ABSTRACT
Pneumocystis remains an important pathogen of immunosuppressed patients, causing a potentially life-threatening pneumonia. Despite its medical importance, the immune responses required to control infection, including the role of interleukin-17 (IL-17), which is important in controlling other fungal infections, have not been clearly defined. Using flow cytometry and intracellular cytokine staining after stimulation with phorbol myristate acetate and ionomycin, we examined gamma interferon (IFN-γ), IL-4, IL-5, and IL-17 production by lung lymphocytes in immunocompetent C57BL/6 mice over time following infection with Pneumocystis murina. We also examined the clearance of Pneumocystis infection in IL-17A-deficient mice. The production of both IFN-γ and IL-17 by pulmonary lymphocytes increased during infection, with maximum production at approximately days 35 to 40, coinciding with peak Pneumocystis levels in the lungs, while minimal changes were seen in IL-4- and IL-5-positive cells. The proportion of cells producing IFN-γ was consistently higher than for cells producing IL-17, with peak levels of ∼25 to 30% of CD3+ T cells for the former compared to ∼15% for the latter. Both CD4+ T cells and γδ T cells produced IL-17. Administration of anti-IFN-γ antibody led to a decrease in IFN-γ-positive cells, and an increase in IL-5-positive cells, but did not impact clearance of Pneumocystis infection. Despite the increases in IL-17 production during infection, IL-17A-deficient mice cleared Pneumocystis infection with kinetics similar to C57BL/6 mice. Thus, while IL-17 production in the lungs is increased during Pneumocystis infection in immunocompetent mice, IL-17A is not required for control of Pneumocystis infection.
KEYWORDS: AIDS, IL-17, pneumocystis, T-cell immunity
INTRODUCTION
Pneumocystis is an opportunistic fungus that causes pneumonia in immunocompromised hosts and infection, but not clinically significant disease, in healthy hosts. Host defense against Pneumocystis infection is critically dependent upon CD4+ T cells, with depletion of CD4+ T cells in animal models resulting in susceptibility to Pneumocystis pneumonia (1–6). CD8+ cells are not required for clearance of Pneumocystis but appear to play a role in decreasing CD4-dependent inflammation (5, 7, 8).
Interleukin-17 (IL-17) is a proinflammatory cytokine secreted by a variety of cells, including CD4+ Th17 cells, γδ T cells, NK and NKT cells, and ILC3 cells (9, 10). IL-23 is a cytokine secreted by antigen-presenting cells that promotes the secretion of IL-17 and maintenance of Th17 cells (11–13). IL-17 induces production of chemokines and cytokines, as well as antibacterial peptides that are important primarily in controlling extracellular bacterial and fungal pathogens (12). IL-17 appears critical to controlling mucocutaneous Candida infections, which are a major manifestation of IL-17 related genetic defects in humans (9, 14).
Although IL-17 plays a role in the control of a variety of fungal infections, the role of IL-17 and CD4+ Th17 cells in immunity to Pneumocystis has not been clearly defined. In one study, IL-23 knockout (KO) mice had higher peak organism loads, as did animals given anti-IL-17 or anti-IL-23p19 neutralizing antibodies, although all mice ultimately cleared infection (15). In another study, mice with defective NF-κB signaling in alveolar epithelial cells showed delayed clearance of Pneumocystis infection and decreased pulmonary Th17 cells (16). However, gamma interferon (IFN-γ) knockout (KO) nude mice had higher organism levels than nude mice, despite higher levels of IL-17 and greater numbers of Th17 cells in bronchoalveolar lavage (BAL) fluid samples (17).
The present study was undertaken to examine the kinetics of Th17 cells, as well as Th1 and Th2 cells, in the lungs of immunocompetent mice infected with Pneumocystis and to clarify the role of IL-17 in control of Pneumocystis infection by utilizing IL-17A KO mice. We also examined the impact of anti-IFN-γ antibody on the different Th subsets, as well as on the clearance of Pneumocystis infection. In these studies, we utilized a cohousing model of infection rather than the transtracheal model used in most of the previous studies because the bolus of organisms and host products used in the latter may induce inflammatory and immune responses that are not representative of those that occur during natural infection.
RESULTS
To better understand the cellular responses to Pneumocystis infection in healthy animals, we examined cell populations in the lungs of immunocompetent C57BL/6 mice over time following exposure. We initially characterized the frequency of NK cells, NKT cells, and γδ T cells because our prior microarray studies in immunocompetent animals had suggested a potential role for these cells in early infection (maximum at ∼14 days) (18). In three separate experiments, we analyzed these cell populations in animals that had been exposed for 7 to 24 days. Although there was some variability in the cell numbers over time, especially for NK cells, we observed no consistent increase in the percentages of any of these cell populations compared to control animals (data not shown).
Given the importance of adaptive immunity in the clearance of Pneumocystis and our prior identification by microarray studies of a large number of genes related to adaptive immunity that showed increased expression that peaked at days 35 to 42 after exposure (18), we next focused on characterizing cell number and function during this period.
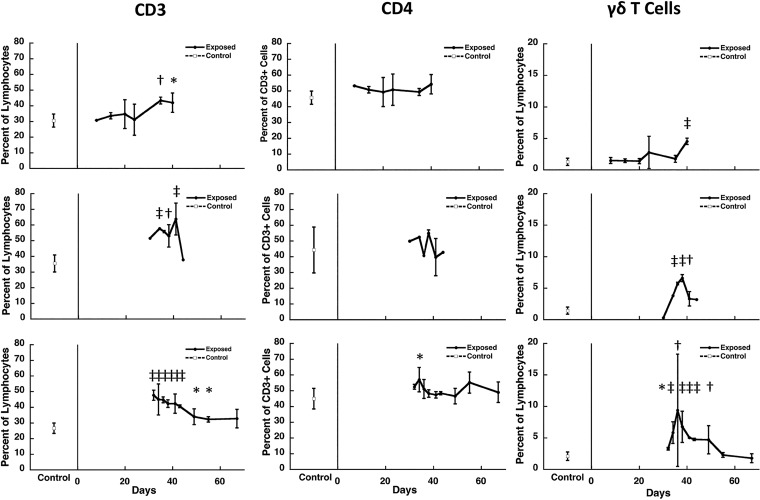
We performed three separate experiments overlapping this period. As shown in Fig. 1, there was a significant increase in CD3+ T lymphocytes in the lungs of immunocompetent animals infected with Pneumocystis that was first seen at days 32 to 35, with a gradual decline to normal levels after day 40. Although there was in general no increase in the proportion of CD3+ T cells that were CD4+ (Fig. 1), this does represent an increase given the overall increase in CD3+ T cells. γδ T cells also showed an increase, with kinetics similar to that seen for CD3+ T cells, although at lower levels (Fig. 1). Consistent with the CD3+ T cell increase, there was a concomitant decrease in the proportion of CD19+ B cells (data not shown). We did not initially analyze CD8+ T cells in these studies, due to limitations in the number of fluorochromes that could be measured in one tube at the time the studies were performed and the limited cell numbers.
FIG 1.
Kinetics of cell subsets in the lungs of Pneumocystis-infected C57BL/6 mice. The percentage of CD3+ T cells and γδ T cells as a percentage of total lymphocytes and of CD4+ T cells as a percentage of CD3 cells isolated from the lungs of Pneumocystis-infected mice over time in three separate studies that cover the period from initial exposure to clearance of infection (days 8 to 76 following start of cohousing with a seeder) was determined. Each row represents the same experiment. The results represent the means and standard deviations for mice from all cages within a single experiment at a given time point. Controls, indicated at the left of each panel, are the mean for uninfected animals from the same experiment that were processed at the same time as the exposed animals. The cells were isolated from lungs at the indicated time points (x axis) after the start of cohousing with Pneumocystis-infected seeders and analyzed by flow cytometry. Due to the limitations in the number of animals that could be housed per cage, two to four exposed animals were studied at each time point per experiment, except as indicated by the absence of error bars, when only one animal was available for analysis. Six to ten controls were included in each experiment. Significant differences compared to uninfected controls are indicated by symbols: *, P < 0.05; †, P < 0.01; and ‡, P < 0.001.
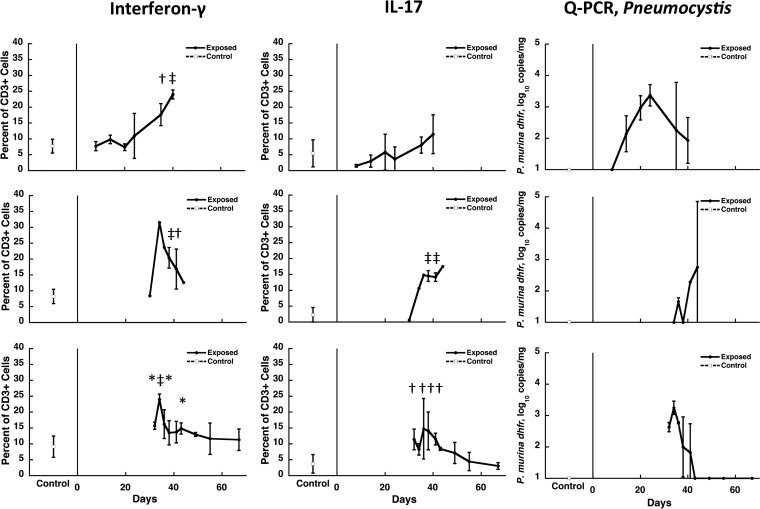
To determine whether Pneumocystis infection in these mice was associated with increases in cells producing IFN-γ and IL-17, we utilized intracellular staining following in vitro stimulation with phorbol myristate acetate (PMA) and ionomycin. As shown in Fig. 2, CD3+ T cells producing both IFN-γ and IL-17 were increased with similar kinetics, peaking at ∼35 to 40 days. Of note, the proportion of IFN-γ+ cells was consistently higher than IL-17+ cells, with a peak of ∼25 to 30% of CD3+ T cells for the former compared to ∼15% for the latter. The kinetics of these cytokine-producing cells also generally tracked the Pneumocystis organism load in the lungs (Fig. 2).
FIG 2.
Kinetics of cytokine-producing cells in the lungs of Pneumocystis-infected C57BL/6 mice. The percentage of CD3+ T cells that produce either IFN-γ or IL-17 (two left sets of panels) and the Pneumocystis burden (right panels) for the same studies are presented as described for Fig. 1. Each row represents the same experiment. The results represent the means and standard deviations for mice from all cages within a single experiment at a given time point. Controls, indicated at the left of each panel, are the means for uninfected animals from the same experiment that were processed at the same time as the exposed animals. The cells were isolated from lungs at the indicated time points (x axis) after the start of cohousing with Pneumocystis-infected seeders and were stimulated in vitro for 4 h with PMA and ionomycin prior to analysis. For Pneumocystis burden analysis, quantitation of the P. murina dihydrofolate reductase gene (dhfr) was performed by real-time PCR. Due to the limitations in the number of animals that could be housed per cage, two to four exposed animals were studied at each time point per experiment, except as indicated by the absence of error bars, when only one animal was available for analysis. Six to ten controls were included in each experiment. Significant differences compared to uninfected controls are indicated by symbols: *, P < 0.05; †, P < 0.01; and ‡, P < 0.001.
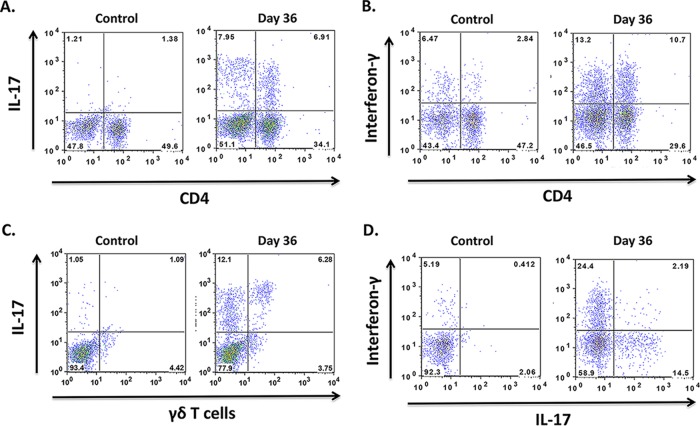
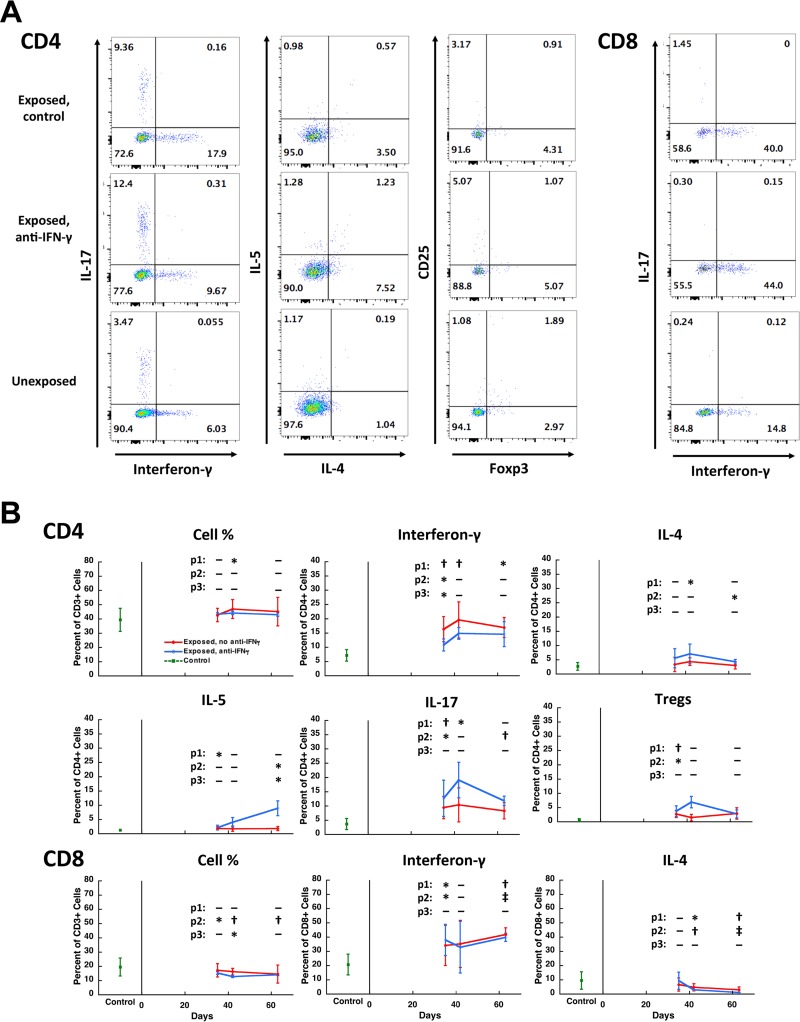
Flow cytometry was used to further characterize the cells producing IFN-γ and IL-17. As shown in Fig. 3A and C, both CD4+ and γδ T cells produced IL-17. Interferon-γ was produced by both CD4+ and CD4− T cells (Fig. 3B; IFN-γ staining was not performed in the same tube as staining for γδ T cells). Among CD4 cells, IL-17 production and IFN-γ production were largely mutually exclusive, which is consistent with a Th1 and Th17 lineage for these cells (Fig. 3D).
FIG 3.
Characterization of cells producing IL-17 and IFN-γ in Pneumocystis-infected lungs. Representative dot plot panels of cells from the lungs of uninfected (control) and Pneumocystis-infected (day 36) mice show the percentages of CD3+ cell subsets that produce IL-17 or IFN-γ. Panels A to C are gated on CD3+ cells, while panel D is gated on CD3+ CD4+ cells. Panels A and C show that both CD4+ cells and γδ T cells produce IL-17, while panel B shows production of IFN-γ by CD4+ and CD4− T cells. As shown in panel D, cytokine-producing CD4+ T cells are positive for either IL-17 or IFN-γ, but rarely both. The numbers within the quadrants indicate the percentages of cells in each quadrant. The mean (standard deviation) day 36 value for IFN-γ production by CD4+ cells was 5.2% (4.8) of CD3+ T cells, while the corresponding values for IL-17 production by CD4+ cells and γδ T cells were, respectively, 3.7% (2.7) and 4.2% (2.4) of CD3+ T cells.
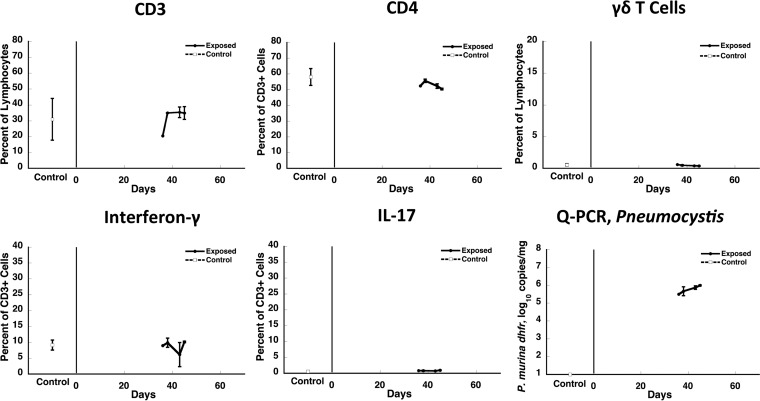
We next examined the parameters described above in CD40L KO mice at 36 to 45 days of cohousing to determine whether IFN-γ and IL-17 were produced in animals that are highly susceptible to Pneumocystis infection. Despite active Pneumocystis infection with organism loads of >105/mg of lung tissue, the percentages of CD3+, CD4+, and γδ T cells and the proportions of cells producing IFN-γ and IL-17 in the lungs of these animals were unchanged compared to uninfected control CD40L KO mice (Fig. 4).
FIG 4.
Kinetics of cell subsets and cytokine-producing cells in the lungs of Pneumocystis-infected CD40L-KO mice. The percentage of CD3+ T cells and γδ T cells as a percentage of total lymphocytes and CD4+ T cells as a percentage of CD3 cells (top row) and the percentage of CD3+ T cells that produce either IFN-γ or IL-17 (bottom row left and middle panels) that were isolated from lungs of Pneumocystis-infected mice over time in one experiment were determined. The Pneumocystis burden from the same animals is shown in the bottom right panel. The results represent the means and standard deviations of mice from all cages within a single experiment at a given time point. The time points studied (days 36 to 45 after start of cohousing with a seeder) are those during which responses in C57BL/6 mice were maximal. Due to the limitations in the number of animals that could be housed per cage, two exposed animals were studied at each time point per experiment, except as indicated by the absence of error bars, when only one animal was available for analysis. Three controls were included in the study. There were no significant differences in any of the cell populations compared to uninfected controls at any time point.
To better characterize the CD4+ T cell subsets responding to Pneumocystis infection, we performed additional studies; we simultaneously examined the effects of anti-IFN-γ antibody on these subsets. These more limited studies focused on days 35 and 42, the days of peak responses, as well as day 63, to determine whether anti-IFN-γ antibody interfered with clearance of Pneumocystis. Figure 5A shows representative flow histograms of these analyses. As shown in Fig. 5B, CD4+ T cell responses in control infected mice were primarily Th1, peaking at 19.6%, and to a lesser extent Th17 (peak, 10.4%), with minimal Th2 responses, as defined by IL-4 (peak, 4.4%) or IL-5 (peak, 1.9%) production. T regulatory cells (Tregs) also showed a modest increase (peak, 2.9%). The administration of anti-IFN-γ antibody was associated with a shift from a Th1 phenotype (peak, 14.9%) to a trend to a greater Th17 (peak, 19.1%) and Th2 (peak, 7.1% for IL-4 and 8.9% for IL-5) phenotype and a further increase in Tregs (peak, 6.9%). Of note, CD8+ T cells also showed increased and high levels of production of IFN-γ (peak, ∼40%) but decreased levels of IL-4, and this was not impacted by anti-IFN-γ antibody (Fig. 5B). The levels of IL-5- and IL-17-producing CD8+ T cells were low (mean < 1 to 2%) in both unexposed controls and infected animals.
FIG 5.
CD4+ and CD8+ T cell subsets during Pneumocystis infection. (A) Representative dot plot panels of lymphocytes from the lungs of three Pneumocystis-infected and uninfected animals showing the percentage of CD4+ (left three columns of panels) and CD8+ (right column of panels) T cell subsets that are producing IFN-γ, IL-17, IL-4, or IL-5 or are Tregs (CD25+/Foxp3+). The top row is from an untreated Pneumocystis-infected animal (day 35), the middle row is from a Pneumocystis-infected animal (day 35) that received anti-IFN-γ antibody twice weekly starting at the beginning of exposure to a Pneumocystis-infected seeder mouse, and the bottom row is from an uninfected control. Numbers within the quadrants indicate the percentage of cells in each quadrant. (B) Mean percent expression of the indicated parameter over time, combining animals from three experiments. CD4 and CD8 cell numbers were gated on CD3+ cells; other parameters were gated on CD4+ or CD8+ T cells. The red line indicates untreated Pneumocystis-infected animals, the blue line indicates anti-IFN-γ antibody treated Pneumocystis-infected animals, and the green square indicates uninfected control animals. Error bars represent standard deviations. Days 35 and 36 were combined for this analysis. P values are indicated as follows: p1, uninfected versus untreated Pneumocystis-infected animals; p2, uninfected versus anti-IFN-γ antibody-treated Pneumocystis-infected animals; p3, untreated versus anti-IFN-γ antibody-treated Pneumocystis-infected animals from the same time point. Significant differences are indicated as follows: *, P < 0.05; †, P < 0.01; and ‡, P < 0.001. Pneumocystis organism load as determined by Q-PCR was not significantly different between the untreated and anti-IFN-γ antibody treated groups at any time point. The mean log10 (standard deviation) dhfr copies/mg of lung tissue for untreated and anti-IFN-γ antibody-treated animals, respectively, were as follows: days 35 to 36, 3.73 (0.32) and 4.04 (0.25); day 42, 2.79 (1.37) and 3.33 (1.35); and day 63, 2.82 (1.87) and 1.36 (0.39). By day 63, one animal in each group had cleared infection, and all animals in both groups had developed antibody responses as determined by ELISA. Two animals with markedly enlarged lungs were excluded from the analysis due to a concern that they were coinfected with another pathogen; inclusion of these animals did not change the results of the statistical analyses.
Previous studies have demonstrated that IFN-γ KO mice can clear Pneumocystis infection with kinetics similar to that in immunocompetent mice (19); consistent with these data, animals that received anti-IFN-γ antibody were clearing Pneumocystis infection by day 63.
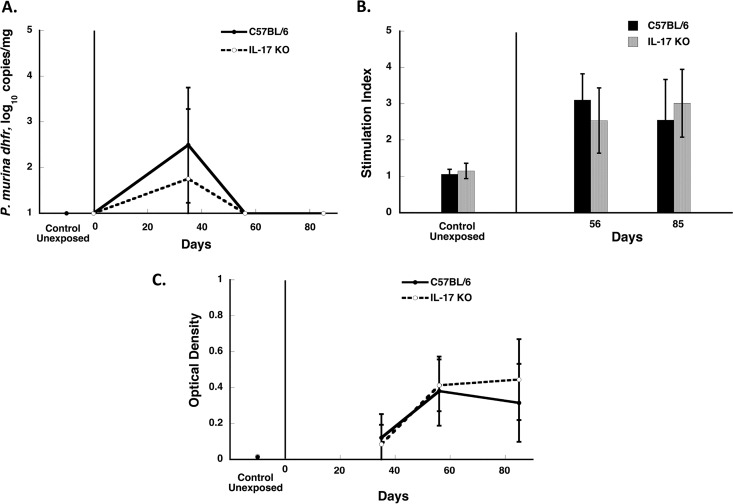
There are limited data on the role of IL-17 in clearance of Pneumocystis. Thus, we undertook studies with IL-17A KO mice to determine their susceptibility to infection. We undertook two studies to look at the kinetics of clearance over 85 days in these animals compared to immunocompetent C57BL/6 mice, using our cohousing model. In both studies, IL-17A KO mice cleared Pneumocystis with kinetics similar to those for wild-type mice (Fig. 6A). Moreover, splenocyte proliferation assays and antibody detection by enzyme-linked immunosorbent assay (ELISA) also showed responses similar to those for wild-type animals (Fig. 6B and C), demonstrating that the cellular and antibody responses to Pneumocystis were intact in these animals. The presence of detectable antibodies also confirms that these animals were in fact infected with Pneumocystis.
FIG 6.
Clearance of Pneumocystis infection in IL-17A KO mice. IL-17A KO and C57BL/6 mice were cohoused with a seeder mouse, and animals from each group were sacrificed at the indicated time points (days 35, days 56 to 58, and days 84 to 86 after the start of cohousing with an infected seeder). The results represent the means and standard deviations for mice from all cages within a single experiment at a given time point. (A) Pneumocystis organism load (as log10 dhfr copies/mg of lung tissue); (B) proliferative responses (indicated as the stimulation index) to crude Pneumocystis antigens; (C) anti-Pneumocystis antibodies (presented as the optical density) detected by ELISA using a crude Pneumocystis antigen preparation. Pneumocystis infection in the lung tissue was quantitated by Q-PCR using the single-copy dhfr gene as the target. There were no significant differences between C57BL/6 and IL-17A KO mice at any time point for any of the parameters.
DISCUSSION
In this study we have shown that both IFN-γ and IL-17 are produced by pulmonary lymphocytes in response to Pneumocystis infection in immunocompetent C57BL/6 mice but not in immunodeficient CD40L KO mice. These responses were temporally associated with the Pneumocystis organism load and peaked at approximately the same time as robust immune responses were detected by our earlier microarray studies (18). Of note, a higher proportion of cells produce IFN-γ compared to IL-17, with the former accounting for ∼25 to 30% of CD3+ T cells compared to ∼15% for the latter. Consistent with their importance in controlling Pneumocystis infection, CD4+ T cells accounted for a substantial proportion of the T cells producing these cytokines. Despite the temporal increase in Th17 and other IL-17-producing cells during Pneumocystis infection, IL-17A is not critical to the clearance of Pneumocystis in otherwise immunocompetent mice, as demonstrated by clearance of infection in IL-17A KO mice with kinetics similar to those of wild-type mice.
IL-17 plays a role in immunity to mucosal or skin infections with bacteria such as Staphylococcus aureus, Klebsiella pneumonia, and Pseudomonas aeruginosa (20–22). IL-17 is also a critical cytokine in controlling mucocutaneous Candida infections based on studies in animal models, as well as in humans with genetic defects (23). Indeed, chronic mucocutaneous candidiasis is a hallmark of patients with decreased IL-17, including IL-17A, production or function (9, 24), whereas, consistent with our studies, Pneumocystis pneumonia is rarely seen in this population (25). IL-17 is important in control of Aspergillus pulmonary infection in a dectin-1-dependent manner (26). In animal models of vaccination against endemic fungi (Coccidioides, Histoplasma, and Blastomyces species) (27), IL-17A is necessary for development of vaccine-induced resistance, and is dependent on Myd88 rather than dectin-1. We and others have shown that Myd88 is not required for the control of Pneumocystis infection, and although dectin-1 deficiency leads to transient increases in organism load, dectin-1 KO mice are able to clear Pneumocystis infection, further highlighting that IL-17 expression mediated by these pathways is not necessary for anti-Pneumocystis immunity (28–30). Although it is possible that other IL-17 family members may be playing a role in the control of Pneumocystis infection, unpublished data discussed in a review of IL-17 indicate that IL-17 receptor-deficient mice cleared infection similarly to wild-type mice, further supporting our observations (31).
Although the role of CD4+ cells in controlling Pneumocystis infection is well documented, it remains unclear whether any CD4+ Th cell subset is absolutely necessary for this control. In previous microarray studies of CD4+ T cells isolated from the lungs of immunocompetent mice, we have identified increases in expression of genes associated with Th1 cells, including IFN-γ, the Th1-specific transcription factor Tbet (Tbx21), and Cxcr3, whereas no or limited increases were seen in Th2- or Th17-specific cytokines or transcription factors (32). The present study supports a predominant Th1 and, to a lesser extent, Th17 response to Pneumocystis infection in immunocompetent animals, with a minimal Th2 response, although it is possible that a more sensitive assay for IL-4, such as the enzyme-linked immunosorbent spot assay, would have detected Th2 responses. In contrast, other studies have suggested that Th2 or Th17 cells may play an important role, although in some studies this is reflected by a transient increase in organism load in KO mice targeting specific pathways, with ultimate clearance of organisms (6, 15, 16, 33, 34). Of note, a previous study has shown that IFN-γ is also unnecessary for host immunity: IFN-γ−/− mice cleared Pneumocystis infection as quickly as IFN-γ+/− mice (19). Our observations in mice administered anti-IFN-γ antibody are consistent with this finding: although there was a shift away from Th1 toward Th2 and Th17 cells, consistent with the role of IFN-γ in augmenting Th1 cells (35), the clearance of Pneumocystis infection was not impacted. It is plausible that there are redundant mechanisms involving multiple Th cells and that the loss of one subclass of Th cells is compensated for by the other subclasses.
An important difference in the studies examining this issue is the animal model used. Most of the studies suggesting Th2-related protection have utilized an intratracheal inoculation model in which a bolus of organisms (together with residual host lung products, which possibly include inflammatory mediators) are intratracheally inoculated to induce infection. This is clearly different from naturally acquired infection which is transmitted via the respiratory route with presumably a very small number of organisms, and the transmission of infection is most closely mimicked by cohousing, the approach we utilized in our studies. Intratracheal inoculation leads to a sudden exposure to high levels of potentially immune-activating Pneumocystis products such as β-glucans and may lead to the activation of different pathways from that seen with inhalation of very small numbers of organisms in which, for example, the β-glucans in large part are masked (36).
Although CD8+ T cells are not critical to clearance of Pneumocystis, we found that a high proportion of pulmonary CD8+ T cells secrete IFN-γ during Pneumocystis infection and that this was unaffected by anti-IFN-γ antibody. Since Pneumocystis is not an intracellular pathogen, it is unlikely that this is the result of classic major histocompatibility complex class I antigen presentation, but it may be due to nonspecific activation of CD8+ T cells following the secretion of chemokines and cytokines in response to infection or to the cross-presentation of antigen by dendritic cells. It is also possible that not all of the cells we measured, especially CD8 cells, are within the lung parenchyma, since we did not stain in vivo with an anti-CD45 antibody, and such staining can distinguish between intravascular and parenchymal cells (37, 38). The modest increase in Tregs observed here is consistent with a prior report in which Tregs were shown to dampen inflammation in Pneumocystis mouse models (39).
Our study has also confirmed that γδ T cells increase during Pneumocystis infection, with kinetics similar to total CD3+ T cells, and that these cells can produce IL-17. Previous studies showed that γδ T cells are elevated in the blood and BAL fluid of individuals with AIDS and Pneumocystis pneumonia (PCP) (40). A recent study examining the role of these cells during Pneumocystis infection, using an intratracheal inoculation model, showed that γδ T cell KO mice actually clear infection more quickly and that γδ T cells appear to reduce excessive tissue inflammation and lung injury during infection through interactions with pulmonary CD8+ T cells and IFN-γ production (41). We also found using the cohousing model that γδ T cell-deficient mice are able to clear infection with kinetics similar to those of wild-type mice (data not shown) (5).
Among the genetic defects that have been studied to date in mouse models that do not lead to global loss of cell populations (e.g., Rag-deficient or scid mice), only the CD40-CD40L interaction has been shown to be absolutely required for ultimate control of Pneumocystis infection, since KO mice deficient in either CD40 or CD40L are highly susceptible to severe infection (18, 32, 42). Further studies are clearly needed to define the critical pathways required for immunity to Pneumocystis infection.
MATERIALS AND METHODS
Animals.
Healthy 8- to 10-week-old, female C57BL/6 mice were obtained from the National Institutes of Health (NIH; Bethesda, MD), The Jackson Laboratory (Bar Harbor, ME), or Envigo RMS, Inc. (Indianapolis, IN). IL-17A KO mice (Il17atm1Yiw) on a B6 background were obtained from Rachel Caspi, NEI, NIH, with the permission of Yoichiro Iwakura, Tokyo University (43); genotypes of animals used in susceptibility studies were confirmed by PCR. CD40L-KO mice (B6;129S2-Tnfsf5tm1Imx/J), which are highly susceptible to Pneumocystis infection (18), were obtained from The Jackson Laboratory. Mice were subsequently bred at the NIH. All studies were carried out under protocols approved by the NIH Clinical Center Animal Care and Use Committee.
Mouse infection model.
To examine the kinetics of cellular responses to Pneumocystis in the lungs of healthy hosts, infection was transmitted to C57BL/6 mice via the respiratory route by cohousing study animals with immunosuppressed seeder animals (scid or CD40L-KO) that had active PCP, which was subsequently verified by a quantitative real-time PCR (Q-PCR) assay; typically the seeders had >106 dhfr copies/mg lung tissue. Unexposed litter-mates served as controls. This cohousing infection model results in infection of animals with predictable kinetics; infection of healthy animals peaks at ca. 35 to 42 days and is cleared by ∼70 days (18, 44). For each experiment, one to two cages housed exposed mice, and one cage housed unexposed mice. Because of the limited number of animals that could be housed per cage, we were unable to conduct a complete kinetic study in a single experiment. Thus, experiments were designed to have overlapping time frames, with analysis ranging from 8 to 76 days after cohousing began. Similarly, we examined the cellular and cytokine responses in CD40L-KO mice at time points comparable to the peak responses in immunocompetent C57BL/6 mice at days 36 to 45 after the start of cohousing. One to four mice per group were sacrificed at each time point.
In a later series of experiments we undertook to better characterize the relative proportion of Th1, Th2, and Th17 CD4+ T cells, as well as Tregs and CD8 cells, in C57BL/6 mice at limited time points and to examine the effects of anti-IFN-γ antibody on these subsets. In the first experiment, we examined CD4+ T cell subsets at days 35 and 42. In the second and third experiments, three mice per time point were administered 0.5 mg of anti-IFN-γ antibody (clone XMG 1.2; rat IgG1 [kindly provided by George Deepe]) intraperitoneally twice weekly starting on the first day of exposure to Pneumocystis and for the duration on the study. Three mice per time point served as controls, two of which were administered 0.5 mg of rat IgG (Sigma-Aldrich, St. Louis, MO) twice weekly, and one of which was left untreated. The animals were sacrificed at days 35 and 42 (experiment 2) or at days 36 and 63 (experiment 3). In all of these studies, the lungs were divided into two portions: one was processed immediately for flow cytometric analysis, and the other was stored at −20°C or −80°C for quantitation of the organism load by Q-PCR.
The cohousing model was also used to examine the kinetics of Pneumocystis infection in IL-17A KO mice. For these studies, approximately equal numbers of IL-17A KO and C57BL/6 mice were cohoused with an infected seeder and sacrificed at days 35, days 56 to 58, and days 84 to 86, at which time the lungs, spleen, and serum were collected. Q-PCR was used to quantitate Pneumocystis organism load, and ELISA and proliferation assays were used to evaluate antibody and cell-mediated responses to Pneumocystis (44, 45). There were 15 animals per strain in experiment 1 and 6 to 7 animals per strain in experiment 2.
Preparation of lung cells.
Two methods were utilized to obtain lung lymphocytes. Initially, the lungs were perfused with phosphate-buffered saline (PBS), harvested, cut into small pieces, incubated with stirring at 37°C for 30 min in PBS with 1% EDTA, and then mashed through a 40-μm-pore size cell strainer. The cells were pelleted and resuspended in 44% Percoll in RPMI, and then 67% Percoll in PBS was gently underlaid. After centrifugation, the cells at the interface were harvested, washed, and incubated in ACK (ammonium-chloride-potassium) lysing buffer for 3 to 4 min. After centrifugation, the pellet was resuspended in RPMI medium containing 10% fetal calf serum (FCS), and 1% penicillin-streptomycin.
In later experiments, the lungs were minced into small pieces, which were incubated for 30 min at 37°C in 5 ml of lung enzyme cocktail (5 ml of RPMI, 12 mg of collagenase type I [Thermo Fisher Scientific, Waltham, MA], and 100 μg of DNase [Roche Diagnostics, Indianapolis, IN]), and passed through a 70-μm-pore size cell strainer. The cells were then incubated in ACK lysing buffer and resuspended in RPMI medium as described above.
Flow cytometry.
Cells were stained with optimal concentrations of the following fluorochrome-conjugated antibodies: CD3e Alexa Fluor 488 (clone 145-2C11), NK1.1 PerCP-Cy5.5 (clone PK136), γδ T cell receptor (clone GL3), and CD19APC (clone 1D3) from BD Biosciences, San Jose, CA, and CD3e PerCP-Vio700 (clone 145-2C11), CD4 APC-Vio770 (clone GK1.5), and CD8a VioGreen (clone 53-6.7) from Miltenyi Biotec, Inc., San Diego, CA, as well as mCD1d tetramers (unloaded or loaded with PBS-57; NIH Tetramer Core Facility, Atlanta, GA), for 30 min at room temperature. The cells were then washed two times with PBS–3% FCS and fixed in formaldehyde. For intracellular cytokine staining, lung cells were incubated with PMA (Sigma-Aldrich, St. Louis, MO) at 50 ng/ml and ionomycin (Sigma-Aldrich) at 500 ng/ml in the presence of BD Golgi Stop (BD Biosciences) for 4 h. The cells were then stained with the antibodies described above for 15 min at room temperature, washed two times with PBS–3% FCS, and fixed and permeabilized with BD Cytofix/Cytoperm (BD Biosciences). After a washing step with BD Perm/Wash buffer (BD Biosciences), the cells were stained with one or more of the following antibodies: anti-IL-17 phycoerythrin (PE) (clone TC11-18H10), anti-IFN-γ allophycocyanin (APC) or Alexa Fluor 488 (clone XMG1.2), IL-4 PE-Cy7 (clone 11B11), and IL-5 APC (clone TRFK5) from BD Biosciences for 15 to 30 min at room temperature. For Treg staining, unstimulated cells were processed using a transcription factor buffer set (BD Biosciences) according to the manufacturer's recommendations and stained with CD25 PE (clone PC61) and Foxp3 Alexa Fluor 488 (clone MF23) from BD Biosciences. For later experiments, dead cells were excluded using a Live/Dead fixable dead cell stain kit (violet fluorescent reactive dye; Thermo Fisher Scientific). Samples were acquired using either a FACSCalibur flow cytometer (BD Pharmingen, San Jose, CA) or a MACSQuant analyzer 10 flow cytometer (Miltenyi Biotec, Inc.), and the data were analyzed using FlowJo software (Tree Star, Inc., San Carlos, CA).
Q-PCR, ELISA, and cell proliferation assays.
DNA extraction from lung homogenates and subsequent quantitation of P. murina dihydrofolate reductase gene (dhfr) copies per mg of lung by means of a real-time PCR were performed as described previously (44). Since dhfr is a single-copy gene, the number of copies reflects the number of nuclei. The lower limit of the assay is 12 dhfr copies/mg of lung tissue.
P. murina antigens for ELISA and cell proliferation assays were prepared from partially purified Pneumocystis organisms or from lungs heavily infected with P. murina (as determined by Diff-Quik staining) by homogenization in PBS, followed by sonication and centrifugation; the collected supernatant was utilized as the antigen preparation (45). Uninfected wild-type lungs processed in a similar manner served as a control antigen. Anti-P. murina serum antibodies were measured by ELISA, and splenocyte proliferation assays were performed as previously described (45).
Statistical analysis.
The flow cytometry results are presented as the mean of exposed animals at a given time point in each experiment and, for statistical analysis, were compared to unexposed animals in the same experiment. For the anti-IFN-γ antibody studies, animals from the three studies were combined by day of exposure, and the anti-IFN-γ antibody recipients and controls (rat IgG and no antibody combined) were compared separately to unexposed animals, as well as to each other. For the IL-17A KO exposure studies, the organism load, ELISA, and proliferation results for the KO mice were compared to those for C57BL/6 control mice from the same experiment. A Student t test was used for these comparisons, with P values of <0.05 considered significant.
ACKNOWLEDGMENTS
This project was supported by the Intramural Research Program of the U.S. National Institutes of Health (NIH) Clinical Center. We have no conflicts of interest.
We thank Yoichiro Iwakura and Rachel Caspi for providing the IL-17A KO mice and George Deepe for providing the anti-IFN-γ antibody. We acknowledge the NIH Tetramer Core Facility (contract HHSN272201300006C) for provision of CD1d tetramers. We thank Rene Costello and Howard Mostowski for providing animal care.
REFERENCES
- 1.Beck JM, Warnock ML, Curtis JL, Sniezek MJ, Arraj-Peffer SM, Kaltreider HB, Shellito JE. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am J Respir Cell Mol Biol 5:186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- 2.Harmsen AG, Stankiewicz M. 1990. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med 172:937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roths JB, Marshall JD, Allen RD, Carlson GA, Sidman CL. 1990. Spontaneous Pneumocystis carinii pneumonia in immunodeficient mutant scid mice: natural history and pathobiology. Am J Pathol 136:1173–1186. [PMC free article] [PubMed] [Google Scholar]
- 4.Shellito J, Suzara VV, Blumenfeld W, Beck JM, Steger HJ, Ermak TH. 1990. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest 85:1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanano R, Reifenberg K, Kaufmann SH. 1996. Naturally acquired Pneumocystis carinii pneumonia in gene disruption mutant mice: roles of distinct T-cell populations in infection. Infect Immun 64:3201–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shellito JE, Tate C, Ruan S, Kolls J. 2000. Murine CD4+ T lymphocyte subsets and host defense against Pneumocystis carinii. J Infect Dis 181:2011–2017. doi: 10.1086/315487. [DOI] [PubMed] [Google Scholar]
- 7.Beck JM, Newbury RL, Palmer BE, Warnock ML, Byrd PK, Kaltreider HB. 1996. Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J Lab Clin Med 128:477–487. doi: 10.1016/S0022-2143(96)90044-X. [DOI] [PubMed] [Google Scholar]
- 8.Roths JB, Sidman CL. 1992. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Invest 90:673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ling Y, Puel A. 2014. IL-17 and infections. Actas Dermosifiliogr 105(Suppl 1):S34–S40. doi: 10.1016/S0001-7310(14)70016-X. [DOI] [PubMed] [Google Scholar]
- 10.Nembrini C, Marsland BJ, Kopf M. 2009. IL-17-producing T cells in lung immunity and inflammation. J Allergy Clin Immunol 123:986–994. doi: 10.1016/j.jaci.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 11.McAleer JP, Kolls JK. 2011. Mechanisms controlling Th17 cytokine expression and host defense. J Leukoc Biol 90:263–270. doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McAleer JP, Kolls JK. 2014. Directing traffic: IL-17 and IL-22 coordinate pulmonary immune defense. Immunol Rev 260:129–144. doi: 10.1111/imr.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mills KH. 2008. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol 38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 14.Lanternier F, Cypowyj S, Picard C, Bustamante J, Lortholary O, Casanova JL, Puel A. 2013. Primary immunodeficiencies underlying fungal infections. Curr Opin Pediatr 25:736–747. doi: 10.1097/MOP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudner XL, Happel KI, Young EA, Shellito JE. 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect Immun 75:3055–3061. doi: 10.1128/IAI.01329-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Nazario N, Rangel-Moreno J, O'Reilly MA, Pasparakis M, Gigliotti F, Wright TW. 2013. Selective ablation of lung epithelial IKK2 impairs pulmonary Th17 responses and delays the clearance of Pneumocystis. J Immunol 191:4720–4730. doi: 10.4049/jimmunol.1301679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu T, Takamoto M, Hida S, Tagawa Y, Sugane K. 2009. IFN-gamma deficiency worsen Pneumocystis pneumonia with Th17 development in nude mice. Immunol Lett 127:55–59. doi: 10.1016/j.imlet.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Hernandez-Novoa B, Bishop L, Logun C, Munson PJ, Elnekave E, Rangel ZG, Barb J, Danner RL, Kovacs JA. 2008. Immune responses to Pneumocystis murina are robust in healthy mice but largely absent in CD40 ligand-deficient mice. J Leukoc Biol 84:420–430. doi: 10.1189/jlb.1207816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garvy BA, Ezekowitz RA, Harmsen AG. 1997. Role of gamma interferon in the host immune and inflammatory responses to Pneumocystis carinii infection. Infect Immun 65:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. 2005. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. J Exp Med 202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubin PJ, Kolls JK. 2007. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol 292:L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan LC, Chaili S, Filler SG, Barr K, Wang H, Kupferwasser D, Edwards JE Jr, Xiong YQ, Ibrahim AS, Miller LS, Schmidt CS, Hennessey JP Jr, Yeaman MR. 2015. Nonredundant roles of interleukin-17A (IL-17A) and IL-22 in murine host defense against cutaneous and hematogenous infection due to methicillin-resistant Staphylococcus aureus. Infect Immun 83:4427–4437. doi: 10.1128/IAI.01061-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conti HR, Gaffen SL. 2015. IL-17-mediated immunity to the opportunistic fungal pathogen Candida albicans. J Immunol 195:780–788. doi: 10.4049/jimmunol.1500909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776. doi: 10.1038/nature06764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freeman AF, Holland SM. 2008. The hyper-IgE syndromes. Immunol Allergy Clin North Am 28:277–291. doi: 10.1016/j.iac.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. 2009. Requisite role for the dectin-1 β-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol 182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuthrich M, Gern B, Hung CY, Ersland K, Rocco N, Pick-Jacobs J, Galles K, Filutowicz H, Warner T, Evans M, Cole G, Klein B. 2011. Vaccine-induced protection against 3 systemic mycoses endemic to North America requires Th17 cells in mice. J Clin Invest 121:554–568. doi: 10.1172/JCI43984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saijo S, Fujikado N, Furuta T, Chung SH, Kotaki H, Seki K, Sudo K, Akira S, Adachi Y, Ohno N, Kinjo T, Nakamura K, Kawakami K, Iwakura Y. 2007. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol 8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 29.Bello-Irizarry SN, Wang J, Johnston CJ, Gigliotti F, Wright TW. 2014. MyD88 signaling regulates both host defense and immunopathogenesis during Pneumocystis infection. J Immunol 192:282–292. doi: 10.4049/jimmunol.1301431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ripamonti C, Bishop LR, Yang J, Lempicki RA, Kovacs JA. 2014. Clearance of Pneumocystis murina infection is not dependent on MyD88. Microbes Infect 16:522–527. doi: 10.1016/j.micinf.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolls JK. 2014. Helper T-cell type 17 cytokines and immunity in the lung. Ann Am Thorac Soc 11(Suppl 5):S284–S286. doi: 10.1513/AnnalsATS.201403-109AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bishop LR, Lionakis MS, Sassi M, Murphy PM, Hu X, Huang DW, Sherman B, Qiu J, Yang J, Lempicki RA, Kovacs JA. 2015. Characterization of chemokine and chemokine receptor expression during Pneumocystis infection in healthy and immunodeficient mice. Microbes Infect 17:638–650. doi: 10.1016/j.micinf.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garvy BA, Wiley JA, Gigliotti F, Harmsen AG. 1997. Protection against Pneumocystis carinii pneumonia by antibodies generated from either T helper 1 or T helper 2 responses. Infect Immun 65:5052–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rapaka RR, Ricks DM, Alcorn JF, Chen K, Khader SA, Zheng M, Plevy S, Bengten E, Kolls JK. 2010. Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina. J Exp Med 207:2907–2919. doi: 10.1084/jem.20100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scott P. 1991. IFN-gamma modulates the early development of Th1 and Th2 responses in a murine model of cutaneous leishmaniasis. J Immunol 147:3149–3155. [PubMed] [Google Scholar]
- 36.Kutty G, Davis AS, Ferreyra GA, Qiu J, Huang DW, Sassi M, Bishop L, Handley G, Sherman B, Lempicki R, Kovacs JA. 2016. β-glucans are masked but contribute to pulmonary inflammation during Pneumocystis pneumonia. J Infect Dis 214:782–791. doi: 10.1093/infdis/jiw249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, Masopust D. 2014. Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9:209–222. doi: 10.1038/nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, Masopust D. 2012. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol 189:2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinley L, Logar AJ, McAllister F, Zheng M, Steele C, Kolls JK. 2006. Regulatory T cells dampen pulmonary inflammation and lung injury in an animal model of Pneumocystis pneumonia. J Immunol 177:6215–6226. doi: 10.4049/jimmunol.177.9.6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agostini C, Zambello R, Trentin L, Semenzato G. 1995. T lymphocytes with γ/δ T-cell receptors in patients with AIDS and Pneumocystis carinii pneumonia. AIDS 9:203–204. doi: 10.1097/00002030-199509020-00014. [DOI] [PubMed] [Google Scholar]
- 41.Steele C, Zheng M, Young E, Marrero L, Shellito JE, Kolls JK. 2002. Increased host resistance against Pneumocystis carinii pneumonia in gamma-delta T-cell-deficient mice: protective role of gamma interferon and CD8+ T cells. Infect Immun 70:5208–5215. doi: 10.1128/IAI.70.9.5208-5215.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Furuta T, Nagata T, Kikuchi T, Kikutani H. 2001. Fatal spontaneous pneumocystosis in CD40 knockout mice. J Eukaryot Microbiol 2001(Suppl):129S–130S. [DOI] [PubMed] [Google Scholar]
- 43.Nakae S, Komiyama Y, Nambu A, Sudo K, Iwase M, Homma I, Sekikawa K, Asano M, Iwakura Y. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 17:375–387. doi: 10.1016/S1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 44.Vestereng VH, Bishop LR, Hernandez B, Kutty G, Larsen HH, Kovacs JA. 2004. Quantitative real-time polymerase chain-reaction assay allows characterization of Pneumocystis infection in immunocompetent mice. J Infect Dis 189:1540–1544. doi: 10.1086/382486. [DOI] [PubMed] [Google Scholar]
- 45.Bishop LR, Helman D, Kovacs JA. 2012. Discordant antibody and cellular responses to Pneumocystis major surface glycoprotein variants in mice. BMC Immunol 13:39. doi: 10.1186/1471-2172-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]