Abstract
Wilson disease is caused by the accumulation of copper in the liver, brain or other organs, due to the mutation in ATP7B gene, which encodes protein that helps in excretion of copper in the bile canaliculus. Clinical presentation varies from asymptomatic elevation of transaminases to cirrhosis with decompensation. Hepatocellular carcinoma is a known complication of cirrhosis, but a rare occurrence in Wilson disease. We present a case of neurological Wilson disease, who later developed decompensated cirrhosis and hepatocellular carcinoma.
Abbreviations: ALP, alkaline phosphatise; ALT, alanine aminotransferase; ANA, anti-nuclear antibody; anti-LKM1, anti-liver kidney microsomal antibody type1; ASMA, anti-smooth muscle antibody; AST, aspartate aminotransferase; HCC, hepatocellular carcinoma; MPCT, multiphasic computed tomography; SAAG, serum-ascites albumin gradient; TACE, trans-arterial chemo-embolization
Keywords: Barcelona clinic liver cancer (BCLC) staging, cirrhosis, decompensation, d-penicillamine
Wilson disease is a rare autosomal recessive disease occurring due to the mutation in ATP7B gene (located on chromosome 13) involved in transporting copper. This leads to progressive copper deposition in liver and other organs like brain, kidneys and cornea.1 The clinical presentation is variable and patients may have hepatic, neurologic or psychiatric manifestations—either alone or in combination. Neurological features predominantly include extra-pyramidal symptoms and cerebellar dysfunction.2 Psychiatric manifestations vary from anti-social behaviour, depression to psychosis and neurosis.2 Hepatic manifestations include asymptomatic elevation of transaminases, chronic hepatitis or cirrhosis—with or without decompensation.1 In advanced Wilson disease, cirrhosis and its sequelae are common. Hepatocellular carcinoma (HCC) usually occurs in patients with cirrhosis; however, it is rarely encountered in patients with Wilson disease. We present a rare case of Wilson disease with development of HCC during the natural course of the disease.
Case
In 2003, a 28-year-old male presented with tremors in bilateral hands, dystonic posturing, dysarthria and drooling of saliva. He denied any prior history of jaundice, abdominal distension, difficulty in vision, joint pains, abnormal skin pigmentation, behavioural and psychiatric symptoms. He did not have any siblings and none of his family members had similar illness. His haemoglobin was 7.8 g/dL, total leucocyte count was 8700/μL, platelet count was 221,000/μL and peripheral smear did not show any evidence of haemolysis. Ocular slit-lamp examination revealed bilateral Kayser–Fleischer rings. Serum copper was 34.42 μg/dL (normal value <15 μg/dL), serum ceruloplasmin was 1.83 mg/dL (normal value 20–40 mg/dL) and 24 h urine copper was 52.5 μg/24 h (normal value <40 μg per 24 h). On the basis of low ceruloplasmin, elevated 24 h urine copper and presence of Kayser–Fleischer rings, he was diagnosed as Wilson disease. His total bilirubin was 0.8 mg/dL, alanine aminotransferase was 12 U/L, aspartate aminotransferase was 28 U/L and alkaline phosphatase was 126 U/L. He was started on trientene, zinc acetate and trihexyphenidyl, after which his symptoms improved. In addition, carbidopa and levodopa were added for the initial 3 years of the disease. He was followed up intermittently till 2011, and thereafter was lost to follow up for 2 years.
In 2013, he presented with abdominal pain in the right upper quadrant, increasing abdominal distension, anorexia and weight loss of 2 months duration. There was no history of worsening of neurological symptoms, gastrointestinal bleeding, jaundice or altered sensorium. He reported that he had continued trientene, trihexyphenidyl and zinc acetate since his last visit to the hospital in 2011. Ultrasound abdomen revealed a shrunken liver with multiple space occupying lesions in both lobes of the liver, splenomegaly, dilated portal vein (15 mm diameter at porta) and ascites. The ascitic fluid evaluation was suggestive of high serum-ascites albumin gradient (SAAG) (>1.1 g/dL), with evidence of spontaneous bacterial peritonitis. His laboratory investigations revealed total bilirubin 1.5 mg/dL, alanine aminotransferase 56 U/L, aspartate aminotransferase 110 U/L and alkaline phosphatise 360 U/L. Hepatitis B surface antigen, total anti-HBcAb, anti-HCV antibody, hepatitis C RNA, ANA, anti-LKM1 and ASMA were negative. Serum alpha feto-protein level was 48,480 ng/mL. Upper gastrointestinal endoscopy did not reveal any varices. Multiphasic computed tomography (MPCT) of the liver showed multiple variable-sized arterial enhancing lesions in both lobes of the liver with washout in the delayed phase. The largest lesion was in the left lobe of the liver (segments II and III), measuring 8.8 cm × 9.2 cm. The main portal vein was normal (Figure 1A and B). Bone scan and positron emission tomography (PET) scan did not reveal any extra-hepatic metastasis. The patient and his relatives were counselled regarding the treatment options. He underwent trans-arterial chemo-embolization (TACE) and was started on salt restricted diet, diuretics and other supportive measures. One month after TACE, his ascites reduced markedly. The liver function tests were as follows—total bilirubin 0.6 mg/dL (normal value 0.8–1.0 mg/dL), AST 29 U/L (normal value up to 50), ALT 23 U/L (normal value up to 50) and albumin were 3 g/dL (4–5.5 g/dL). Follow-up MPCT liver showed partial tumour response to TACE (Figure 2A–C). The patient was planned for a repeat session of TACE for residual disease. However, he did not follow-up for the planned visit, despite repeat attempts at communication via telephone and mail.
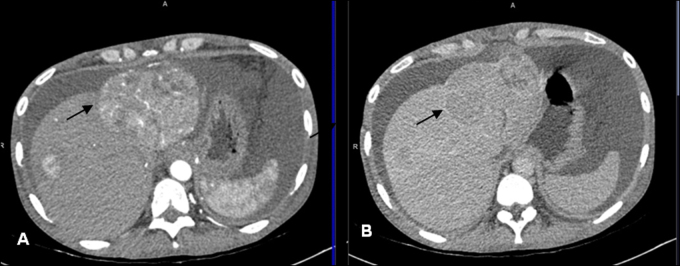
Figure 1.
Axial sections of multiphasic CT liver showing multiple variable-sized mass lesions in the segments 2, 4a and 8 of the liver, enhancing in the arterial phase (A) and showing washout in the delayed phase (B) suggestive of multifocal HCC. The largest lesion is seen in segment 2 of the liver (arrow), is well defined, heterogenously enhancing and exophytic with small satellite nodules around. Liver is small in size and ascites are present.
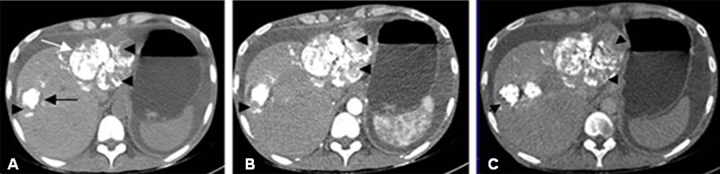
Figure 2.
Axial sections of multiphasic CT liver performed at 1 month following first session of trans-arterial chemo-embolization (TACE). Non-contrast axial CT image (A) depicting radiodense lipiodol deposited in the multiple liver lesions. The tiny lesions are completely covered with lipiodol while the two larger lesions in segments 2 (white arrow) and 8 (black arrow) are showing lipiodol defects (arrow heads) which are enhancing on the arterial phase (B) with washout in the delayed phase (C) CT images (arrow heads), suggesting partial response to TACE.
Discussion
Wilson disease is a metabolic disorder which predominantly presents with hepatic or neurologic symptoms—either alone or in combination. It is a rare disease with worldwide prevalence of approximately 30/million population.3 Mutation in ATP7B gene causes impairment of copper secretion into the bile canaliculus and copper binding into ceruloplasmin. This results in toxic levels of copper in the hepatocytes and spill into other organs. Hepatocyte apoptosis and mitochondrial oxidative injury is postulated to be the mechanism of copper-induced injury in hepatocytes.4, 5 Due to availability of chelating agents, life expectancy has now significantly increased.6
In a recent study, of the 130 patients of Wilson disease followed for a median duration of 15 years, only 2 cases of Wilson disease developed HCC; annual HCC risk was 0.09% for all patients and 0.14% for cirrhotic patients.7 Another large study of 1186 patients of Wilson disease reported the development of malignancy in 14 patients (HCC-8; intrahepatic cholangiocellular carcinomas-6), indicating an incidence of 0.28 per 1000 person years for hepatobiliary malignancies.8 In both these studies, risk of HCC was low even in cirrhotic patients, and this led the authors to recommend that regular surveillance for HCC is not required in patients with Wilson disease. In another retrospective study of 363 patients with Wilson disease, 11 patients developed malignancies, including HCC, hepatoma, cholangiocarcinoma and poorly differentiated adenocarcinomas of undetermined primary site.9 The duration between the diagnosis of Wilson disease and HCC diagnosis is variable, with some reports stating it to be as long as 50 years.8 In another study, Pfeiffenberger reported median age of HCC diagnosis as 53 (range 33–72) years. The median time duration between Wilson disease diagnosis and HCC diagnosis was 20.5 (range 2–41) years.8 Our patient developed HCC after 10 years of onset of symptoms of Wilson disease. Ours being a tertiary care centre, we treat a large number of HCC patients. Between 1989 and 2015, of the 1321 HCC patients registered in our Liver Clinic, this was the first case of HCC developing in a patient with Wilson disease.
Various hypotheses have been put forward for the development of hepatocarcinogenesis in Wilson disease. Recent data in animal (rat) models suggest that accumulation of iron and synergistic radical formation with copper leads to hepatic fibrosis and hepatocarcinogenesis.10 Also, treatment with d-penicillamine leads to iron accumulation and subsequent injury to hepatocytes.11 In a mouse model, copper causes oxidative injury and various enzyme inhibition and death of hepatocytes. Thiamine supplementation has shown to attenuate the HCC in ATP7B mouse model of Wilson disease.12 There is lack of data in humans on role of thiamine and anti-oxidants.
There are no specific guidelines for treatment of HCC in cases of Wilson disease. Standard management protocols are followed as per the Barcelona Clinic Liver Cancer (BCLC) staging. Since our patient had intermediate stage of HCC (BCLC B), trans-arterial chemo-embolization was considered as a suitable therapeutic option. The patient tolerated the first session of TACE well and had partial response. Thereafter, he was lost to follow-up.
In conclusion, HCC is a rare complication that may develop in Wilson disease. Familiarity with this association is important to facilitate early diagnosis and management.
Author Contributions
Deepak Gunjan—drafting of manuscript.
Shalimar—drafting of manuscript, critical revision.
Neeti Nadda—drafting of manuscript.
Saurabh Kedia—patient management.
Baibaswata Nayak—patient management.
Shashi Bala Paul—patient management.
Shivanand Ramachandra Gamanagatti—patient management.
Subrat Kumar Acharya—critical revision.
Conflicts of Interest
The authors have none to declare.
References
- 1.Ala A., Walker A.P., Ashkan K., Dooley J.S., Schilsky M.L. Wilson's disease. Lancet. 2007;369(9559):397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- 2.Bandmann O., Weiss K.H., Kaler S.G. Wilson's disease and other neurological copper disorders. Lancet Neurol. 2015;14(1):103–113. doi: 10.1016/S1474-4422(14)70190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frydman M. Genetic aspects of Wilson's disease. J Gastroenterol Hepatol. 1990;5:483–490. doi: 10.1111/j.1440-1746.1990.tb01427.x. [DOI] [PubMed] [Google Scholar]
- 4.Sauer S.W., Merle E., Opp S. Severe dysfunction of respiratory chain and cholesterol metabolism in Atp7b−/− mice as a model for Wilson disease. Biochim Biophys Acta. 2011;1812:1607–1615. doi: 10.1016/j.bbadis.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Zischka H., Lichtmannegger J., Schmitt S. Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Investig. 2011;121:1508–1518. doi: 10.1172/JCI45401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts E.A., Schilsky M.L., American Association for Study of Liver Diseases (AASLD) Diagnosis and treatment of Wilson disease: an update. Hepatology. 2008;47(6):2089–2111. doi: 10.1002/hep.22261. [DOI] [PubMed] [Google Scholar]
- 7.van Meer S., de Man R.A., van den Berg A.P. No increased risk of hepatocellular carcinoma in cirrhosis due to Wilson disease during long-term follow-up. J Gastroenterol Hepatol. 2015;30(3):535–539. doi: 10.1111/jgh.12716. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffenberger J., Mogler C., Gotthardt D.N. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015;35(5):1615–1622. doi: 10.1111/liv.12727. [DOI] [PubMed] [Google Scholar]
- 9.Walshe J.M., Waldenström E., Sams V., Nordlinder H., Westermark K. Abdominal malignancies in patients with Wilson's disease. QJM. 2003;96(9):657–662. doi: 10.1093/qjmed/hcg114. [DOI] [PubMed] [Google Scholar]
- 10.Kato J., Kobune M., Kohgo Y. Hepatic iron deprivation prevents spontaneous development of fulminant hepatitis and liver cancer in Long-Evans Cinnamon rats. J Clin Investig. 1996;98(4):923–929. doi: 10.1172/JCI118875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medici V., Di Leo V., Lamboglia F. Effect of penicillamine and zinc on iron metabolism in Wilson's disease. Scand J Gastroenterol. 2007;42(12):1495–1500. doi: 10.1080/00365520701514495. [DOI] [PubMed] [Google Scholar]
- 12.Sheline C.T. Thiamine supplementation attenuated hepatocellular carcinoma in the Atp7b mouse model of Wilson's disease. Anticancer Res. 2011;31(10):3395–3399. [PMC free article] [PubMed] [Google Scholar]