M, Mori S, Yagi K, Yamagishi M. Clinical characteristics of primary hyperaldosteronism due to adrenal microadenoma. Steroids. 2011;76:1363–1366. doi: 10.1016/j.steroids.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Ishidoya S, Kaiho Y, Ito A, Morimoto R, Satoh F, Ito S, Ishibashi T, Nakamura Y, Sasano H, Arai Y. Single-center outcome of laparoscopic unilateral adrenalectomy for patients with primary aldosteronism: lateralizing disease using results of adrenal venous sampling. Urology. 2011;78:68–73. doi: 10.1016/j.urology.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 16.Satoh F. Localization of aldosterone-producing adrenocortical adenomas- significance of adrenal venous sampling. Hypertens Res. 2007;30:1083–1095. doi: 10.1291/hypres.30.1083. [DOI] [PubMed] [Google Scholar]
- 17.Czarkowski M, Staszków M, Kostyra K, Shebani Z, Niemczyk S, Matuszkiewicz-Rowińska J. Determining the accuracy of blood pressure measurement by the Omron HEM-907 before and after hemodialysis. Blood Press Monit. 2009;14:232–238. doi: 10.1097/mbp.0b013e328331d5b5. [DOI] [PubMed] [Google Scholar]
- 18.Matsuura T, Takase K, Ota H, Yamada T, Sato A, Satoh F, Takahashi S. Radiologic anatomy of the right adrenal vein: preliminary experience with MDCT. AJR Am J Roentgenol. 2008;191:402–408. doi: 10.2214/AJR.07.3338. [DOI] [PubMed] [Google Scholar]
- 19.Sasano H, Mason JI, Sasano N, Nagura H. Immunolocalization of 3β-hydroxysteroid dehydrogenase in human adrenal cortex and in its disorders. Endocr Pathol. 1990;1:94–101. doi: 10.1007/BF02915624. [DOI] [PubMed] [Google Scholar]
- 20.Sasano H, Mason JI, Sasano N. Immunohistochemical study of cytochrome P-45017 alpha in human adrenocortical disorders. Hum Pathol. 1989;20:113–117. doi: 10.1016/0046-8177(89)90174-3. [DOI] [PubMed] [Google Scholar]
- 21.Gomez-Sanchez CE, Qi X, Velarde-Miranda C, Plonczynski MW, Parker CR, Rainey W, Satoh F, Maekawa T, Nakamura Y, Sasano H, Gomez-Sanchez EP. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Mol Cell Endocrinol. 2014;383:111–117. doi: 10.1016/j.mce.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budwit-Novotny DA, McCarty KS, Cox EB, Soper JT, Mutch DG, Creasman WT, Flowers JL, McCarty KS., Jr Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986;46:5419–5425. [PubMed] [Google Scholar]
- 23.Bassett MH. The orphan nuclear receptors Nurr1 and NGFIB regulate adrenal aldosterone production. Mol Endocrinol. 2003;18:279–290. doi: 10.1210/me.2003-0005. [DOI] [PubMed] [Google Scholar]
- 24.Ye P, Nakamura Y, Lalli E, Rainey WE. Differential effects of high and low steroidogenic factor-1 expression on CYP11B2 expression and aldosterone production in adrenocortical cells. Endocrinology. 2009;150:1303–1309. doi: 10.1210/en.2008-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vincent JM, Morrison ID, Armstrong P, Reznek RH. The size of normal adrenal glands on computed tomography. Clin Radiol. 1994;49:453–455. doi: 10.1016/s0009-9260(05)81739-8. [DOI] [PubMed] [Google Scholar]
- 26.Rossi GP, Auchus RJ, Brown M, Lenders JW, Naruse M, Plouin PF, Satoh F, Young WF., Jr An expert consensus statement on use of adrenal vein sampling for the subtyping of primary aldosteronism. Hypertension. 2014;63:151–160. doi: 10.1161/HYPERTENSIONAHA.113.02097. [DOI] [PubMed] [Google Scholar]
- 27.Azizan EA, Lam BY, Newhouse SJ, Zhou J, Kuc RE, Clarke J, Happerfield L, Marker A, Hoffman GJ, Brown MJ. Microarray, qPCR, and KCNJ5 sequencing of aldosterone-producing adenomas reveal differences in genotype and phenotype between zona glomerulosa- and zona fasciculata-like tumors. J Clin Endocrinol Metab. 2012;97:E819–E829. doi: 10.1210/jc.2011-2965. [DOI] [PubMed] [Google Scholar]
- 28.Scholl UI, Goh G, Stölting G, et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat Genet. 2013;45:1050–1054. doi: 10.1038/ng.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams TA, Monticone S, Schack VR, et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension. 2014;63:188–195. doi: 10.1161/HYPERTENSIONAHA.113.01733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.