ABSTRACT
Polioviruses (PVs) are members of the genus Enterovirus. In the Netherlands, the exclusion of PV circulation is based on clinical enterovirus (EV) surveillance (CEVS) of EV-positive cases and routine environmental EV surveillance (EEVS) conducted on sewage samples collected in the region of the Netherlands where vaccination coverage is low due to religious reasons. We compared the EEVS data to those of the CEVS to gain insight into the relevance of EEVS for poliovirus and nonpolio enterovirus surveillance. Following the polio outbreak in Syria, EEVS was performed at the primary refugee center in Ter Apel in the Netherlands, and data were compared to those of CEVS and EEVS. Furthermore, we assessed the feasibility of poliovirus detection by EEVS using measles virus detection in sewage during a measles outbreak as a proxy. Two Sabin-like PVs were found in routine EEVS, 11 Sabin-like PVs were detected in the CEVS, and one Sabin-like PV was found in the Ter Apel sewage. We observed significant differences between the three programs regarding which EVs were found. In 6 sewage samples collected during the measles outbreak in 2013, measles virus RNA was detected in regions where measles cases were identified. In conclusion, we detected PVs, nonpolio EVs, and measles virus in sewage and showed that environmental surveillance is useful for poliovirus detection in the Netherlands, where live oral poliovirus vaccine is not used and communities with lower vaccination coverage exist. EEVS led to the detection of EV types not seen in the CEVS, showing that EEVS is complementary to CEVS.
IMPORTANCE We show that environmental enterovirus surveillance complements clinical enterovirus surveillance for poliovirus detection, or exclusion, and for nonpolio enterovirus surveillance. Even in the presence of adequate surveillance, only a very limited number of Sabin-like poliovirus strains were detected in a 10-year period, and no signs of transmission of oral polio vaccine (OPV) strains were found in a country using exclusively inactivated polio vaccine (IPV). Measles viruses can be detected during an outbreak in sewage samples collected and concentrated following procedures used for environmental enterovirus surveillance.
KEYWORDS: enterovirus, environmental, measles, poliovirus, sewage, surveillance studies
INTRODUCTION
Acute flaccid paralysis (AFP) surveillance is the current WHO standard for polio surveillance for the Global Polio Eradication Initiative (GPEI). In the Netherlands, this type of surveillance has not been implemented successfully, and the current practice for the exclusion of poliovirus (PV) circulation is based on clinical enterovirus (EV) surveillance (CEVS) (1, 2) and environmental enterovirus surveillance (EEVS) (3). The EEVS is performed among the orthodox Protestant population who refuse vaccination based on religious grounds. The orthodox Protestant population live geographically clustered and isolated in what is known as the Dutch Bible Belt. The orthodox Protestant population in the Bible Belt is estimated at 2.2 × 105 persons with a vaccination coverage of a minimum of 60% (4). Of concern, several orthodox Protestant schools, especially secondary schools, are very large and have a regional function, attracting mostly unvaccinated children from a wide area, resulting in vaccination coverage of, in some cases, less than 20% (4; Wilhelmina L. M. Ruijs, personal communication).
EEVS in the Dutch Bible Belt was performed quite successfully during the polio outbreak in 1992-1993 (5, 6). After that, in 1997 a routine EEVS system was implemented based on collection of grab samples from sewages at secondary schools and villages in the Dutch Bible Belt where PV cases were identified in the 1992-1993 outbreak. The EEVS was halted in 2004 after Europe was declared polio free by the WHO in 2002. In September 2005, the EEVS was started up again after the detection of poliovirus in Indonesia and among the Amish in the United States: both of these populations had contacts with orthodox Protestants in the Dutch Bible Belt (7, 8). The routine EEVS continues to date and is performed by analysis of 80 to 110 sewage grab samples collected every year at secondary schools and in residential areas in the Dutch Bible Belt.
Any possible new polio outbreak worldwide with the possibility of importation to the Netherlands, or detection of wild-type polioviruses (WPVs) or vaccine-derived polioviruses (VDPVs) in the Netherlands, is a reason to assess the need for intensification of the surveillance program. Therefore, following the polio outbreak in Syria (9), EEVS was additionally employed at the primary refugee center (PRC) in Ter Apel, the Netherlands, from November 2013 to April 2015.
Poliovirus is excluded in CEVS and EEVS by a negative result in EV real-time reverse transcription (RT)-PCR, detection of nonpolio EVs (NPEVs) by sequencing of EV-positive samples and/or lack of growth on L20B cells of clinical and sewage samples. As a result, many NPEV sequences have been collected, allowing analysis and comparison of EEVS and CEVS.
Since no polio outbreaks have occurred in the Netherlands since 1993, we questioned whether detection of measles virus (MV) was suitable as a proxy for detection of poliovirus in EEVS, as two large measles outbreaks have occurred in the Bible Belt since 1993. In the Netherlands, the vaccination coverage for measles parallels that for polio, and measles outbreaks specifically affect the orthodox Protestant population as well (10). MV is shed from the nasopharynx and in urine (11). Therefore, detection of measles virus RNA in environmental samples may be feasible in locations where clusters of infections occur, provided that sufficient amounts are shed in the environment and that viral RNA persists.
In this study, we compared the routine EEVS data to those of the CEVS to gain insight into the relevance of EEVS for EV surveillance, as well as that of EEVS performed at the PRC-Ter Apel. Furthermore, we assessed the feasibility of poliovirus detection in the targeted population by EEVS by using measles virus detection during a measles outbreak as a proxy.
RESULTS
Poliovirus detection.
Between September 2005 and February 2015, polioviruses were detected in a total of 14 samples from the three surveillance programs (EEVS in the Bible Belt, EEVS in PRC-Ter Apel, and CEVS), all of which were characterized as Sabin-like (SL) vaccine strains. No WPVs or VDPVs were detected during the study period. Poliovirus was found twice in the period studied in the Bible Belt sewage: a PV2 SL strain with 3 mutations in 2006 and a PV1 SL strain with 2 mutations in 2008 (Table 1). These polioviruses were detected in sewage samples from two different school sites, and neither was related to poliovirus-positive cases. PV SL strains were also found in 11 CEVS samples (Table 1). All PV SL strains were isolated from children without AFP and with a reported history of recent oral polio vaccine (OPV) vaccination abroad. One PV1 SL strain with 2 mutations was found in January 2015 in PRC-Ter Apel sewage.
TABLE 1.
Polioviruses detected in sewage and clinical samples
| Surveillance program | Location | Sampling date (mo and yr) | Virus typea | Mutations (n)b |
|---|---|---|---|---|
| EEVS | Gouda | Oct 2006 | PV2 SL | 3 |
| CEVS | Zoetermeer | Jul 2007 | PV3 SL | 1 |
| CEVS | Rotterdam | Feb 2007 | PV1 SL | 0 |
| CEVS | Rotterdam | Nov 2007 | PV1 SL | 5 |
| EEVS | Amersfoort | Jun 2008 | PV1 SL | 2 |
| CEVS | Delft | Sept 2008 | PV1 SL | 1 |
| CEVS | Maastricht | Dec 2008 | PV1 SL | 0 |
| CEVS | Rotterdam | Apr 2009 | PV3 SL | 2 |
| CEVS | Heeswijk-Dinther | Oct 2010 | PV3 SL | 2 |
| CEVS | Groningen | Jan 2011 | PV3 SL | 2 |
| CEVS | Rotterdam | May 2011 | PV1 SL | 0 |
| CEVS | Haarlem | Jun 2011 | PV1 SL | 0 |
| CEVS | Rotterdam | Jul 2011 | PV3 SL | 3 |
| EEVS | Ter Apel | Jan 2015 | PV1 SL | 2 |
Routine EEVS in the Bible Belt.
For the routine EEVS in the Bible Belt, 856 sewage samples were collected between September 2005 and February 2015; 426 (49.8%) samples were collected from sewage pits at secondary schools, and 430 (50.2%) samples were collected from sewage pits in residential areas (Fig. 1, Table 2). In 371 (43.4%) samples, one EV was isolated, and in 56 (6.5%) samples, two EVs were isolated (Table 2). The total positivity rate and the number of double positives were significantly higher in residential samples than in samples collected at secondary schools (P = 0.0001).
FIG 1.
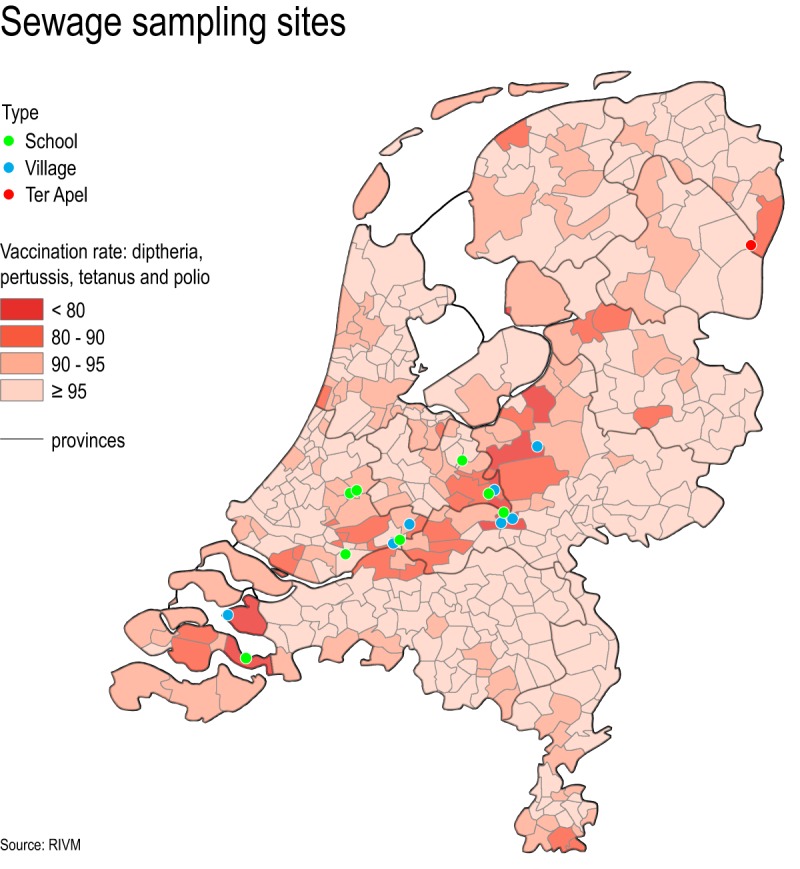
Map of the Netherlands showing the vaccination rates per community (basic immunity of birth cohort 2010 at the age of 2 years) and the sewage sampling sites. The Dutch Bible Belt stretches from the province of Zeeland in the southwest to Overijssel in the mid-northeast.
TABLE 2.
Viruses detected in EEVS sewage samples from the Bible Belt and Ter Apela
| Location | Samples analyzed (n) | Samples snPCR positive for EV after culture on RD and/or Ht-29 cells (n [%])b,c |
||
|---|---|---|---|---|
| EV | EV+EV | Total | ||
| Bible Belt | ||||
| School | 426 | 144 (33.8) | 8 (1.9) | 152 (35.7) |
| Residential area | 430 | 227 (52.8) | 48 (11.2) | 275 (64.0) |
| Total | 856 | 371 (43.4) | 56 (6.5) | 427 (49.9) |
| PRC-Ter Apel | 186 | 159 (85.5) | 9 (4.8) | 168 (90.3) |
Bible Belt samples collected September 2005 to February 2015; Ter Apel samples collected November 2013 to February 2015.
snPCR, seminested PCR as published by Nix et al. (25).
EV, one EV type detected; EV+EV, two EV types detected.
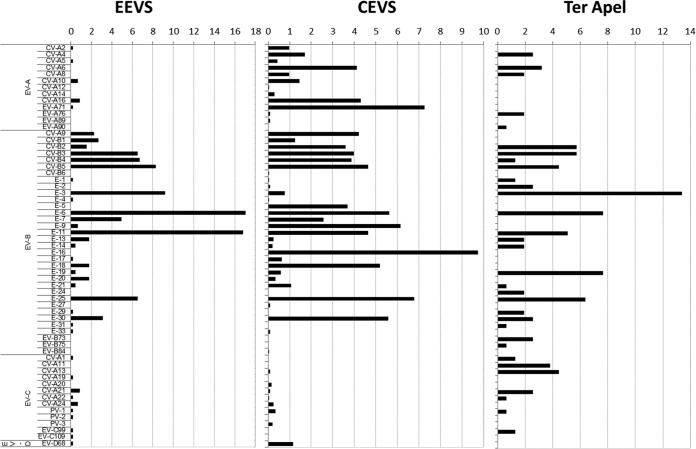
Counting both the single- and double-positive samples, 483 EVs were isolated, and 446 (92.3%) could be characterized by sequencing. Ten (2.2%) were EV cluster A (EV-A), 422 (94.6%) EV-B, 14 (2.9%) EV-C, and 1 (0.1%) EV-D (Fig. 2). The dominant NPEV types identified in the Bible Belt sewage were echovirus type 6 (E-6) (n = 76, 17%) and E-11 (n = 75, 16.8%) (Fig. 2). E-6 was frequently detected in the years 2009 and 2014 and E-11 in the years 2006-2007 and 2012 in the EEVS.
FIG 2.
Distribution of enteroviruses detected in the environmental enterovirus surveillance (EEVS) in the Dutch Bible Belt (September 2005 to February 2015), Ter Apel (November 2013 to February 2015), and the clinical enterovirus surveillance (CEVS) (January 2007 to February 2015).
Environmental enterovirus surveillance at the primary refugee center in Ter Apel.
In the period from November 2013 to February 2015, 186 sewage samples from the primary refugee center (PRC)-Ter Apel were collected and analyzed. In total, 159 (85.5%) samples were positive for one EV (Table 2), and in 9 (4.8%) samples, two different EVs were detected, bringing the total of EV isolates found in the PRC-Ter Apel sewage to 177 EV isolates. Of these isolates, 158 (88.7%) could be characterized; 16 (10.2%) were EV-A, 119 (75.8%) EV-B, and 23 (14.0%) EV-C (Fig. 2). In contrast to findings in the Bible Belt, the dominant EV type identified was E-3 (n = 21, 13.3%) (Fig. 2).
Clinical enterovirus surveillance.
For the CEVS, analysis was performed on 2,802 clinical samples sent to the National Institute for Public Health and the Environment (RIVM) between January 2007 and February 2015 for the exclusion of poliovirus and further characterization. A total of 2,190 (78.2%) samples were found positive for an EV, of which 2,064 (94.2%) could be characterized; 451 (21.8%) were EV-A, 1,565 (75.8%) EV-B, 24 (1.2%) EV-C, and 24 (1.2%) EV-D (Fig. 2). The dominant NPEV type identified in clinical samples was E-16 (n = 201, 10%).
Distribution and diversity of NPEV types.
RNA extracted from CEVS EV-positive samples and the routine EEVS EV-positive samples in the Bible Belt was sequenced and characterized. Both surveillance systems were dominated by the detection of EV-B viruses (Fig. 2). However, in CEVS, we found a significantly higher percentage (P <0.001) of EV-A viruses (21.7%) than in the Bible Belt sewage (2.2%). More specifically, EV-A viruses CV-A4, A6, A8, A12, A14, EV-A76, and A89 were found only in the CEVS and not in the Bible Belt sewage. Similarly, several EV-B viruses (CV-B6, E-2, E-5, E-16, and E-27) and EV-C viruses (CV-A13 and A20) were found only in the CEVS and not in the EEVS. In contrast, EV-B virus E-31 and the EV-C viruses CV-A1, CV-A19, EV-C99, and EV-C109 were found in the Bible Belt sewage only and not in the CEVS. Several EV-A viruses (CV-A2, A5, A10, A16, and EV-A71), all EV-B viruses with the exception of E-31, some EV-C viruses (CV-A21, A22, A24), and EV-D68 were found in both the Bible Belt sewage and the CEVS (Fig. 2).
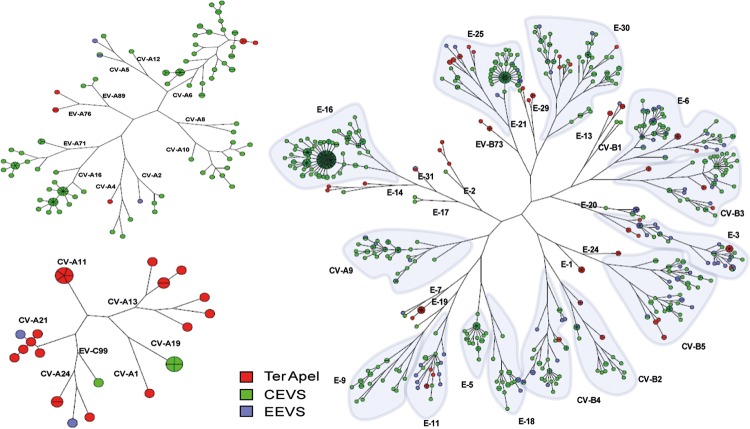
At the PRC in Ter Apel, the sewage was also dominated by EV-B strains. In the short study period at the PRC, the fraction of EV-A and -C viruses was significantly higher in the Ter Apel sewage than in the Bible Belt sewage (P <0.001). Interestingly, none of the EV-A viruses found in the Bible Belt sewage were found in Ter Apel sewage and, vice versa, none of the EV-A viruses found in Ter Apel sewage were found in the Bible Belt sewage. The EV-A viruses (CV-A4, A6, A8, and EV-A76), EV-B virus E-2, and EV-C virus CV-A13 were found in Ter Apel sewage and the CEVS but not in the Bible Belt sewage. Compared to EEVS in both the Bible Belt and Ter Apel, several EV-A viruses (CV-A12, A14, EV-A89), several EV-B viruses (CV-B6, E-5, E-16, and E-27), and the EV-C virus CV-A20 were exclusively found in CEVS, and the EV-A virus EV-A90, the EV-B viruses E-24, EV-B73, and B75, and the EV-C virus CV-A11 were found exclusively at the PRC. Several EV-B viruses (CV-B2-5, E-1, E-6, E-11-14, E-19, E-25, E-30) and EV-C viruses (CV-A21, CV-A22) could be detected in all three surveillance programs. Phylogenetic analysis shows a degree of genetic diversity, which ranged between 0.3% and the type-defining threshold of 25% (Fig. 3).
FIG 3.
Maximum parsimony tree based on partial VP1 genes (25) of EV-A (upper left), EV-B (right), and EV-C (lower left) typed in the environmental enterovirus surveillance (EEVS) in the Dutch Bible Belt (September 2005 to February 2015, blue circles), Ter Apel (November 2013 to February 2015, red circles), and the clinical enterovirus surveillance (CEVS) (January 2007 to February 2015, green circles). Note that sequences were omitted from analysis when sequence length was too short (<250 nucleotides) or only partially overlapping within the fragment length defined by the primers used (25).
Based on the partial VP1 gene sequences used for phylogenetic analysis, only for E-3 was a genetic homologue cluster of 4 identical strains (2 EEVS, 1 CEVS, 1 Ter Apel) from all three surveillance programs found (Fig. 3). The 2 EEVS samples in this cluster came from one city in the middle of the country and were collected in September 2013 and February 2014; the E-3 in this cluster from Ter Apel was collected in January 2014, and the CEVS sample was from February 2014. Based on the partial VP1 gene sequences used for phylogenetic analysis we also found several genetic homologue clusters (E-1, E-3, E-6, E-19, and CV-A11) spanning periods of 2 weeks or more (red clusters in Fig. 3) due to the frequent sampling at the PRC-Ter Apel. Identical strain detection is very uncommon in follow-up samples for the EEVS where sewage pits were sampled roughly once every 6 weeks. One genetic homologue cluster of 5 E-20 strains originated from one Bible Belt sewage pit that was sampled three times a week in that period, due to the reported release of poliovirus in Belgium (12). Four E-20 strains of this cluster were collected in October 2014 and one in January 2015. Another small cluster of 6 E-3 strains originated from 5 EEVS samples from 4 different sites in the Bible Belt collected between December 2013 and March 2014 and one CEVS sample from June 2014.
Measles virus detections.
We analyzed sewage samples collected in the Bible Belt during the measles outbreak in 2013 for measles virus RNA to validate if outbreaks can be detected and to confirm that we sample the correct target population for early poliovirus detection if PV is introduced in the Netherlands.
Sewage samples collected for poliovirus exclusion in the Bible Belt between 4 May 2013 and 3 December 2013 were analyzed by RT-PCR for the presence of measles virus.
Due to significant inhibition of the RT-PCR, samples had to be diluted 10-fold compared to the standard input concentration to obtain proper target amplification. Of the 56 samples tested, 6 (10.7%) were positive for measles virus RNA. The first measles-positive sewage sample was obtained on 18 June 2013 from a secondary school sampling site in the midwestern part of the Bible Belt, which was also one of the first regions where measles cases were reported and detected by clinical surveillance, including cases attending this specific school (data not shown and anonymized, aggregated data published by Knol et al. [10] and Woudenberg et al. [13]). The measles virus RNA detection rates are shown in Table 3. The measles viruses in this outbreak belonged to the D8 strain (strain MVs/Alblasserdam.NLD/22.13, WHO/MeaNS Id 50730, GenBank accession number KM066606) and in one sewage sample collected 9 July 2013 the measles virus (MV) could be sequenced. This strain was 100% identical, over the N-terminal 450-nucleotide fragment of the measles virus nucleocapsid gene, to the outbreak strain mentioned above and was therefore confirmed as belonging to the outbreak.
TABLE 3.
Measles virus RNA detections in concentrated sewage samples collected at (secondary) schools or residential areas during the 2013 measles epidemic in the Dutch Bible Belt
| 2013 sampling date | No. positive/no. tested |
Reported no. of cases during measles epidemic in Bible Belt (2013 period)a | |
|---|---|---|---|
| Schools | Residential areas | ||
| May 4 | 0/1 | 0/0 | 0 (May 1–7) |
| Jun 18 | 1/2 | 0/4 | 69 (Jun 17–23) |
| Jul 9 | 0/2 | 2/5b | 180 (Jul 8–14) |
| Jul 30 | 0/0 | 2/6 | 124 (Jul 29–Aug 4) |
| Sep 10 | 0/4 | 0/2 | 108 (Sep 9–15) |
| Sep 24 | 0/2 | 0/4 | 93 (Sep 23–29) |
| Oct 8 | 0/4 | 0/2 | 113 (Oct 7–13) |
| Nov 5 | 0/4 | 0/2 | 50 (Nov 4–10) |
| Nov 19 | 0/3 | 1/3 | 33 (Nov 18–24) |
| Dec 3 | 0/3 | 0/3 | 31 (Dec 2–8) |
| Totals | 1/25 | 5/31 | |
Data from Woudenberg et al. (13).
One of these samples was sequenced and confirmed as belonging to the D8 outbreak strain.
Detection limits of poliovirus and measles virus.
The analytical detection limits in clean water were estimated in a spiking experiment at approximately 500 RT-PCR detectable units (RT-PCRDU)/liter for poliovirus and at 50 to 500 RT-PCRDU/liter for measles virus. For spiked sewage samples the detection limits were 103 to 104 RT-PCRDU/liter for measles virus, <5 × 103 RT-PCRDU/liter for poliovirus, and 25 50% cell culture infectious doses (CCID50)/liter for culture for poliovirus.
These analytical detection limits—the viruses shed per person and the water volumes discharged per day, assuming complete mixing in the sewage system—result in the detectability of 1 average poliovirus-shedding person per approximately 103 persons in residential settings and 1 in approximately 7 × 103 persons at schools (assuming 1 bolus per day, excreted at school). For measles virus, these rates would be 1 measles virus shedder in 5 × 102 to 5 × 103 persons in residential settings and schools.
DISCUSSION
This study describes a comparison between different EV surveillance systems in the Netherlands and detection of measles viruses in sewage samples during a well-documented measles outbreak between May 2013 and March 2014 (10, 13). In the absence of AFP surveillance in the Netherlands, environmental EV surveillance in the Dutch Bible Belt and nationwide clinical EV surveillance are the two pillars of the surveillance activities to prove the absence of poliovirus circulation in the country.
Poliovirus.
In the period studied, we detected PV SL strains in 2 Bible Belt sewage samples, 1 PRC-Ter Apel sewage sample, and 11 clinical samples. The great similarity of the strains detected in this study to the reference Sabin strain and the lack of secondary detections of these strains suggest that transmission of the OPV strains was absent or very limited in the Netherlands in the period studied. Poliovirus detection in a sewage sample was never preceded or followed by a clinical detection or secondary poliovirus detection in the same sewage 3 to 6 weeks later. The populations covered by the sewage sampling sites in our surveillance were small relative to the detection limits; within the current system of EEVS, we are likely to detect single poliovirus shedders per sampling site and characterize the virus within 7 to 10 days. The current schedule of sampling each site approximately once every 6 weeks would result in a time to detection of clinically unnoticed poliovirus transmission in the Bible Belt at an estimated average of less than 50 days. Bencsko and Ferenci estimated the time to detection of an AFP case based on AFP surveillance at approximately 500 days after poliovirus introduction in a high vaccine coverage–high IPV use country such as the Netherlands (14). Since silent transmission of PV can be severe in our country, the number of infected persons (not ill) can exceed 1,000 before detection by AFP surveillance. We show that in our setup the detection of poliovirus by environmental surveillance will curtail the time to detection and the number of infected persons dramatically, as was suggested for OPV types 1 and 3 in the Dutch population (15) and for wild poliovirus type 1 spread in Israel (16). This clearly supports the aim of WHO for the expansion of environmental surveillance as a tool to strengthen polio surveillance during the endgame of poliovirus eradication (17).
Since EEVS is anonymous, it is not clear who shed the virus. With a generation time of 10 days for poliovirus transmission, and the small populations per sampling site, the current setup allows for a focused primary response if a wild-type poliovirus or VDPV is detected by environmental surveillance.
We detected only one PV1 SL in PRC-Ter Apel sewage samples. The PRC samples were collected 3 times per week, and considering the detection limit and population size at the PRC, we estimated detection of single shedders at the average level of shedding (2.3 × 104 CCID50 per g in a seronegative population [15]). Lower levels of shedding indeed result in reduced likelihood of detection and in reduced likelihood of continued transmission. The WPV1 strain that caused 36 confirmed polio cases in Syria in 2013-2014 was not detected in the Netherlands (this paper) or in Germany (18).
Nonpolio enterovirus.
The data from routine EEVS and CEVS were used to investigate the value of routine EEVS complementary to CEVS for poliovirus exclusion and EV surveillance. In addition, data collected from the Ter Apel sewage were analyzed to investigate the circulation of NPEV strains originating primarily through importation from Syria, Iran, and Iraq.
Even though the CEVS and the routine EEVS were dominated by detection of EV-B viruses, the EV species distribution is clearly different for both types of surveillance. EV-A strains were detected 10-fold more often in the CEVS than in the EEVS, and EV-C viruses were detected slightly more often than EV-A viruses in Bible Belt sewage, while being detected in only 2% of CEVS samples. The difference in detection could be related to the methodology used, as sewage samples are assayed through culture and CEVS samples primarily through molecular methods, and analyses of sewage data will reflect only cultivable types. However, types such as CV-A4, 6, 8, 12-14, and E-16, which were found only in CEVS and not in the Bible Belt sewage, were previously found to grow well in culture (19, 20). The fact that most of these types were also found in Ter Apel sewage confirms their cultivability and detection through EEVS. For types such as E-5 and E-16, the difference could be related to the targeted age group at risk (<4 years old [2]), of which the youngest are largely still in diapers, and therefore these NPEVs are less likely to be detected in sewage samples. On the other hand, several types exclusively found in CEVS (e.g., CV-A12 and A20) can be a reflection of low circulation frequency (1, 2) or imported cases. In contrast to types found exclusively in CEVS, types exclusively found in EEVS (e.g., CV-A1 and A19) suggest, for example, silent circulation due to low pathogenicity, as characterized by a low case-to-infection rate, as is the case for poliovirus (21). Furthermore, as the Bible Belt community is considered quite isolated, we should consider that CEVS types detected across the country may rarely be transmitted to the Bible Belt community (and vice versa) and therefore are not seen in the Bible Belt sewage surveillance.
The EEVS in the Bible Belt and Ter Apel show a high diversity of NPEV types in the sewage, similar to previous reports about sewage waters (22). In the Bible Belt and Ter Apel surveillance EV-B strains prevailed, and in the Ter Apel sewage EV-C viruses were detected relatively frequently. Several types found in Ter Apel (e.g., EV-A90 and CV-A11) were never found in Bible Belt sewage or in the CEVS in over 20 years or were rarely found (e.g., E-24 was found only 4 times in CEVS since 1996 [1]). The PRC population originated mostly from Syria, Iran, and Iraq, and the viruses found most likely originate from that region or were contracted during the travels of this population through Asia and Europe. However, Bottcher et al. did not detect any of these Ter Apel-specific types in Syrian refugees. Vice versa, several types found in Syrian refugees in Germany were not detected in the Ter Apel sewage (18). While Bottcher et al. screened stool samples from all ages, our study was restricted to sewage and the difference could be related to diaper usage as mentioned above.
The several clusters detected in the Ter Apel surveillance most likely indicate that the transmission of these viruses occurred within the PRC, since most refugees stayed in the PRC for only 3 to 4 days at that time and, for example, the E-19 cluster spans a period of over 4 weeks. These findings show that directed frequent sampling for environmental surveillance may be a functional approach for outbreak detection in defined settings.
Measles: a proxy for assessing the value of the EEVS in the Bible Belt.
By the detection of measles virus RNA in several sewage samples and the ability to confirm the presence of the outbreak strain in one of these samples, we showed that indeed measles virus was detectable by environmental surveillance during an outbreak of measles in the Dutch Bible Belt. Moreover, the locations where measles virus RNA was detected in sewage samples corresponded to the locations where cases were reported by routine clinical surveillance. Since this measles outbreak was confined to the nonvaccinated orthodox Protestant community, as was the polio outbreak in 1992-1993, we consider this a confirmation that the correct target population for poliovirus circulation exclusion is being sampled in our EEVS system.
More often, MV was not detected in sewage samples, whereas measles cases were reported from around the sampling sites around the moment of sampling. For school sites this may be explained by the short prodromal phase during which pupils shed virus in urine followed by absence from school after this phase because of measles illness, thus limiting the likelihood of virus detection in the school sewage samples. Additionally, most likely only the younger (11- to 15-year-old) pupils at the schools were susceptible to MV infection, since those older than 15 years were mostly immune as a result of previous measles encounters (23). Sick pupils staying at home would consequently increase the shedding of MV into the sewage sampling sites in residential settings. The low detection rate in these samples does not conform to expectations based on the measles epidemic description and the estimated detection limits. The low number of MV detections may result from the infrequent sampling and reduced detectable viral load due to lower or less sustained amounts of measles viral RNA shed via urine in the environment compared to the shedding of poliovirus RNA in feces from single patients. We also assume that the enveloped measles virus is less stable in the environment than the nonenveloped poliovirus and therefore less likely to be detected by environmental sampling.
In conclusion, EEVS is suitable for poliovirus detection in sewage in the Netherlands where poliovirus circulation is absent and complements clinical enterovirus surveillance. Several PV SL strains were detected throughout the years but continued transmission was never found. In addition, EEVS led to the detection of additional NPEV types not seen in the CEVS, which shows a high frequency of different NPEV types circulating in the Netherlands. Detection of several NPEVs and MV in EEVS was shown to be concomitant with clinical detection, enabling more specific analysis of the extent of the circulation frequency among the population. Currently, data from our routine environmental surveillance are included in a VIRO-TypeNed database (2, 24), enabling analysis of clinical and sewage data in parallel for better monitoring of outbreaks as well as monitoring of (asymptomatic) strains frequently found in sewage that may evolve to cause severe disease consequently appearing in the clinic.
MATERIALS AND METHODS
Sampling and sampling sites in the Bible Belt and primary refugee center.
Between September 2005 and February 2015, routine EEVS sampling sites were located in communities with relatively low vaccination rates. These sites contained sewage originating from a secondary school or a residential district (with or without primary school), were at least once positive for poliovirus type 3 during the Dutch 1992-1993 epidemic, and had sewage pits that were accessible for regular sampling. Sampling sites are indicated on the map in Fig. 1, which also shows the vaccination coverage of the community's population.
From 2010 to October 2014 the sewage sampling covered approximately 104 secondary school students (aged 11 to 19 years), 800 primary school children (aged 4 to 12 years), and 2 × 103 persons in residential areas. Following the reporting of an accidental poliovirus release by GSK Belgium (12), 2 sampling sites in the southwestern province of Zeeland (1 secondary school with around 500 students, 1 residential area with a population of 2 × 103 persons) were added to the routine surveillance in October 2014.
The eight sampling sites at secondary schools (500 to 3,000 pupils per school) covered a population of approximately 104, mostly nonvaccinated, pupils aged 11 to 19 years. The residential sampling sites covered 100 to 1,800 persons per pit and a total population of approximately 4 × 103 persons, of which over 50% were vaccinated. Overall, an estimated 1 to 5% of the orthodox-Reformed population living in the Bible Belt is sampled once every 6 weeks.
Grab samples of 1 liter were collected manually from sewage pits and transported to the RIVM the same day. The samples were collected from school sites on Tuesday mornings but not during holidays. The samples were stored at 2 to 6°C until concentration was started, usually the next day.
The sewage grab samples at the PRC-Ter Apel were collected every Monday, Wednesday, and Friday starting 15 November 2013 and continued to 10 April 2015. The PRC sheltered a maximum of 1,200 refugees up to June 2014, when its capacity was increased to a maximum of 1,800. In the period from November 2013 to February 2015, the daily inflow of refugees varied between 70 and 300 persons. Overall, 54% of the refugees sheltered in 2014 in Ter Apel came from Syria, Iran, and Iraq, and 3% came from Afghanistan and Pakistan (Agata Hinc, personal communication, 2014).
Sample concentration.
The 1-liter sewage samples were first centrifuged for 10 min at 3,000 rpm (1,800 × g) to remove solids from the water phase. The pellet was extracted with 10% (vol/vol) chloroform and centrifuged for 10 min at 3,000 rpm (1,800 × g), the water phase was added to the cleared sewage sample, and the chloroform phase was discarded. The first step was not performed if the samples appeared unclogged. Subsequently, the (cleared) sewage sample (>950 ml) was concentrated to a volume of 1 to 3 ml by ultrafiltration using Amicon ultrafiltration membranes PM10 in Amicon stirred ultrafiltration cells at 50 to 75 lb/in2 pressure and 4°C. When the target volume of 1 to 3 ml was reached, the pressure was released, and the membrane was rinsed with a 2-ml pipette to resuspend the viruses. The concentrated fraction was collected in 15-ml tubes.
Enterovirus culture of sewage samples.
About 3 ml of the concentrate was extracted with 25% (vol/vol) chloroform and centrifuged for 10 min at 3,000 rpm (1,800 × g). The water phase was collected and used for inoculation immediately or stored at −20°C until further analysis. For the standard EV surveillance for poliovirus exclusion, a 9 × 100-μl concentrated sewage suspension was inoculated on 3- to 7-day-old cells (3× L20B, 3× Rd, and 3× Ht29 [Ht29 cells replaced the HEp2 cells in 2012]) in roller tubes in a total volume of 1 ml. The cells were incubated at 37°C, either rotating at 3 rpm (RD and Ht29) or stationary (L20B). The cytopathic effect (CPE) was monitored by light microscopy every working day for 7 days. If CPE was complete, the tubes were frozen and thawed twice, and the suspensions were used for RNA extraction. If the concentrated sewage suspension proved cytotoxic, 100 μl of the first-passage culture suspension was inoculated on fresh cells for a second passage. If no CPE was found in any of the cultures, the concentrated sewage samples were considered negative for infectious enterovirus and consequently also for infectious poliovirus. In 2014, all cultures, with or without CPE, progressed to further analysis by RT-PCR to determine if enteroviruses that did replicate but did not cause CPE could be detected.
Clinical enterovirus surveillance (CEVS).
The clinical EV surveillance was performed as described by van der Sanden et al. (1). In short, clinical samples, mostly stools, diagnosed as positive for an EV infection by 5′ untranslated region (UTR) RT-PCR by Dutch virology laboratories were sent to the RIVM for exclusion of poliovirus and/or further characterization of nonpolio enteroviruses. Samples collected between January 2007 and February 2015 were analyzed for this study.
Molecular detection and typing of enterovirus.
RNA was isolated from CPE-positive sewage cultures and 5′ UTR RT-PCR-positive clinical samples by automated extraction using the LC Nucleic Acid isolation kit (MagnaPure96, Roche). RNA was eluted in 50 μl elution buffer and amplified in the seminested RT-PCR described by Nix et al. (25). The sensitivity of this RT-PCR, defined as the equivalent of the lowest dose of cultured infectious virus detected by this RT-PCR, was 0.252 50% tissue culture infective dose (TCID50) for EV-A71, 0.126 TCID50 for coxsackievirus B3, 0.69 TCID50 for PV Sabin 1, 100 TCID50 for coxsackievirus A24, and 0.002 TCID50 for EV-D68. The 350 to 400-bp fragments of the VP1 gene were purified using ExoSAP-IT and sequenced at Baseclear (Leiden). The partial VP1 sequences were edited using BioNumerics version 7.1 and used as input in the EV genotyping tool (see http://www.rivm.nl/mpf/enterovirus/typingtool/), which has an automated algorithm to assign the species and (sub)type of the sequences entered (26). For characterization of the full VP1 gene of poliovirus, eluted RNA was amplified as described in reference 27.
Phylogeny.
Phylogenetic trees were constructed using the maximum parsimony method of BioNumerics version 7.1 on partial VP1 gene sequences collected since 2007. Sequences were omitted from analysis when sequence length was too short (<250 nucleotides) or only partially overlapping within the fragment length defined by the primers used (25). Genetic diversity and clustering of viruses were based on pairwise distribution calculated by BioNumerics version 7.1. Strains with 0.3% diversity were considered genetic homologues when clustered together in a single sphere.
Measles virus: molecular virus detection and typing.
RNA was isolated from concentrated sewage samples by automated extraction using the LC Nucleic Acid isolation kit (MagNA Pure96, Roche). RNA was eluted in 50 μl elution buffer, and detection of the measles virus RNA was performed by real-time RT-PCR in TaqMan format, using primer pair N1F (CGATGACCCTGACGTTAGCA) and N1R (GCGAAGGTAAGGCCAGATTG), as previously described (28). Hybridization was carried out with a specific probe (FaM-GGCTGTTAGAGGTTGTCCAGAGTGACCAG-BHQ1). The sensitivity of this RT-PCR, defined as the equivalent of the lowest dose of infectious virus strain MV-BIL cultured on human cells and detected by this RT-PCR, was 0.01 TCID50 in cell culture suspensions (11). For analysis of concentrated sewage samples a 10-fold dilution was required, resulting in a sensitivity of 0.1 TCID50.
Specific sequences were generated from RT-PCR-positive samples using primers amplifying the N-terminal 450-nucleotide fragment of the measles virus nucleocapsid gene according to procedures for genotyping as approved by WHO and CDC (29). The generated sequences were compared with the sequences derived from the clinical specimens of confirmed measles cases and with the consensus sequences representing the different genotypes of measles virus (29).
Detection limits.
For determination of the analytical detection limits, 1-liter water samples were spiked in duplicate with 500, 5,000, or 50,000 RT-PCRDU of PV1 Sabin and measles virus MV-BIL, and 2 different sewage samples were spiked in duplicate with 103, 104, or 105 measles virus and 5 × 103, 5 × 104, or 5 × 105 polioviruses. Samples were concentrated and analyzed by RT-PCR and culture in duplicate.
For the estimation of virus detection in sewage samples we used the following data: water discharge per person per day, 125 liters at home and 20 liters at school; urine excretion per person per day, 1,250 ml at home and 250 ml at school; average fecal excretion per person per day, 128 g; MV excretion, 5 × 105 RT-PCRDU/ml urine; and poliovirus excretion, 2.3 × 104 CCID50 per g (seronegative population [15]).
Statistics.
Statistical analyses were performed using the univariate chi-square test. A two-sided P value of <0.01 was considered statistically significant.
Accession number(s).
All sequences used in our analysis were deposited in GenBank; the accession numbers for NPEV partial sequences are KY865753 to KY866664 and for PV sequences full length VP1 and partial are KY884680 to KY884693.
ACKNOWLEDGMENTS
We thank the laboratory staff of the RIVM (Ron Altena, Dani Atto, Gokhan Uslu, Annemarie van den Brandt, Bas van den Veer, Lisa Wijsman, Daphne Gijselaar, Sharon van den Brink, and Jeroen Cremer) for screening and typing all of the samples. We thank all schools and communities for their willingness to participate in the routine environmental surveillance and Jan van der Have (Public Health Services Groningen) and the board of the PRC-Ter Apel for facilitating the environmental surveillance at their premises and providing quantitative data on their population. We also thank the virologists participating in the EV clinical surveillance for collecting and providing EV-positive samples, Henriette Giesbers for preparing Fig. 1, and Helma Ruijs for detailed information on vaccination levels in the Bible Belt population.
REFERENCES
- 1.van der Sanden SM, Koopmans MP, van der Avoort HG. 2013. Detection of human enteroviruses and parechoviruses as part of the national enterovirus surveillance in the Netherlands, 1996-2011. Eur J Clin Microbiol Infect Dis 32:1525–1531. doi: 10.1007/s10096-013-1906-9. [DOI] [PubMed] [Google Scholar]
- 2.Benschop KS, Rahamat-Langendoen JC, van der Avoort HG, Claas EC, Pas SD, Schuurman R, Verweij JJ, Wolthers KC, Niesters HG, Koopmans MP, VIRO-TypeNed . 2016. VIRO-TypeNed, systematic molecular surveillance of enteroviruses in the Netherlands between 2010 and 2014. Euro Surveill 21 (39):pii=30352 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hovi T, Shulman LM, van der Avoort H, Deshpande J, Roivainen M, De Gourville EM. 2012. Role of environmental poliovirus surveillance in global polio eradication and beyond. Epidemiol Infect 140:1–13. doi: 10.1017/S095026881000316X. [DOI] [PubMed] [Google Scholar]
- 4.Ruijs WL, Hautvast JL, van Ansem WJ, Akkermans RP, van't Spijker K, Hulscher ME, van der Velden K. 2012. Measuring vaccination coverage in a hard to reach minority. Eur J Public Health 22:359–364. doi: 10.1093/eurpub/ckr081. [DOI] [PubMed] [Google Scholar]
- 5.Mulders MN, van Loon AM, van der Avoort HG, Reimerink JH, Ras A, Bestebroer TM, Drebot MA, Kew OM, Koopmans MP. 1995. Molecular characterization of a wild poliovirus type 3 epidemic in the Netherlands (1992 and 1993). J Clin Microbiol 33:3252–3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Avoort HG, Reimerink JH, Ras A, Mulders MN, van Loon AM. 1995. Isolation of epidemic poliovirus from sewage during the 1992-3 type 3 outbreak in the Netherlands. Epidemiol Infect 114:481–491. doi: 10.1017/S0950268800052195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander JP, Ehresmann K, Seward J, Wax G, Harriman K, Fuller S, Cebelinski EA, Chen Q, Jorba J, Kew OM, Pallansch MA, Oberste MS, Schleiss M, Davis JP, Warshawsky B, Squires S, Hull HF, Vaccine-Derived Poliovirus Investigations Group . 2009. Transmission of imported vaccine-derived poliovirus in an undervaccinated community in Minnesota. J Infect Dis 199:391–397. doi: 10.1086/596052. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 2006. Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 countries, 2002–2005. MMWR Morb Mortal Wkly Rep 55:145–150. [PubMed] [Google Scholar]
- 9.Eichner M, Brockmann SO. 2013. Polio emergence in Syria and Israel endangers Europe. Lancet 382:1777. doi: 10.1016/S0140-6736(13)62220-5. [DOI] [PubMed] [Google Scholar]
- 10.Knol M, Urbanus A, Swart E, Mollema L, Ruijs W, van Binnendijk R, Te Wierik M, de Melker H, Timen A, Hahne S. 2013. Large ongoing measles outbreak in a religious community in the Netherlands since May 2013. Euro Surveill 18:pii=20580 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20580. [DOI] [PubMed] [Google Scholar]
- 11.van Binnendijk RS, van den Hof S, van den Kerkhof H, Kohl RH, Woonink F, Berbers GA, Conyn-van Spaendonck MA, Kimman TG. 2003. Evaluation of serological and virological tests in the diagnosis of clinical and subclinical measles virus infections during an outbreak of measles in the Netherlands. J Infect Dis 188:898–903. doi: 10.1086/377103. [DOI] [PubMed] [Google Scholar]
- 12.Duizer E, Rutjes S, de Roda Husman AM, Schijven J. 2016. Risk assessment, risk management and risk-based monitoring following a reported accidental release of poliovirus in Belgium, September to November 2014. Euro Surveill 21:30169 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=21414. [DOI] [PubMed] [Google Scholar]
- 13.Woudenberg T, van Binnendijk RS, Sanders EA, Wallinga J, de Melker HE, Ruijs WL, Hahne SJ. 2017. Large measles epidemic in the Netherlands, May 2013 to March 2014: changing epidemiology. Euro Surveill 22(3). pii: 30443 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bencsko G, Ferenci T. 2016. Effective case/infection ratio of poliomyelitis in vaccinated populations. Epidemiol Infect 144:1933–1942. doi: 10.1017/S0950268816000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lodder WJ, Buisman AM, Rutjes SA, Heijne JC, Teunis PF, de Roda Husman AM. 2012. Feasibility of quantitative environmental surveillance in poliovirus eradication strategies. Appl Environ Microbiol 78:3800–3805. doi: 10.1128/AEM.07972-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anis E, Kopel E, Singer SR, Kaliner E, Moerman L, Moran-Gilad J, Sofer D, Manor Y, Shulman LM, Mendelson E, Gdalevich M, Lev B, Gamzu R, Grotto I. 2013. Insidious reintroduction of wild poliovirus into Israel, 2013. Euro Surveill 18(49):pii/20651 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20586. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 2015. Polio environmental surveillance expansion plan: global expansion plan under the endgame strategy 2013–2018. http://polioeradication.org/wp-content/uploads/2016/07/GPLN_ExpansionPlanES.pdf. Accessed 7 February 2017
- 18.Bottcher S, Neubauer K, Baillot A, Rieder G, Adam M, Diedrich S. 2015. Stool screening of Syrian refugees and asylum seekers in Germany, 2013/2014: identification of Sabin like polioviruses. Int J Med Microbiol 305:601–606. doi: 10.1016/j.ijmm.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Benschop K, Minnaar R, Koen G, van Eijk H, Dijkman K, Westerhuis B, Molenkamp R, Wolthers K. 2010. Detection of human enterovirus and human parechovirus (HPeV) genotypes from clinical stool samples: polymerase chain reaction and direct molecular typing, culture characteristics, and serotyping. Diagn Microbiol Infect Dis 68:166–173. doi: 10.1016/j.diagmicrobio.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Duizer E, Benschop K, Uslu G, Jusic E, Schalk M, Koen G, van Eijk H, de Haan K, van der Sanden S, van der Avoort H, Koopmans M, Wolthers K. 2013. Verleden, heden en toekomst van de enterovirus en parechovirusdiagnostiek en surveillance in Nederland. Ned Tijdschr Med Microbiol 21:48–55. [Google Scholar]
- 21.Mueller S, Wimmer E, Cello J. 2005. Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event. Virus Res 111:175–193. doi: 10.1016/j.virusres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Harvala H, Calvert J, Van Nguyen D, Clasper L, Gadsby N, Molyneaux P, Templeton K, McWilliams Leitch C, Simmonds P. 2014. Comparison of diagnostic clinical samples and environmental sampling for enterovirus and parechovirus surveillance in Scotland, 2010 to 2012. Euro Surveill 19 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20772. [DOI] [PubMed] [Google Scholar]
- 23.van den Hof S, Meffre CM, Conyn-van Spaendonck MA, Woonink F, de Melker HE, van Binnendijk RS. 2001. Measles outbreak in a community with very low vaccine coverage, the Netherlands. Emerg Infect Dis 7(3 Suppl):593–597. doi: 10.3201/eid0707.010743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niesters HG, Rossen JW, van der Avoort H, Baas D, Benschop K, Claas EC, Kroneman A, van Maarseveen N, Pas S, van Pelt W, Rahamat-Langendoen JC, Schuurman R, Vennema H, Verhoef L, Wolthers K, Koopmans M. 2013. Laboratory-based surveillance in the molecular era: the TYPENED model, a joint data-sharing platform for clinical and public health laboratories. Euro Surveill 18:20387 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20387. [DOI] [PubMed] [Google Scholar]
- 25.Nix WA, Oberste MS, Pallansch MA. 2006. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol 44:2698–2704. doi: 10.1128/JCM.00542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kroneman A, Vennema H, Deforche K, van der Avoort H, Penaranda S, Oberste MS, Vinje J, Koopmans M. 2011. An automated genotyping tool for enteroviruses and noroviruses. J Clin Virol 51:121–125. doi: 10.1016/j.jcv.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2006. Polio Lab Network quarterly update. New test algorithm to be implemented in 2007. Vol XXXIII Vaccines and Biologicals, Global Poliomyelitis Eradication Initiative, World Health Organization, Geneva, Switzerland: http://polioeradication.org/polio-today/polio-now/surveillance-indicators/the-global-polio-laboratory-network-gpln/. [Google Scholar]
- 28.Hummel KB, Lowe L, Bellini WJ, Rota PA. 2006. Development of quantitative gene-specific real-time RT-PCR assays for the detection of measles virus in clinical specimens. J Virol Methods 132:166–173. doi: 10.1016/j.jviromet.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Bankamp B, Byrd-Leotis LA, Lopareva EN, Woo GK, Liu C, Jee Y, Ahmed H, Lim WW, Ramamurty N, Mulders MN, Featherstone D, Bellini WJ, Rota PA. 2013. Improving molecular tools for global surveillance of measles virus. J Clin Virol 58:176–182. doi: 10.1016/j.jcv.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]