Abstract
Introduction
Environments associated with smoking may promote lapse and relapse in smokers attempting to quit. Here we examined the effects of exposure to visual smoking environment cues on smoking urge and the ability to resist smoking, as measured with a delay-to-smoking task in which monetary contingencies are provided for resisting smoking.
Methods
Adult daily smokers (n=22) completed two experimental sessions, each following 6 hr smoking abstinence. Sessions differed only in the type of cue participants were exposed to (smoking environments vs. nonsmoking environments). Participants completed subjective ratings of smoking urge, withdrawal and other reactions (i.e. craving, affect). Behavioral outcomes on the delay-to-smoking task included latency to first cigarette, number of cigarettes smoked and average number of puffs per cigarette.
Results
Across cue exposure sessions, 64% of participants initiated smoking (no effect of condition was observed). However, exposure to smoking environments as compared to the nonsmoking environments resulted in greater craving, faster initiation of smoking, and more smoked cigarettes. Greater craving was associated with a shorter time to initiate smoking, but this effect did not differ across sessions. In contrast, withdrawal was more strongly associated with number of cigarettes smoked during smoking environment sessions.
Conclusion
Together, these results suggest smoking environments increase smoking urge and promote smoking behavior. Further research is necessary to examine the specific and interactive effects of smoking-related environments on real-world smoking lapse and relapse.
Keywords: Environment, Tobacco Smoking, Nicotine Withdrawal, Cue Reactivity, Craving, Lapse and Relapse
1. Introduction
Drug dependent individuals experience craving following exposure to cues associated with past or current drug taking (Carter & Tiffany 1999, Conklin 2006, Drummond et al 1995, McClernon et al 2016). Despite recognition of environments as influential determinants of drug use and relapse in animal models (Fuchs et al 2008, Marchant et al 2015), only limited research has specifically evaluated the effects of drug-environments on drug self-administration in humans. Conklin and colleagues (Conklin et al 2010, Conklin et al 2008) found that pictures of smoking environments (e.g. park bench, bus stop) provoked craving at levels similar to proximal cues (e.g. lit cigarette). The current study seeks to extend this prior work by examining whether smoking environment cues decrease the ability of smokers to resist smoking. To test this, we used a well-validated analogue of smoking lapse, i.e. the delay to smoking task (DST).
The DST models smoking lapse and relapse by measuring how long a smoker can delay initiation of smoking in exchange for monetary reward while exposed to smoking paraphernalia (i.e. cigarette, ashtray, and lighter) (McKee et al 2011, McKee et al 2012). In this task, smokers are presented cigarettes and smoking paraphernalia and then instructed they can initiate smoking at any point, but will receive a monetary incentive for every five minutes they resist smoking. Latency to smoke and number of cigarettes smoked during the DST are associated with duration of nicotine abstinence and the magnitude of the monetary reinforcer (McKee et al 2012). Here we examined how smoking environment cues (relative to nonsmoking environment cues) impacted DST outcomes. We hypothesized that exposure to smoking environments would decrease latency to first cigarette and increase ad lib smoking once smoking is initiated. We also examined the effects of smoking environment exposure on indices of smoking urge and other reactions (e.g. craving, affect) and whether craving or withdrawal predicted smoking behavior during the task.
2. Materials and Methods
2.1 Participants
Twenty-two adult smokers were recruited. Participants were required to be ages 18 to 55, generally healthy (i.e. not currently ill, ambulatory), smoke at least 5 cigarettes per day (CPD) for ≥ 1 year, and have no interest in quitting smoking for the duration of the study. Participants were excluded if they used smokeless tobacco, or were currently abusing alcohol or other drugs (verified with breath and urine samples). All participants provided informed consent and all procedures were approved by the Duke University IRB.
2.2 Procedures and Measures
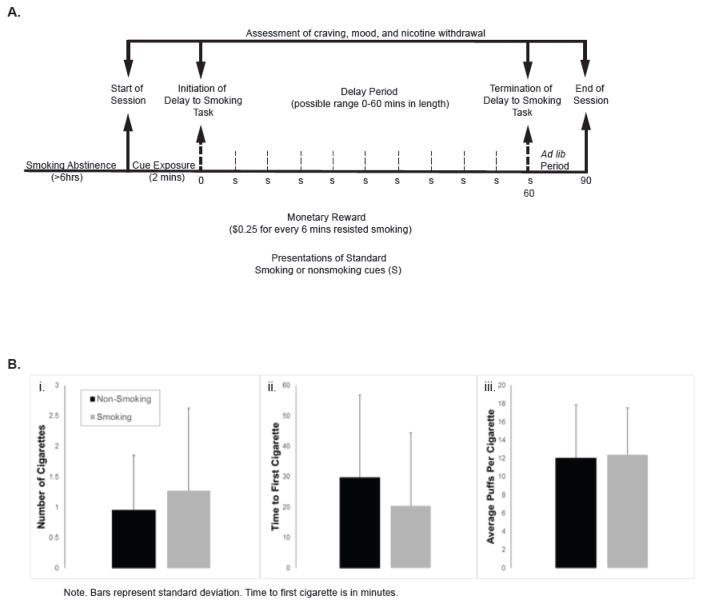
Following a screening/training visit, participants completed two separate cue exposure sessions approximately one week apart following ≥ 6 hrs smoking abstinence (confirmed by a 40% reduction in breath carbon monoxide (CO) level from the value obtained at either the screening or training visits) (Conklin et al 2010, McClernon et al 2016). The two sessions differed only on the environmental cues presented (smoking or nonsmoking; order randomly assigned and counterbalanced). One session involved exposure to validated images (Conklin 2006, Conklin et al 2008, McClernon et al 2016) of standard smoking environment cues (e.g. car, bus stop, restaurant, and bars); the other exposure to standard nonsmoking environment cues (e.g. gym, church, daycare, and auditorium). For each cue type (smoking, nonsmoking) cues consisted of 10 different environments, each presented from 4 different angles. Each session began with a 2 minute cue exposure phase (see Figure 1A), in which environment cues (smoking or nonsmoking images depending on session) were presented continuously for two minutes; for 3 seconds each. Afterwards, participants were shown 8 unlit cigarettes of their preferred brand and informed of the opportunity to initiate smoking at any time over a 60-minute period. A monetary reinforcer ($0.25) was provided for each 6-minute period that a participant resisted smoking. Participants were allowed to read books or magazines. However, during the final minute of each 6 minute period, a tone alerted them to view additional smoking or nonsmoking environment cues (four images shown for 15 seconds each). Each of the ten environments viewed during the cue reactivity phase were thus repeated during this phase. After the delay period ended (60 mins) or as soon as the participant decided to initiate smoking by pushing a button, they could then smoke ad lib for the next 30 minutes. During this phase, they were provided a $4.00 smoking “tab” and for each cigarette they smoked, $0.50 was deducted from their tab. In cases where participants initiated smoking prior to the end of the delay period, environment cue presentation continued during the ad lib period until all cues were shown. This ensured all participants were exposed to the full set of cues regardless of when they initiated smoking. All participants were required to remain in the room for a full 90 minutes regardless of smoking behavior.
Figure 1.
Participants completed the Minnesota Nicotine Withdrawal Scale (MNWS) upon arrival for each experimental session (Hughes & Hatsukami, 1986, 1998). Subjective ratings of positive affect (3 items), negative affect (1 item) and craving (4 items) were obtained before and after the cue-reactivity phase, as well as at the end of the delay period (i.e. either prior to lighting the first cigarette or the end of 60 minutes, whichever came first). Using an established scale (Conklin et al., 2010), participants rated their agreement with each statement (ranging from do not agree to strongly agree) using a 0–100 scale. Participants were video recorded during each session to assess smoking behavior (see below). Breath CO levels were measured at the beginning and end of each session to assess CO boost (post – pre) using a Vitalograph CO monitor (Vitalograph, Inc.; Lenexa, KS).
2.3 Data Processing and Analysis
Video recordings of behavior during the DST were coded by two independent raters in order to assess latency to first puff (in seconds), number of cigarettes smoked, and average number of puffs per cigarette. All analyses were conducted in SPSS Version 24.0 (IBM Corp., Armonk, NY) through application of a mixed models framework with a repeated statement and compound symmetry covariance matrix (analogous to repeated measures ANOVA/ANCOVA). First, we examined the effect of Environment Type (smoking vs. non-smoking) on craving and affective responses during the initial two-minute exposure period. Time (Pre-Exposure vs. Post-Exposure) was included as a factor and the Environment Type x Time interaction was of primary interest. Next, we examined the effect of Environment Type on smoking behavior during the DST (latency to first cigarette, number of cigarettes smoked). Lastly, we examined whether (A) Withdrawal (at arrival); (B) Cue-Induced Craving (post-exposure craving minus pre-exposure craving); and (C) Peak-Provoked Craving (craving immediately prior to entering the ad lib portion of the task) predicted smoking behavior. Each variable was introduced separately into a model with smoking behavior indices (latency to first cigarette, number of cigarettes smoked) as the dependent variable and both the main effect and its interaction with Environment were examined.
3. Results
3.1 Sample Characteristics and Coding Reliability
Sample characteristics are presented in Table 1. CO levels were equivalent at the beginning of the smoking (mean=7.05, SD=4.1) and nonsmoking (mean=7.05, SD= 5.1) cue sessions, t21=0, p=1. Coding of smoking behavior videos by two raters was highly reliable across indices (ICC’s 0.99–1.00). The average of the two coder ratings was used in analyses of smoking behavior.
Table 1.
Sample Characteristics
| Variable | Mean (SD) or % |
|---|---|
| Demographics | |
| Age | 38.0(11.0) |
| Years Education | 13.8(1.9) |
| Gender (% Female) | 40.9 |
| Income (% <$16,000/yr) | 40.9 |
| Race | |
| White | 22.7 |
| Black | 63.6 |
| Asian | 9.1 |
| Multiracial | 4.5 |
| Smoking Characteristics | |
| FTND | 4.0 (2.3) |
| Years Smoking | 18.1 (10.8) |
| Cigarettes Per Day | 11.8(6.4) |
| Menthol Preference (%Yes) | 68.2 |
| # of Quit Attempts | 2.5 (2.6) |
| Motivation to Quit | 7.0 (2.5) |
Note: FTND = Fagerstrom Test for Nicotine Dependence. Motivation to Quit was assessed using a modified version of the Quit Ladder (Biener & Abrams, 1991). Scores range from 1 to 11 with higher scores indicating greater motivation.
3.2 Effects of Environment Type on Craving and Affect
During the initial two-minute exposure to environment cues, there were main effects of both Environment [F(1,63)=7.41, p=.008] and Time [F(1,63)=16.89, p<.001] on craving. However, these were qualified by a significant Environment x Time interaction [F(1,63)=6.70, p =.012]. Post-hoc analyses indicated this effect was driven by a significant reduction in craving following presentation of nonsmoking environment cues [F(1,21) =19.31, p <.001], with no change in craving occurring during presentation of smoking environment cues (p =.247). No other main effects or interactions for craving reached significance (all p’s >.2). There were no main effects or interactions for either positive (all p’s >.4) or negative affect (all p’s >.3).
3.3 Effects of Environment Type on Smoking Behavior
Eight (36%) participants did not smoke any cigarettes during the DST in either session. The remaining 14 (64%) participants smoked at least one cigarette during both sessions. During the smoking environment sessions, 12 participants initiated smoking during the delay period (mean delay: 14.8 min); an additional 2 delayed for the full 60 minutes but smoked during the subsequent 30 minute ad lib period. During the nonsmoking environment session, 10 participants initiated smoking during the delay period (mean delay: 16.3 min); an additional 4 participants smoked during the ad lib period. A McNemar’s Chi-Square Test (X2=2.00, p= .16) did not reach significance, indicating Environment was not related to the phase of the task during which participant’s initiated smoking. Individuals who did not smoke at all were excluded from this test. Environment was significantly related to latency to first puff [F(1,21) =8.28, p =.013] and the number of cigarettes smoked during the DST [F(1,21) =5.33, p =.031]. As shown in Figure 1B, participants initiated smoking sooner and smoked more cigarettes when viewing smoking compared with nonsmoking environments.
3.4 Predictors of Smoking Behavior
Latency to First Cigarette
A significant main effect of craving was found [F(1,12.1), p =.002] indicating greater craving was associated with a shorter time to first puff. However, this effect did not interact with Environment (p>.5). There were no significant effects or interactions for either nicotine withdrawal or Environment-Provoked Craving (all p’s>.05).
Number of Cigarettes
A significant Withdrawal x Environment interaction emerged [F(1,18.9)=8.27, p =.010], indicating a stronger positive relationship between withdrawal and number of cigarettes smoked during smoking environment sessions (p=.010) relative to nonsmoking environment sessions (p=.063). There were no significant main effects or interactions for either Cue- or Peak-Provoked Craving (all p’s>.15).
4. Discussion
The main finding of this study is that exposure to smoking environments reduces the ability to resist smoking in the context of an analogue lapse and relapse task. Specifically exposure to smoking environment cues, compared to nonsmoking cues, resulted in shorter latencies to smoke and greater cigarette consumption. Even though the majority of participants initiated smoking, no effect of condition was observed. Further, environment was not related to which phase of the task smoking was initiated. These results directly inform our understanding of how environments impact smoking behavior. These findings extend previous research showing that proximal cues affect craving, withdrawal and smoking behavior (Conklin et al 2015, Droungas et al 1995, Payne et al 1991) to reveal similar effects of environment cues in the context of a laboratory model of lapse and relapse (i.e. DST).
Interestingly, in the present study, we observed a reduction in craving following exposure to nonsmoking environments. In a previous report, exposure to nonsmoking cues (i.e. people in which smokers chose not to smoke in front of) attenuated craving (Conklin et al 2013). The present report demonstrated similar results, indicating that nonsmoking cues may influence craving following exposure to proximal smoking cues. These results provide additional evidence that a combination of cigarette availability and environmental cues influence craving and smoking behavior and suggest the need for greater attention to the role that cues associated with smoking abstinence play in smoking behavior.
The majority of quit attempts end in lapse which in turn often portend relapse (Brandon et al 1986, Kirchner et al 2012, Piasecki 2006). The DST attempts to model both by assessing the latency to smoke a first cigarette (i.e. lapse) and smoking beyond a first cigarette (i.e. relapse). In the present study we demonstrated that latency to smoke is affected by environments and self-reported craving which may suggest that real-world lapses are also influenced by these same factors. Moreover, in the present study, number of total cigarettes smoked was associated with environment and withdrawal suggesting the interaction of these factors may be critical in transitioning from lapse to relapse. Future studies of real world lapse and relapse are needed to evaluate the interactive influence of environment and self-reported craving and withdrawal as predictors of lapse and relapse.
This study has limitations. First, a neutral- or no- cue condition was not included as in other cue reactivity studies (Payne et al 1991, Carter and Tiffany 1999). The lack of such a condition makes it difficult to evaluate whether nonsmoking environment cues truly suppressed craving. Second, a small sample size was used to examine the effect environment has on the ability to resist smoking. Nonetheless, the results of this study can assist in the design of future studies that examine environments associated with prior drug use.
The present study provides further evidence of the influence of smoking related environments on craving and smoking behavior. Ongoing studies will evaluate the effect of incorporating smoking related contexts into an extinction-based treatment on smoking cessation outcomes. Further research is necessary to examine the specific and interactive effects of smoking-related environments on real-world smoking lapse and relapse and research on the development of novel interventions to minimize these influences.
Highlights.
Smoking environments increase smoking urge and promote smoking behavior.
Subjects initiated smoking sooner and smoked more after exposure to smoking cues.
Greater craving associated with a shorter time to initiate smoking.
Reduced craving after exposure to nonsmoking environments.
Withdrawal associated with cigarettes smoked during smoking environment sessions.
Acknowledgments
This research was supported by the National Institute on Drug Abuse [grant numbers R01 DA038442 (FJM) and R01 DA038442-S1 (FJM)].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brandon TH, Tiffany ST, Baker TB. The process of smoking relapse. NIDA Res Monogr. 1986;72:104–17. [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Meta analysis of cue reactivity in addiction research. Addiction. 1999;92:15–26. [PubMed] [Google Scholar]
- Conklin CA. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp Clin Psychopharmacol. 2006;14:12–9. doi: 10.1037/1064-1297.14.1.12. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Robin N, McClernon FJ, Salkeld RP. Bringing the real world into the laboratory: personal smoking and nonsmoking environments. Drug Alcohol Depend. 2010;111:58–63. doi: 10.1016/j.drugalcdep.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ. Proximal versus distal cues to smoke: the effects of environments on smokers’ cue-reactivity. Exp Clin Psychopharmacol. 2008;16:207–14. doi: 10.1037/1064-1297.16.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Salkeld RP, Perkins KA, Robin N. Do people serve as cues to smoke? Nicotine Tob Res. 2013;15:2081–7. doi: 10.1093/ntr/ntt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklin CA, Vella EJ, Joyce CJ, Salkeld RP, Perkins KA, Parzynski CS. Examining the relationship between cue-induced craving and actual smoking. Exp Clin Psychopharmacol. 2015;23:90–6. doi: 10.1037/a0038826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droungas A, Ehrman RN, Childress AR, O’Brien CP. Effect of smoking cues and cigarette availability on craving and smoking behavior. Addict Behav. 1995;20:657–73. doi: 10.1016/0306-4603(95)00029-c. [DOI] [PubMed] [Google Scholar]
- Drummond S, Tiffany ST, Glautier S, Remington B, editors. Cue exposure in understanding and treating addictive behaviors. New York: Wiley; 1995. pp. 1–17. [Google Scholar]
- Fuchs RA, Lasseter HC, Ramirez DR, Xie X. Relapse to drug seeking following prolonged abstinence: the role of environmental stimuli. Drug Discov Today Dis Models. 2008;5:251–58. doi: 10.1016/j.ddmod.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–3. doi: 10.1136/tc.7.1.92a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, Wileyto EP. Relapse dynamics during smoking cessation: recurrent abstinence violation effects and lapse-relapse progression. J Abnorm Psychol. 2012;121:187–97. doi: 10.1037/a0024451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Kaganovsky K, Shaham Y, Bossert JM. Role of corticostriatal circuits in context-induced reinstatement of drug seeking. Brain Res. 2015;1628:219–32. doi: 10.1016/j.brainres.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClernon FJ, Conklin CA, Kozink RV, Adcock RA, Sweitzer MM, et al. Hippocampal and Insular Response to Smoking-Related Environments: Neuroimaging Evidence for Drug-Context Effects in Nicotine Dependence. Neuropsychopharmacology. 2016;41:877–85. doi: 10.1038/npp.2015.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, et al. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. J Psychopharmacol. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine Tob Res. 2012;14:1362–71. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TJ, Schare ML, Levis DJ, Colletti G. Exposure to smoking-relevant cues: effects on desire to smoke and topographical components of smoking behavior. Addict Behav. 1991;16:467–79. doi: 10.1016/0306-4603(91)90054-l. [DOI] [PubMed] [Google Scholar]
- Piasecki TM. Relapse to smoking. Clin Psychol Rev. 2006;26:196–215. doi: 10.1016/j.cpr.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, Hickcox M. First lapses to smoking: within-subjects analysis of real-time reports. J Consult Clin Psychol. 1996;64:366–79. doi: 10.1037//0022-006x.64.2.366. [DOI] [PubMed] [Google Scholar]
- Tiffany ST. A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev. 1990;97:147–68. doi: 10.1037/0033-295x.97.2.147. [DOI] [PubMed] [Google Scholar]