Abstract
Cytomegalovirus (CMV) infection is the most common viral infection in liver transplant recipients, affecting post-transplant patients and graft survival. Recent advances in diagnosis and management of CMV have led to marked reduction in incidence, severity, and its associated morbidity and mortality. CMV DNA assay is the most commonly used laboratory parameter to diagnose and monitor CMV infection. Current evidence suggests that both pre-emptive and universal prophylaxis approaches are equally justified in liver transplant recipients. Intravenous ganciclovir and oral valganciclovir are the most commonly used drugs for treatment of CMV disease. Most of the centre use valganciclovir prophylaxis for prevention of CMV disease in liver trasplant recipient. The aim of this article is to review the current standard of care for diagnosis and management of CMV disease in liver transplant recipients.
Abbreviations: CMV, cytomegalovirus; HCV, hepatitis C virus; HHV, human herpes virus; IV, intravenous; LT, liver transplantation; NAT, nucleic acid test
Keywords: cytomegalovirus, liver transplantation, infection, CMV disease
Cytomegalovirus (CMV) is a ubiquitous double-stranded DNA virus that infects 50–100% of humans depending upon the population studied. It is the most common viral infection in liver transplant recipients and influences the outcome of liver transplantation.1, 2
Types of CMV infection:
CMV infection can be primary CMV infection, CMV reactivation, or CMV disease. CMV infection is defined as evidence of CMV replication regardless of symptoms (differs from latent CMV and reactivation).
Primary infection is defined as occurrence of CMV viremia in a previously unexposed transplant recipient. Transplant recipients with donor seropositive and recipient seronegative status are at higher risk of primary CMV infection.
CMV disease is defined as evidence of CMV infection with attributable symptoms. CMV disease can be further categorized as a viral syndrome with fever, malaise, leukopenia, and/or thrombocytopenia or as tissue-invasive disease.
CMV reactivation is defined as evidence of CMV replication in patients who were previously positive for CMV serology.
Overall, 18–29% of all liver transplant recipients will develop CMV disease in the absence of prevention strategy.3 In the absence of antiviral preventive strategy, CMV disease among liver recipients occurs most commonly during the first 3 months after transplantation.4 Its incidence varies widely depending upon donor and recipient CMV serologic status; the incidence is as high as 44–65% in CMV D+/R−, 8–19% among CMV-seropositive (CMV R+), and 1–2% among CMV D−/R− patients. The CMD D−/R− patients usually acquire the virus from natural transmission or through blood transfusion.3, 5, 6
Pathophysiology of CMV Infection
Primary infection results in viral latency mainly in lymphoid and myloid cells and ensures the persistence of the virus throughout the life of the host. This viral latency plays an important role in liver transplant recipients who develop CMV infection. The cellular sites of viral latency become reservoirs for reactivation during periods of inflammation (such as allograft rejection and critical illness) and immunosuppression.
Clinical Manifestation of CMV Infection
The classic illness caused by CMV after liver transplantation is CMV disease in the form of fever and bone marrow suppression (most commonly, leukopenia and neutropenia) and accounts for 60% of CMV diseases after liver transplantation. Occasionally, CMV infection may manifest as tissue-invasive disease, which mainly involves the gastrointestinal tract (in the form of CMV gastritis, esophagitis, enteritis, and colitis). Gastrointestinal CMV disease accounts for more than 70% of tissue-invasive CMV disease cases in liver and other solid organ transplant recipients.7 The transplanted liver allograft is also susceptible to develop CMV hepatitis, and this often manifests with symptoms that may be clinically indistinguishable from acute rejection.8
CMV has not only direct effects on tissue that it infects but also has indirect effects resulting from its ability to modulate the immune system (Table 1). CMV is a potent upregulator of alloantigen, which increases the risk of acute rejection and chronic allograft dysfunction.9, 10, 11, 12 A higher incidence of vascular and hepatic artery thrombosis has been reported in liver transplant recipients with CMV disease and thought to be due to infection of the vascular endothelial cells.13, 14 CMV infection/reactivation is associated with increased risk of bacterial, other viruses, and invasive fungal infection.15, 16 CMV-infected transplant recipients are more likely to develop Epstein–Barr virus-associated post-transplant lymphoid disorder or coinfections with other viruses such as human herpes virus (HHV) 6 and HHV7.15, 16, 17 Similarly, there is significant association between CMV infection and accelerated course of HCV recurrence and allograft loss after liver transplant.18, 19, 20, 21, 22, 23 In a study of 347 HCV-infected liver recipients, CMV infection increased the risk of allograft fibrosis by 1.5 times and CMV disease increased the risk of allograft inflammation by 3.4 times.24 Recent evidence has suggested possible role of CMV infection in post-transplant metabolic diseases such as post-transplant diabetes mellitus.25 Therefore, the strategies to reduce the risk of CMV reactivation may help to reduce the risk of related infections, acute or chronic rejection, or HCV recurrence.
Table 1.
Effect of CMV on Liver Transplant Recipients.
| Direct effects | Indirect effects |
|---|---|
| CMV syndrome | Acute allograft rejection |
| Fever | Chronic allograft rejection |
| Myelosuppression | Vanishing bile duct syndrome |
| Tissue-invasive CMV disease | Opportunistic bacterial and viral infections |
| Gastrointestinal disease | Epstein–Bar virus and PTLD |
| CMV hepatitis | HHV-6 and HHV-7 infections |
| CMV pneumonitis | New-onset diabetes mellitus |
| CNS disease, retinitis | Vascular thrombosis |
Adapted from Bruminhent et al.50
Diagnosis
The diagnostic modalities for CMV infection include serology, qualitative and quantitative polymerase chain reaction (PCR), pp65 antiginemia, culture, and histopathology.
Viral culture of blood and urine has limited clinical utility for prediction, diagnosis, and management of CMV disease in adult liver transplant recipients.26 Similarly, because of immunosuppression, liver transplant recipients have delayed or impaired ability to mount an antibody response and, hence, CMV serology to detect IgG and IgM antibody has limited role for diagnosis in post liver transplant recipients.27 Although histopathology confirms the presence of tissue-invasive CMV disease, it is not routinely used due to its invasive nature. It may be useful in some cases where CMV is suspected, but CMV testing in blood is negative especially in the case of gastrointestinal CMV disease.28
There are several studies supporting the clinical utility of CMV replication assays, particularly plasma or whole blood quantitative PCR assay in managing CMV disease.27 The combination of viral load in the initial phase of infection and the rate of increase in viral load may help to identify patients at risk of CMV disease. It is commonly used in many centers to diagnose active CMV disease, screen for pre-emptive antiviral therapy and monitor response to antiviral therapy. Quantitative PCR test and CMV pp65 antigenemia test are available for detecting viral DNA and antigen, respectively. Antigenemia has higher sensitivity than culture and is comparable to PCR.29, 30 It is useful to guide pre-emptive therapy for rapid and sensitive diagnosis of CMV disease and to guide treatment response.29 However, quantitative PCR assays are more commonly used than the antigenemia test because CMV DNA PCR assay has better standardization, increased stability of the specimen, smaller specimen volume, and ability to test patients with leukopenia.31 Quantitative CMV PCR is useful to guide pre-emptive therapy for rapid and sensitive diagnosis of CMV infection and to guide response to treatment.31 However, lack of an international reference standard limited the generation and implementation of viral threshold for pre-emptive therapy, disease prognostication, and therapeutic monitoring. Therefore, it recommended that each transplant center should work within their clinical laboratories to define their relevant viral threshold for their clinical applications.26 In 2011, WHO released the first international reference standard for the quantification of CMV DNA, and commercially available CMV DNA assays should now be calibrated to this standard.32, 33
Prevention of CMV Disease After Liver Transplant—Universal Prophylaxis vs Pre-emptive Therapy
Universal prophylactic and pre-emptive therapy are the two most commonly used strategies to prevent CMV infection/reactivation in liver transplant recipient. In prophylactic therapy, anti-CMV drug is given to all who are at increased risk of CMV reactivation, whereas in pre-emptive therapy, anti-CMV drug is given only when there is evidence of CMV replication. Both of these strategies are similarly effective in preventing CMV disease after liver transplantation.3, 34, 35, 36, 37, 38 However, no large, prospective, well-controlled, randomized trial with head-to-head comparison of pre-emptive therapy and prophylaxis has been done in liver transplant recipients so far. In a retrospective study comparing the two approaches in liver transplant recipients, antiviral prophylaxis was more effective in prevention of CMV disease in high-risk D+/R−, but there were no differences in acute rejection, opportunistic infections, or rate of mortality.39, 40 Onor et al. have reported 4.9% and 50.0% (P < 0.001) incidence of CMV viremia at 3 months in the universal antiviral prophylaxis and pre-emptive therapy groups, respectively, but the rates were reversed, at 24.6% and 8.3% (P = 0.026), respectively at 6 months, and the reversal of the rates during the latter period accounts for the higher rates of late-onset CMV disease with antiviral prophylaxis.41
The prophylactic strategy depends on donor and recipient CMV serological status.
-
CMV D+/R−: Universal prophylaxis is the standard of care in this group of patients (duration—3–6 months).
It has been used by the majority of American and European transplant centers for preventing primary CMV disease in high-risk CMV D+/R− liver transplant recipients.42, 43 Moreover, it has the added benefit of reduction in bacterial and fungal opportunistic infections and mortality.44, 45, 46, 47 However, according to the recently updated American Society of Transplantation (AST) and The Transplantation Society (TTS) guidelines, pre-emptive therapy may be an option in CMV D+/R− liver transplant recipients.26, 31 The main reason for this preference for antiviral prophylaxis is the rapidity of CMV replication in CMV D+/R− liver recipients, which may escape detection with once-weekly CMV surveillance.
CMV D+ or D−/R+: Universal prophylactic or Pre-emptive therapy. Duration—3 months. This group of patients is commonly encountered in India. The details of both strategies have been discussed in detail later on.
-
CMV D−/R−: This group of patients is at low risk of CMV primary infection and routine use of prophylaxis not recommended.
Universal prophylaxis may be preferred in other high-risk patients, including those on recent antilymphocyte therapy, potent immunosuppression including desensitization, or ABO-incompatible protocols (including those on rituximab, bortezomib, eculizumab, and plasmapheresis/immunoadsorption).26, 31
Pre-emptive Therapy
The basic principle of pre-emptive therapy is to detect the presence of early CMV replication prior to the onset of clinical symptoms, so that antiviral therapy is administered early in order to prevent the progression of asymptomatic infection to clinical disease.35, 36, 38, 48, 49, 50 Pre-emptive therapy has the potential advantage of targeting therapy to the patient's with highest risk and thereby decreasing drug costs and toxicity, but this is offset by the cost of frequent laboratory monitoring and increased logistic in order to obtain, receive, and act upon the result in timely fashion. The success of this approach depends upon the optimal laboratory test and frequency and duration of monitoring, appropriate patient selection, and choosing the type, dose, and duration of an antiviral drug. Quantitative nucleic acid test (NAT) is now the preferred method for detecting CMV after transplantation29 and commonly used for diagnosing and monitoring of CMV infection. A recent study by Reasonable et al. using this assay in the plasma samples of 267 solid organ (including liver) transplant recipients demonstrated that patients with pre-treatment CMV DNA of less than 18200 [4.3 log(10)] IU/mL have 1.5-fold higher chance for CMV disease resolution. Similarly, CMV suppression to less than 137 [2.1 log(10)] IU/mL is predictive of clinical response to antiviral treatment.51 The recommended optimal interval and duration of monitoring for pre-emptive therapy is once-weekly CMV NAT for 12 weeks after liver transplantation. If a patient shows viremia above a defined threshold during the surveillance period, antiviral therapy (with oral valganciclovir or intravenous ganciclovir) should be initiated and continued until two consecutive CMV DNA is not detectable for two weeks.26, 31 Studies have shown that both IV ganciclovir or oral valganciclovir are effective for pre-emptive treatment of CMV infection in liver transplant recipients, including high-risk CMV D+/R− patients.37, 49 However, some studies have indicated that pre-emptive therapy may not be completely effective in CMV D+/R− liver recipients, since the replication kinetics of CMV in immune-deficient individuals is so rapid52 that it may escape detection with once-weekly surveillance.5, 35, 53
Patients with low CMV viral load may have a transient viremia that generally resolves without treatment.54 Some authors have suggested that just reduction in immunosuppression leads to clearance of CMV. Wadhwan et al.55 have reported CMV reactivation and CMV disease rate of 13% and 2.9%, respectively without prophylaxis in CMV immunoglobulin G-positive living donor liver transplant recipients. Only patients with CMV disease were treated while CMV reactivated patients were not treated. All the patients with CMV reactivation (without treatment) and CMV disease (with treatment) became negative for CMV during follow-up. CMV reactivation or disease did not affect recipient's survival during median follow-up at 28 months. Although this approach seems attractive, its applicability is limited by the fact that further study validating this approach is lacking currently.
Several clinical trials have demonstrated the efficacy of pre-emptive therapy in CMV disease prevention.35, 36, 37, 49 When conducted properly, pre-emptive therapy, with the use of IV ganciclovir or oral valganciclovir, resulted in the reduction of CMV disease by about 70%46, 47, 56 and was less likely associated with late-onset CMV disease as compared to antiviral prophylaxis.35, 36 In a survey of CMV prevention strategies by Levitsky et al., the authors found that valganciclovir is the most commonly used drug for pre-emptive therapy due to its better bioavailability and ease of oral administration,42 In addition, pre-emptive therapy is also beneficial in reducing the indirect effects of CMV, although to a much lesser degree compared to antiviral prophylaxis. In one study by Singh et al., the incidence of major opportunistic infections, bacteremia, bacterial infection, HCV recurrence, and rejection were not significantly different between liver transplant patients who received pre-emptive therapy and those who did not have CMV reactivation.57
Universal Antiviral Prophylaxis
Antiviral prophylaxis is highly effective in preventing both the direct and indirect effects of CMV after liver transplantation.3, 34, 46, 47, 56 Universal prophylaxis has additional benefit, since both ganciclovir and valganciclovir are active against other herpes viruses including VZV, HSV, and EBV.47 Similarly, the use of CMV prophylaxis also reduces the risk of other infections and complications by reducing infections that occur more commonly in patients with CMV reactivations. CMV prophylaxis reduced the risk of biopsy-proven rejection in liver transplant recipients. Compared to placebo or no treatment, patients who received antiviral prophylaxis had lower incidence of CMV disease (58–80% reduction) and CMV infection (about 40% reduction)46; reduction in all-cause mortality was observed,46, 47 mainly due to a decline in CMV-related death.46 The recent AST and TTS guidelines also prefer antiviral prophylaxis in CMV D+/R− liver recipients.26, 31
Ganciclovir and valganciclovir are the two most commonly used drugs for CMV prophylaxis and treatment in liver transplant recipients. The other commonly available drugs are shown in Table 2.
Table 2.
Currently Available Antiviral Drugs for Cytomegalovirus Prophylaxis and Treatment in Liver Transplant Recipients.
| Drug | Route | Usual adult prophylaxis dose | Usual adult treatment dose | Major toxicity |
|---|---|---|---|---|
| Ganciclovir | Intravenous | 5 mg/kg once daily | 5 mg/kg twice daily | Bone marrow suppression |
| Ganciclovir | Oral | 1 g three times daily | Not applicable | Low oral bioavailability; high pill burden |
| Valganciclovir | Oral | 900 mg once daily | 900 mg twice daily | Ease of administration; leukopenia |
| Foscarnet | Intravenous | Not recommended | 60 mg/kg every 8 h (or 90 mg/kg every 12 h) | Second-line drug intravenous access; nephrotoxicity |
| Cidofovir | Intravenous | Not recommended | 5 mg/kg once weekly × 2 then every 2 weeks thereafter | Third-line drug intravenous access; nephrotoxicity |
Adapted from Bruminhent et al.50
Valganciclovir vs Ganciclovir
Ganciclovir-based regimen is more effective than acyclovir or immunoglobulins in reducing the incidence of CMV disease after liver transplantation.3, 58, 59 However, Oral ganciclovir is poorly absorbed resulting in low systemic ganciclovir levels with oral administration.60 Valganciclovir provides systemic ganciclovir levels that are comparable to IV ganciclovir.60, 61 Pharmacokinetic studies indicate that a 900 mg dose of valganciclovir achieves a similar daily area under the concentration time curve (AUC24) as an IV dose of 5 mg/kg of ganciclovir.60
The role of valganciclovir in the prevention of CMV disease after liver transplantation was evaluated in a multicentre randomized noninferiority clinical trial that compared it with oral ganciclovir in a cohort of 364 CMV D+/R− solid organ recipients including liver transplant recipients. Among all solid organ transplant recipients, the 6-month incidence of CMV disease was 12% and 15% in the valganciclovir and oral ganciclovir groups and 12-month incidence was 17% and 18% with valganciclovir and oral ganciclovir, respectively.34 However, on subgroup analysis of the 177 liver transplant recipients, the incidence of CMV disease was 19% in the valganciclovir group as opposed to only 12% in the ganciclovir group. There was also a higher incidence of tissue-invasive CMV disease in the valganciclovir group.34 Based on these results, valganciclovir did not gain approval from the United States Food and Drug Administration (US-FDA) for prophylaxis against CMV disease after liver transplantation. A recent meta-analysis of 5 controlled clinical studies, including 380 liver transplant recipients who received valganciclovir (450 or 900 mg daily) prophylaxis, showed that the overall CMV disease rate was 12%, and 20% among D+/R− patients. The risk of CMV disease with valganciclovir was 1.81-fold higher than ganciclovir. For high-risk CMV D+/R− patients, the risk of CMV disease was 2-fold higher than ganciclovir. The risk of leukopenia with valganciclovir was 1.9-fold higher than those using ganciclovir.62 Despite these results and non-FDA-approval of valganciclovir for this indication, valganciclovir remains as the most widely used drug for CMV prophylaxis after liver transplantation.42
Similarly, the optimal duration of CMV prophylaxis in LT recipient is not defined. Since there was concern for late-onset CMV disease with 3 months of antiviral prophylaxis in CMV D+/R− patients, another study was done to compare 100 days vs 200 days of valganciclovir prophylaxis in kidney transplant recipients and incidence of CMV disease was 16.1% vs 36.8% (P < 0.001) at the end of one year in the 200 vs 100 days groups, respectively and the result persisted up to 2 years after transplantation (21.3% vs 38.7% P < 0.001).61, 62 Although a similar comparative study has not been done in LT recipient, many centers have extrapolated this result in prevention of CMV disease in high-risk liver transplant recipients. However, it must be emphasized that prolonged prophylaxis is associated with theoretical risk of ganciclovir resistance and drug toxicity such as leukopenia and also increases the cost of drug considerably.63, 64
Therefore, in summary, duration of prophylaxis in D+/R− liver recipients should be between 3 and 6 months. For seropositive recipients, most of the centers recommend 3 months of prophylaxis.
Based on available evidence and guidelines in literature, our institutional post liver transplant CMV prophylaxis strategy is described below.
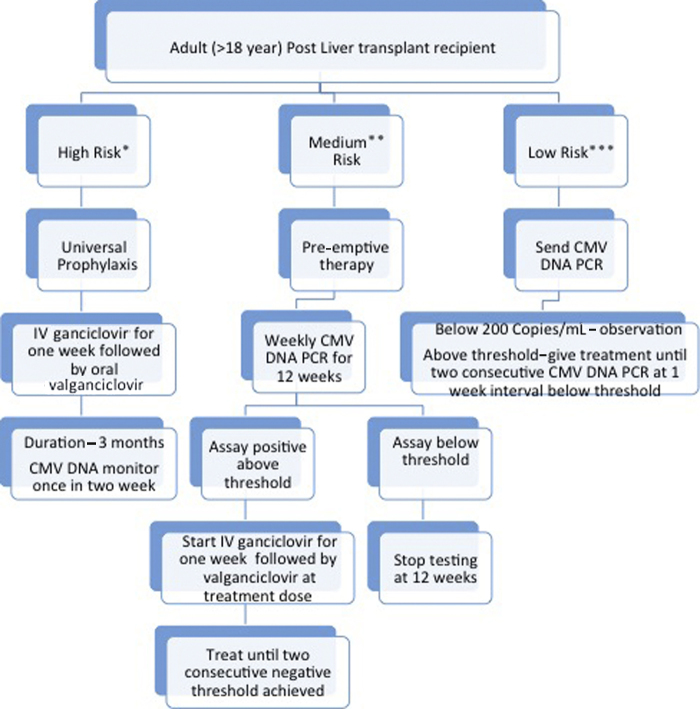
We classify liver transplant recipients into high, medium, and low risk of CMV infection. High-risk group includes D+/R−, patients receiving steroid pulse therapy or antithymocyte globulin for biopsy-proven rejection or ABO-incompatible liver transplant. We use universal prophylaxis for the high-risk group.26, 31 Medium-risk group includes D+ or D−/R+ patients, patients transplanted for acute liver failure, retransplantation patients, or who receive bolus steroid on empirical basis for suspected rejection but not in range of steroid pulse therapy. We use pre-emptive approach in this group of patients.26, 31 Low-risk group includes D−/R− patients or those with clinical suspicion of CMV reactivation. Here, we send CMV DNA and start intravenous ganciclovir if the level is above threshold (>200 copies per mL in our institute) (Figure 1). Our strategy allows universal prophylaxis in high-risk group, whereas it avoids over treatment of CMV infection and associated drug related side effect, cost for medium and low-risk recipients. In our experience of 272 living donor liver transplant recipients from January 2012 to April 2013, 55 (20.5%) out of 272 recipients (all CMV IgG positive before transplant) were found to have CMV reactivation at median time of 25 days (range 2–90 days) after liver transplant. The 90 days survival was significantly low in patients with CMV reactivation as compared to those without CMV reactivation (73.2% vs 92.6%, P = 0.001).65
Figure 1.
Approach to CMV prophylaxis in adult post liver transplant recipients. *High risk—D+/R− status. Patient receiving steroid pulse therapy or antithymocyte globulin for biopsy-proven rejection ABO-incompatible liver transplant. **Medium risk—or D−/R+ status. Patients who receive bolus steroid on empirical ground for suspected rejection but not in range of steroid pulse therapy. Acute liver failure. Re-transplantation. ***Low risk—D−/R− status. Clinical suspicion of CMV reactivation alone.
However, it is important to note that patients who complete CMV prophylaxis after liver transplantation are at risk for late-onset CMV disease, which may be associated with graft loss and increased mortality.44 Late-onset CMV disease is relatively uncommon in patients who are managed with pre-emptive CMV therapy.26
A Hybrid strategy of prophylaxis followed by pre-emptive monitoring after prophylaxis has been used at some centers but adequate data are lacking for such an approach. In a study of 71 high-risk recipients by Lisboa et al., weekly virology monitoring of patients who completed prophylaxis was ineffective at predicting CMV disease.66
Treatment of CMV Disease After LT
The current evidence suggests that CMV disease after liver transplantation should be treated with either IV ganciclovir or valganciclovir.49, 67 However, oral ganciclovir should not be used for the treatment of CMV disease because of its poor bioavailability.15 The degree of pharmacologic immunosuppression should be reduced whenever possible during treatment of CMV disease.15
In a multicenter noninferiority trial, 321 solid organ (including liver) transplant recipients with nonsevere CMV disease were randomized to valganciclovir (900 mg twice daily) or IV ganciclovir (5 mg/kg twice daily) for a fixed 21-day course, followed by valganciclovir (900 mg once daily) maintenance treatment for 4 weeks. There was no significant difference in viral eradication at 21 and 49 days between the groups.68 The overall time to viral eradication was 21 days with valganciclovir and 19 days with IV ganciclovir. The study population in this trial were mostly CMV-seropositive kidney recipients with nonsevere CMV disease; this pivotal trial now supports the use of valganciclovir for oral treatment of CMV disease, at least in selected transplant patients.68 IV ganciclovir is preferable to valganciclovir in patients with severe or life-threatening disease, or in patients who may have a problem with gastrointestinal absorption of the oral drug. In many instances, valganciclovir is used as a step-down treatment when the clinical symptoms have resolved after an initial induction treatment with IV ganciclovir.
The duration of treatment of CMV disease is based on viral load monitoring. The persistence of the virus at the end of therapy (by PCR or pp65 antigenemia) is associated with a higher risk of clinical relapse.29 It is now generally accepted that multiple (at least two) weekly negative CMV PCR results should be obtained before antiviral therapy is discontinued.31 Similarly, patients should also be monitored for side effect of these medicines particularly leucopenia.31
Conclusion
CMV infection is the most common viral infection in liver transplant recipients. Recent advances in diagnosis and management of CMV has led to marked reduction in incidence, severity, and associated morbidity and mortality. CMV DNA assay is the most commonly used laboratory parameter to diagnose and monitor CMV infection. Current evidence suggests both pre-emptive and universal prophylaxis approaches are equally justified in liver transplant recipients. IV ganciclovir and oral valganciclovir are the most commonly used drugs for treatment of CMV disease. Most of the centers use valganciclovir prophylaxis for prevention of CMV disease in liver transplant recipients. The duration of treatment should be individualized and is usually 3–6 months.
Conflicts of Interest
The authors have none to declare.
Acknowledgement
Mr Yogesh Saini (research coordinator).
References
- 1.Razonable R.R., Paya C.V. Herpesvirus infections in transplant recipients: current challenges in the clinical management of cytomegalovirus and Epstein–Barr virus infections. Herpes J IHMF. 2003;10(3):60–65. [PubMed] [Google Scholar]
- 2.Razonable R.R., Emery V.C. 11th Annual Meeting of the IHMF (International Herpes Management Forum). Management of CMV infection and disease in transplant patients. Herpes J IHMF. 2004;11(3):77–86. [PubMed] [Google Scholar]
- 3.Gane E., Saliba F., Valdecasas G.J. Randomised trial of efficacy and safety of oral ganciclovir in the prevention of cytomegalovirus disease in liver-transplant recipients. The Oral Ganciclovir International Transplantation Study Group [corrected] Lancet Lond Engl. 1997;350(9093):1729–1733. doi: 10.1016/s0140-6736(97)05535-9. [DOI] [PubMed] [Google Scholar]
- 4.Ljungman P., Griffiths P., Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 5.Razonable R.R., van Cruijsen H., Brown R.A. Dynamics of cytomegalovirus replication during preemptive therapy with oral ganciclovir. J Infect Dis. 2003;187(11):1801–1808. doi: 10.1086/375194. [DOI] [PubMed] [Google Scholar]
- 6.Singh N., Wannstedt C., Keyes L., Wagener M.M., Cacciarelli T.V. Who among cytomegalovirus-seropositive liver transplant recipients is at risk for cytomegalovirus infection? Liver Transplant. 2005;11(6):700–704. doi: 10.1002/lt.20417. [DOI] [PubMed] [Google Scholar]
- 7.Fica A., Cervera C., Pérez N. Immunohistochemically proven cytomegalovirus end-organ disease in solid organ transplant patients: clinical features and usefulness of conventional diagnostic tests. Transpl Infect Dis. 2007;9(3):203–210. doi: 10.1111/j.1399-3062.2007.00220.x. [DOI] [PubMed] [Google Scholar]
- 8.Paya C.V., Hermans P.E., Wiesner R.H. Cytomegalovirus hepatitis in liver transplantation: prospective analysis of 93 consecutive orthotopic liver transplantations. J Infect Dis. 1989;160(5):752–758. doi: 10.1093/infdis/160.5.752. [DOI] [PubMed] [Google Scholar]
- 9.Razonable R.R., Paya C.V. Infections and allograft rejection—intertwined complications of organ transplantation. Swiss Med Wkly. 2005;135(39–40):571–573. doi: 10.4414/smw.2005.10984. [DOI] [PubMed] [Google Scholar]
- 10.O’Grady J.G., Alexander G.J., Sutherland S. Cytomegalovirus infection and donor/recipient HLA antigens: interdependent co-factors in pathogenesis of vanishing bile-duct syndrome after liver transplantation. Lancet Lond Engl. 1988;2(8606):302–305. doi: 10.1016/s0140-6736(88)92356-2. [DOI] [PubMed] [Google Scholar]
- 11.Noack K.B., Wiesner R.H., Batts K., van Hoek B., Ludwig J. Severe ductopenic rejection with features of vanishing bile duct syndrome: clinical, biochemical, and histologic evidence for spontaneous resolution. Transplant Proc. 1991;23(1 Pt 2):1448–1451. [PubMed] [Google Scholar]
- 12.Ludwig J., Wiesner R.H., Batts K.P., Perkins J.D., Krom R.A. The acute vanishing bile duct syndrome (acute irreversible rejection) after orthotopic liver transplantation. Hepatol Baltim Md. 1987;7(3):476–483. doi: 10.1002/hep.1840070311. [DOI] [PubMed] [Google Scholar]
- 13.Pastacaldi S., Teixeira R., Montalto P., Rolles K., Burroughs A.K. Hepatic artery thrombosis after orthotopic liver transplantation: a review of nonsurgical causes. Liver Transplant. 2001;7(2):75–81. doi: 10.1053/jlts.2001.22040. [DOI] [PubMed] [Google Scholar]
- 14.Madalosso C., de Souza N.F., Ilstrup D.M., Wiesner R.H., Krom R.A. Cytomegalovirus and its association with hepatic artery thrombosis after liver transplantation. Transplantation. 1998;66(3):294–297. doi: 10.1097/00007890-199808150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Eid A.J., Razonable R.R. Cytomegalovirus disease in solid organ transplant recipients: advances lead to new challenges and opportunities. Curr Opin Organ Transplant. 2007;12(6):610–617. [Google Scholar]
- 16.Peleg A.Y., Husain S., Qureshi Z.A. Risk factors, clinical characteristics, and outcome of Nocardia infection in organ transplant recipients: a matched case–control study. Clin Infect Dis. 2007;44(10):1307–1314. doi: 10.1086/514340. [DOI] [PubMed] [Google Scholar]
- 17.Mendez J.C., Dockrell D.H., Espy M.J. Human beta-herpesvirus interactions in solid organ transplant recipients. J Infect Dis. 2001;183(2):179–184. doi: 10.1086/317929. [DOI] [PubMed] [Google Scholar]
- 18.Humar A., Washburn K., Freeman R. An assessment of interactions between hepatitis C virus and herpesvirus reactivation in liver transplant recipients using molecular surveillance. Liver Transplant. 2007;13(10):1422–1427. doi: 10.1002/lt.21266. [DOI] [PubMed] [Google Scholar]
- 19.Humar A., Kumar D., Raboud J. Interactions between cytomegalovirus, human herpesvirus-6, and the recurrence of hepatitis C after liver transplantation. Am J Transplant. 2002;2(5):461–466. doi: 10.1034/j.1600-6143.2002.20511.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosen H.R., Chou S., Corless C.L. Cytomegalovirus viremia: risk factor for allograft cirrhosis after liver transplantation for hepatitis C. Transplantation. 1997;64(5):721–726. doi: 10.1097/00007890-199709150-00010. [DOI] [PubMed] [Google Scholar]
- 21.Razonable R.R., Burak K.W., van Cruijsen H. The pathogenesis of hepatitis C virus is influenced by cytomegalovirus. Clin Infect Dis. 2002;35(8):974–981. doi: 10.1086/342911. [DOI] [PubMed] [Google Scholar]
- 22.Singh N., Husain S., Carrigan D.R. Impact of human herpesvirus-6 on the frequency and severity of recurrent hepatitis C virus hepatitis in liver transplant recipients. Clin Transplant. 2002;16(2):92–96. doi: 10.1034/j.1399-0012.2002.1o096.x. [DOI] [PubMed] [Google Scholar]
- 23.Burak K.W., Kremers W.K., Batts K.P. Impact of cytomegalovirus infection, year of transplantation, and donor age on outcomes after liver transplantation for hepatitis C. Liver Transplant. 2002;8(4):362–369. doi: 10.1053/jlts.2002.32282. [DOI] [PubMed] [Google Scholar]
- 24.Bosch W., Heckman M.G., Pungpapong S., Diehl N.N., Shalev J.A., Hellinger W.C. Association of cytomegalovirus infection and disease with recurrent hepatitis C after liver transplantation. Transplantation. 2012;93(7):723–728. doi: 10.1097/TP.0b013e3182472876. [DOI] [PubMed] [Google Scholar]
- 25.Van Laecke S., Desideri F., Geerts A. Hypomagnesemia and the risk of new-onset diabetes after liver transplantation. Liver Transplant. 2010;16(11):1278–1287. doi: 10.1002/lt.22146. [DOI] [PubMed] [Google Scholar]
- 26.Razonable R.R., Humar A., AST Infectious Diseases Community of Practice Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13(suppl 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 27.Clinical Utility of Cytomegalovirus (CMV) Serology Testing in High-Risk CMV D+/R− Transplant Recipients. PubMed—NCBI [Internet; cited 04.08.16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=clinical+utility+ov+CMV+serology+testing+in+high+risk+CMV+D%2B%2FR-+recipient. [DOI] [PubMed]
- 28.Eid A.J., Arthurs S.K., Deziel P.J., Wilhelm M.P., Razonable R.R. Clinical predictors of relapse after treatment of primary gastrointestinal cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2010;10(1):157–161. doi: 10.1111/j.1600-6143.2009.02861.x. [DOI] [PubMed] [Google Scholar]
- 29.Razonable R.R., Paya C.V., Smith T.F. Role of the laboratory in diagnosis and management of cytomegalovirus infection in hematopoietic stem cell and solid-organ transplant recipients. J Clin Microbiol. 2002;40(3):746–752. doi: 10.1128/JCM.40.3.746-752.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caliendo A.M., St George K., Kao S.Y. Comparison of quantitative cytomegalovirus (CMV) PCR in plasma and CMV antigenemia assay: clinical utility of the prototype AMPLICOR CMV MONITOR test in transplant recipients. J Clin Microbiol. 2000;38(6):2122–2127. doi: 10.1128/jcm.38.6.2122-2127.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kotton C.N., Kumar D., Caliendo A.M. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96(4):333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 32.A Collaborative Study to Establish the 1st WHO International Standard for Human Cytomegalovirus for Nucleic Acid Amplification Technology [Internet; cited 06.08.16]. Available from: http://www.sciencedirect.com/science/article/pii/S1045105616300070. [DOI] [PubMed]
- 33.An International Multicenter Performance Analysis of Cytomegalovirus Load Tests. PubMed—NCBI [Internet; cited 06.08.16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=an+international+multicenter+performance+analysis+of+cytomegalovirus+load+tests. [DOI] [PMC free article] [PubMed]
- 34.Paya C., Humar A., Dominguez E. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4(4):611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 35.Paya C.V., Wilson J.A., Espy M.J. Preemptive use of oral ganciclovir to prevent cytomegalovirus infection in liver transplant patients: a randomized, placebo-controlled trial. J Infect Dis. 2002;185(7):854–860. doi: 10.1086/339449. [DOI] [PubMed] [Google Scholar]
- 36.Singh N., Wannstedt C., Keyes L., Gayowski T., Wagener M.M., Cacciarelli T.V. Efficacy of valganciclovir administered as preemptive therapy for cytomegalovirus disease in liver transplant recipients: impact on viral load and late-onset cytomegalovirus disease. Transplantation. 2005;79(1):85–90. doi: 10.1097/01.tp.0000146844.65273.62. [DOI] [PubMed] [Google Scholar]
- 37.Singh N., Paterson D.L., Gayowski T., Wagener M.M., Marino I.R. Cytomegalovirus antigenemia directed pre-emptive prophylaxis with oral versus I.V. ganciclovir for the prevention of cytomegalovirus disease in liver transplant recipients: a randomized, controlled trial. Transplantation. 2000;70(5):717–722. doi: 10.1097/00007890-200009150-00002. [DOI] [PubMed] [Google Scholar]
- 38.Singh N., Yu V.L. Preemptive therapy for cytomegalovirus. Liver Transplant. 2006;12(2):327. doi: 10.1002/lt.20676. [DOI] [PubMed] [Google Scholar]
- 39.Winston D.J., Imagawa D.K., Holt C.D., Kaldas F., Shaked A., Busuttil R.W. Long-term ganciclovir prophylaxis eliminates serious cytomegalovirus disease in liver transplant recipients receiving OKT3 therapy for rejection. Transplantation. 1995;60(11):1357–1360. [PubMed] [Google Scholar]
- 40.Bodro M., Sabé N., Lladó L. Prophylaxis versus preemptive therapy for cytomegalovirus disease in high-risk liver transplant recipients. Liver Transplant. 2012;18(9):1093–1099. doi: 10.1002/lt.23460. [DOI] [PubMed] [Google Scholar]
- 41.Onor I.O., Todd S.B., Meredith E. Evaluation of clinical outcomes of prophylactic versus preemptive cytomegalovirus strategy in liver transplant recipients. Transpl Int. 2013;26(6):592–600. doi: 10.1111/tri.12101. [DOI] [PubMed] [Google Scholar]
- 42.Levitsky J., Singh N., Wagener M.M., Stosor V., Abecassis M., Ison M.G. A survey of CMV prevention strategies after liver transplantation. Am J Transplant. 2008;8(1):158–161. doi: 10.1111/j.1600-6143.2007.02026.x. [DOI] [PubMed] [Google Scholar]
- 43.Vandecasteele E., De Waele J., Vandijck D. Antimicrobial prophylaxis in liver transplant patients—a multicenter survey endorsed by the European Liver and Intestine Transplant Association. Transpl Int. 2010;23(2):182–190. doi: 10.1111/j.1432-2277.2009.00974.x. [DOI] [PubMed] [Google Scholar]
- 44.Arthurs S.K., Eid A.J., Pedersen R.A. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46(6):840–846. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 45.Limaye A.P., Bakthavatsalam R., Kim H.W. Impact of cytomegalovirus in organ transplant recipients in the era of antiviral prophylaxis. Transplantation. 2006;81(12):1645–1652. doi: 10.1097/01.tp.0000226071.12562.1a. [DOI] [PubMed] [Google Scholar]
- 46.Hodson E.M., Jones C.A., Webster A.C. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: a systematic review of randomised controlled trials. Lancet Lond Engl. 2005;365(9477):2105–2115. doi: 10.1016/S0140-6736(05)66553-1. [DOI] [PubMed] [Google Scholar]
- 47.Kalil A.C., Levitsky J., Lyden E., Stoner J., Freifeld A.G. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med. 2005;143(12):870–880. doi: 10.7326/0003-4819-143-12-200512200-00005. [DOI] [PubMed] [Google Scholar]
- 48.Mattes F.M., Hainsworth E.G., Hassan-Walker A.F. Kinetics of cytomegalovirus load decrease in solid-organ transplant recipients after preemptive therapy with valganciclovir. J Infect Dis. 2005;191(1):89–92. doi: 10.1086/425905. [DOI] [PubMed] [Google Scholar]
- 49.Singh N., Wannstedt C., Keyes L. Valganciclovir as preemptive therapy for cytomegalovirus in cytomegalovirus-seronegative liver transplant recipients of cytomegalovirus-seropositive donor allografts. Liver Transplant. 2008;14(2):240–244. doi: 10.1002/lt.21362. [DOI] [PubMed] [Google Scholar]
- 50.Bruminhent J., Razonable R.R. Management of cytomegalovirus infection and disease in liver transplant recipients. World J Hepatol. 2014;6(6):370–383. doi: 10.4254/wjh.v6.i6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Razonable R.R., Åsberg A., Rollag H. Virologic suppression measured by a cytomegalovirus (CMV) DNA test calibrated to the World Health Organization international standard is predictive of CMV disease resolution in transplant recipients. Clin Infect Dis. 2013;56(11):1546–1553. doi: 10.1093/cid/cit096. [DOI] [PubMed] [Google Scholar]
- 52.Emery V.C., Sabin C.A., Cope A.V., Gor D., Hassan-Walker A.F., Griffiths P.D. Application of viral-load kinetics to identify patients who develop cytomegalovirus disease after transplantation. Lancet Lond Engl. 2000;355(9220):2032–2036. doi: 10.1016/S0140-6736(00)02350-3. [DOI] [PubMed] [Google Scholar]
- 53.Cytomegalovirus Infection in Transplant Recipients. The Role of Tumor Necrosis Factor. PubMed—NCBI [Internet; cited 06.08.16]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/?term=Cytomegalovirus+infection+in+transplant+recipients.+The+role+of+tumor+necrosis+factor.+Transplantation+1994. [PubMed]
- 54.Razonable R.R., Hayden R.T. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. 2013;26(4):703–727. doi: 10.1128/CMR.00015-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wadhawan M., Gupta S., Goyal N. Cytomegalovirus infection: its incidence and management in cytomegalovirus-seropositive living related liver transplant recipients: a single-center experience. Liver Transplant. 2012;18:1448–1455. doi: 10.1002/lt.23540. [DOI] [PubMed] [Google Scholar]
- 56.Small L.N., Lau J., Snydman D.R. Preventing post-organ transplantation cytomegalovirus disease with ganciclovir: a meta-analysis comparing prophylactic and preemptive therapies. Clin Infect Dis. 2006;43(7):869–880. doi: 10.1086/507337. [DOI] [PubMed] [Google Scholar]
- 57.Singh N., Wannstedt C., Keyes L., Wagener M.M., Gayowski T., Cacciarelli T.V. Indirect outcomes associated with cytomegalovirus (opportunistic infections, hepatitis C virus sequelae, and mortality) in liver-transplant recipients with the use of preemptive therapy for 13 years. Transplantation. 2005;79(10):1428–1434. doi: 10.1097/01.tp.0000157867.98649.f5. [DOI] [PubMed] [Google Scholar]
- 58.Winston D.J., Wirin D., Shaked A., Busuttil R.W. Randomised comparison of ganciclovir and high-dose acyclovir for long-term cytomegalovirus prophylaxis in liver-transplant recipients. Lancet Lond Engl. 1995;346(8967):69–74. doi: 10.1016/s0140-6736(95)92110-9. [DOI] [PubMed] [Google Scholar]
- 59.Winston D.J., Busuttil R.W. Randomized controlled trial of oral ganciclovir versus oral acyclovir after induction with intravenous ganciclovir for long-term prophylaxis of cytomegalovirus disease in cytomegalovirus-seropositive liver transplant recipients. Transplantation. 2003;75(2):229–233. doi: 10.1097/01.TP.0000040601.60276.96. [DOI] [PubMed] [Google Scholar]
- 60.Razonable R.R., Paya C.V. Valganciclovir for the prevention and treatment of cytomegalovirus disease in immunocompromised hosts. Expert Rev Anti Infect Ther. 2004;2(1):27–41. doi: 10.1586/14787210.2.1.27. [DOI] [PubMed] [Google Scholar]
- 61.Pescovitz M.D., Rabkin J., Merion R.M. Valganciclovir results in improved oral absorption of ganciclovir in liver transplant recipients. Antimicrob Agents Chemother. 2000;44(10):2811–2815. doi: 10.1128/aac.44.10.2811-2815.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalil A.C., Mindru C., Botha J.F. Risk of cytomegalovirus disease in high-risk liver transplant recipients on valganciclovir prophylaxis: a systematic review and meta-analysis. Liver Transplant. 2012;18(12):1440–1447. doi: 10.1002/lt.23530. [DOI] [PubMed] [Google Scholar]
- 63.Humar A., Lebranchu Y., Vincenti F. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10(5):1228–1237. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 64.Humar A., Limaye A.P., Blumberg E.A. Extended valganciclovir prophylaxis in D+/R− kidney transplant recipients is associated with long-term reduction in cytomegalovirus disease: two-year results of the IMPACT study. Transplantation. 2010;90(12):1427–1431. doi: 10.1097/tp.0b013e3181ff1493. [DOI] [PubMed] [Google Scholar]
- 65.Kandolkar V.P., Saigal S., Kumar N., Yadav S.K., Saraf N., Choudhary N.S. CMV infection in living donor liver transplant recipients significantly impacts the early post-transplant outcome: a large single center experience (ILTS Abstract-P-549) Transplantation. 2015;99 doi: 10.1111/tid.12905. 7S-1. [DOI] [PubMed] [Google Scholar]
- 66.Lisboa L.F., Preiksaitis J.K., Humar A., Kumar D. Clinical utility of molecular surveillance for cytomegalovirus after antiviral prophylaxis in high-risk solid organ transplant recipients. Transplantation. 2011;92(9):1063–1068. doi: 10.1097/TP.0b013e31822fa4b7. [DOI] [PubMed] [Google Scholar]
- 67.Ontejo M., Montejo E., Gastaca M. Prophylactic therapy with valgancyclovir in high-risk (cytomegalovirus D+/R−) liver transplant recipients: a single-center experience. Transplant Proc. 2009;41(6):2189–2191. doi: 10.1016/j.transproceed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 68.Asberg A., Humar A., Rollag H. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7(9):2106–2113. doi: 10.1111/j.1600-6143.2007.01910.x. [DOI] [PubMed] [Google Scholar]